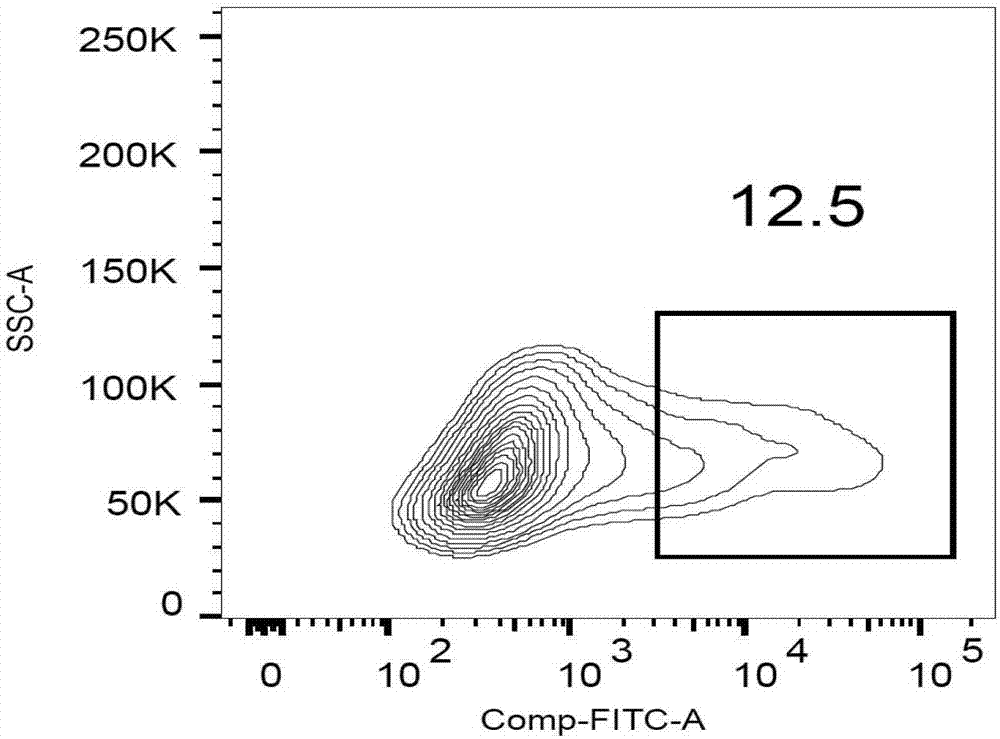
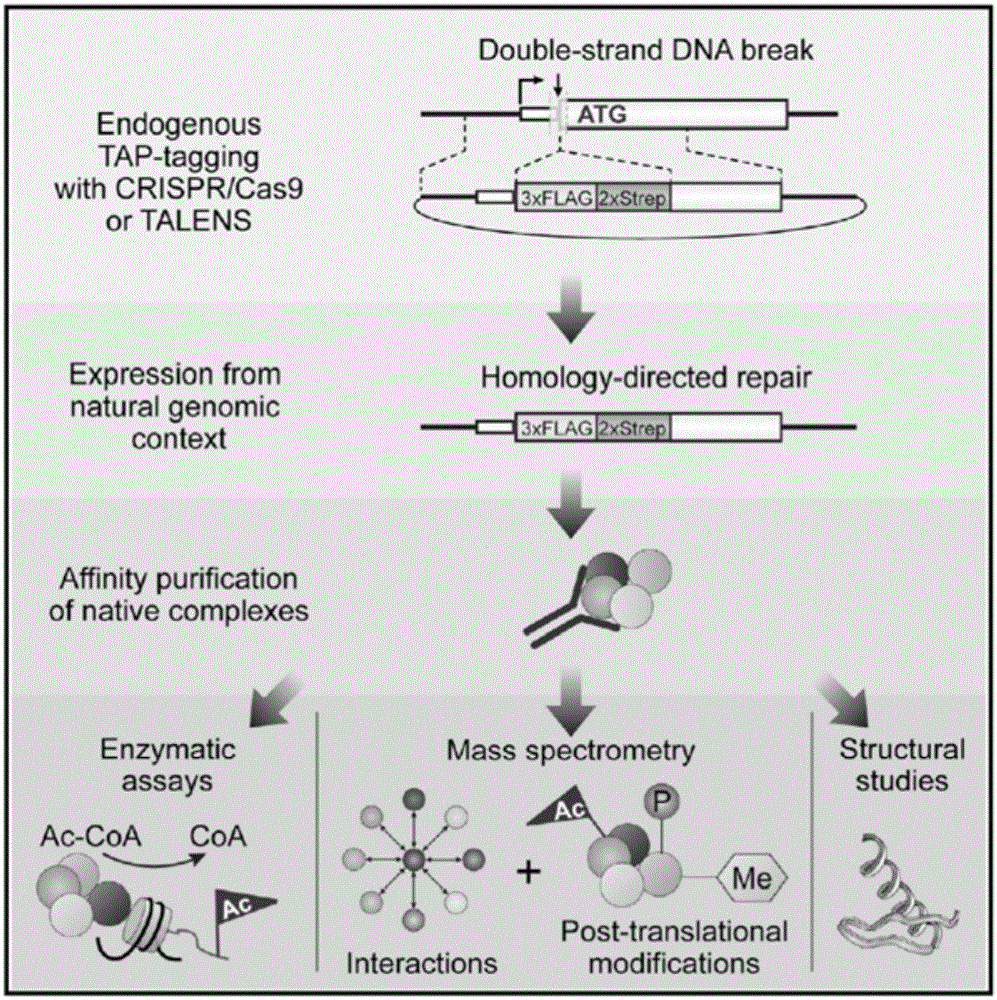
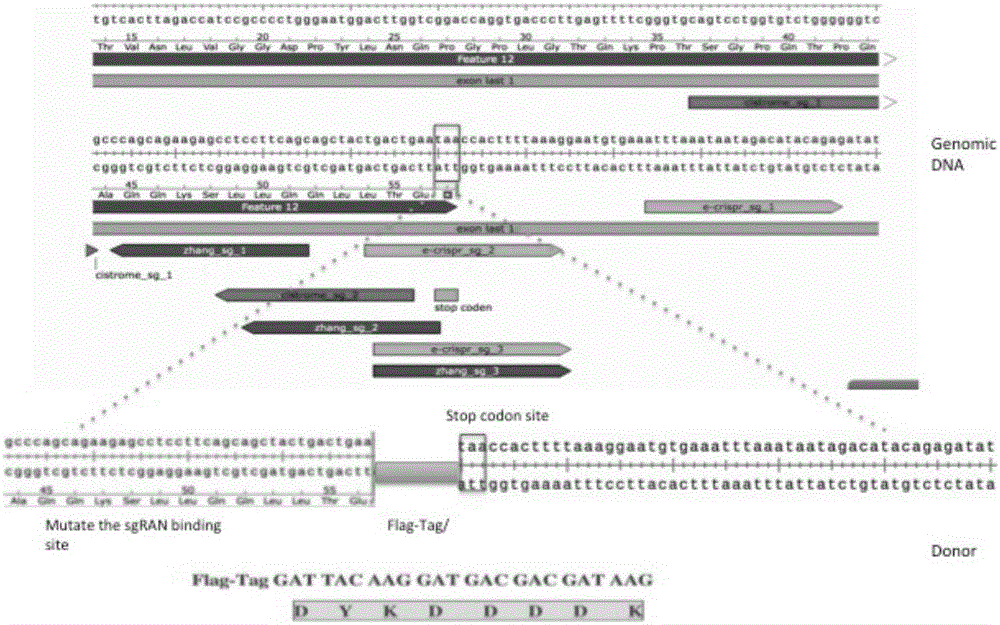
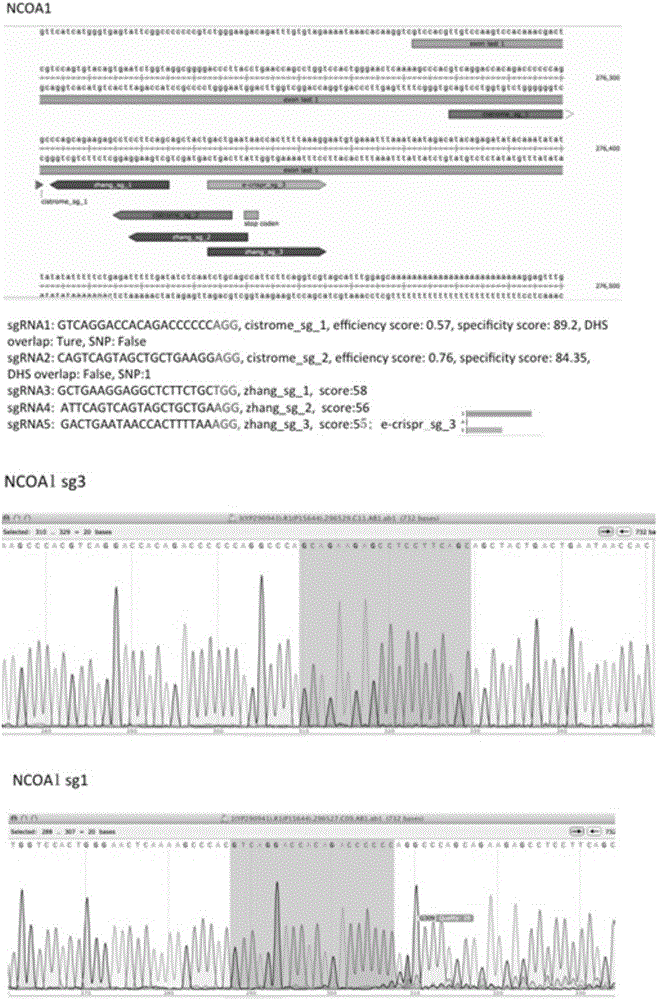
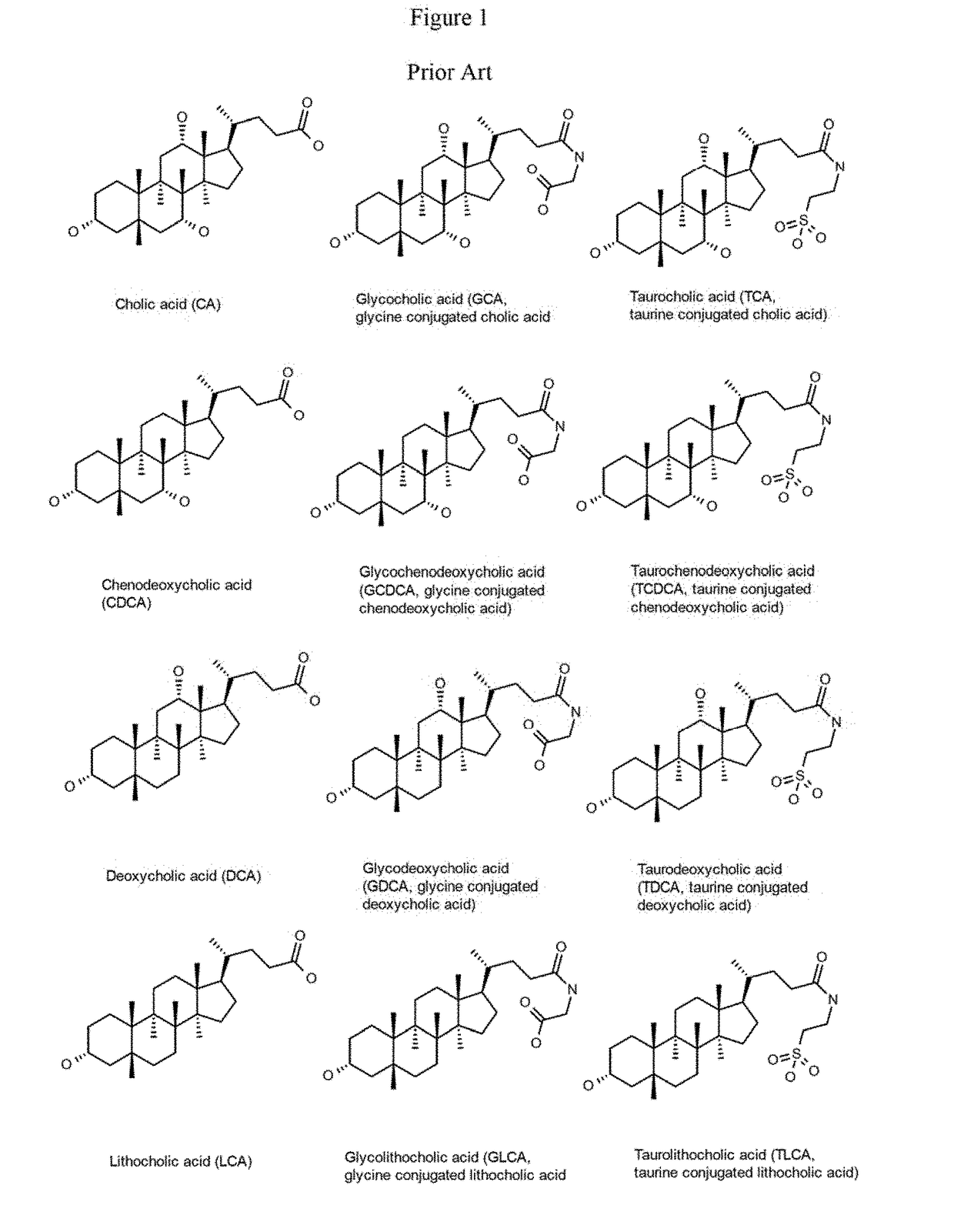
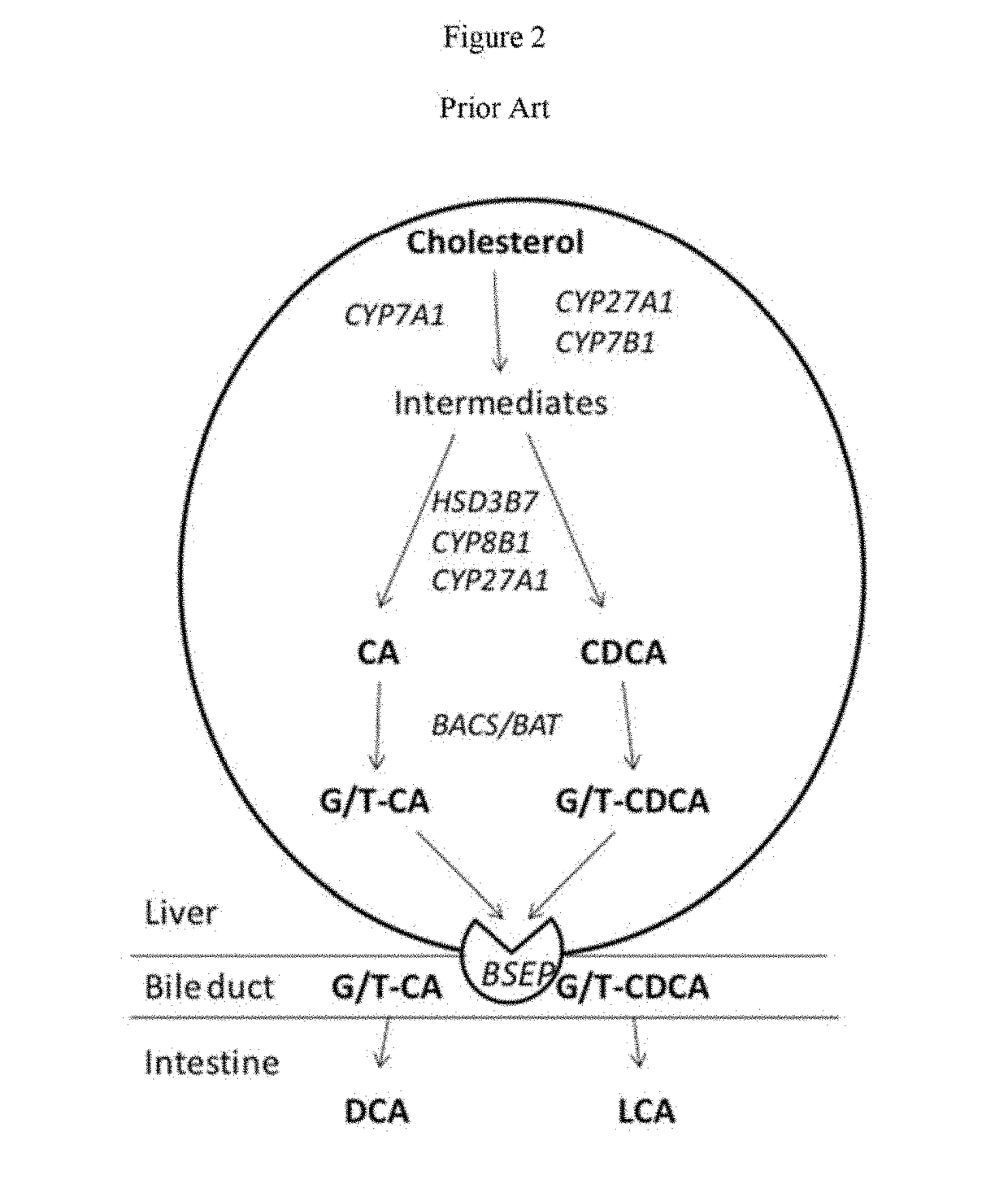
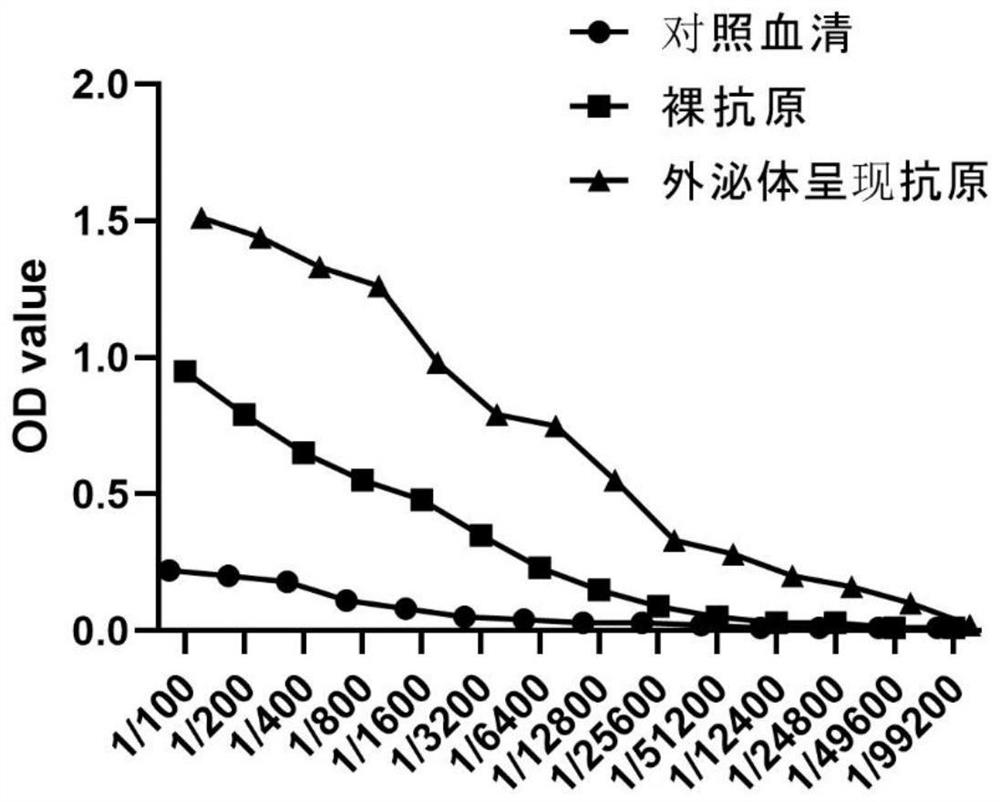
Patents
Literature
171 results about "Stable cell line" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Stable cell lines are used in a variety of research applications such as recombinant protein and antibody production, assay development, gene editing, functional studies and more.
