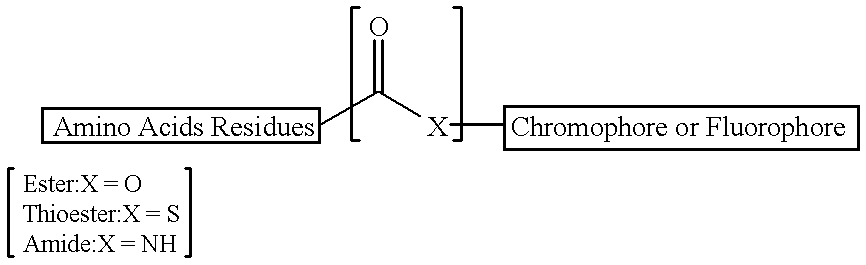
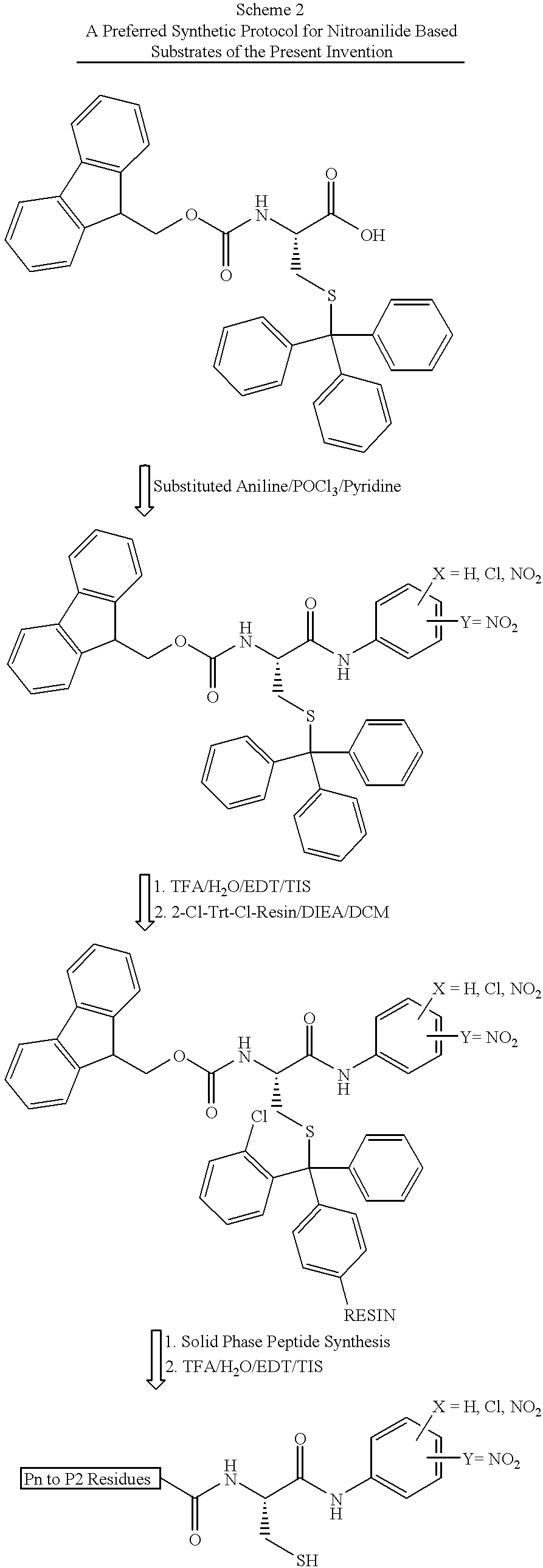
