Pharmaceutical formulations for adalimumab
a technology of adalimumab and adalimumab, which is applied in the direction of immunoglobulins against cytokines/lymphokines/interferons, pharmaceutical non-active ingredients, inorganic non-active ingredients, etc., can solve problems such as troublesome finding the right buffer, and achieve good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
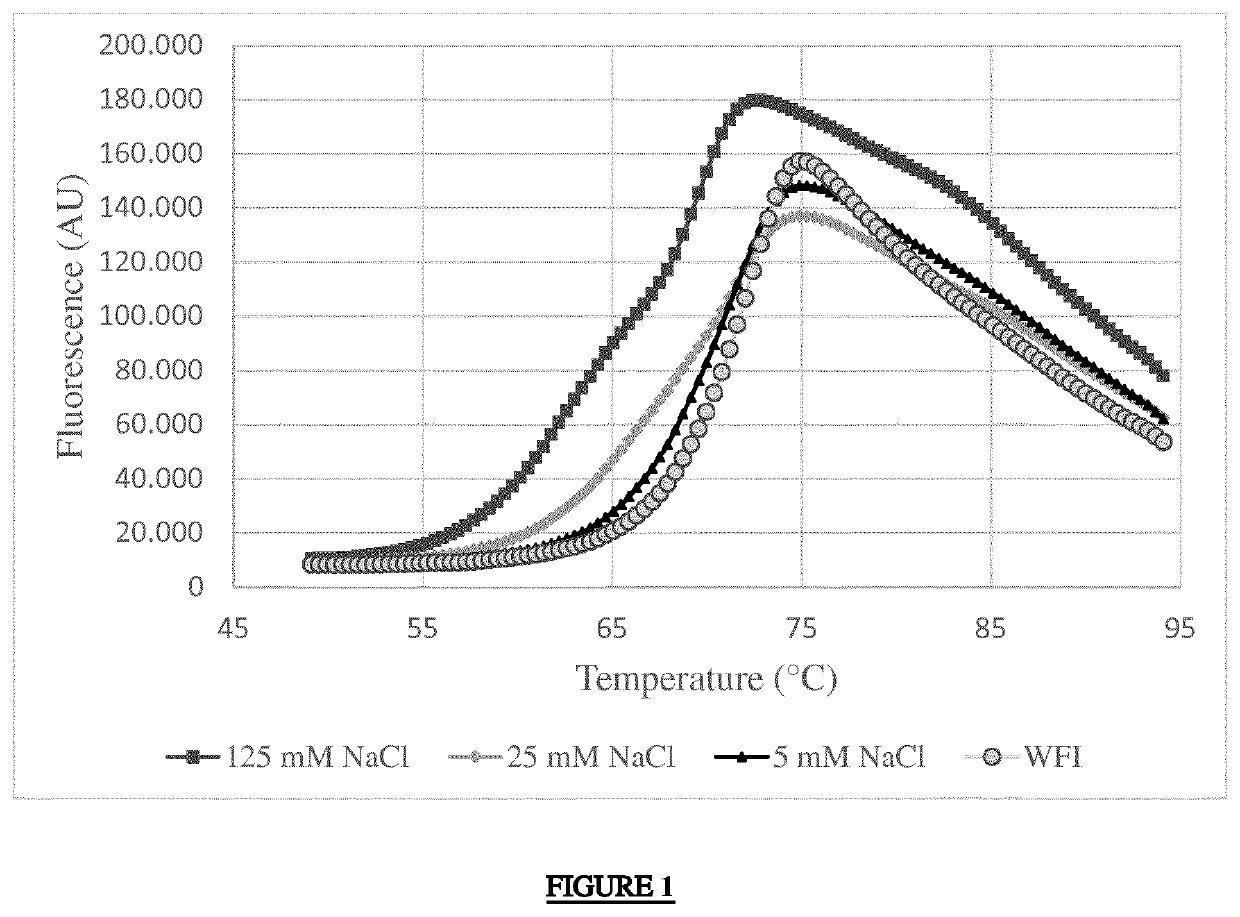
[0026]The effect of NaCl on the thermal stability of adalimumab was tested in a 7500 Real Time PCR thermocycler system from Applied Biosystems by a qPCR-based Thermofluor Assay using SYPRO Orange dye (Invitrogen, Cat. no. S6651) to assess the apparent melting temperature (Tm) of adalimumab protein. The amount of fluorescence, which correlates with the degree of denaturation or melting of the protein, was determined as a function of temperature for several adalimumab samples having different concentrations of NaCl. The procedures are set forth below.
[0027]Adalimumab protein was supplied frozen in a matrix that contained phosphate buffer. The material was defrosted and then buffer-exchanged with water for injection (about 6 volumes) and concentrated to 108 mg / ml using UF / DF apparatus. Samples for testing were then prepared with a final concentration of 0.2 mg / ml adalimumab protein, 1:2000 dilution of the supplied SYPRO orange dye stock, and the indicated concentration of NaCl: 0, 5, 2...
example 2
[0030]The effect of other salts on the thermal stability of adalimumab was tested in accordance with the procedures described in Example 1. The melting point as determined according to the above procedure are reported below in Table 3 for the salts MgCl2, KCl, and CaCl2, each at three different concentrations.
TABLE 3Tm lowTm highCondition(° C.)(° C.)No salt (WFI)N / A72.3 ± 0.15 mM MgCl2N / A71.1 ± 0.025 mM MgCl2N / A71.8 ± 0.1125 mM MgCl261.7 ± 0.269.9 ± 0.25 mM KClN / A71.6 ± 0.125 mM KClN / A71.9 ± 0.1125 mM KCl64.4 ± 0.270.1 ± 0.25 mM CaCl2N / A70.5 ± 1.125 mM CaCl266.9 ± 0.872.7 ± 0.8125 mM CaCl261.4 ± 0.170.8 ± 0.2
[0031]All three salts developed a second, lower melting point at a concentration of 125 mM. Thus, at such concentrations, the thermal stability of adalimumab becomes a concern. The salt CaCl2 developed a second melting point also in the 25 mM concentration, which renders it less preferred from a thermal stability perspective.
example 3
[0032]The following formulations in Table 4 were made and placed on stability as described below. Two formulations correspond to the present invention (Samples 1 and 2) and a Control Sample corresponds to the formulation used in the approved 100 mg / ml HUMIRA® composition.
TABLE 4IngredientSample 1Sample 2Control SampleAdalimumab100mg / ml100mg / ml100mg / mlNaCl10mM10mM—Sucrose255mM——Mannitol—230mM230mMPolysorbate 800.1% (w / v)0.1% (w / v)0.1% (w / v)
[0033]The pH of each Sample (1, 2, and Control Samples) was 5.2±0.2.
[0034]The Samples were prepared as follows. Frozen adalimumab material was defrosted, concentrated, and buffer-exchanged with water for injection in like manner as described in Example 1 to obtain adalimumab in WFI in a concentration of 108 mg / ml. The adalimumab protein in water was then dialyzed against a 230 mM solution of Mannitol for Sample 2 and the Control sample and against 255 mM sucrose for Sample 1. Stock solutions of NaCl (4 M), and Polysorbate 80 (20% w / v) were added as...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com