Methods for increasing the efficiency of recombinant AAV product
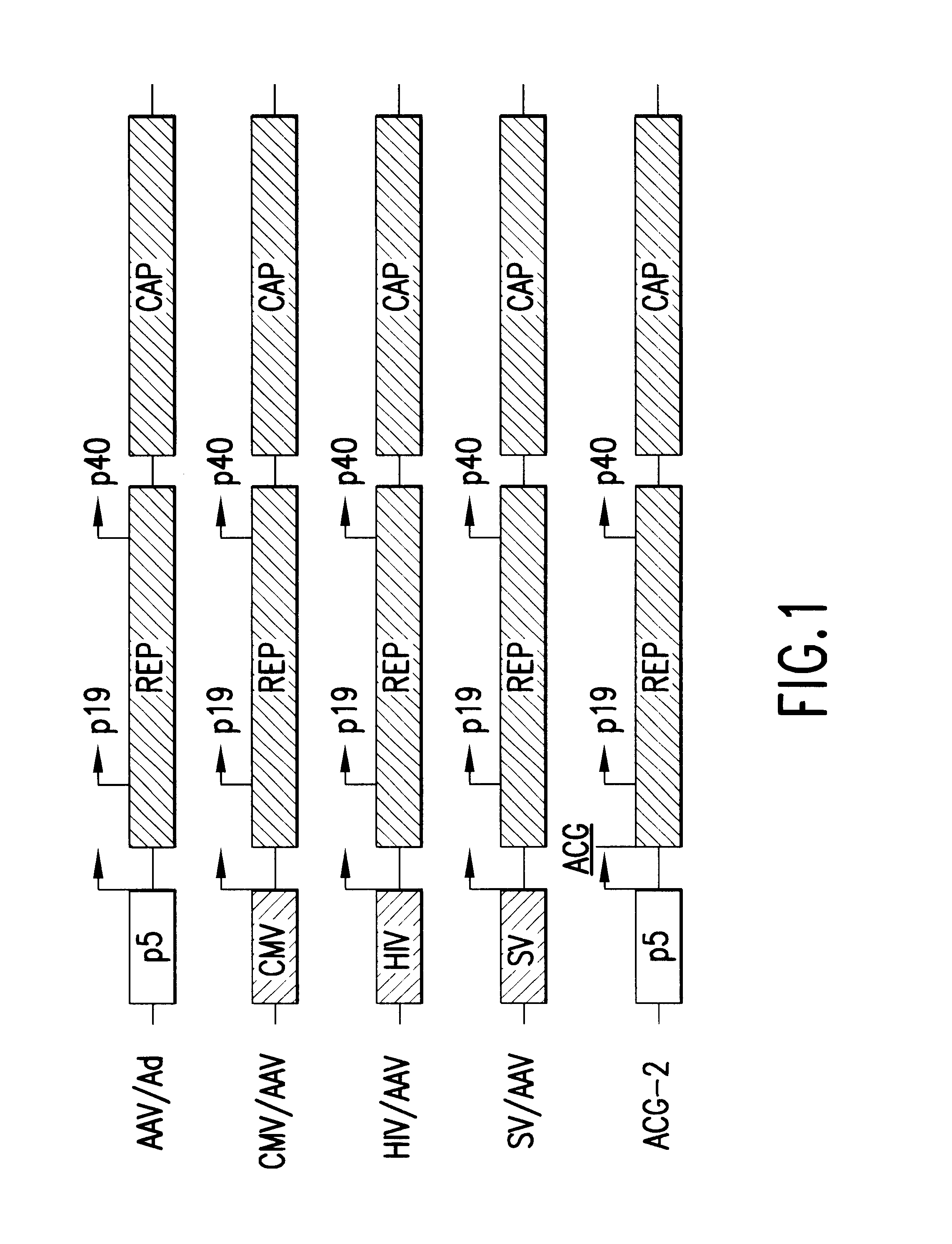
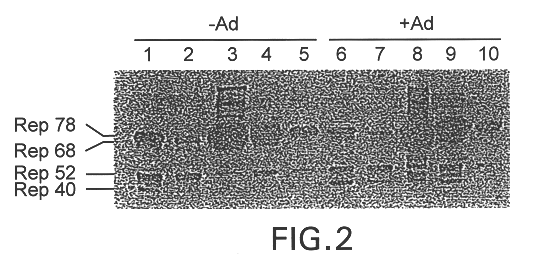
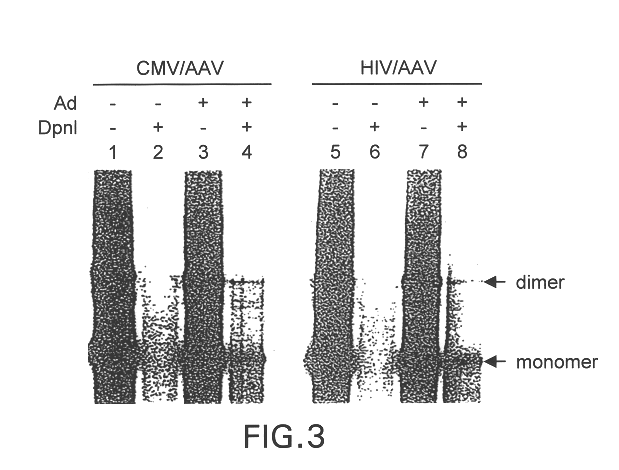
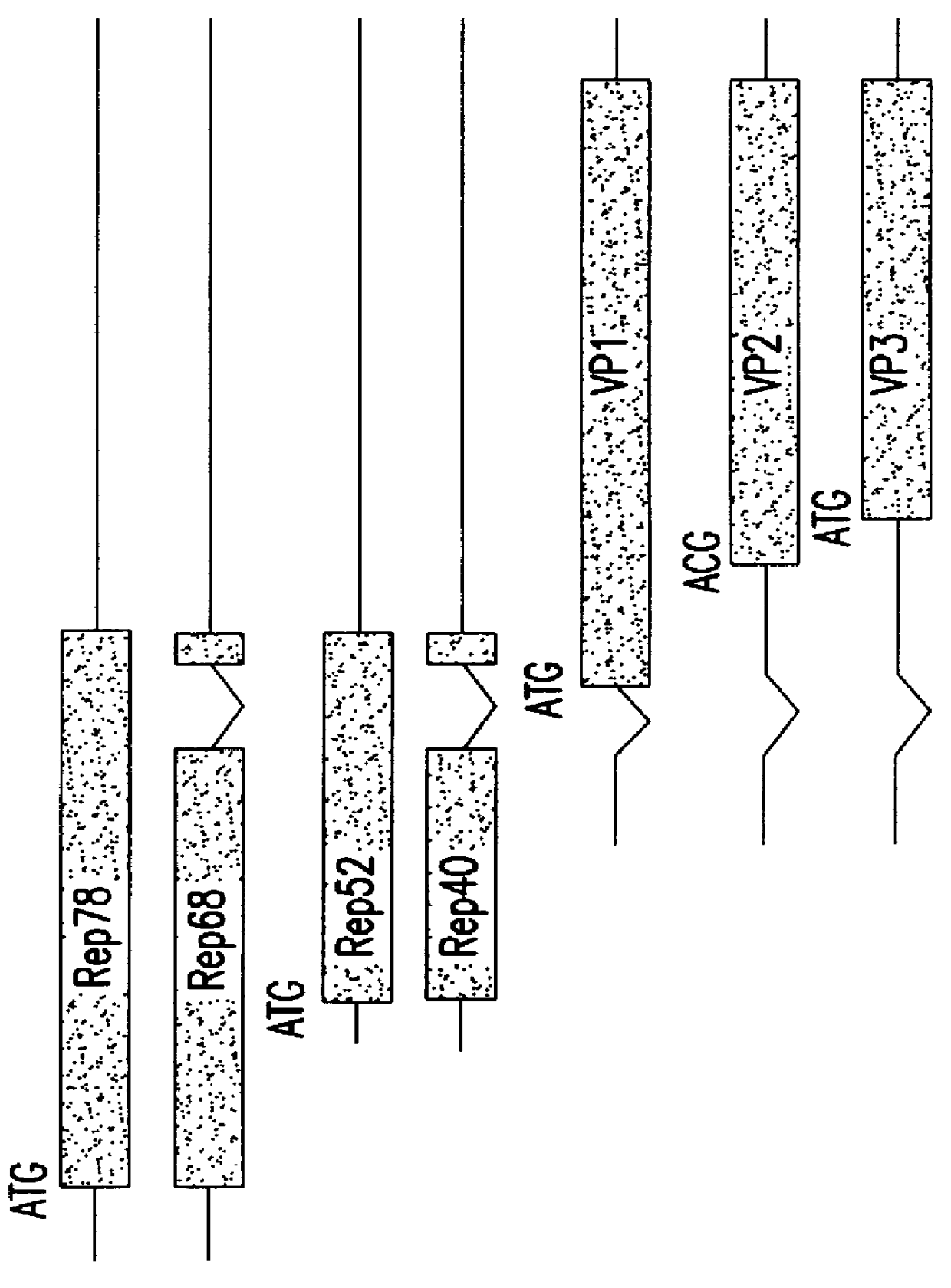
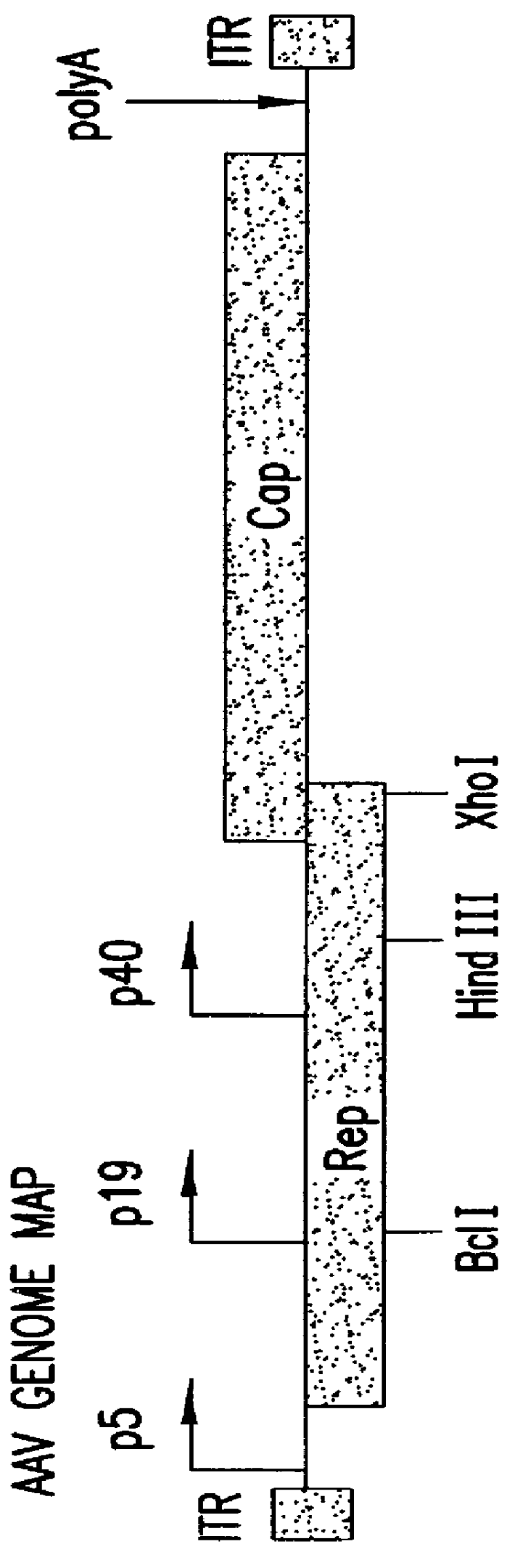
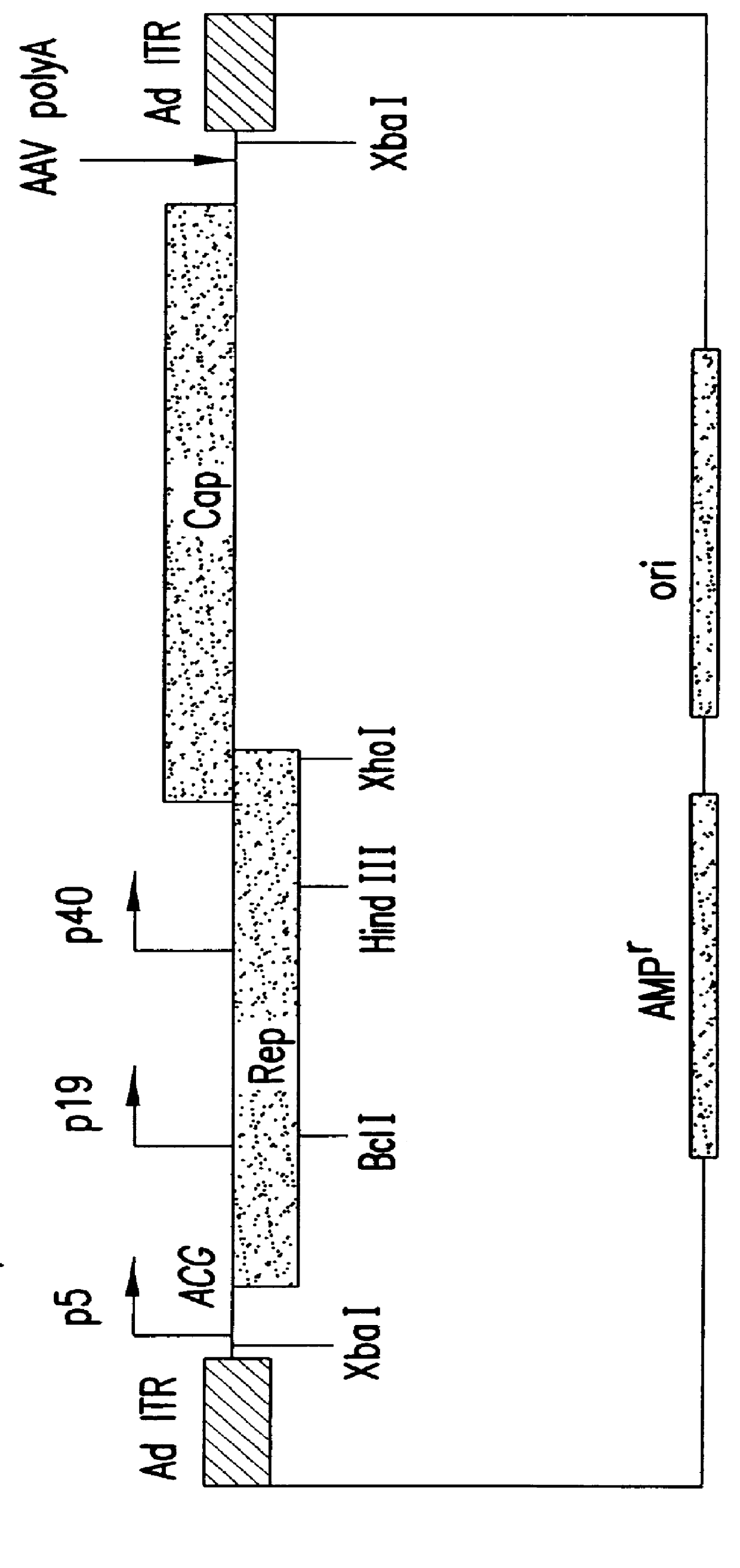
InactiveUS6548286B1Altering the stability of REP mRNAShort lifeGenetic material ingredientsVirus peptidesPost translationalStable cell line
The present invention relates to methods and compositions for increasing the production of high titre stocks of recombinant AAV (rAAV) through regulation of expression of the AAV REP and CAP proteins. The methods and compositions of the invention are based on the observation that the low level expression of the AAV REP protein increases the production of AAV viral capsid protein and efficiency of packaging resulting in production of higher titre recombinant viral stocks. The invention encompasses recombinant AAV vectors that direct the expression of AAV REP and CAP proteins and the use of such vectors for the production of novel stable cell lines capable of generating high titre rAAV vectors. The invention provides methods for regulating the expression of the AAV REP gene at the transcriptional and post-translational level. The methods and compositions of the invention can be used to produce high titre stocks of rAAV which can be used in gene therapy for the purpose of transferring genetic information into appropriate host cells for the management and correction of human diseases including inherited and acquired disorders.
Owner:CELLS GENESYS INC +1
Method for increasing the efficiency of recombinant AAV production
InactiveUS6037177AReduce expressionIncreased of replicationArtificial cell constructsVertebrate cellsPost translationalTiter
The present invention relates to methods and compositions for increasing the production of high titre stocks of recombinant AAV (rAAV) through regulation of expression of the AAV REP and CAP proteins. The methods and compositions of the invention are based on the observation that the low level expression of the AAV REP 78 / 68 protein increases the production of AAV viral capsid protein and efficiency of packaging resulting in production of higher titre recombinant viral stocks. The invention encompasses recombinant AAV vectors that direct the expression of AAV REP and CAP proteins and the use of such vectors for the production of novel stable cell lines capable of generating high titre rAAV vectors. The invention provides methods for regulating the expression of the AAV REP 78 / 68, REP 40 / 52 and CAP gene at the transcriptional and post-translational level. The methods and compositions of the invention can be used to produce high titre stocks of rAAV which can be used in gene therapy for the purpose of transferring genetic information into appropriate host cells for the management and correction of human diseases including inherited and acquired disorders.
Owner:CELLS GENESYS INC
Method for rapidly obtaining CRISPR/Cas9 (clustered regularly interspersed short palindromic repeats/Cas9) gene knockout stable cell line through monoclonal cell sorting
InactiveCN107418974AImprove knockout productivitySave the trouble of screeningVectorsStable introduction of DNAFluorescenceHigh survival rate
The invention relates to a method for rapidly obtaining a CRISPR / Cas9 (clustered regularly interspersed short palindromic repeats / Cas9) gene knockout stable cell line through monoclonal cell sorting and belongs to the technical field of genetic engineering and genetic modification. According to the method, a CRISPR / Cas9 system, single-cell sorting by a flow cytometer and fluorescent protein screening on an expression vector are combined, positive monoclonal cells can be obtained in short time, and gene knockout work efficiency of the cell line is greatly improved. Compared with a traditional cell line gene knockout method, the flow cytometer is utilized for single-cell sorting, on one hand, tedious work of antibiotic screening is omitted, so that a large quantity of single cells can be obtained in quite short time, and on the other hand, the condition that cells in each culture hole are single cells can be guaranteed and false positive rate is reduced. Sorting is performed 40-80 h after cell transfection, at the time point, the highest survival rate of the cells after sorting can be guaranteed, and the screening efficiency is improved accordingly.
Owner:XINXIANG MEDICAL UNIV
Endogenous protein marking method used for Chip-seq genome-wide binding spectrum
InactiveCN106399311AMeet the packaging ratioHigh infection efficiencyMicrobiological testing/measurementBiological testingMaterial resourcesStable cell line
Owner:TONGJI UNIV
Stable knockout single plasmid vector with coordination of transposon and CRISPR/Cas9 system and application of stable knockout single plasmid vector
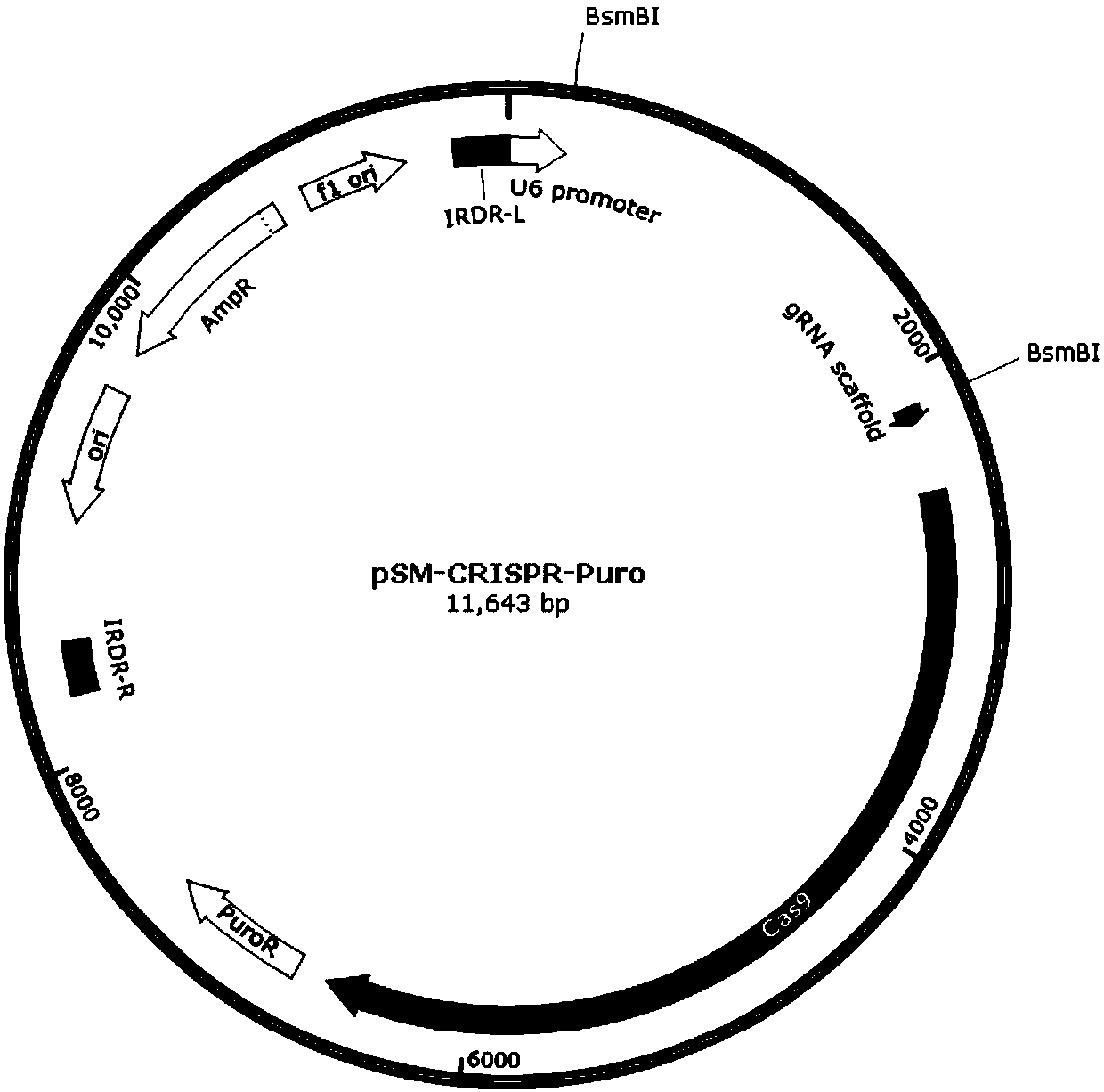
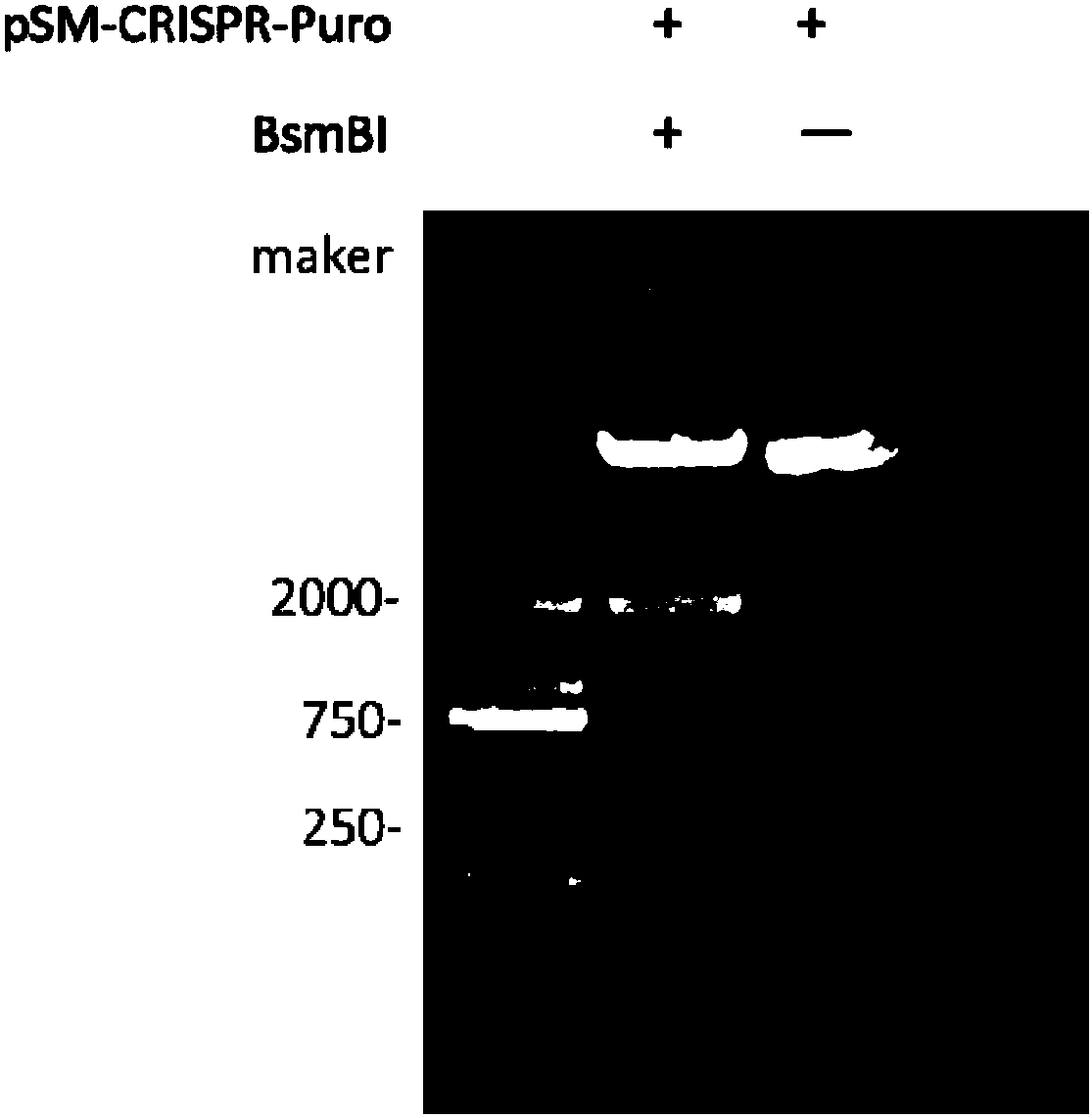
InactiveCN107686848ASimplify the build processThe process is simpleHydrolasesStable introduction of DNAStable cell lineBiology
The invention discloses a stable knockout single plasmid vector with coordination of transposon and a CRISPR / Cas9 system and application of stable knockout single plasmid vector and belongs to the field of gene engineering. The single plasmid vector is a double-stranded circular plasmid containing an IRDR-L-IRDR-R box, and the IRDR-L-IRDR-R box comprises an IRDR-L sequence, a promoter, a gRNA scaffold sequence, a Cas9 protein sequence, a resistance screening gene sequence and an IRDR-R sequence. The single plasmid vector provided by the invention can realize expression of sgRNA and a Cas9 protein only needing once construction and once transfection. A method is simple in process, is efficient and rapid, greatly simplifies a plasmid construction flow, shortens an experiment period and improves the working efficiency. The vector provided by the invention carries a transposase recognition sequence, does not use a virus, can conveniently, rapidly and safely establish a gene knockout stablecell line and is beneficial to screening stable cell lines by comprising the puromycin screening resistance.