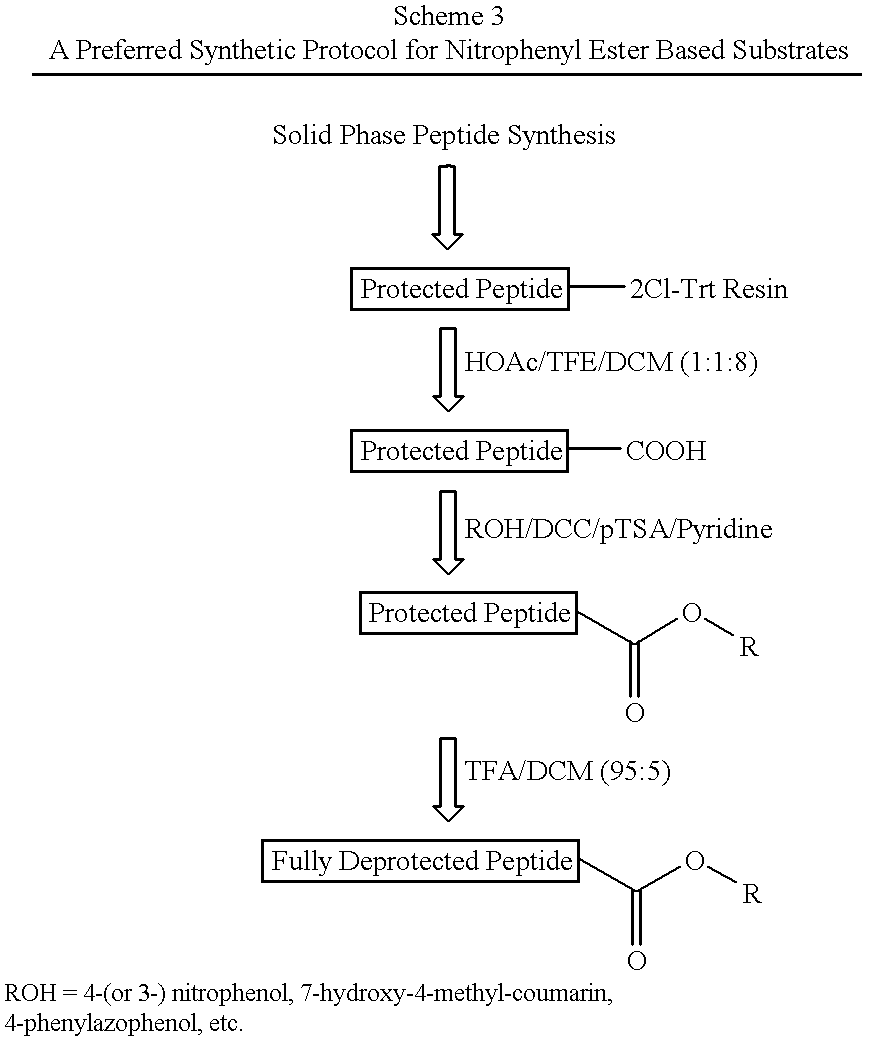
