Liquid formulation of Anti-tnf alpha antibody
A liquid preparation and antibody technology, applied in the direction of antibodies, antibody medical components, medical preparations with non-active ingredients, etc., can solve the problems of difficult drug application and viscosity production for patients, reduce aggregation and particle formation, and improve patient convenience. sex, pain relief
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
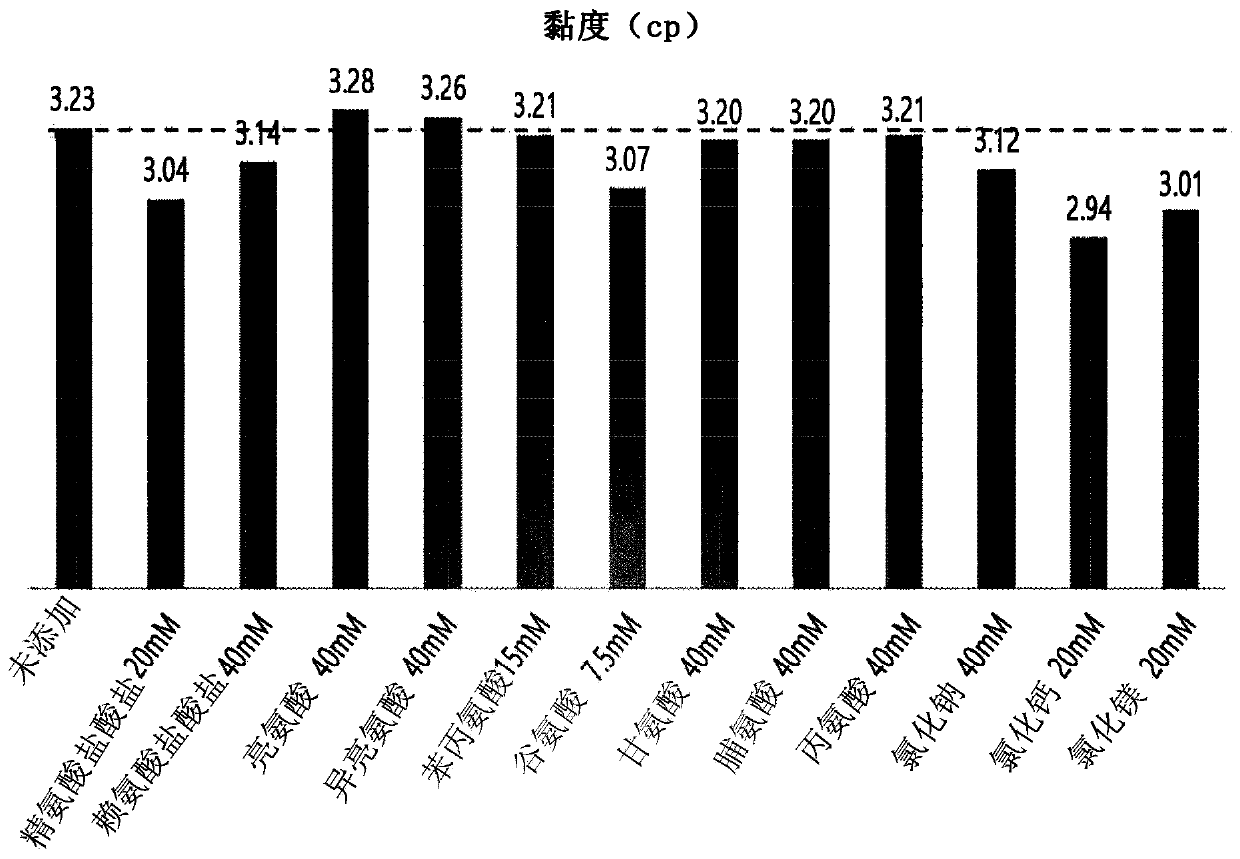
[0071] Confirmation of the effect and stability of reducing the viscosity of adalimumab solution by additives
[0072] To identify the additives to be used in the preparation of the liquid formulation of adalimumab, a pH 5.2 composition consisting of sucrose (55 mg / mL), methionine (5 mM), polysorbate 80 (1 mg / mL) and adalimumab was prepared Formulation 1 of mAb (100 mg / mL).