Owner:SUN YAT SEN MEMORIAL HOSPITAL SUN YAT SEN UNIV
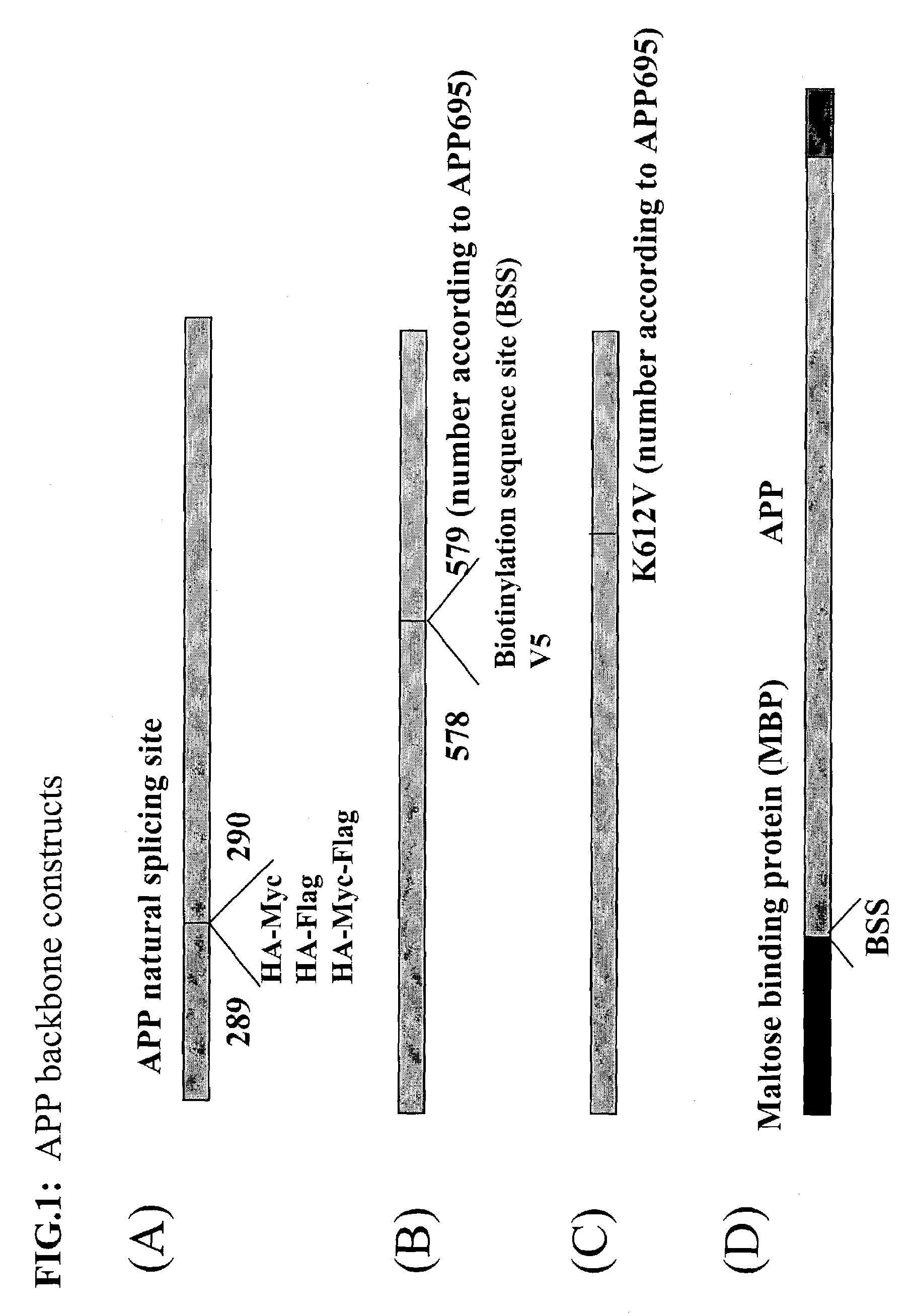
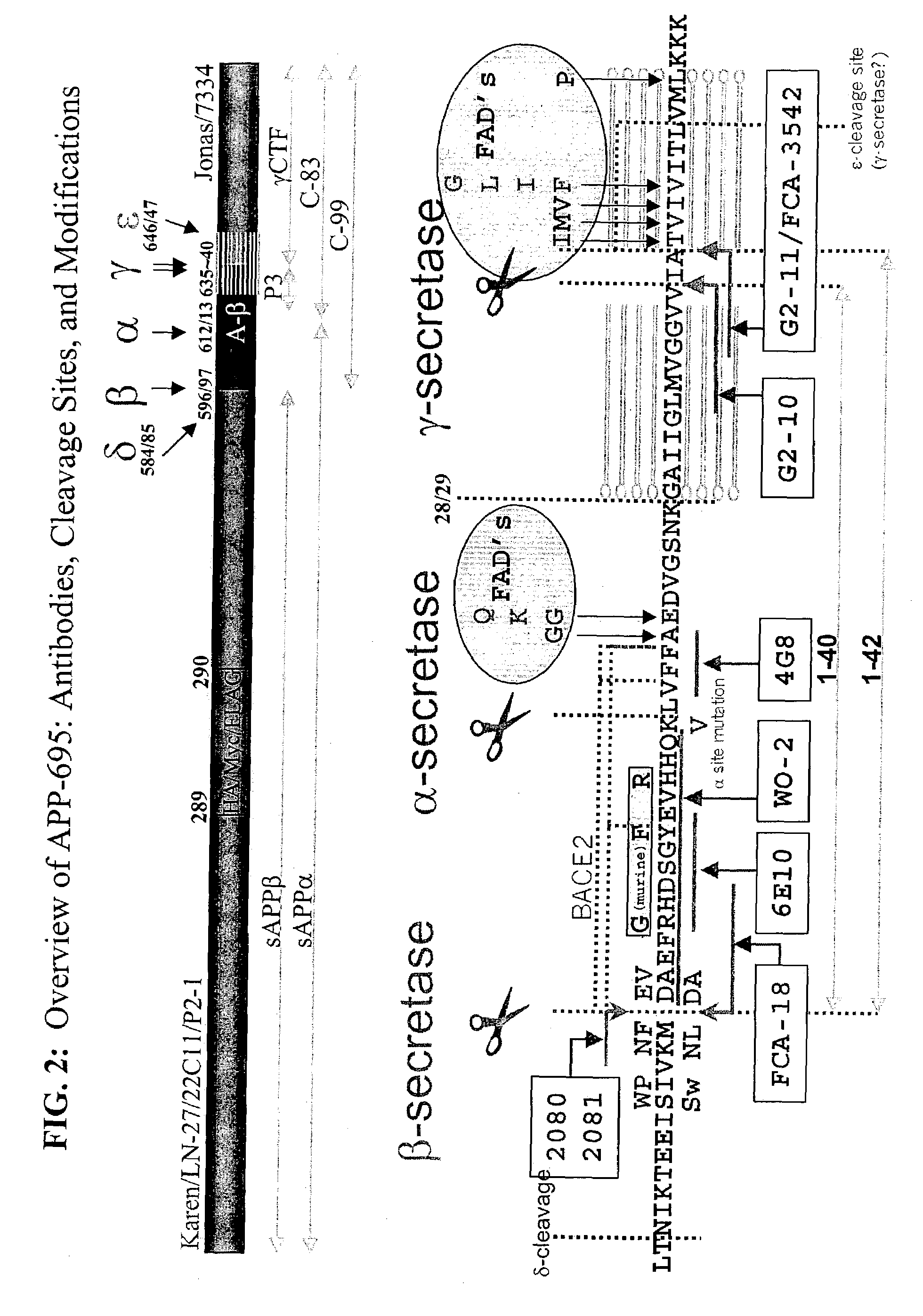
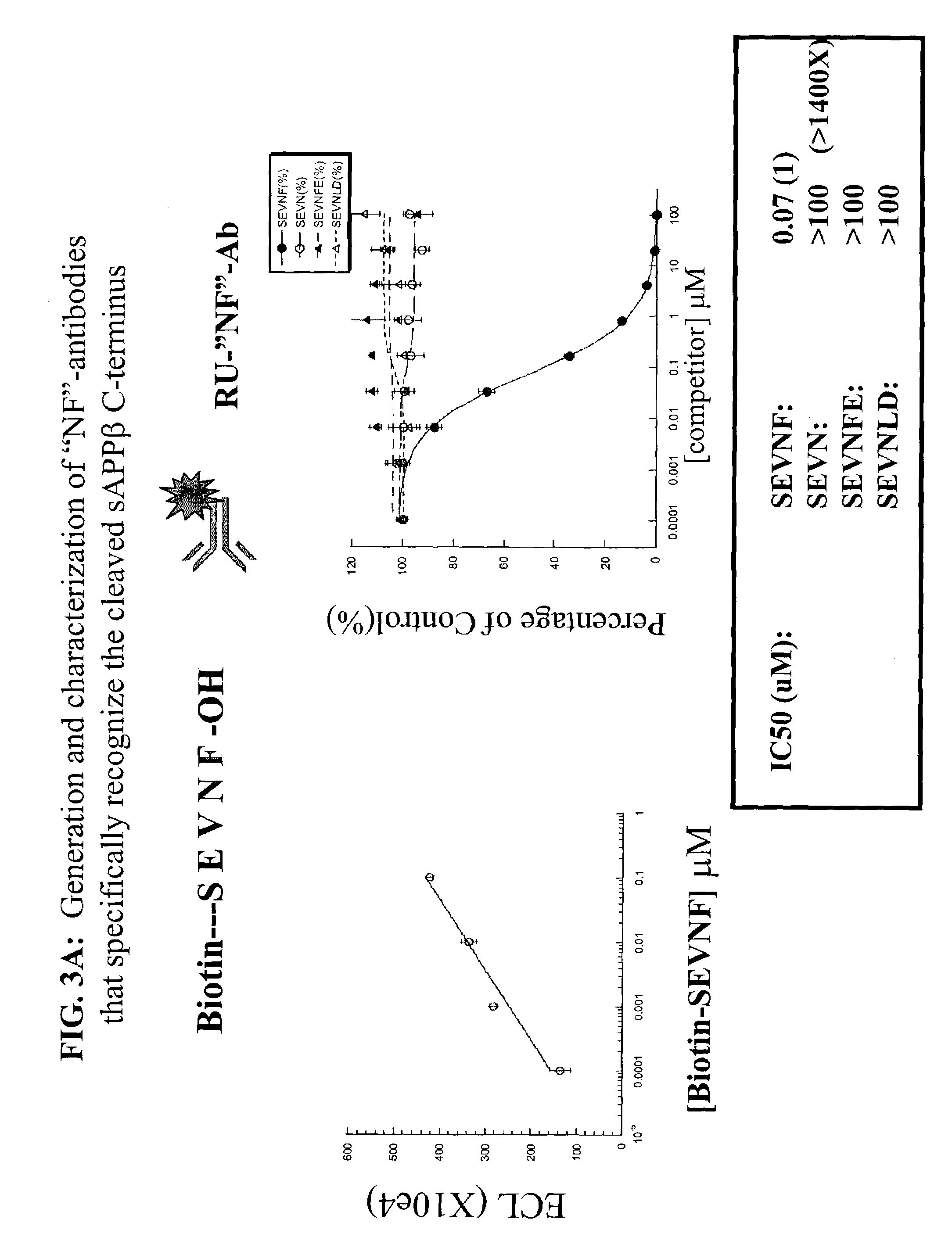
Assays using amyloid precursor proteins with modified beta-secretase cleavage sites to monitor beta-secretase activity
InactiveUS7196163B2More productiveOrganic active ingredientsPeptide/protein ingredientsWild typeAmyloid
Provided are methods of identifying inhibitors of β-secretase that employ modified β-secretase substrates. The modified β-secretase substrates have β-secretase cleavage sites that are altered from wild type. The amino acid sequences of the altered β-secretase cleavage sites contain different amino acids in at least one of the positions P2-P1-P1′-P2′ of the β-secretase cleavage site. Many of the modified β-secretase substrates are more efficient substrates for β-secretase than are corresponding substrates having wild-type sequences, that is, these modified substrates are more susceptible to enzymatic breakdown by β-secretase. Recombinant polynucleotide molecules encoding the modified β-secretase substrates are provided. Antibodies that recognize cleavage products of the modified β-secretase substrates are provided. Stable cell lines expressing the modified β-secretase substrates are provided. Transgenic animals expressing the modified β-secretase substrates are provided.
Owner:MERCK SHARP & DOHME CORP
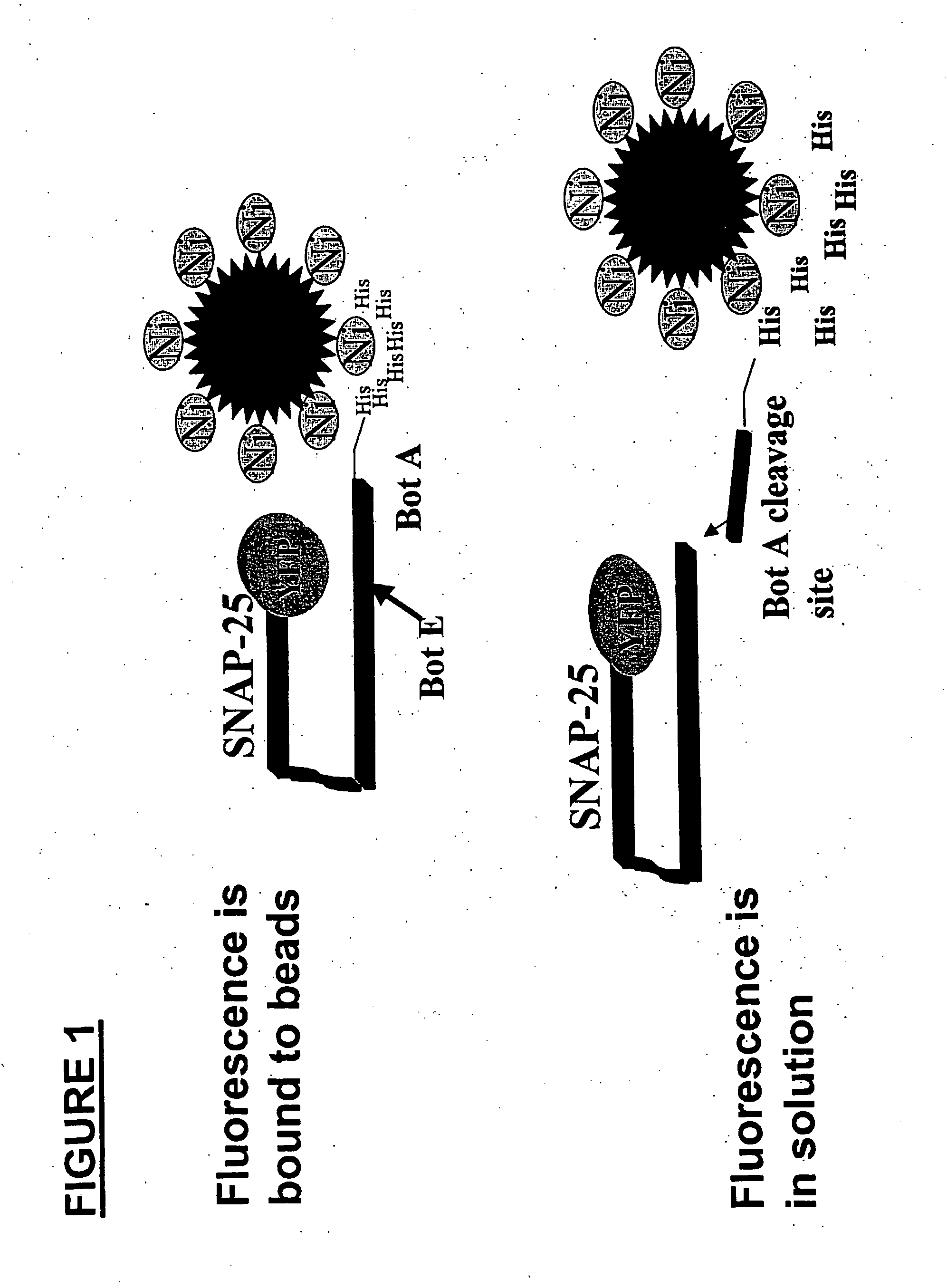
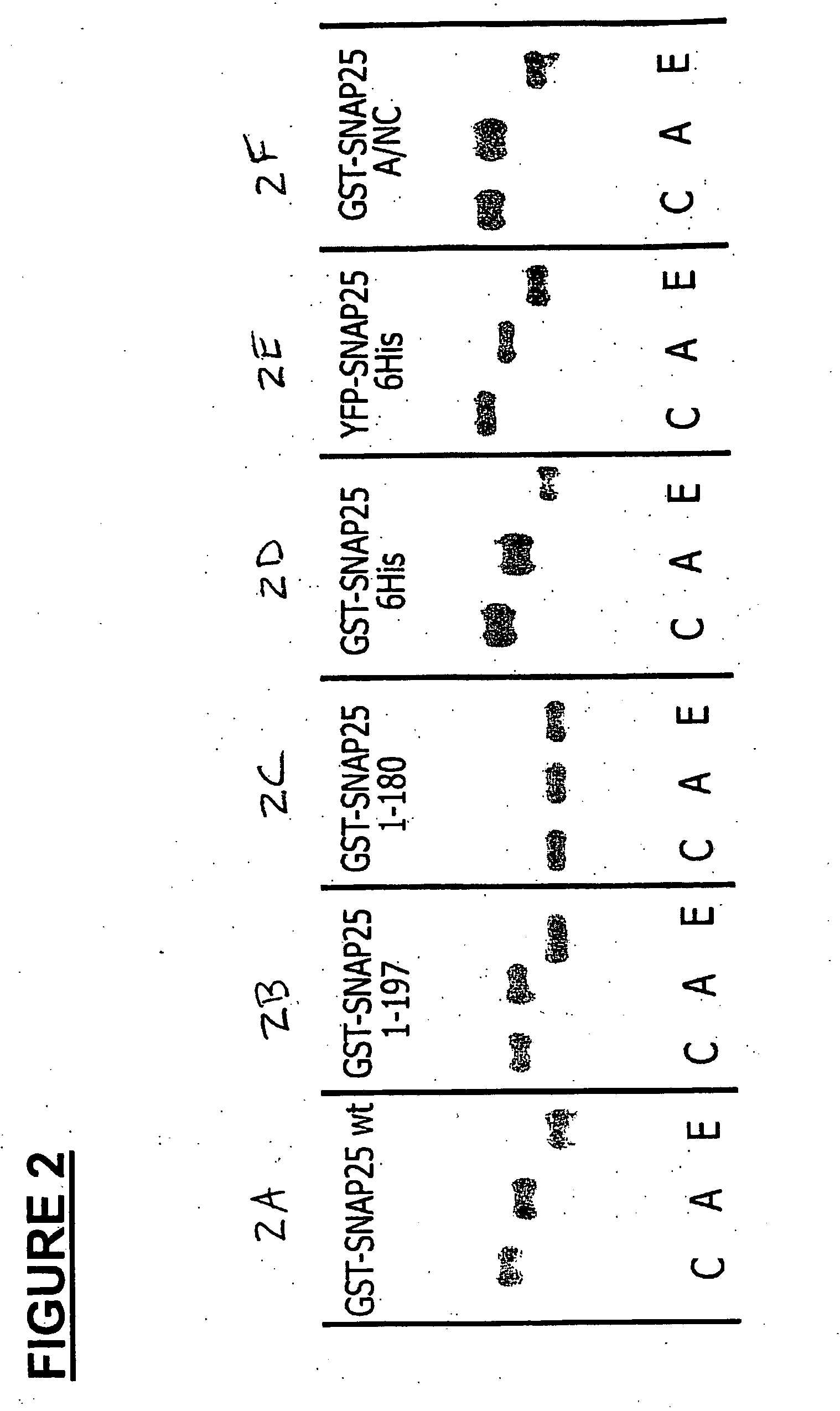
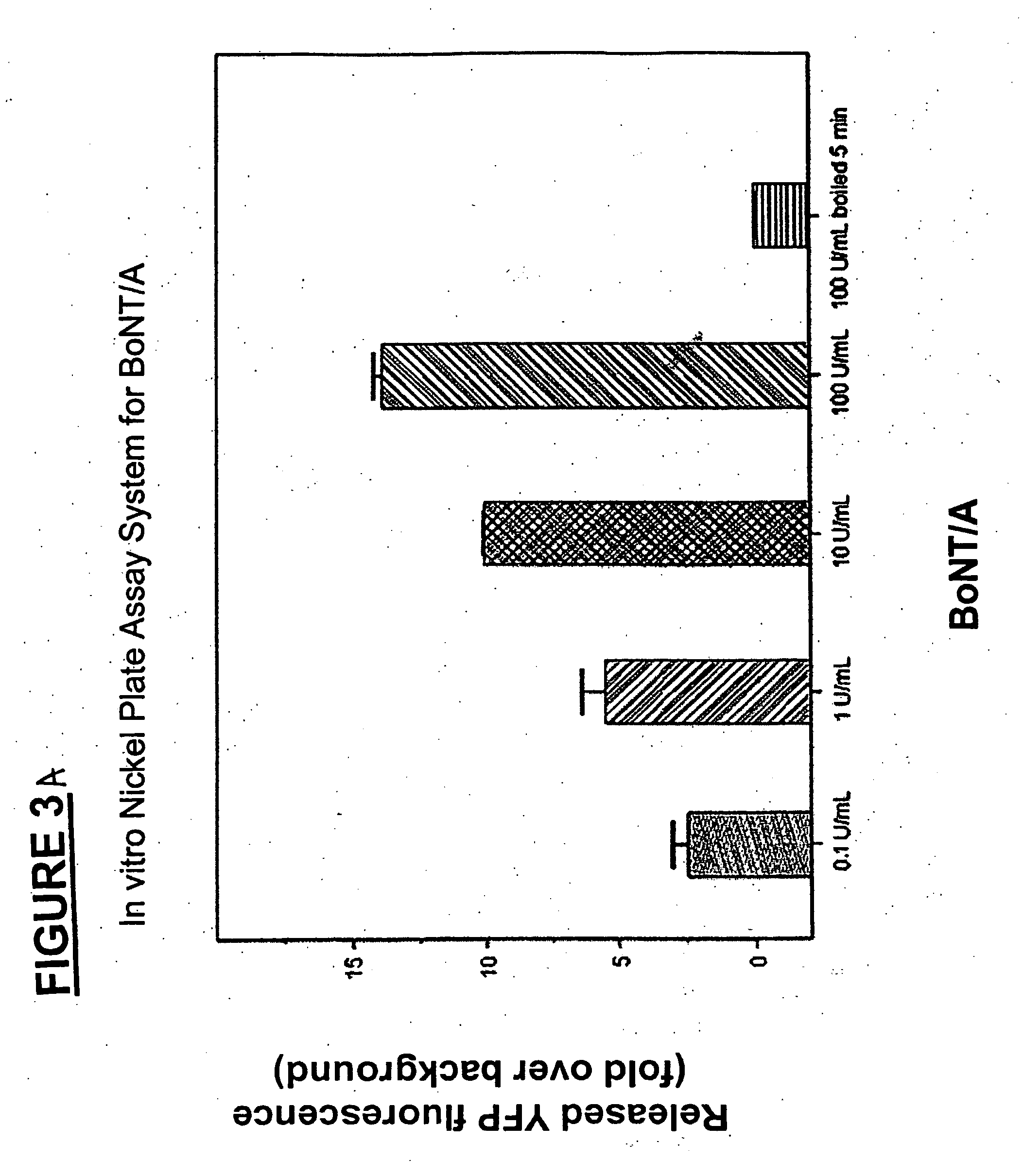
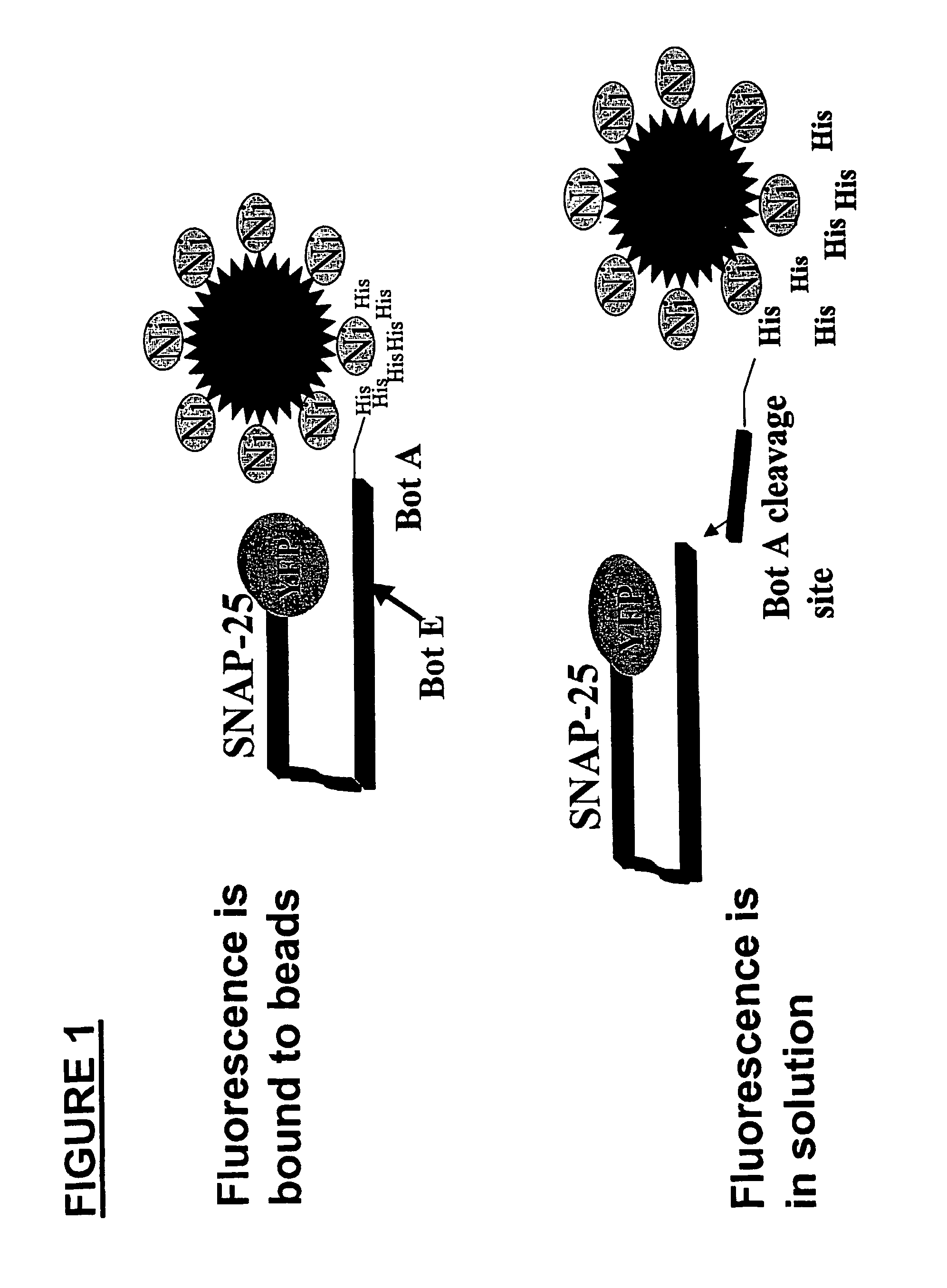
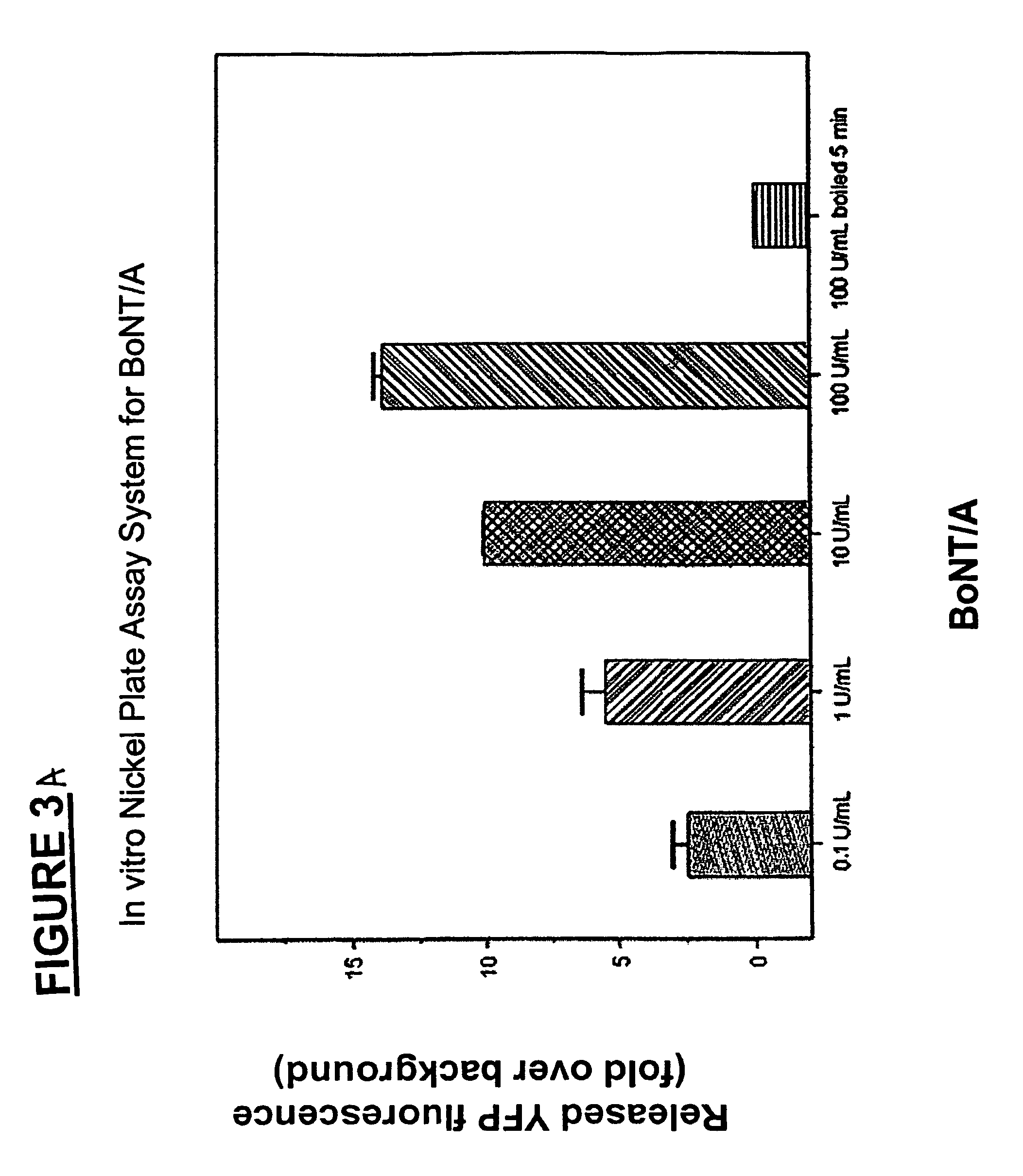
Methods for identifying inhibitors of botulinum neurotoxins
InactiveUS20060233836A1Compound screeningBacterial antigen ingredientsPeptide substrateStable cell line
A system and method for identifying a botulinum neurotoxin inhibitor employing a botulinum neurotoxin substrate complex having a peptide substrate, preferably SNAP-25, a reporter domain on one side of said peptide substrate and an immobilization domain on the opposite side of said peptide substrate. The botulinum neurotoxin inhibitor is identified by its ability to decrease the relative amount of cleaved complex, detected through measuring a decrease in complex bound to a solid support. The method of the present invention also utilizes novel cells that express a botulinum neurotoxin substrate complex. The methods of the present invention are adapted for cell based screening to monitor the catalytic activity of a BoNT in living cells and to identify molecules that inhibit the catalytic activity of a BoNT in living cells. Also provided are novel stable cell lines that express the botulinum toxin substrate complex and viral vectors capable of efficiently expressing an active light chain of the BoNT within mammalian cells.
