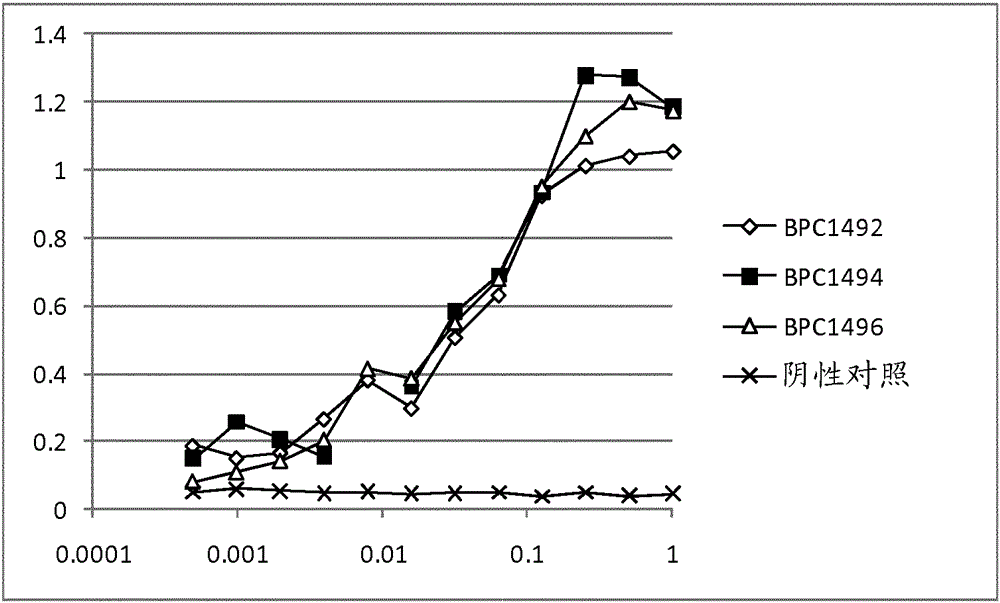
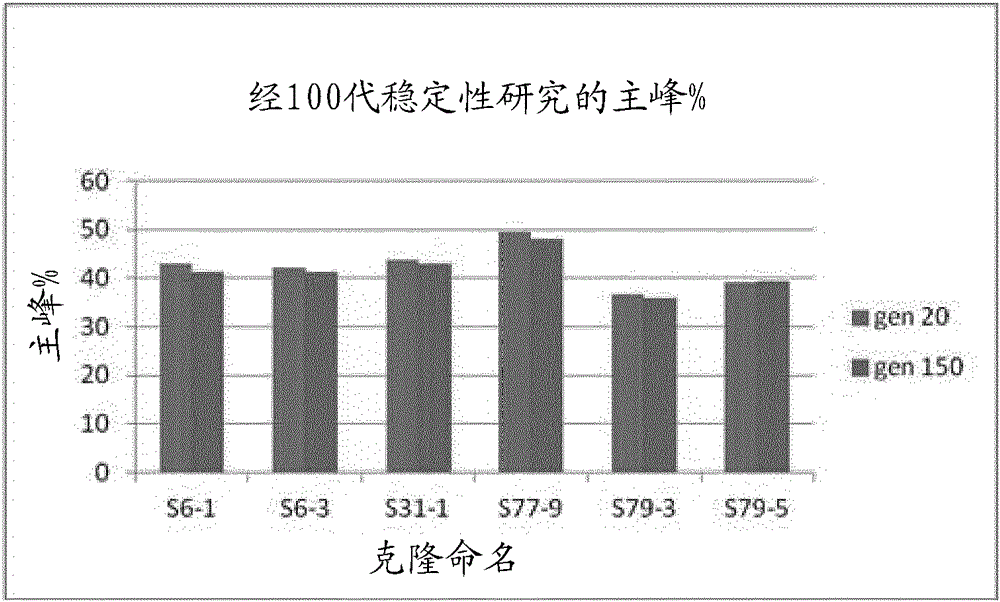
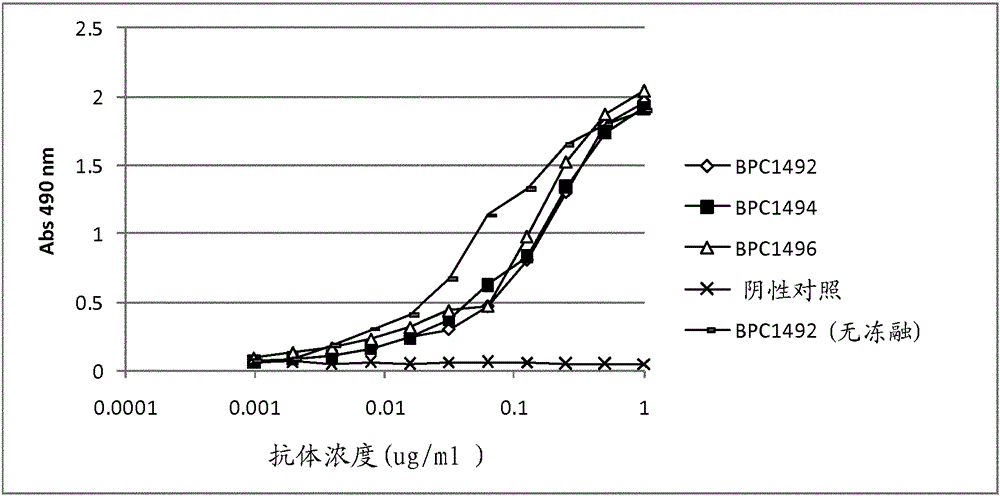
Tnf-alpha antigen-binding proteins
A protein and antigen binding technology, applied in the direction of anti-animal/human immunoglobulin, immunoglobulin, anti-cytokine/lymphokine/interferon immunoglobulin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0161] Example 1: Cloning of antibody expression vector
[0162] DNA expression constructs encoding the variable heavy (VH) and variable light (VL) domains of the anti-TNFα antibody were previously prepared de novo and included restriction sites for cloning into mammalian expression vectors. Both the heavy and light chain variable region sequences were sequence optimized for expression in mammalian cells (for methodology see WO2009024567 and Kotsopoulou et al. J Biotechnol (2010) 146:186-193). Information describing the heavy and light chain variable region sequences can be found in US Pat. No. 6,090,382. To generate the constructs used in this study, variable heavy domain (VH) sequences were amplified using PCR. PCR primers contain HindIII and A Spel restriction site to form the VH domain framework containing the signal sequence for cloning into the pTT mammalian expression vector containing the human γ1 constant region. Similarly, the VL domain sequence was amplified b...
Embodiment 2
[0164] Example 2: Engineering Transformation of the Fc Region