[0073] In addition, by further adding arginine hydrochloride, lysine hydrochloride, leucine, isoleucine, phenylalanine, glutamic acid, glycine, proline, alanine to the composition of formulation 1, Formulations 2 to 13 were prepared for each of sodium chloride, calcium chloride, and magnesium chloride, and the viscosity of each formulation was measured using an mVROC device (Rheosense Inc.). The types and concentrations of additives added to the formulations and the viscosity of each formulation are shown in Table 1 below and figure 1 middle.
[0074] [Table 1]
[0075] Preparation number ...
Embodiment 2
[0085] Stability assessment based on arginine hydrochloride content1
[0086] In order to evaluate the stability of adalimumab formulations according to the content of arginine hydrochloride, the following formulations were prepared.
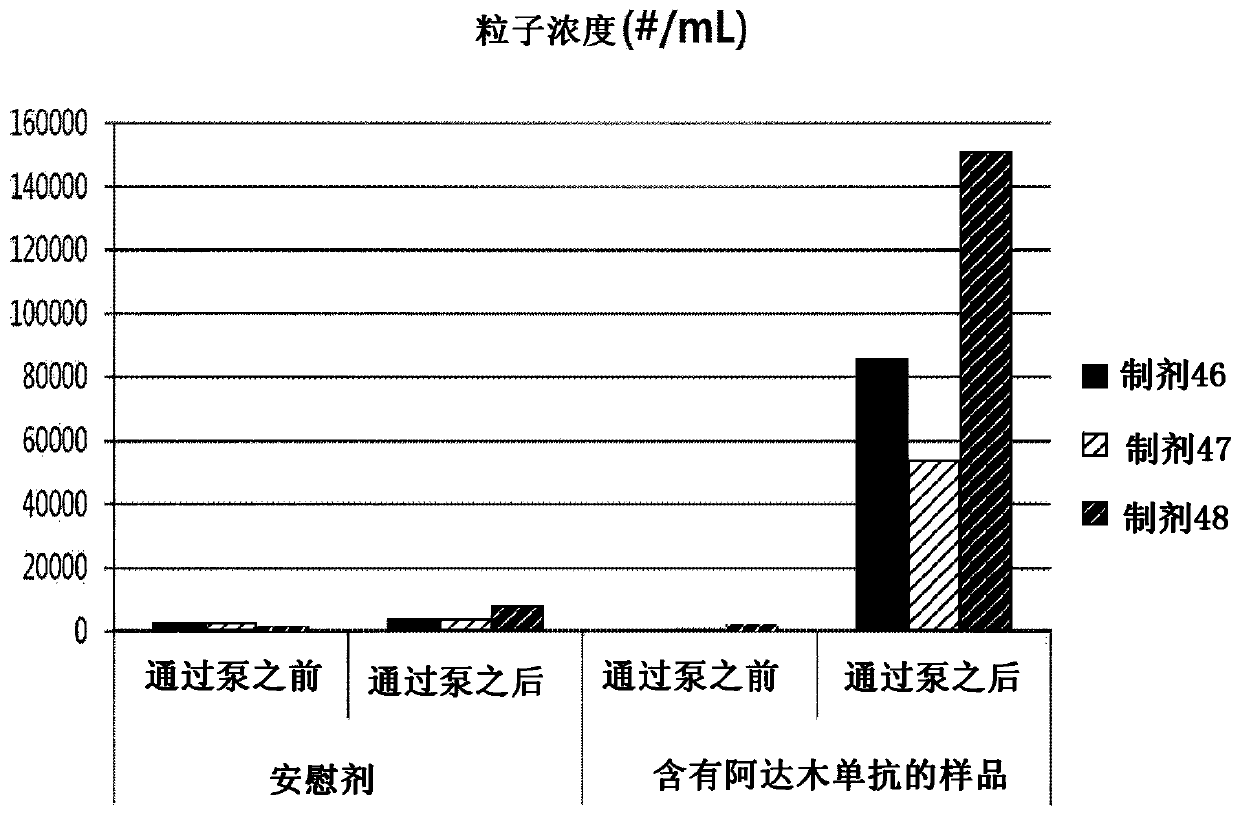
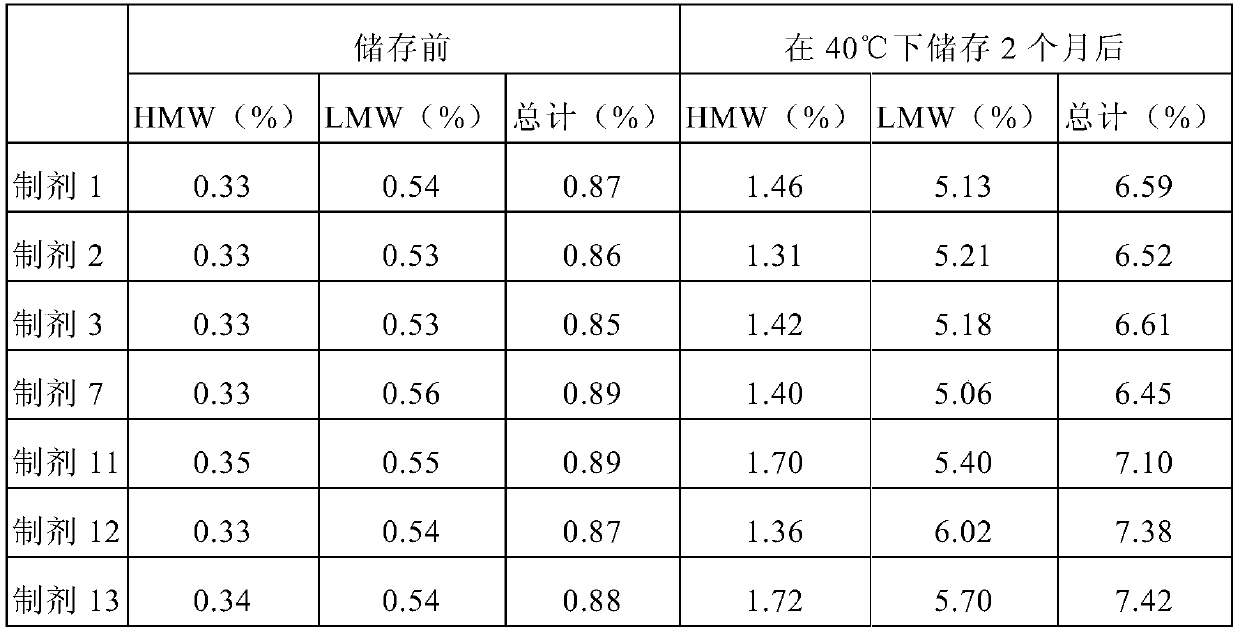
[0087] Formulation 14 was prepared to contain sucrose (55 mg / mL), methionine (5 mM), polysorbate 80 (1 mg / mL) and adalimumab (100 mg / mL). Formulations 15 and 16 were prepared by adding arginine hydrochloride (20 mM) and arginine hydrochloride (40 mM) to the composition of formulation 14, respectively, and filling 0.4 mL into 1 mL glass syringes, respectively. Each syringe was stored at 40°C for 2 months and then subjected to SE-HPLC analysis for stability analysis. The composition of each formulation and the HMW contained therein before and after storage are shown in Table 4 below.
[0088] [Table 4]
[0089]
[0090] With reference to the SE-HPLC results in Table 4, when the content of arginine hydrochloride was increased from 0 mM (prepa...
Embodiment 3
[0092] Comparison of the formation of charged variants of antibodies according to arginine content
[0093] To compare the level of formation of charged variants of the antibody according to arginine content, formulations containing arginine and formulations without arginine were prepared, stored at 40°C for 1 month, and compared by CEX-HPLC. Characteristics of charged variants. The content of charged variants before and after each formulation and storage is shown in Table 5 below.
[0094] [table 5]
[0095]
[0096] Formulation A was prepared to contain sucrose (55mg / mL), methionine (5mM), polysorbate 80 (PS80: 1mg / mL) and adalimumab (100mg / mL), and formulation B was prepared to contain The composition of A also contained arginine hydrochloride (20 mM). The content of the acidic variant was similar for both formulations prior to storage at 40°C for 1 month. After comparing the two formulations after storage, it was confirmed that formulation B containing arginine hydr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com