Owner:BOTX TECH LLC
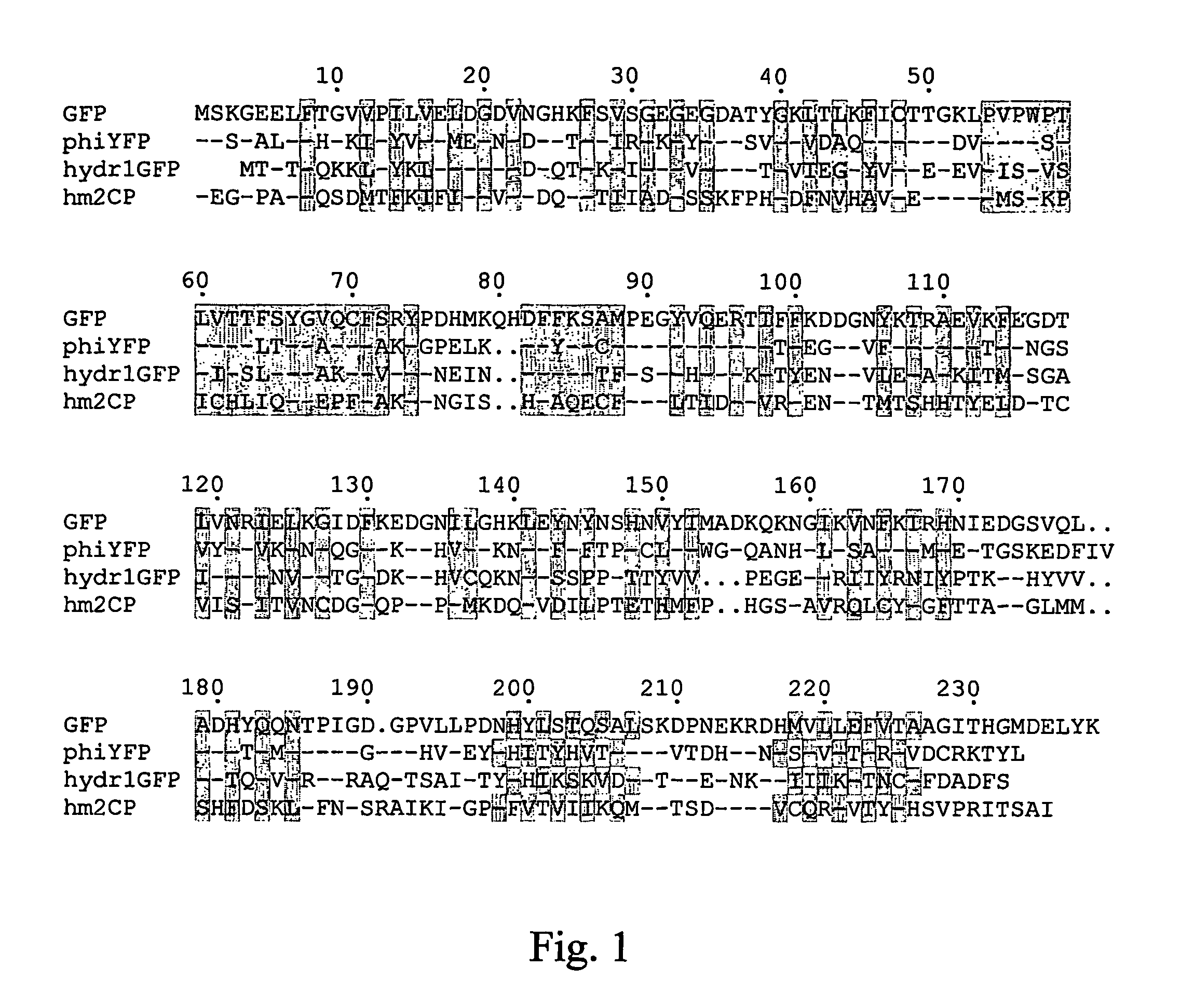
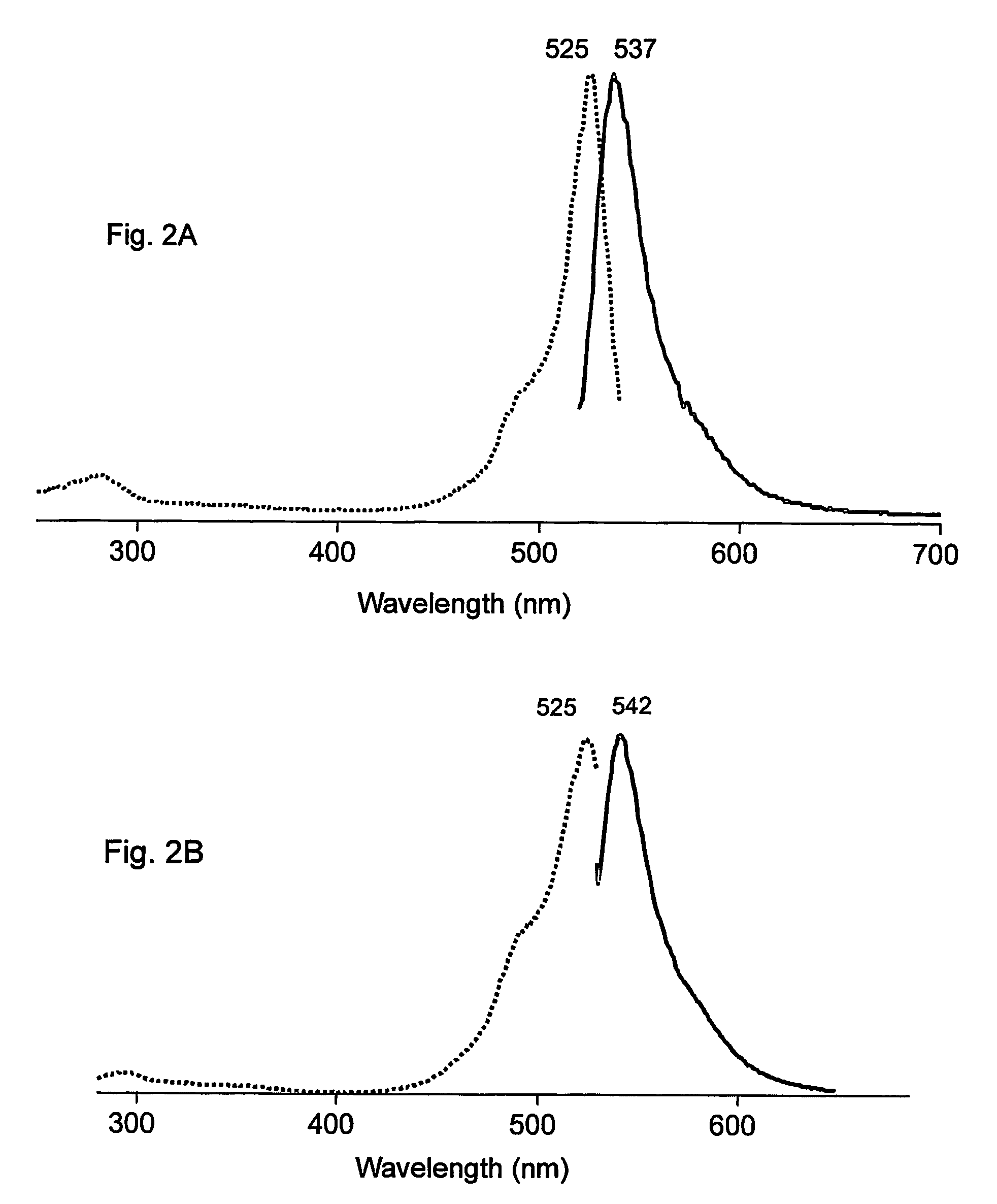
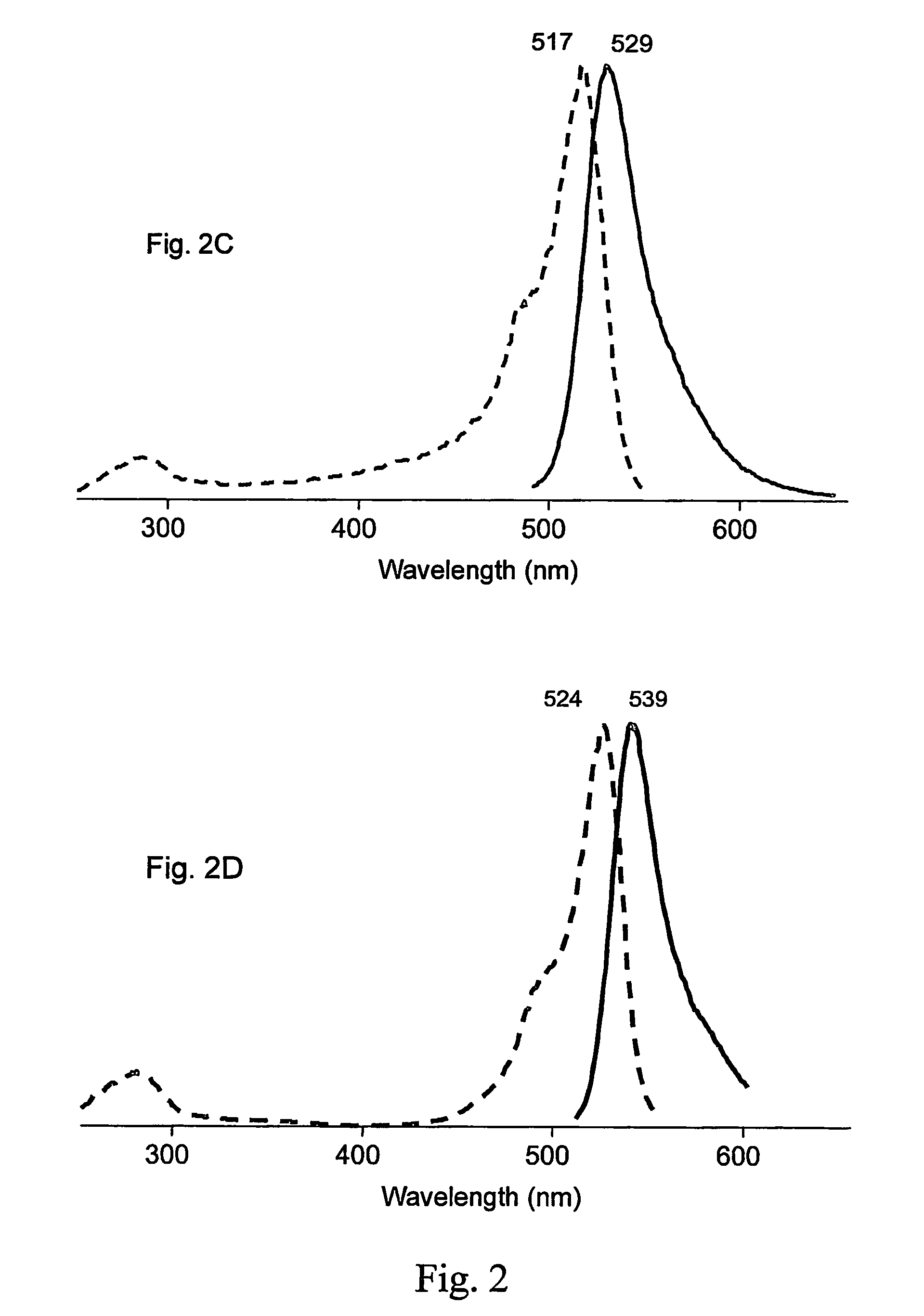
Fluorescent proteins and chromoproteins from non-Aequorea hydrozoa species and methods for using same
The present invention provides nucleic acid molecules encoding a fluorescent and chromo-proteins and mutants, variants and derivatives thereof, as well as proteins and peptides encoded by these nucleic acids. The nucleic acid molecules and proteins of interest are isolated from non-Aequorea Hydrozoa species. The proteins of interest include yellow fluorescent protein, phiYFP, from Phialidium sp., green fluorescent protein hydr1GFP and purple chromoprotein, hm2CP from hydroid medusae of sub-order Anthomedusae. Also of interest are proteins that are substantially similar to, or derivatives, or homologues, or mutants of, the above-referenced specific proteins. Also provided are fragments of the nucleic acids and the peptides encoded thereby, as well as antibodies specific to the proteins and peptides of the invention. In addition, host-cells, stable cell lines and transgenic organisms comprising above-referenced nucleic acid molecules are provided. The subject protein and nucleic acid compositions find use in a variety of different applications and methods, particularly for labeling of biomolecules, cell or cell organelles. Finally, kits for use in such methods and applications are provided.
Owner:ZAKRYTOE AKTSIONERNOE OBSCHESTVO EVROGEN
Method for producing a recombinant protein at high specific productivity, high batch yield and high volumetric yield by means of transient transfection
InactiveUS20080145893A1Increase speedImprove versatilityPeptide/protein ingredientsGenetically modified cellsBiotechnologyEngineering
Recombinant proteins are of great commercial interest. Yet, most current production methods in mammalian cells involve the time- and labor-consuming step of creating stable cell lines. Production methods based on transient gene expression are advantageous in terms of speed and versatility, yet, thus far, those methods have not shown the specific productivity, batch yield and volumetric yield to be an economic alternative to stable cell lines. The inventors improved on the methodology of transient transfection and achieved commercially relevant yields in terms of specific productivity (exceeding 35 pg per cell per day), batch yield (exceeding 700 mg / l) and volumetric yield.