[0165] Forward and reverse priming primers were used to introduce modifications (M252Y / S254T / T256E and T250Q / M428L) in the human γ1 constant region of the plasmid encoding the heavy chain of pascolizumab (anti-IL-4 antibody) using the Quikchange protocol (Promega).
[0166] A PCR fragment encoding the VH domain of an anti-TNFα antibody was generated using a previously constructed codon-optimized vector as a template as described in Example 1 above. The fragment obtained using HindIII and SpeICloned into the pTT expression vector containing the modified human gamma 1 constant region as described in the paragraph above. The plasmid encoding the heavy chain of anti-TNFα antibody with M252Y / S254T / T256E modification was named SJC324. The plasmid encoding the heavy chain with T250Q / M428L modification was named SJC323.
[0167] Forward and reverse priming primers were used to introduce modifications in the human γ1 c...
Embodiment 3
[0168] Example 3: Antibody expression in HEK2936 cells using pTT episomal vector
[0169] Expression plasmids encoding the above heavy and light chains were transiently co-transfected into HEK2936E cells. Expressed antibodies were purified from supernatants by affinity chromatography using 1 mL HiTrap protein A columns (GE Healthcare). Table 1 below shows a list of the antibodies produced.
[0170] Some antibodies are also expressed in CHO cells using a different set of expression vectors. See Examples 13, 14 and 15 for descriptions of use in molecular biology, expression and purification.
[0171] Table 1: List of expressed antibodies
[0172]
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com