Method of removing recombinant expression antibody aggregates and degradation products
A technology for degradation products and antibodies, applied in peptide preparation methods, chemical instruments and methods, anti-animal/human immunoglobulins, etc., can solve problems such as difficult removal of antibody aggregates and degradation fragments, increase of antibody protein retention time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1: Recombinant human anti-TNF-α monoclonal antibody protein sequence.
[0080] A human antibody for treating TNF-α-related diseases is recombinant human anti-TNF-α monoclonal antibody is expressed in CHO cells, and is purified by a series of standard chromatography steps. In this embodiment, the Ada-wood single-sequence of the Adta has a heavy chain variable region of SEQ ID NO: 1; and has the light chain variable region of SEQ ID NO: 2. Its molecular weight is about 148 kDa, consisting of 2 IgG1 heavy chains and 2 kappa light chains. Each heavy chain contains 451 amino acids, the molecular weight is about 49 kDa, and its sequence is shown in SEQ ID NO: 5; each light chain contains 214 amino acids, the molecular weight is about 24 kDa, and the sequence is shown in SEQ ID NO: 6.
Embodiment 2
[0081] Example 2: Recombinant expression and purification of recombinant human Ada Tumi.
[0082] The method of specific binding of TNF-α monoclonal antibody anti-TNF-α antibody is expressed in CHO cells. The expression vector containing the antibody gene was constructed by conventional molecularcloning, a derivative cell line of CHO-K1 cells (ATCCCCCL61) as a host cell expression. The construction process of high-yield stable cell line is briefly described as follows: Host cell suspension growth in CD-CHO medium (GIBCO, CA), takes place in a long-term host cell centrifugation, resuspended in fresh CD-CHO medium, count And regulate the cell density to 1.43 × 10 7 A / ml, take 600 ul of the cell suspension to add an electric shock cup, then add a linear plasmid 40 ug, and mix the cells and plasmids uniform with a pipette. Use the BIO-RAD radiograph to convert, instrument parameter setting To: Capacitor: 960ufd, voltage: 300V. Usually the electric shock time is 15-20 milliseconds. T...
Embodiment 3
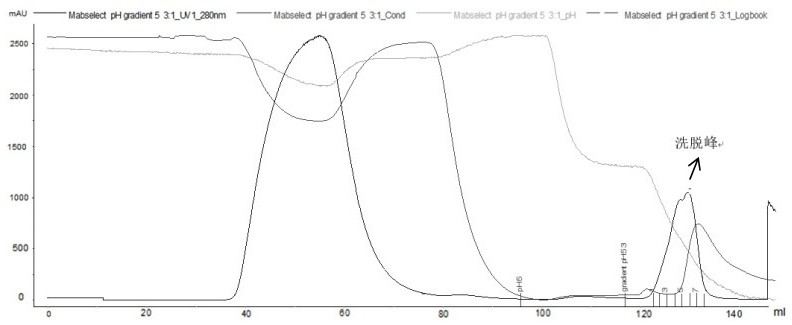
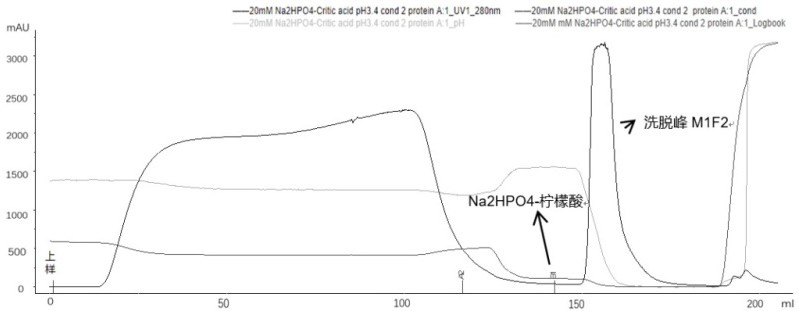
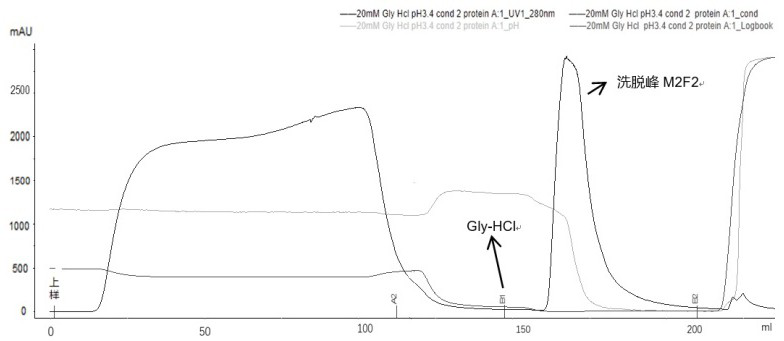
[0083] Example 3: The effect of recombinant Ada Habitan common proximity and chromatography pre-washing conditions.
[0084] By adding pre-washing steps in affinity chromatography, it is mainly to add different pre-wash additives to examine the removal effect of pre-washing conditions for the polymer. If the pre-wash condition is inappropriate, the focus is focused on the optimization of the eluting step.
[0085] Affinity chromatography (different pre-wash):
[0086] Filler: Ge MabSelect 5ml, Chromatography: Bo Gron BXP10mm / 20cm, column height: 6.4cm;
[0087] Buffer:
[0088] Balance buffer buffer A1 (20 mM Pb + 0.15 M NaCl, pH 7.0);
[0089] Rebalance buffer Buffer A2 (20 mM Pb, pH 7.0);
[0090] 3 pre-wash dense Buffer B1-1 (20mm Pb + 1m urea, pH 7.0);
[0091] Buffer B1-2 (0.2 m Arg-HCl + 1M NaCl, pH 7.0);
[0092]Buffer B1-3 (10 mm EDTA + 1M NaCl, pH 7.0);
[0093] Elite buffer B2 (20mm citrate-Na 2 HPO 4 , PH3.4).
[0094] experiment procedure:
[0095] First use buffer A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com