Owner:HILDINGER MARKUS
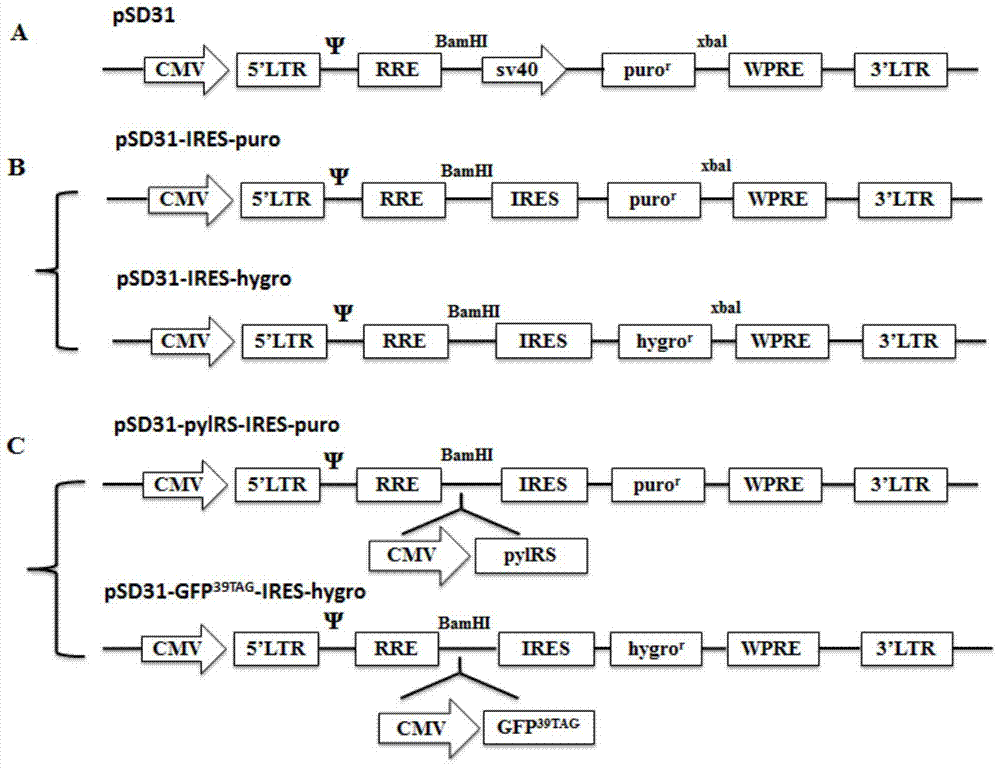
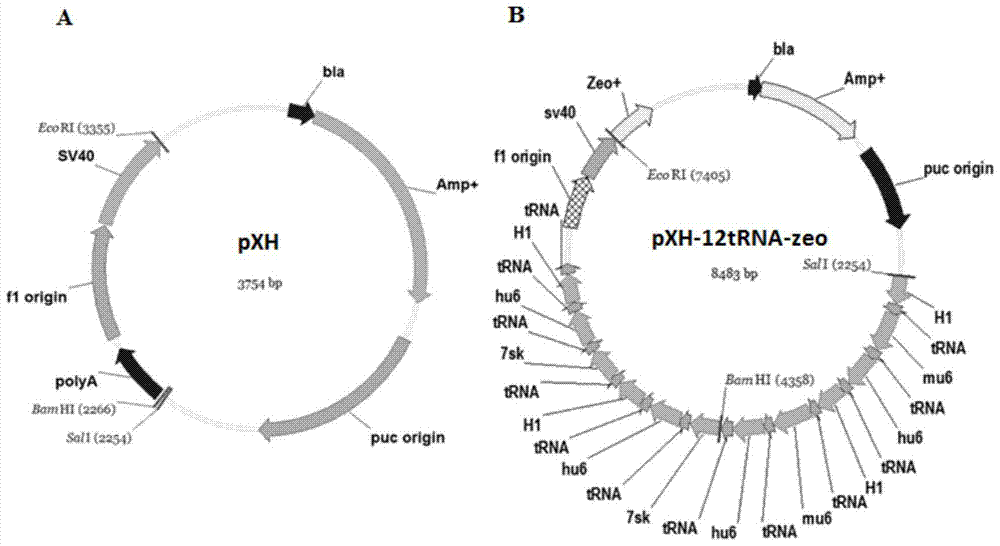
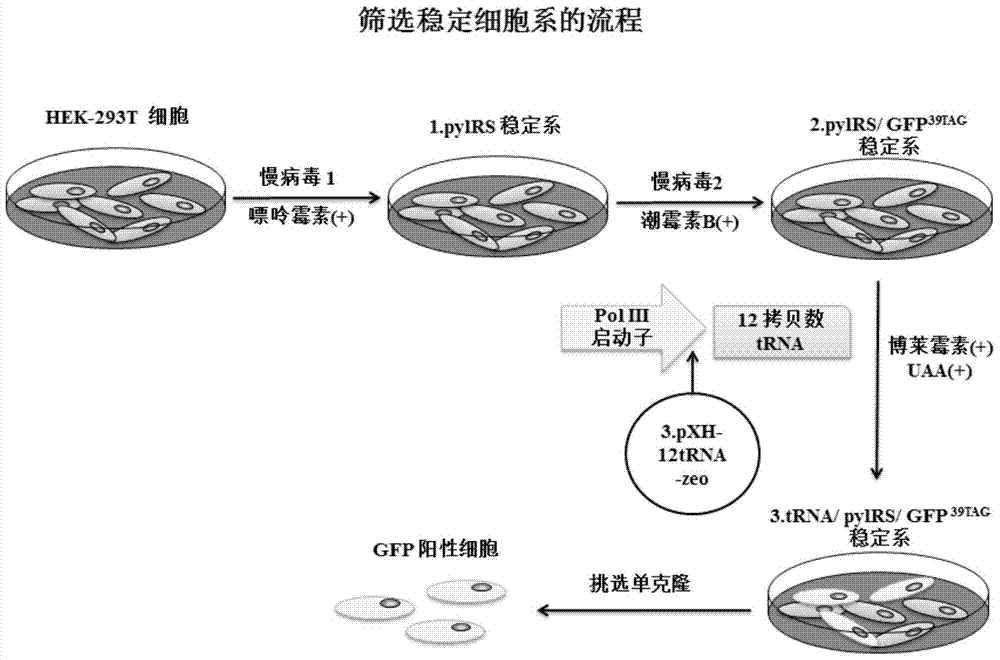
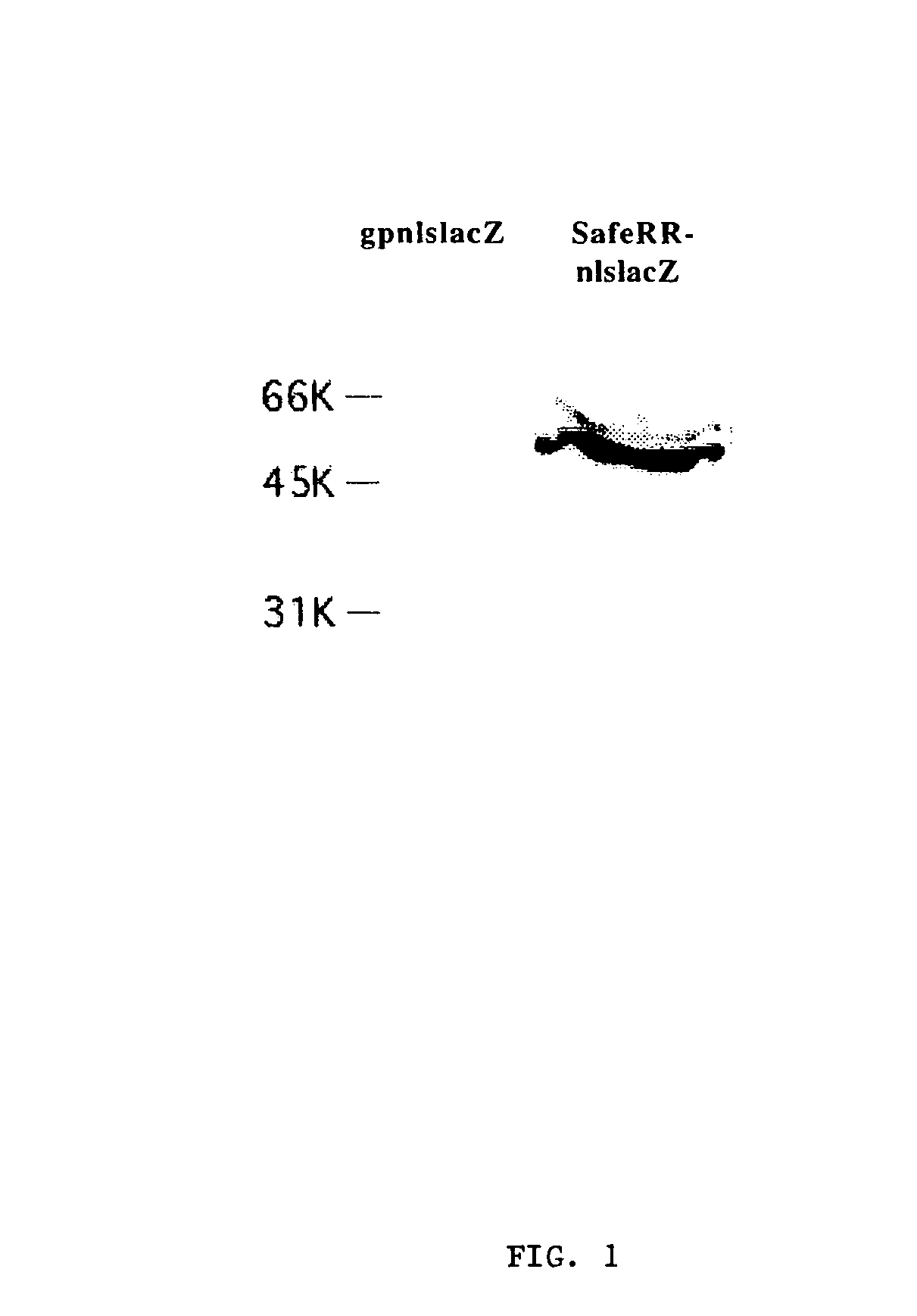
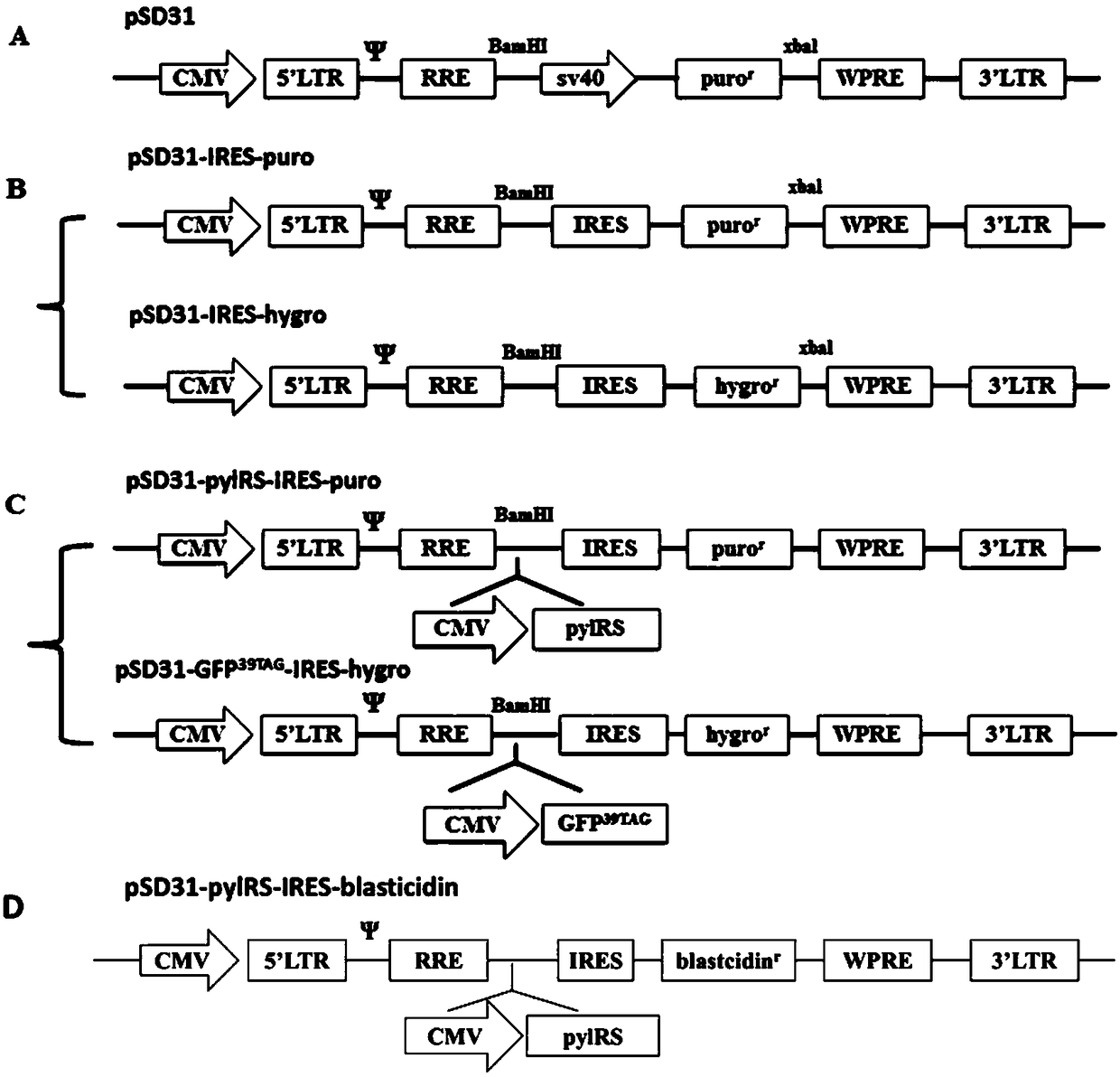
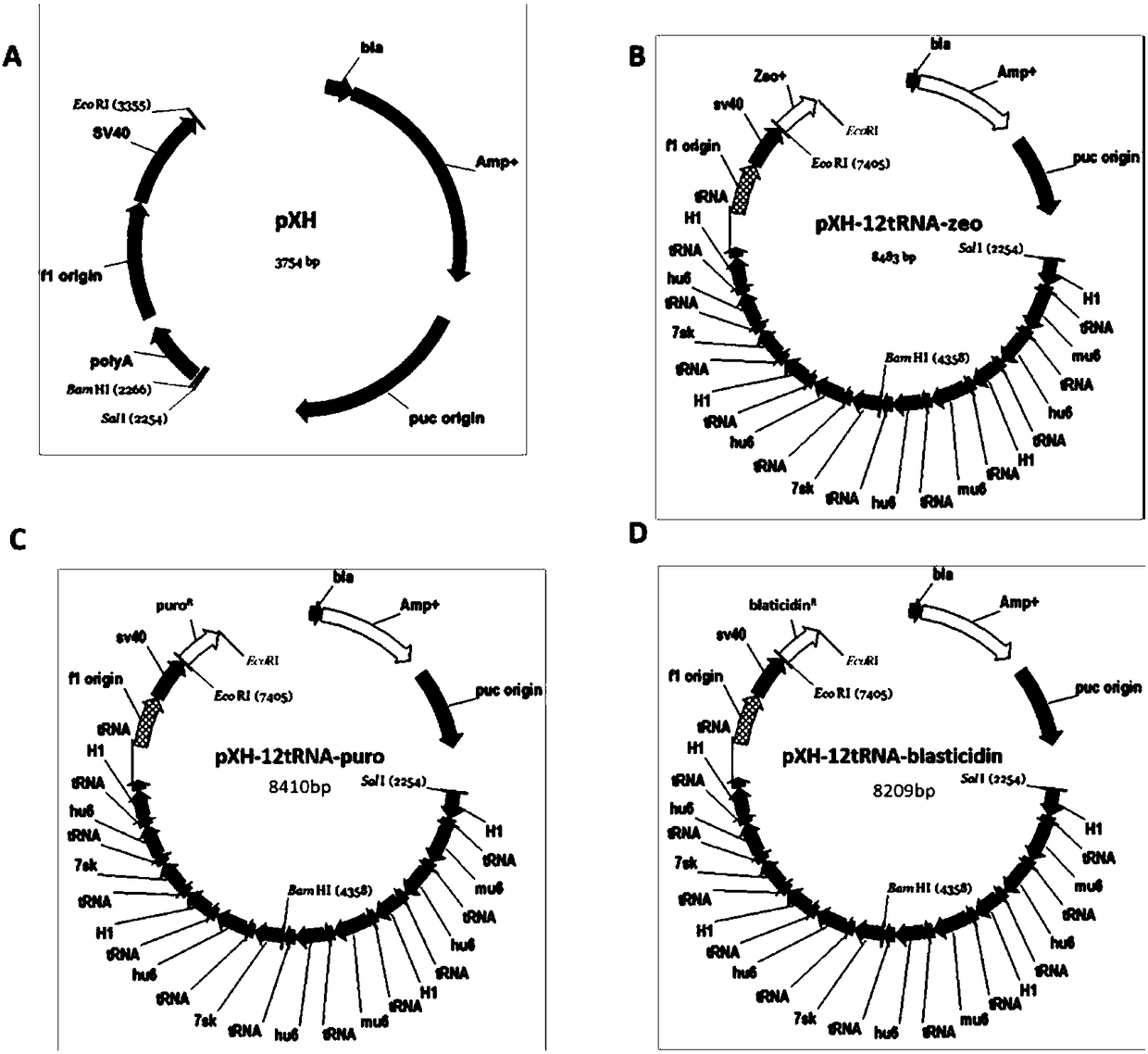
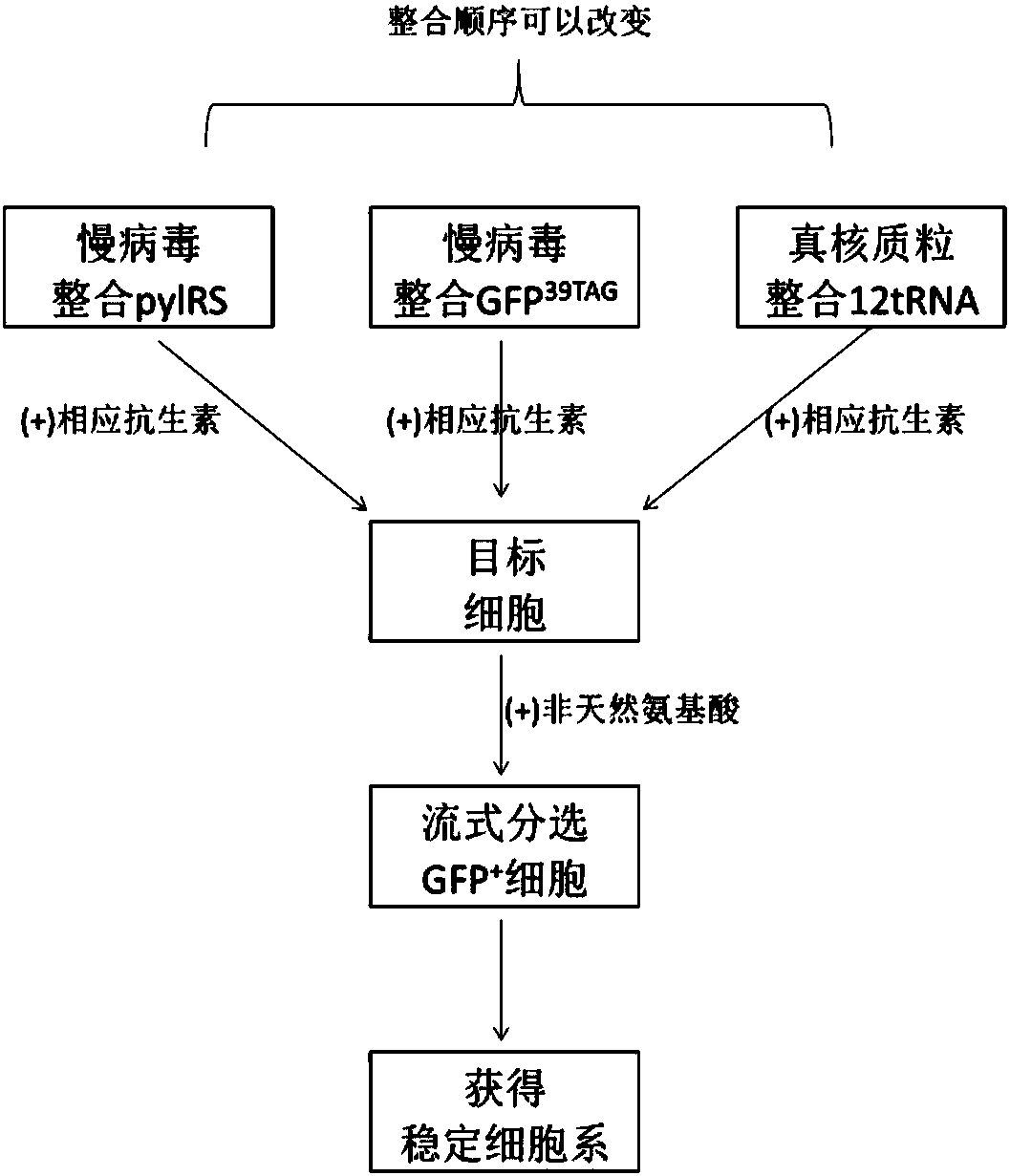
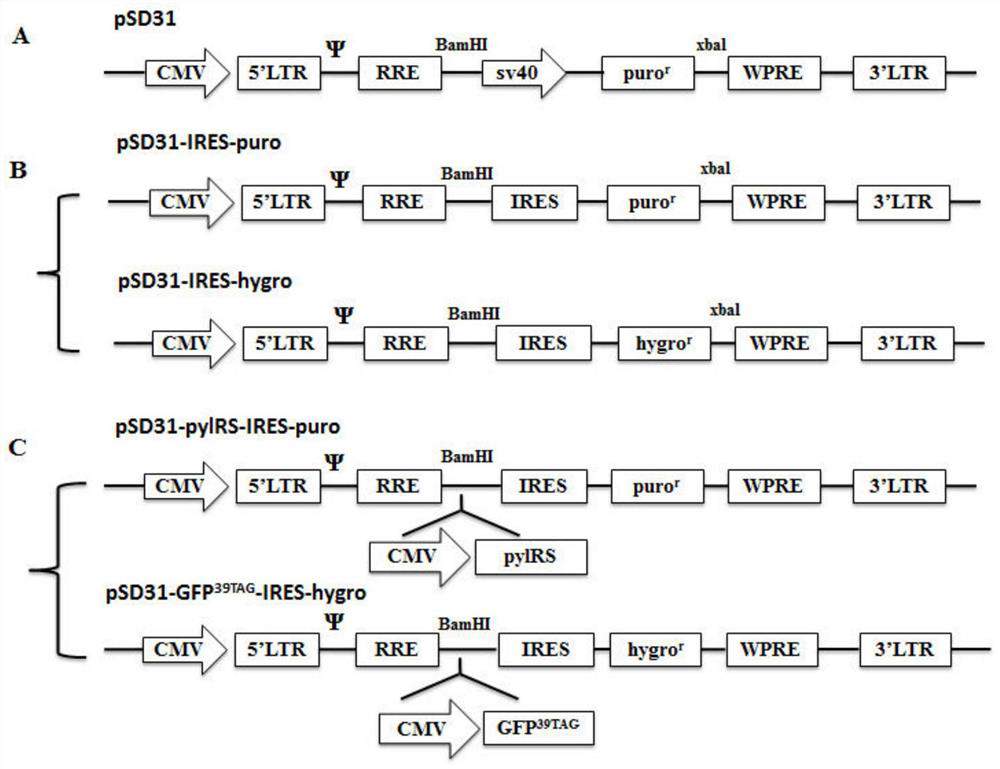
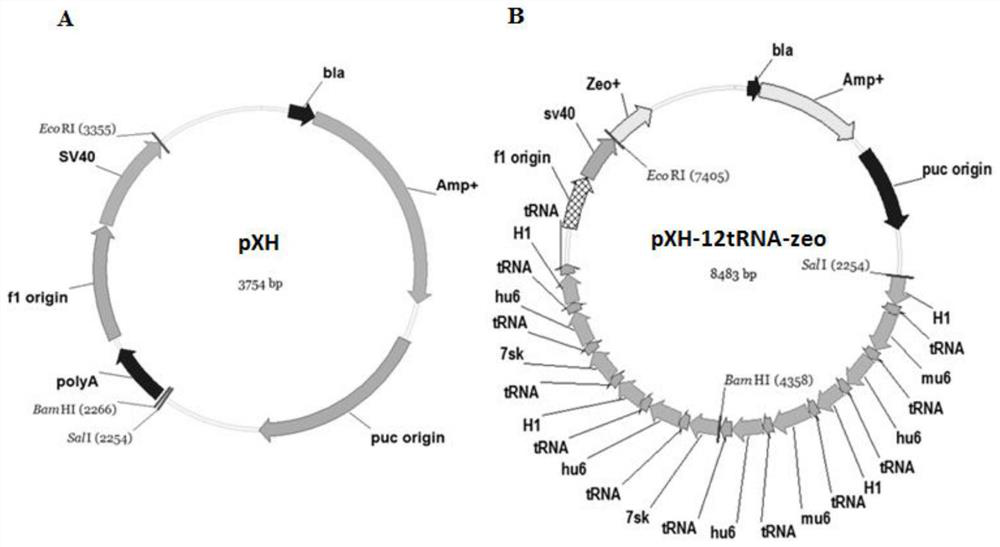
Building of stable cell line carrying orthogonal tRNA/arginyl tRNA synthetase
The invention relates to a building method of a stable cell line carrying orthogonal tRNA / arginyl tRNA synthetase. On the basis of a gene codon expansion technology, a pair of orthogonal tRNA / arginyl tRNA synthetase is used for introducing unnatural amino acid into a fixed point of protein. The invention also relates to a building method of double lentiviral vectors, a building method of a multi-copy-number tRNA carrying carrier and a method for stably integrating the orthogonal tRNA / arginyl tRNA synthetase gene into the cell genome through double lentiviral stable transduction and plasmid stable transfection. The invention further relates to application of the stable cell line, such as the purpose of expressing the target protein containingunnatural amino acid.
Owner:PEKING UNIV
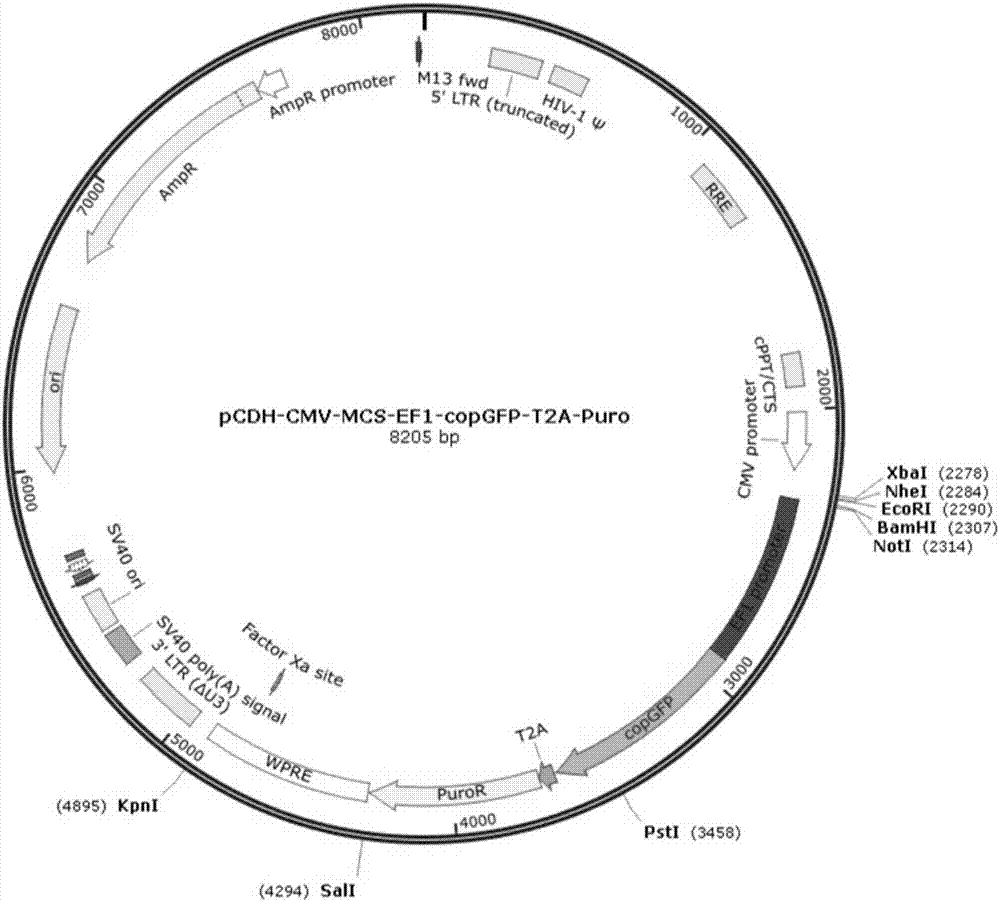
Method for constructing stable cell strain secreting expression protein
PendingCN107043786ALow costImprove efficiencyGenetically modified cellsNucleic acid vectorProtein targetLentivirus
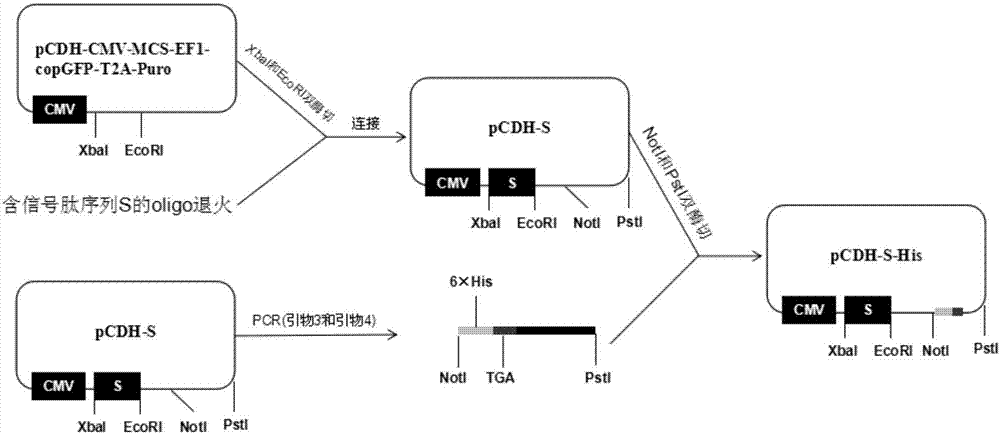
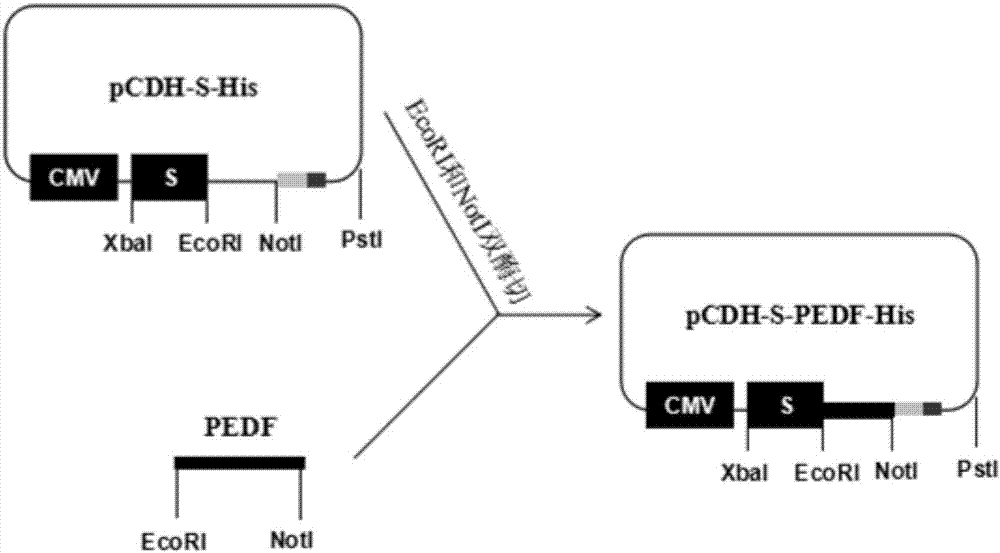
The invention belongs to the field of biotechnology and particularly relates to a method for constructing a stable cell strain secreting an expression protein. The method comprises the following steps: with a recombinant lentiviral vector pCDH-S-His as a skeleton, constructing a target protein gene to the recombinant lentiviral vector pCDH-S-His to obtain a recombinant plasmid pCDH-S-P-His; co-transfecting the recombinant plasmid and helper plasmids pSPAX2 and pMD2.G with 293T cells by using a PEI transfection method to obtain a recombinant lentivirus; transducing the obtained lentivirus to the 293T cells, and performing puromycin screening to obtain a plurality of monoclonal cell strains; detecting the content of target protein in each cell supernatant by an Elisa method; determining a high-expression cell strain. Compared with a traditional plasmid transfection method, the method provided by the invention has the advantages of short experimental period, high positive rate and good stability of the cell strain.
Owner:合肥知恩生物技术有限公司
Canine distemper virus (CDV) sensitive cell line and establishment method and application thereof
InactiveCN102807970AMicrobiological testing/measurementMicroorganism based processesMadin Darby canine kidney cellCanine distemper virus CDV
The invention discloses a canine distemper virus (CDV) sensitive cell line and an establishment method and application thereof, and belongs to the technical field of biology. According to the hundestaupe virus sensitive cell line, lentivirus four-plasmid packaging system is adopted to obtain a strain of stable cell line madin-darby canine kidney-cell isolate (MDCK-CSL) which expresses canine source signal lymphocyte activating molecules (SLAM), wherein the conservation number of strains is CGMCC No. 5881. The comparison of the propagation conditions of a CDV standard strain on the MDCK-CSL and MDCK cells indicates that after the CDV-Snyder Hill standard strain infects the MDCK-CSL cell line for 24 hours, cytopathy of the fusion, rounding contraction, obvious death and the like of cells can be observed; and the MDCK cells infected for 6 days continuously grow slowly, and the cytopathy does not occur. The CDV sensitive cell line MDCK-CSL provides a technical platform for the separation of CDV wild strains and the complete study of biological properties of the CDV wild strains, and also establishes a foundation for the prevention and control of canine distemper.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
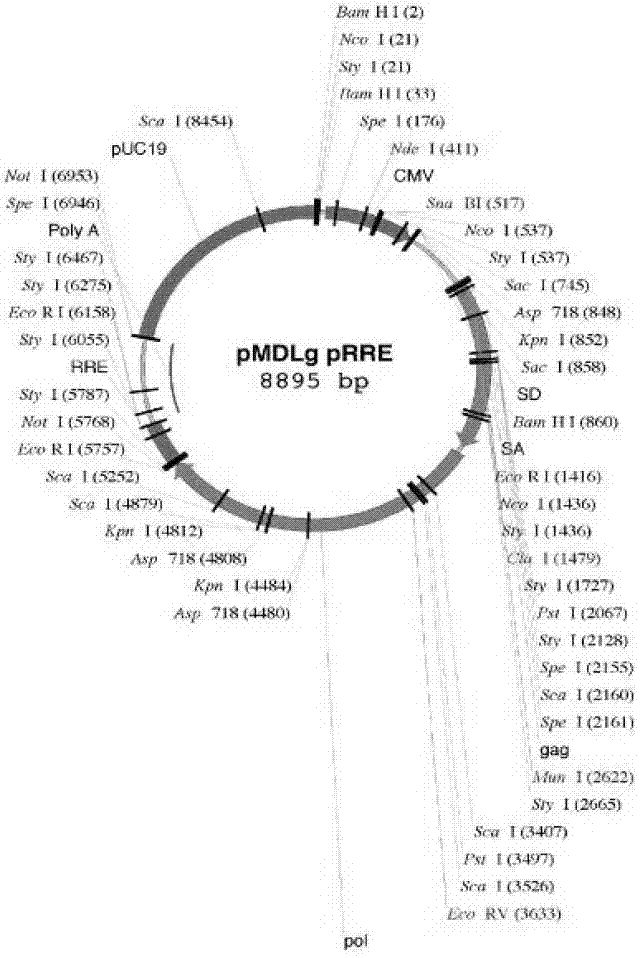
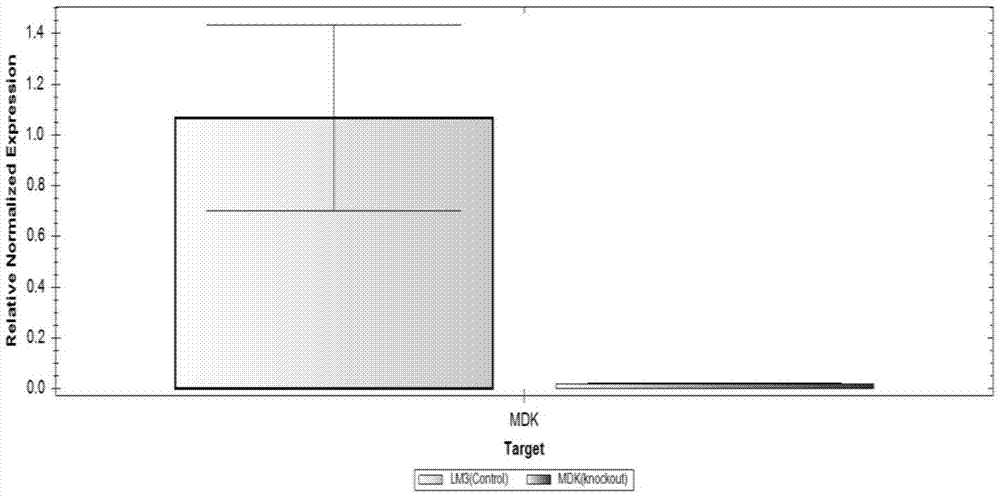
LM3 cell line with Midkine stable low expression and construction method of LM3 cell line
InactiveCN106867967AStable expressionHigh transfection efficiencyTumor/cancer cellsGenetic engineeringLiquid mediumStable cell line
The invention relates to the field of biomedical research, and in particular to a construction method of an LM3 stable cell line with Midkine low expression. The LM3 cell line with Midkine stable low expression is constructed by virtue of an RNA interference technology. The construction method comprises the following steps: conducting primer design and target fragment amplification, and constructing Midkine shRNA recombinant plasmid; then, collecting a liquid medium containing viruses, filtering the liquid medium, adding the filtered liquid medium to a culture dish inoculated with LM3 cells in advance and conducting co-culture; and finally, conducting Puromycin resistance screening and sub-culturing on the LM3 cells accepting transfection, so that the LM3 stable cell line with Midkine low expression is finally obtained. With the establishment of the cell line, a new experimental material is provided for researching a molecular action mechanism of Midkine in tumors and a regulatory effect of the Midkine in tumor energy metabolism.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
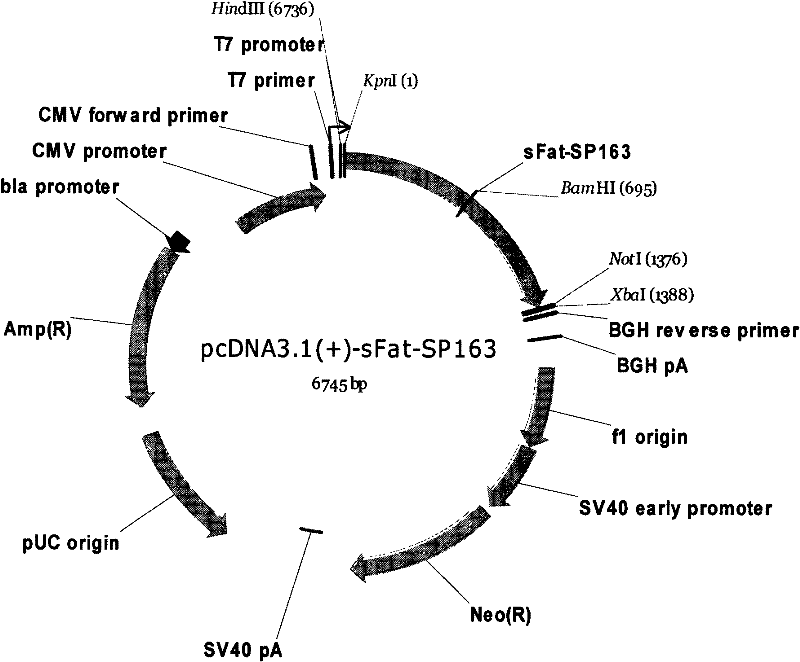
Novel vector for raising content of polyunsaturated fatty acids in animal body
InactiveCN102206675AIncrease contentVector-based foreign material introductionAnimal husbandryBiotechnologyOMEGA-3 POLYUNSATURATED FATTY ACIDS
The invention discloses a novel vector for raising the content of polyunsaturated fatty acids in an animal body. The expression vector provided by the invention can simultaneously transcribe and express omega-3 fatty acid dehydrogenase and delta12 fatty acid dehydrogenase, and the vector sequence contains gene sequences of Sequence NO.1 and Sequence NO.2. In addition, the sequences of the enhancer SP163 and IRES are added between the sFat and Fat2 genes, and the vector provided by the invention can highly express two types of dehydrogenase at the same time. The vector provided by the invention is also transfected into the CHO cells, followed by the screening of antibiotics to obtain a stable cell line for highly expressing the sFat and Fat2 genes with the gene expression amount being 105 times that of untransfected cells. The invention also discloses a concrete method for raising offspring animals having high content of polyunsaturated fatty acids by using the vector. The vector provided by the invention lays a foundation for producing animal products having high content of omega-3 polyunsaturated fatty acids and improving the quality of meat products or dairy products by producing transgenic livestock.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
Stable cell line MDCK for amplifying recombinant canine adenovirus (CAV2) and construction method of stable cell line
InactiveCN108342362ASolve problems that are difficult to expressImprove stabilityGenetically modified cellsVirus peptidesLentivirusTiter
The invention provides a stable cell line for expressing canine adenovirus genes E1A and E1B and a construction method and application of the stable cell line. The two genes E1A and E1B in an E1 region are constructed in a lentivirus carrier, then an MDCK cell stably expresses the genes E1A and E1B respectively, and the MDCK-E1A-E1B stable cell line is constructed. The E1 gene which is long in segment and not easy to construct is split into the genes E1A and E1B which are short in segment and easy to construct, so that the success rate of constructing stable cells is increased; the genes E1A and E1B carry fluorescent proteins with different colors so that the stability of the stable cells can be monitored in real time. Experiments prove that the MDCK-E1A-E1B stable cell line is higher in titer of the amplified canine adenovirus than that of an MDCK-E1 stable cell line, and a good foundation is laid for application of the canine adenovirus in the fields of virus live carrier vaccines, gene therapy, cancer treatment and the like.
Owner:BRAINVTA (WUHAN) CO LTD
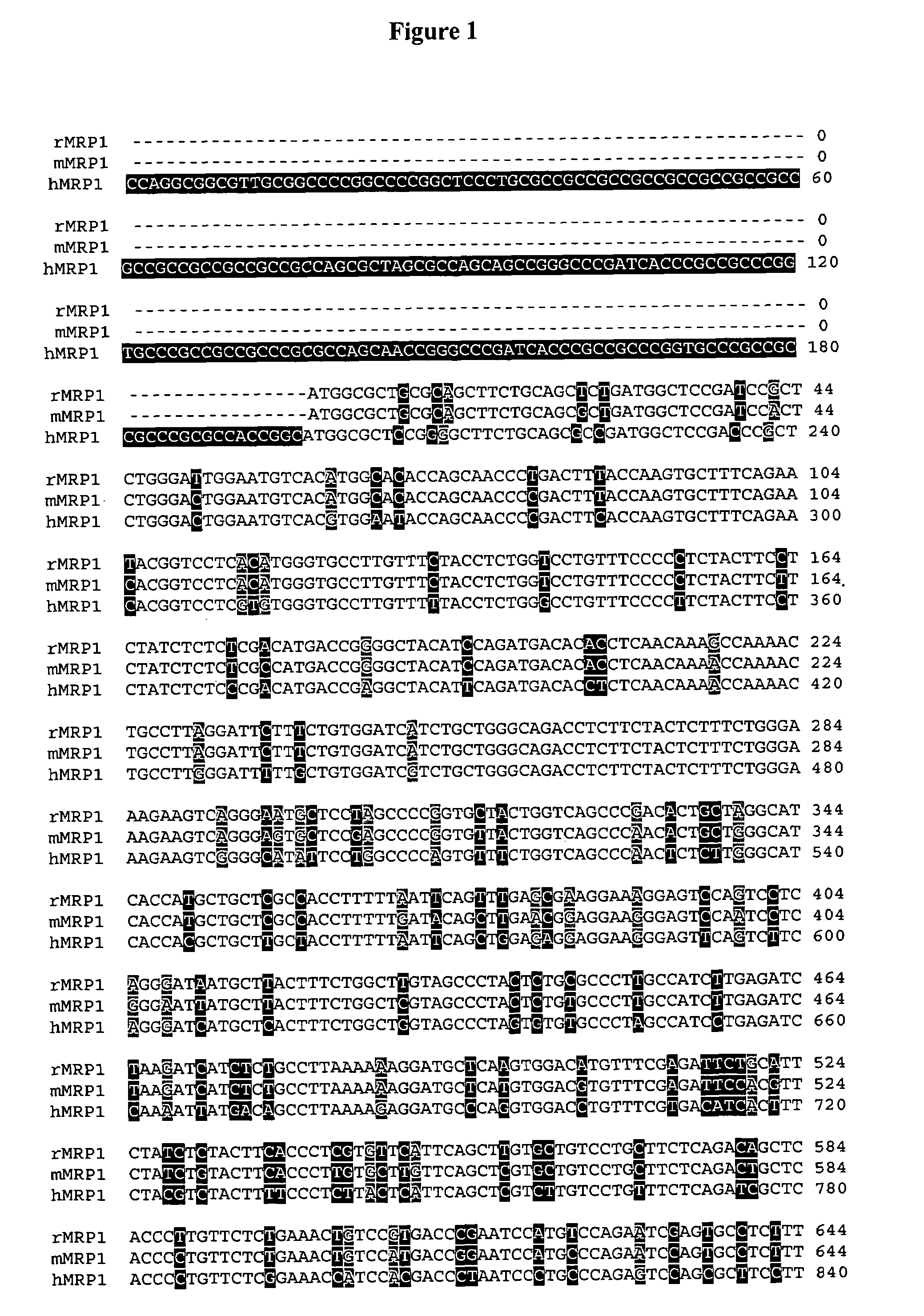
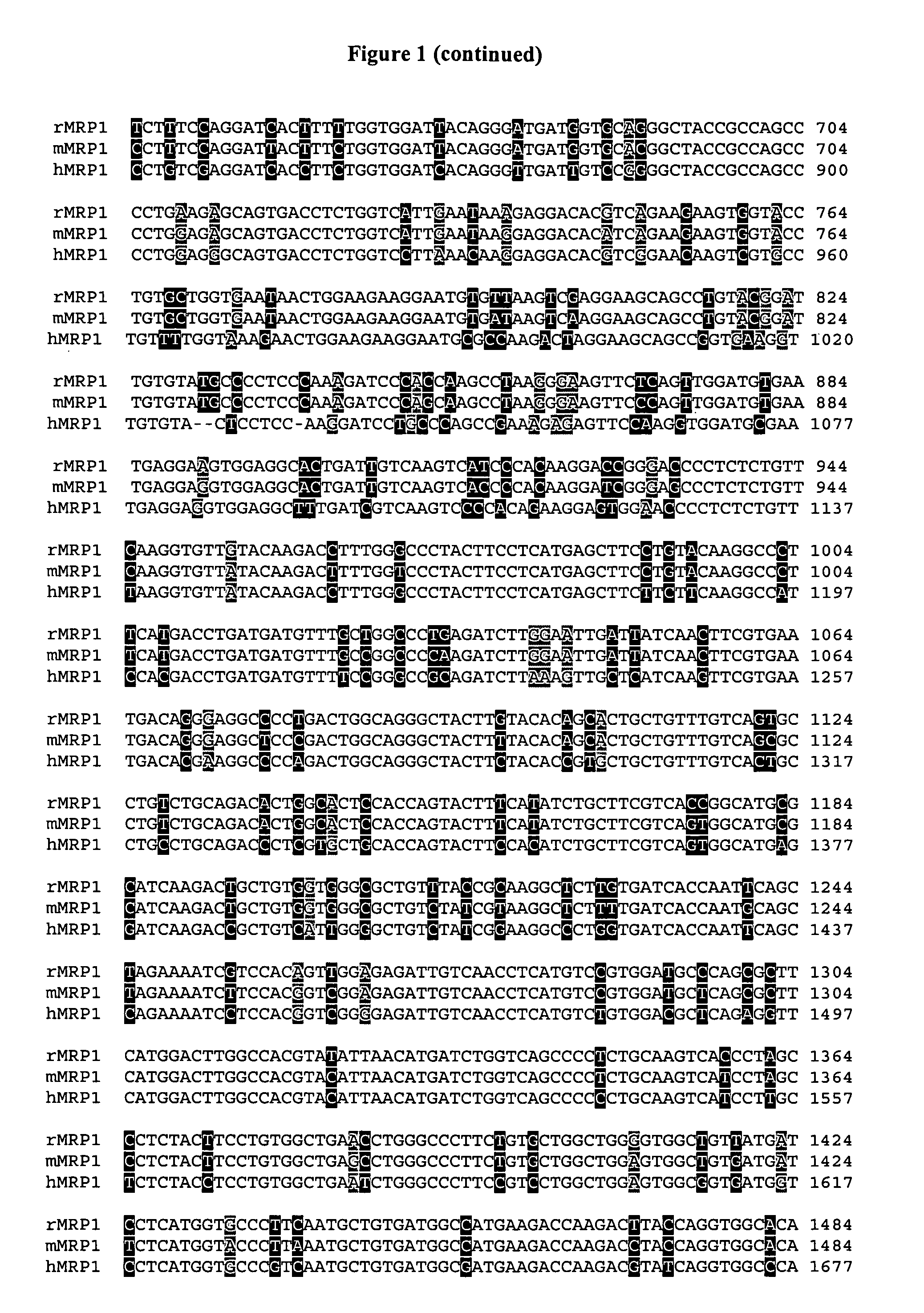
Sequence variants of multi-drug resistance genes, MDR1 and MRP1, and methods for assessment of drug penetration and disposition
InactiveUS7445897B2Cell receptors/surface-antigens/surface-determinantsMicrobiological testing/measurementMRP1 ProteinMammal
Owner:UNIV OF WASHINGTON
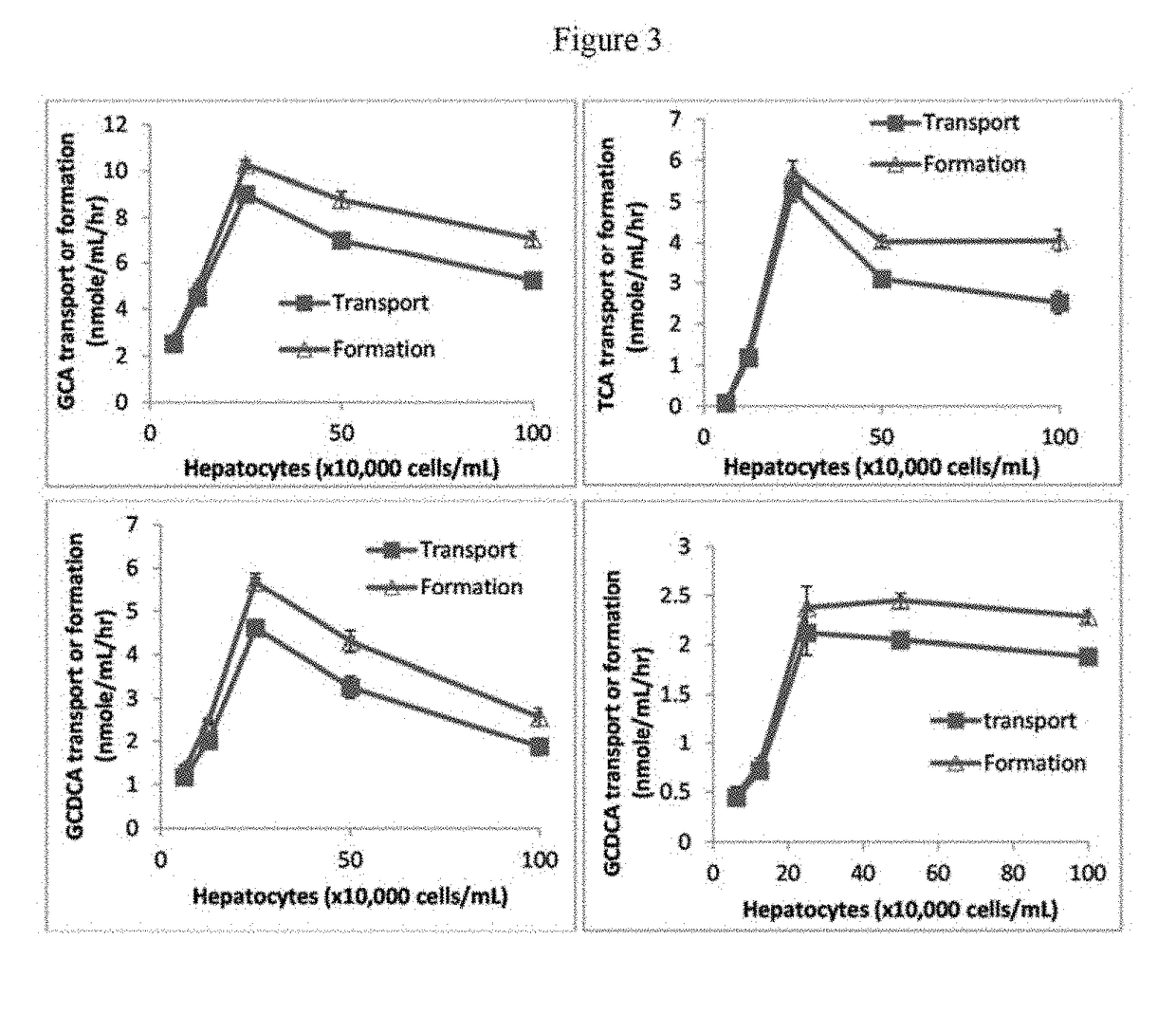
Method for measuring bile salt export transport and/or formation activity
A method is provided to measure modulation of bile salt export transport and / or formation activity in hepatocyte or stable cell line preparations by test agents including but not limited to drugs, drug candidates, biologicals, food components, herb or plant components, proteins, peptides, DNA, RNA. Furthermore, the method is to determine modulation of bile salt export transport and / or formation activity not only by said test agents, but further their metabolites or biotransformed products formed in situ. The bile salt export transport and / or formation activity modulation includes but not limited to inhibition, induction, activation and / or regulation. The method can be practiced to identify test agents, which have potential to cause liver injury, drug-drug interactions, and / or can be used as therapeutic agents for the treatment of cholestasis, abnormality of bile salt metabolism, liver diseases and cholesterol abnormality.
Owner:BIOTRANEX
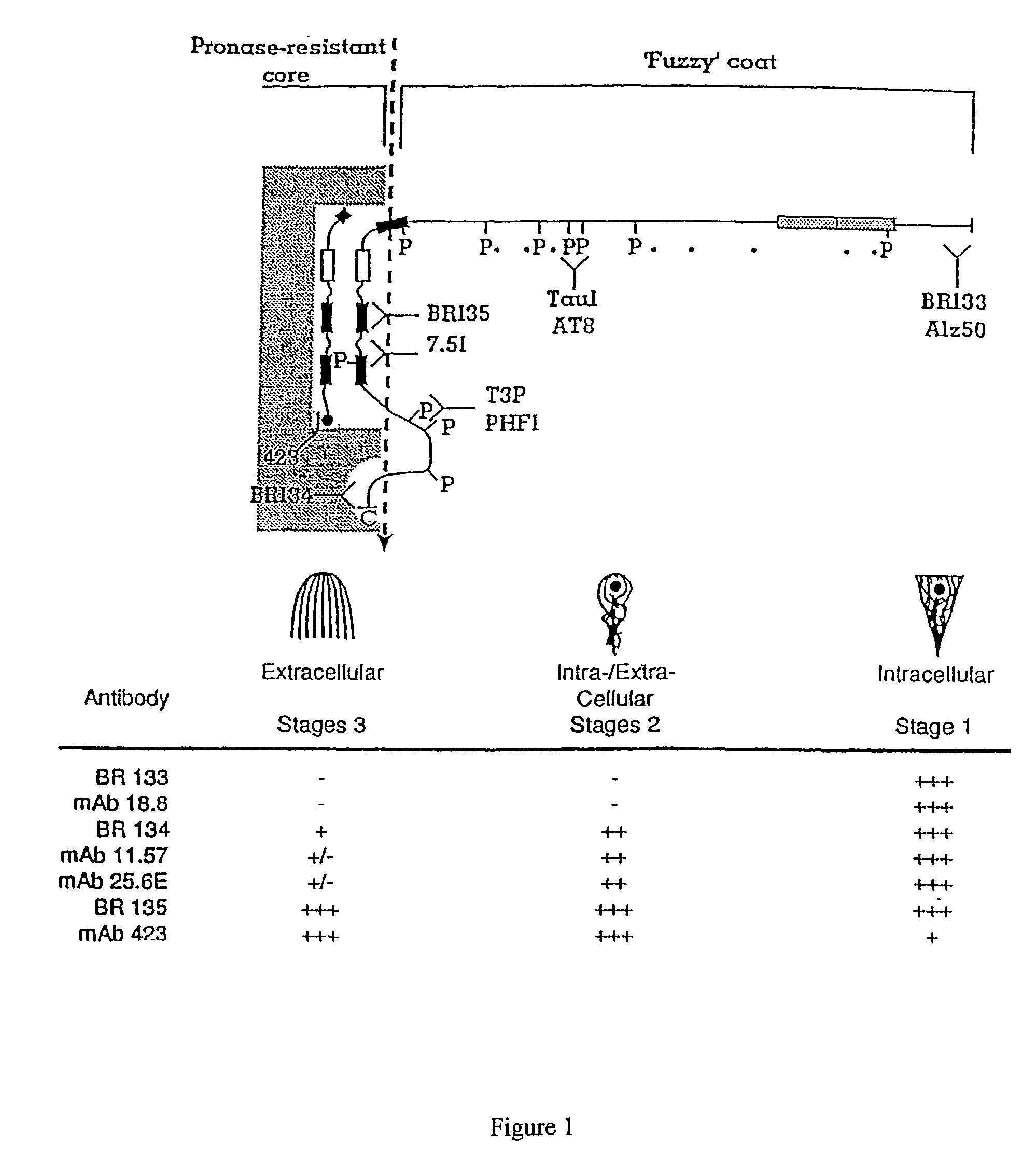
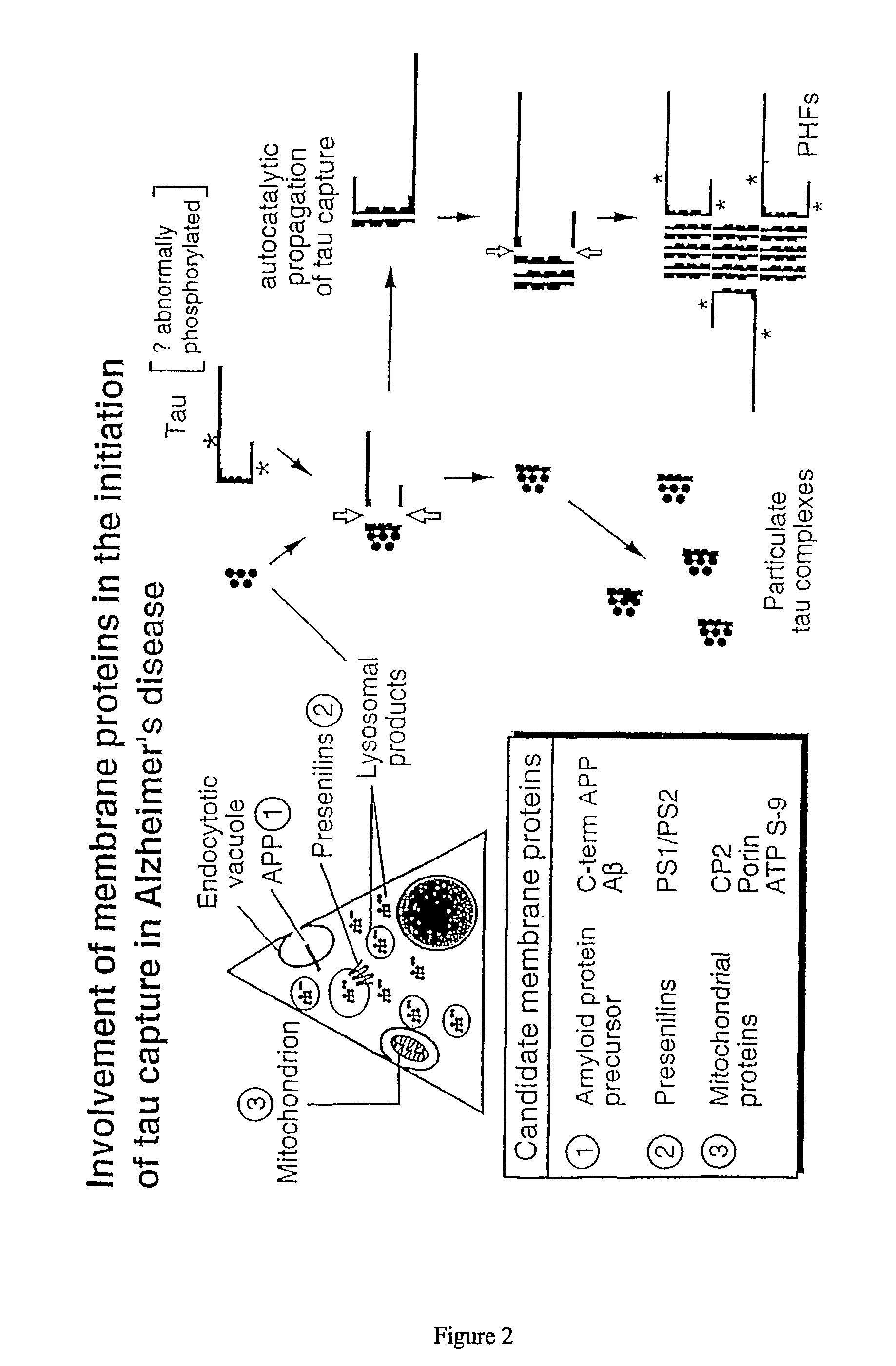
Materials and methods relating to protein aggregation in neurodegenerative disease
InactiveUS7335505B2Bioreactor/fermenter combinationsOrganic active ingredientsCell AggregationsProtein aggregation
The present invention provides methods of proteolytically converting a precursor protein (e.g. tau) to a product fragment (e.g., a 12 kd fragment) in a stable cell line, wherein the precursor protein is associated with a disease state in which the precursor protein aggregates pathologically (e.g. a tauopathy), and the methods comprise: (a) providing a stable cell line transfected with nucleic acid encoding: (i) a template fragment of the precursor protein such that the template fragment is constitutively expressed in the cell at a level which is not toxic to the cell; and (ii) the precursor protein, which protein is inducibly expressed in the cell in response to a stimulus, whereby interaction of the template fragment with the precursor protein causes a conformational change in the precursor protein such as to cause aggregation and proteolytic processing of the precursor protein to the product fragment. The method is preferably used to screen for modulators of the aggregation process by monitoring production (or modulation of production) of the product band or bands. Also provided are materials for used in the assays, plus medicaments, and related uses and processes, based on compounds which show high activity in the assay of the invention e.g. reduced diaminophenothiazines.
Owner:WISTA LAB LTD
Methods for identifying inhibitors of botulinum neurotoxins
A system and method for identifying a botulinum neurotoxin inhibitor employing a botulinum neurotoxin substrate complex having a peptide substrate, preferably SNAP-25, a reporter domain on one side of said peptide substrate and an immobilization domain on the opposite side of said peptide substrate. The botulinum neurotoxin inhibitor is identified by its ability to decrease the relative amount of cleaved complex, detected through measuring a decrease in complex bound to a solid support. The method of the present invention also utilizes novel cells that express a botulinum neurotoxin substrate complex. The methods of the present invention are adapted for cell based screening to monitor the catalytic activity of a BoNT in living cells and to identify molecules that inhibit the catalytic activity of a BoNT in living cells. Also provided are novel stable cell lines that express the botulinum toxin substrate complex and viral vectors capable of efficiently expressing an active light chain of the BoNT within mammalian cells.
Owner:BOTX TECH LLC
Rabies virus labeling whole brain area neural network structure, and preparation and application thereof
ActiveCN108359640AStable passageIncrease productionGenetically modified cellsFermentationInjection siteLentivirus
The invention belongs to the field of construction of stable cell lines, and more specifically, relates to a rabies virus labeling a whole brain area neural network structure, and preparation and an application thereof. A third-generation lentivirus technology is used for completing preparation of a BHK-N2C(G) cell line, that is to say, through construction of an FUGW-H2B-GFP-P2A-N2C(G) lentivirusvector and virus packaging, BHK cells are infected to obtain BHK-N2C(G) cells with nuclear localization fluorescence, and the cells are used for production of the defective recombinant rabies virus.The BHK-N2C(G) cells are infected with an RV-[delta]G-X virus solution, and then an RV-N2C(G)-[delta]G-X product is prepared by amplification and purification. In-vivo test results indicate that the prepared recombinant defective rabies virus RV-N2C(G)-[delta]G-X has no obvious inflammation reaction at an injection site, and has more reverse projection areas and high reverse projection efficiency.
Owner:BRAINVTA (WUHAN) CO LTD
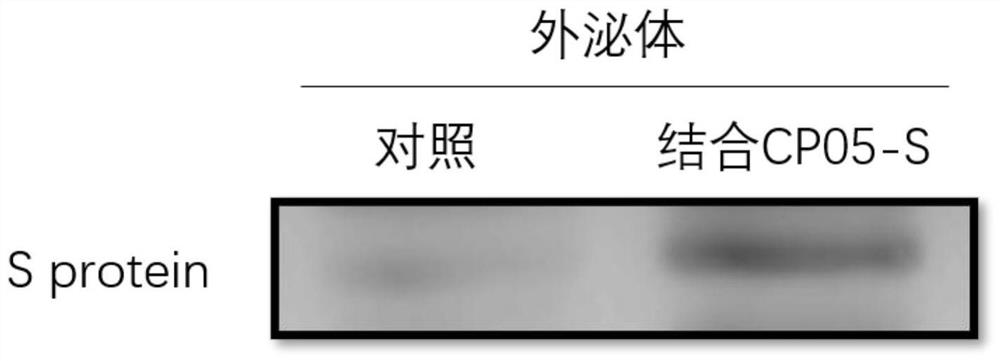
Exosome coupled with coronavirus S protein on surface and preparation method for exosome and application of exosome
InactiveCN111647557AImprove securityAvoid the risk of infectionSsRNA viruses positive-senseViral antigen ingredientsCD63Receptor
The invention provides an exosome coupled with coronavirus S protein on the surface and a preparation method for the exosome and application of the exosome, and belongs to the technical field of biological pharmacy. A stable cell line is obtained through transient transfection of human cells or infection with a recombinant lentivirus to achieve recombinant expression of aptamer CP05-S protein, andthe aptamer CP05-S protein is mixed with human plasma for incubation, and the exosome is bound to an aptamer CP05 through surface CD63 membrane molecules to form the exosome coupled with the S protein on the surface. The exosome provided by the invention is employed to present antigen S protein, so that a prepared vaccine avoids the toxicity of an immunologic adjuvant and the risk of virus vaccine infection, and loading the S protein to the surface of the exosome to prepare the vaccine is an ideal vaccine development strategy, and is also an ideal strategy for blocking a virus invasion route;and meanwhile, the S protein is delivered through the exosome, and thus, competitive inhibition can be performed on binding of the S protein of the novel coronavirus to a receptor, the blocking action is exerted, and invasion of the novel coronavirus is effectively blocked, and accordingly, as an inhibitor for novel coronavirus infection, the exosome coupled with the S protein can be used for treatment of acute novel coronavirus pneumonia.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Mammalian cell expression vectors and utilization
An improved mammalian expression vector system which allows: (1) highly expressing exogenous proteins in host mammalian cells; (2) rapidly and efficiently screening recombinant stable cell lines expressing the gene of interest; (3) maintain sustainable expression and prevent gene silencing; and (4) effectively secreting proteins into media in some of cases. The entire expression vector system includes optimized promoters and core promoters, the use of special internal ribosome entry sites, integration of the bacterial backbone into the mammalian expression unit, multiple choices of selection markers, artificial matrix attachment region elements, effective secreting lead sequences and their 5′ and 3′ UTRs, and proper combinations of these expression elements.
Owner:ATGCELL
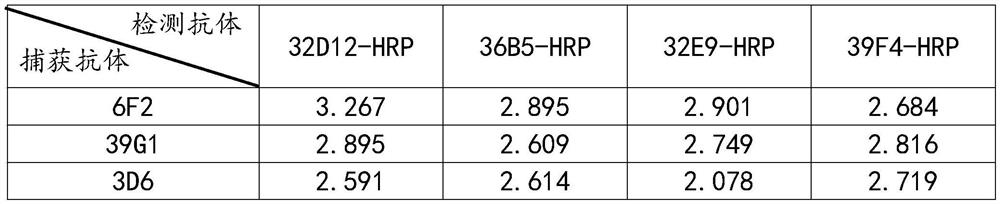
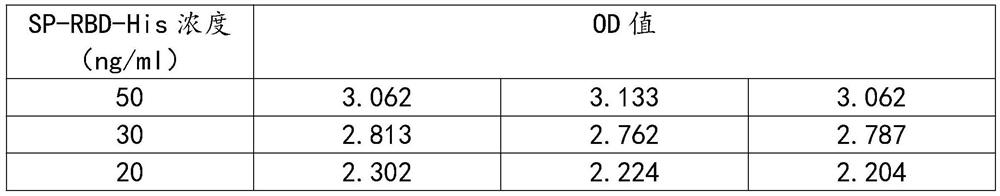
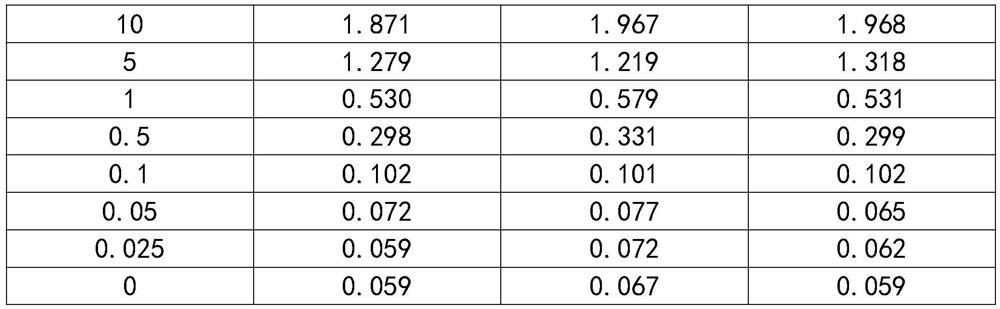
Method for preparing monoclonal antibody of spike protein of novel coronavirus
The invention discloses a method for preparing a monoclonal antibody of a spike protein of novel coronavirus. The method comprises the following steps: antigen preparing, animal immunizing, cell fusing, screening detecting, stable cell line establishing and monoclonal antibody producing. The spike protein is a superficial membrane protein of SARS-CoV-2 and contains two subunits S1 and S2, the S1 mainly comprises one receptor binding domain and is responsible for identifying a cell surface receptor, and the S2 comprises fundamental elements required for membrane fusion. The S protein reacts with an antibody and T cells in induction and plays a crucial role in protective immunization, so that the monoclonal antibody of the spike protein can be excellently bound to the spike protein to form acomplex, the complex can be digested or degraded, then, the SARS-CoV-2 virus can be deactivated, and the effect of killing the SARS-CoV-2 virus is achieved.
Owner:通用生物(安徽)股份有限公司
Methods for packaging propagation-defective vesicular stomatitis virus vectors using a stable cell line that expresses g protein
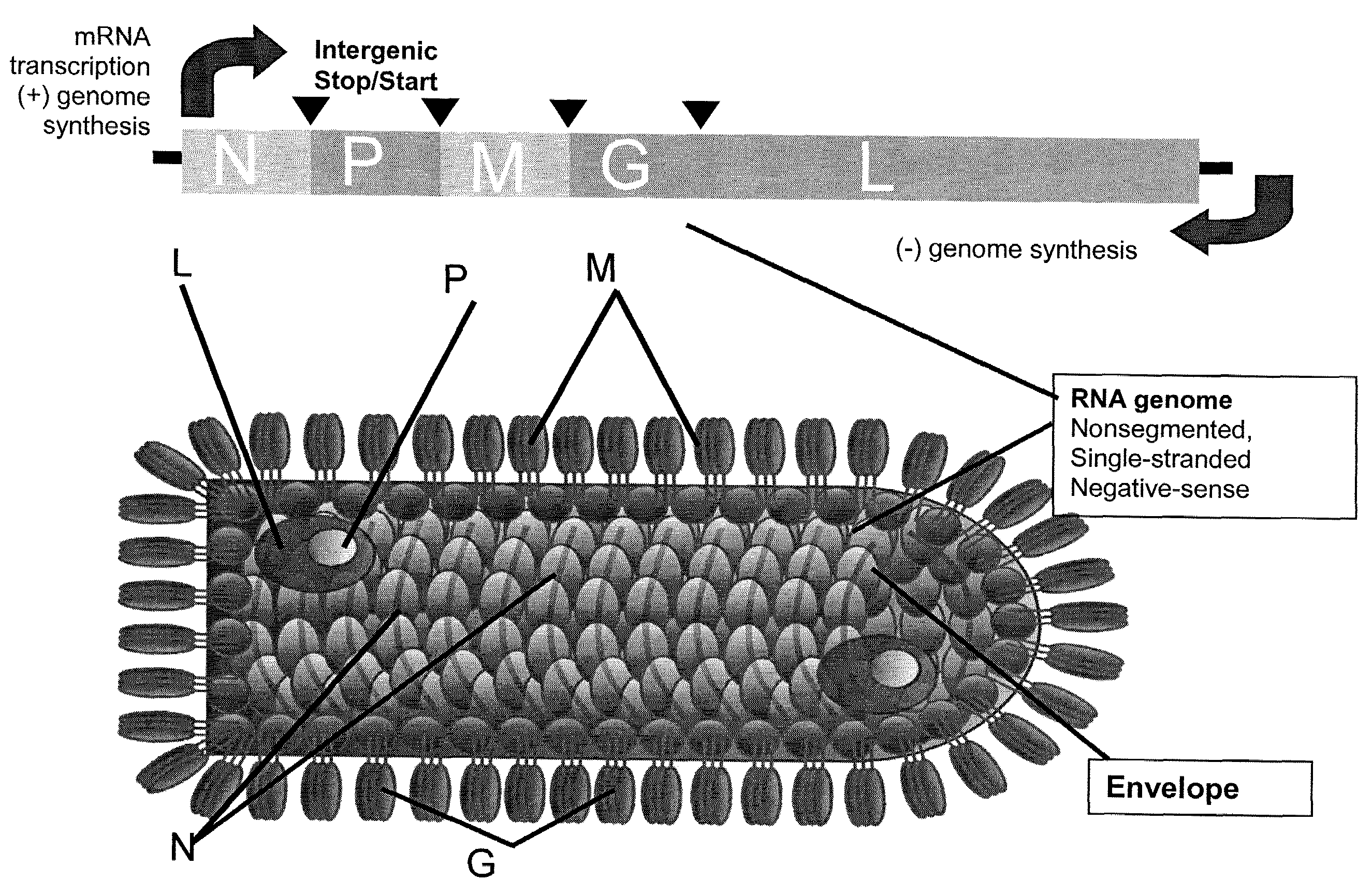
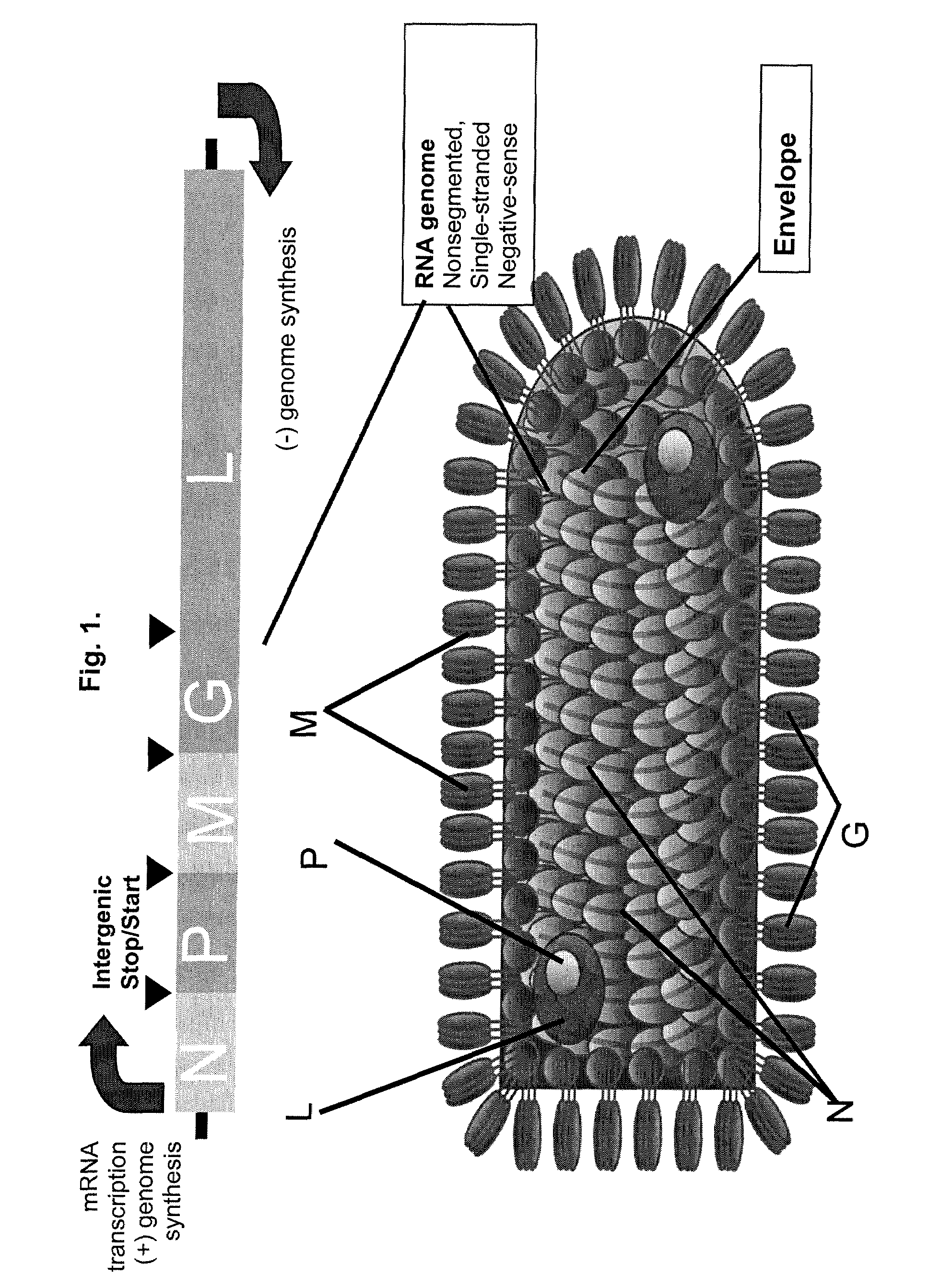
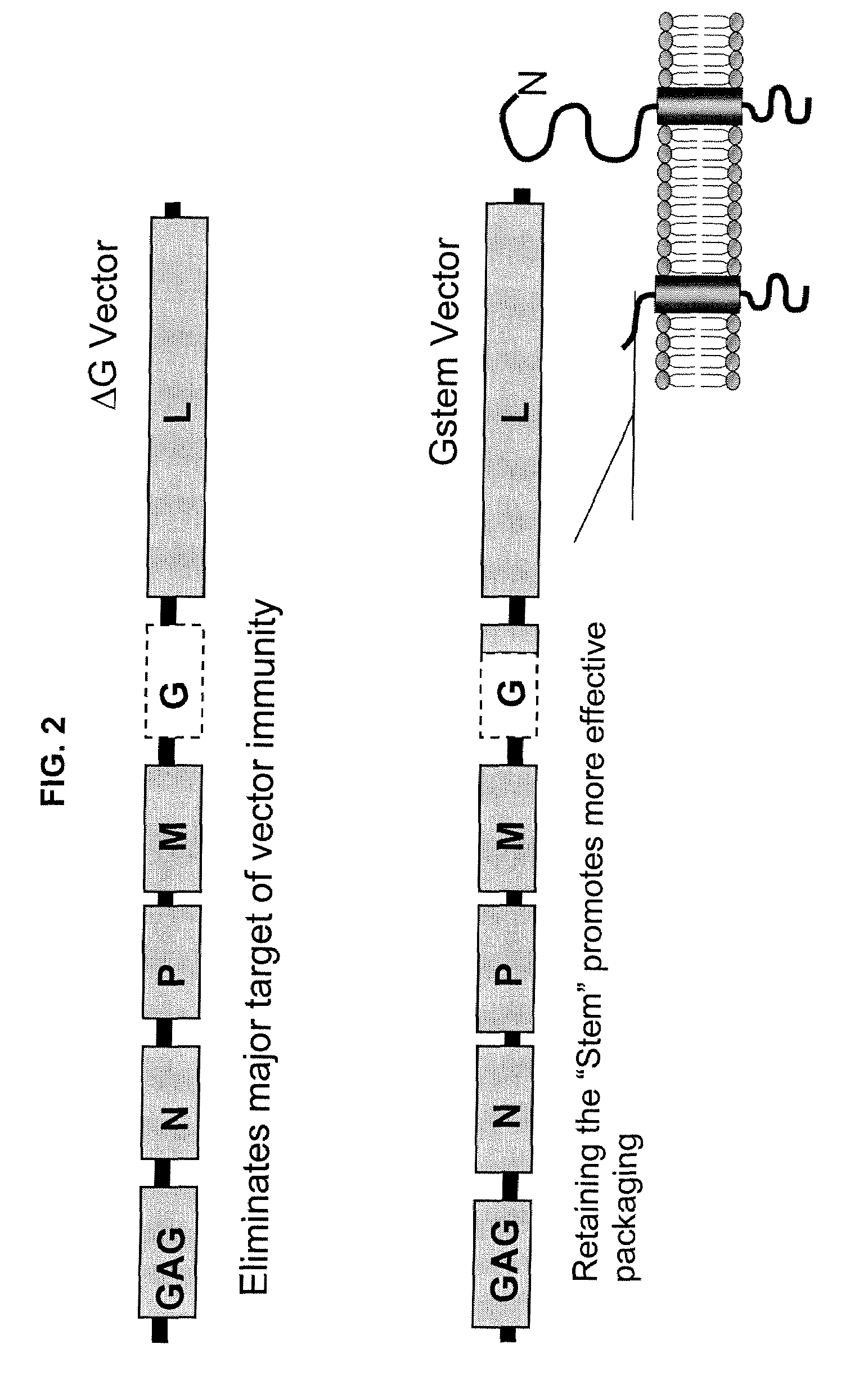
InactiveUS20090162321A1Improving the packaging of a propagation-defective Vesicular Stomatitis VirusSsRNA viruses negative-senseBiocideInfected cellStable cell line
A method of producing propagation-defective Vesicular Stomatitis Virus (VSV) is provided. The method involves providing a cell that includes an optimized VSV G gene, wherein expression of VSV G protein from the optimized VSV G gene is inducible; and inducing the cell to express VSV G protein from the optimized VSV G gene. The method also involves infecting the induced cell with an attenuated VSV; growing the infected cells in culture; and recovering attenuated VSV from the culture.
Owner:WYETH LLC
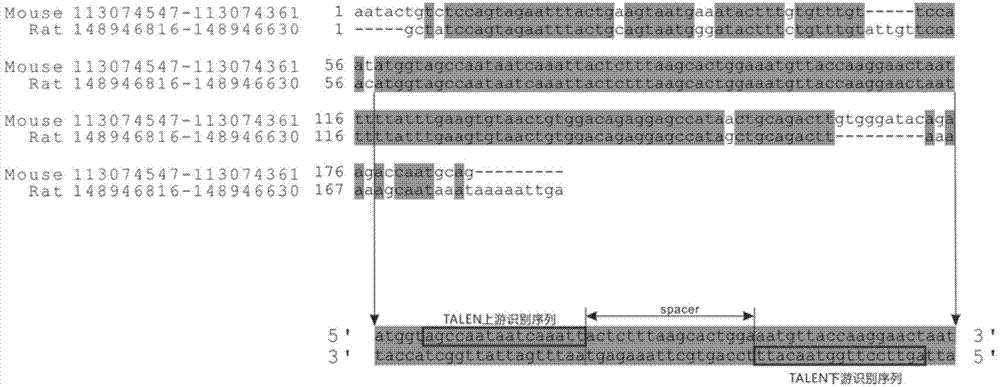
Method of target-integrating foreign DNA (Deoxyribonucleic Acid) sequence to Rosa26 sites of rat and mouse as well as application thereof
ActiveCN103540587AClear genetic backgroundStable exogenous expressionMicrobiological testing/measurementVector-based foreign material introductionCell strainStable cell line
The invention discloses a method of target-integrating a foreign DNA (Deoxyribonucleic Acid) sequence to Rosa26 sites of rats and mice as well as application thereof. According to the invention, a stable cell line can be obtained for expressing various foreign genes through constructed TALEN plasmid, rat homologous recombination plasmid and mouse homologous recombination plasmid by means of respectively transferring corresponding plasmids to cells of rats and mice. The stable cell line obtained can be stably handed from generation to generation with clear genetic background, so that the biological function of the foreign gene is accurately represented.
Owner:OBIO TECH SHANGHAI CORP LTD
Methods and Compositions for Generating Stable Transfected Cells
Methods and compositions are provided involving high producing cell lines. Embodiments concern efficient methods for screening for such cell lines and for creating such cell lines. These cell lines can be used to create large amounts of protein. To quickly generate large quantity of recombinant proteins or vaccines for both pre-clinical study and clinical trials, almost all drug development will face the same challenging obstacle of rapidly generating a high stable producer. Developing and identifying a stable cell line is a critical part of biopharmaceutical development.
Owner:MAXCYTE
Pseudotyped retroviruses and stable cell lines for their production
InactiveUS7033595B1Effective blockingSsRNA viruses positive-senseVectorsViral glycoproteinNucleotide
Cells that produce inventive pseudotyped retroviruses having a broad host range have been produced. In one aspect of the invention, the cells produce retroviruses pseudotyped with at least two different viral glycoproteins, such as togaviral glycoproteins. In alternative embodiments, the cells produce retroviruses pseudotyped with filoviral glycoproteins. Methods of producing the above-described cells, as well as the pseudotyped retroviruses thus produced, are also provided. In other embodiments, methods of screening agents effective in blocking viral entry into a cell, including filoviral entry or entry of viruses having at least two different viral glycoproteins disposed in their lipid bilayer, such as togaviruses, are provided. Moreover, methods of using the inventive pseudotyped retroviruses for introducing nucleotide sequences into target cells, and kits for forming the inventive pseudotyped retroviruses, are also provided.
Owner:PURDUE RES FOUND INC
Construction of stable cell line carrying orthogonal tRNA/aminoacyl tRNA synthetase
InactiveCN109295100AAchieve stable expressionStable expressionPeptide/protein ingredientsGenetically modified cellsAminoacyl-tRNA synthetasesAminoacyl-tRNA
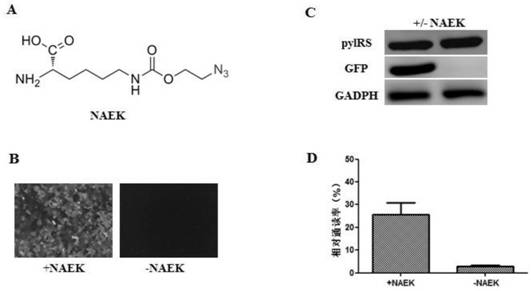
The present invention relates to a method for constructing a stable cell line carrying an orthogonal tRNA / aminoacyl tRNA synthetase, and the method stably integrates an orthogonal tRNA / aminoacyl tRNAsynthetase gene into cell genomes by double lentiviral stable transduction and plasmid stable transfection. The invention further relates to the stable cell line obtained by the method and the use ofthe stable cell line in the expression of a protein containing non-natural amino acids, and by utilizing unique reactive groups on the non-natural amino acids, the purpose of high-efficiency and specific protein modification can be achieved.
Owner:PEKING UNIV
Method
InactiveUS20090311695A1Rapid investigationPotential utilitySugar derivativesMicrobiological testing/measurementInteinExon
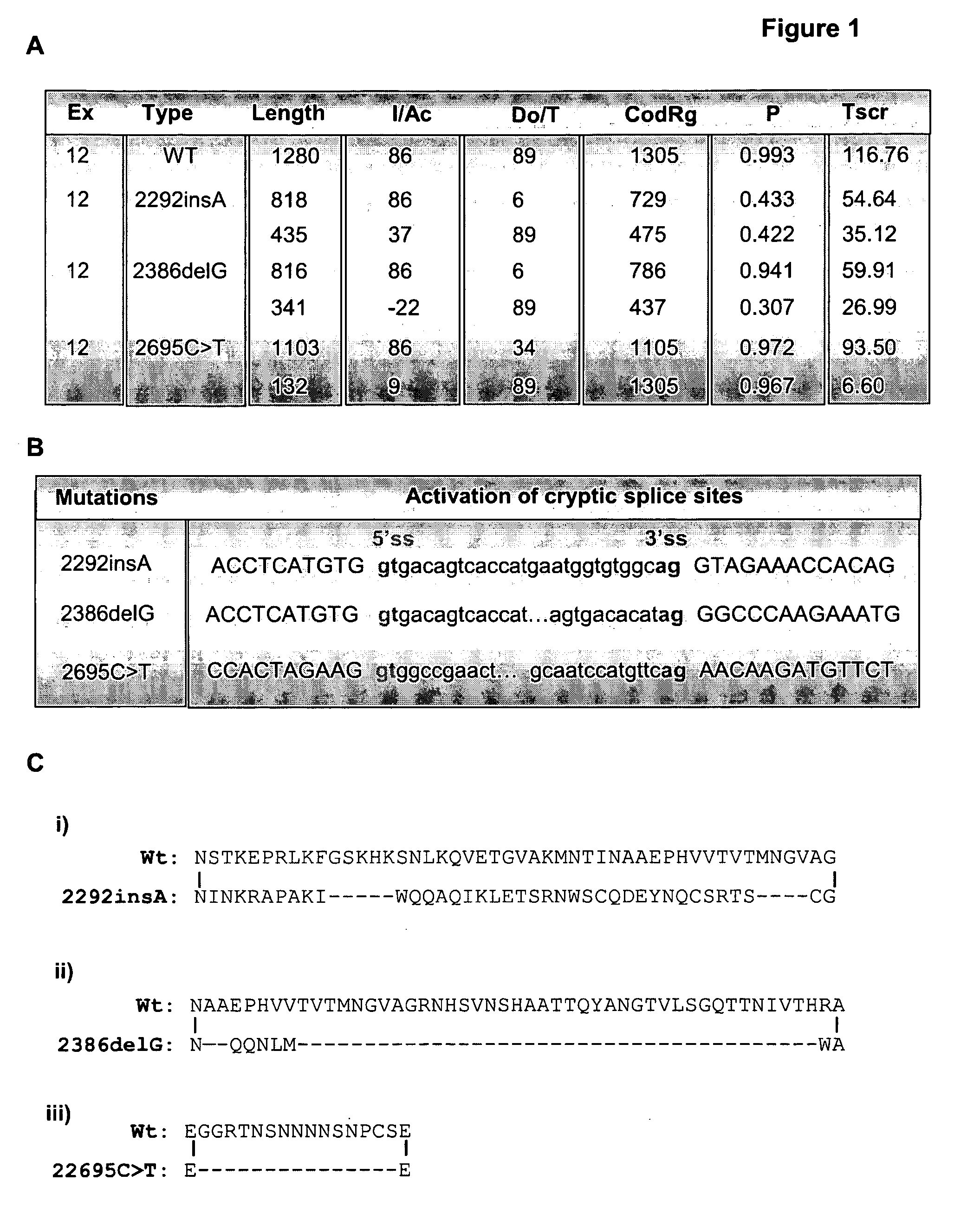
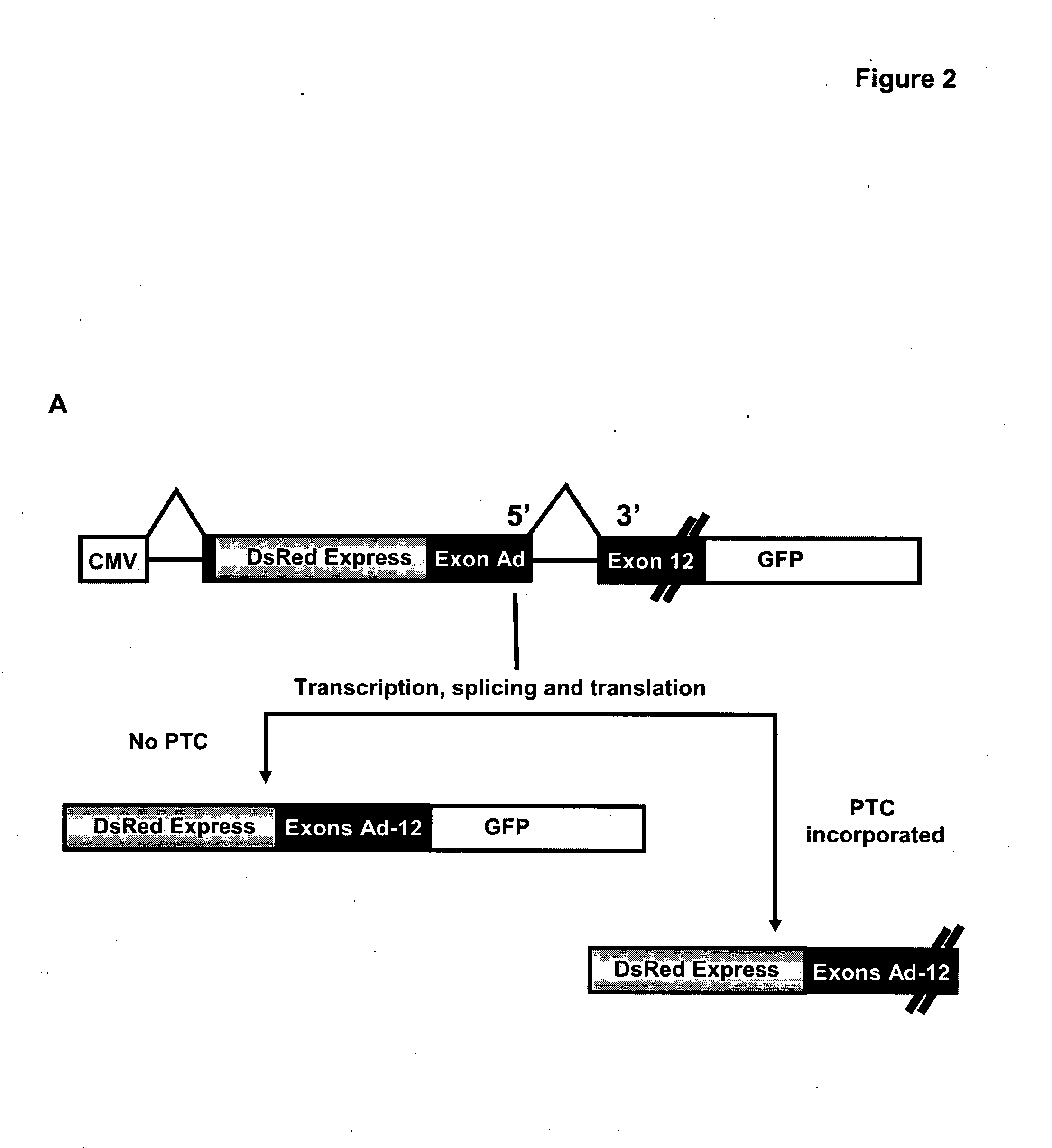
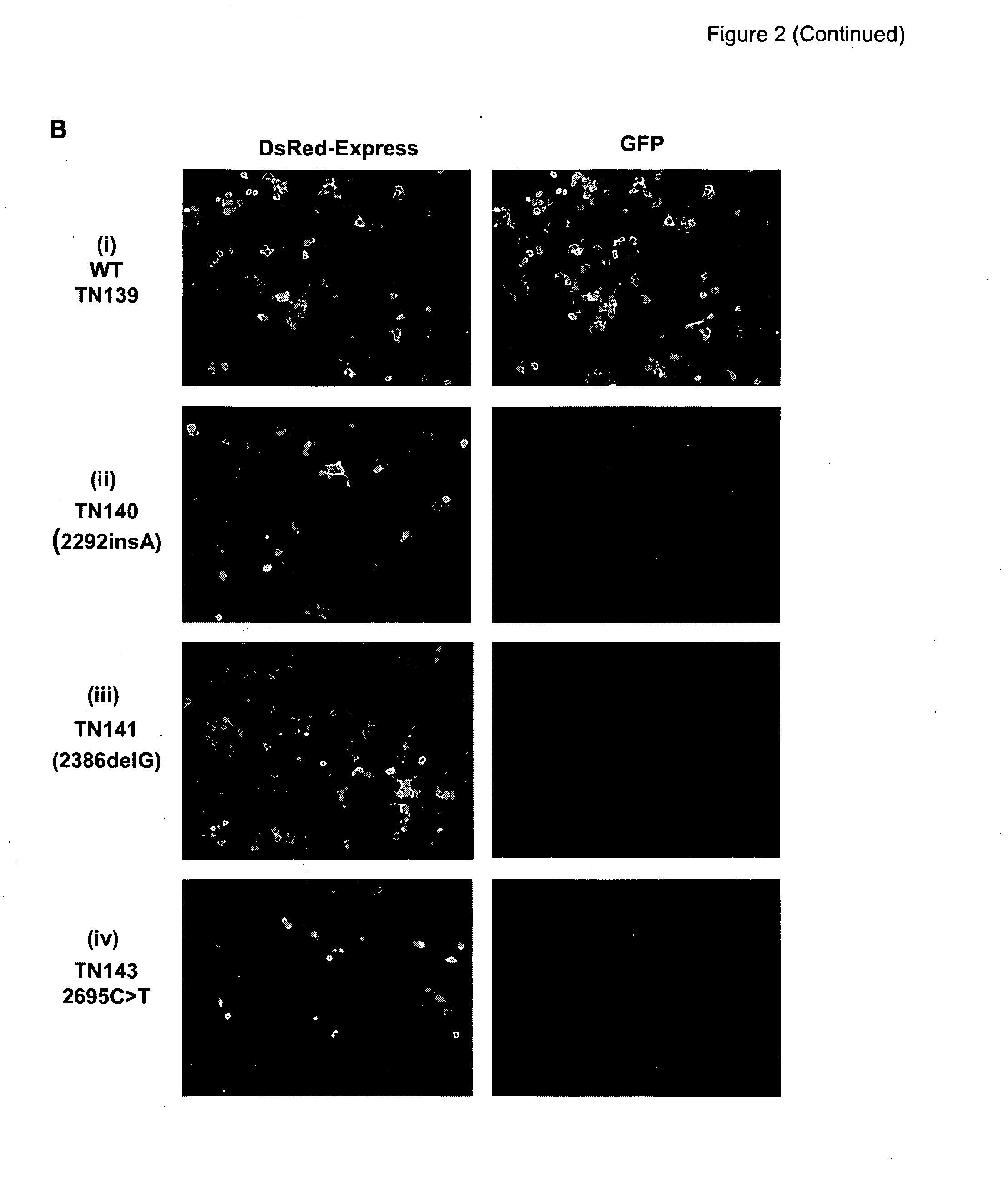
In one aspect, there is described a method for determining the effect of a genetic variation or mutation on the integrity of an RNA transcript comprising the steps of: (a) providing a nucleic acid construct comprising two different reporters separated by an in frame splicing unit, wherein said splicing unit comprises at least two exons separated by at least one intron and wherein at least one exon (eg. the exon downstream of said intron) comprises the genetic variation; (b) transfecting said construct into a cell and / or generating a stable cell line; (c) culturing said cell; and (d) determining the effect of said genetic variation on the integrity of the RNA transcript, wherein a difference in reporter activity in comparison to a cell comprising the nucleic acid construct without the genetic variation is indicative that said genetic variation affects the integrity of the RNA transcript. Assays—such as high throughput assays—are also described for identifying agents (nucleic acids, peptides and small molecules) that modulate the integrity of the RNA transcript and / or are involved in modulating RNA metabolism.
Owner:KING'S COLLEGE LONDON
Construction of stable cell line carrying orthogonal tRNA/aminoacyl tRNA synthetases
InactiveCN112725282AGenetically modified cellsMicroorganism based processesStable cell lineAmino acid
The invention relates to a construction method of a stable cell line carrying orthogonal tRNA / aminoacyl tRNA synthetases. On the basis of a gene codon extension technology, a pair of orthogonal tRNA / aminoacyl tRNA synthetases is used for introducing non-natural amino acid into protein at a fixed point. The invention also relates to a construction method of a double lentiviral vector, a construction method of an orthogonal tRNA carrier carrying multiple copy numbers and a method for stably integrating orthogonal tRNA / aminoacyl tRNA synthetase genes into a cell genome by virtue of stable transduction of double slow viruses and stable transfection of plasmids. The invention further relates to the use of the stable cell line, such as the use for the expression of interest proteins containing non-natural amino acids.
Owner:PEKING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com