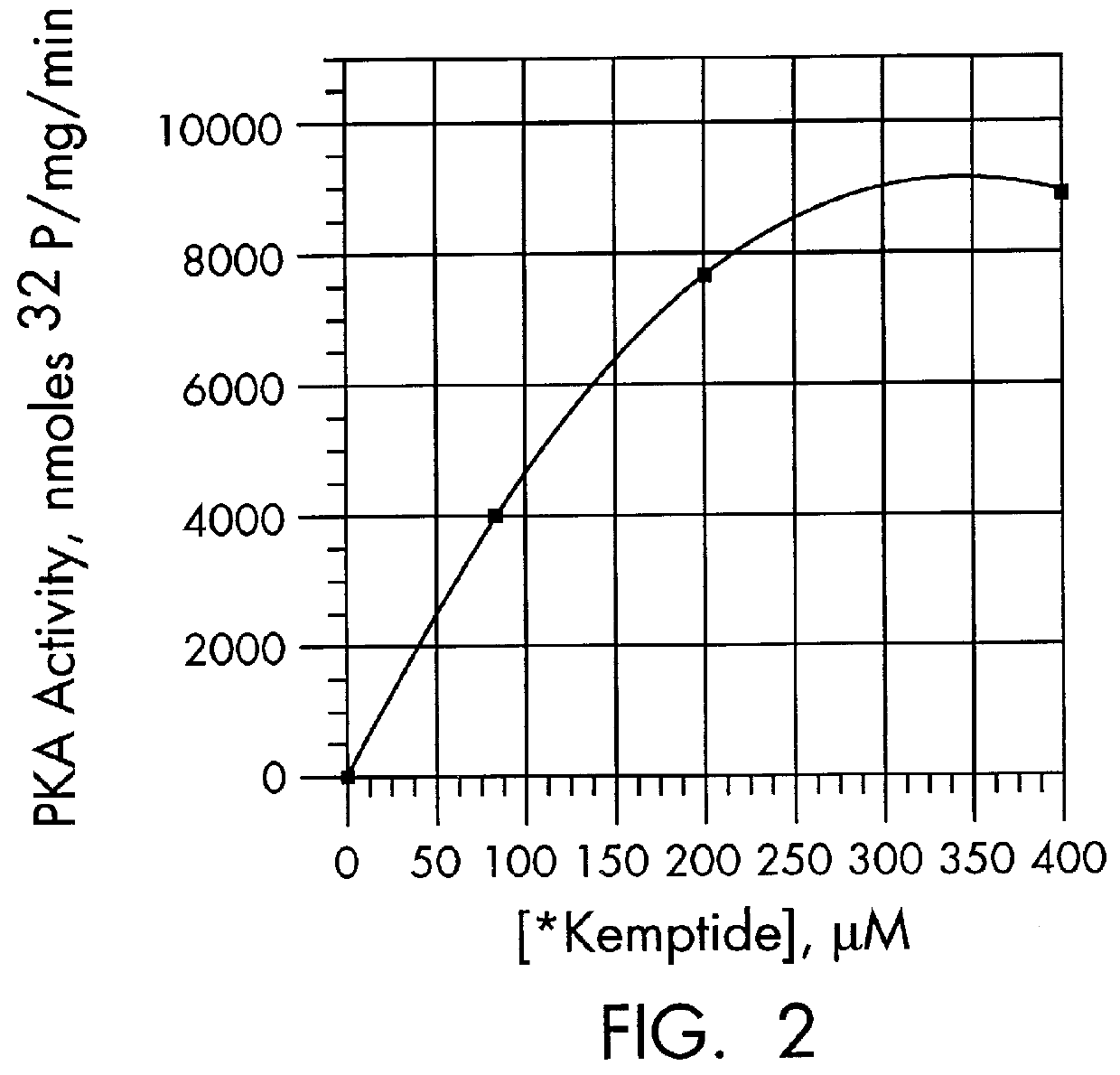
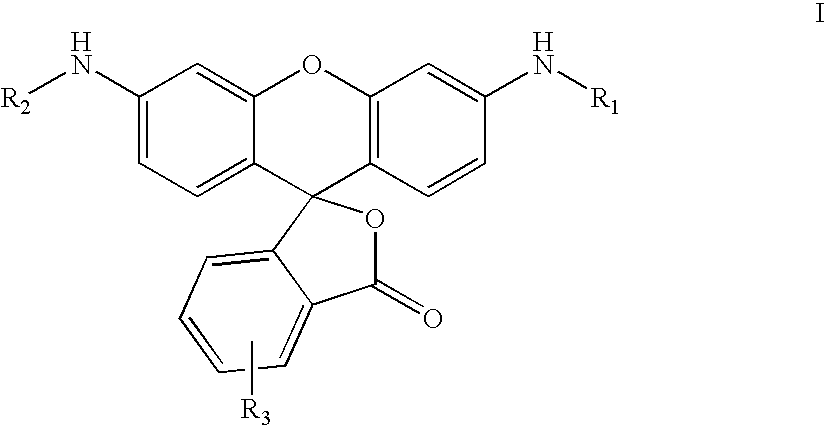
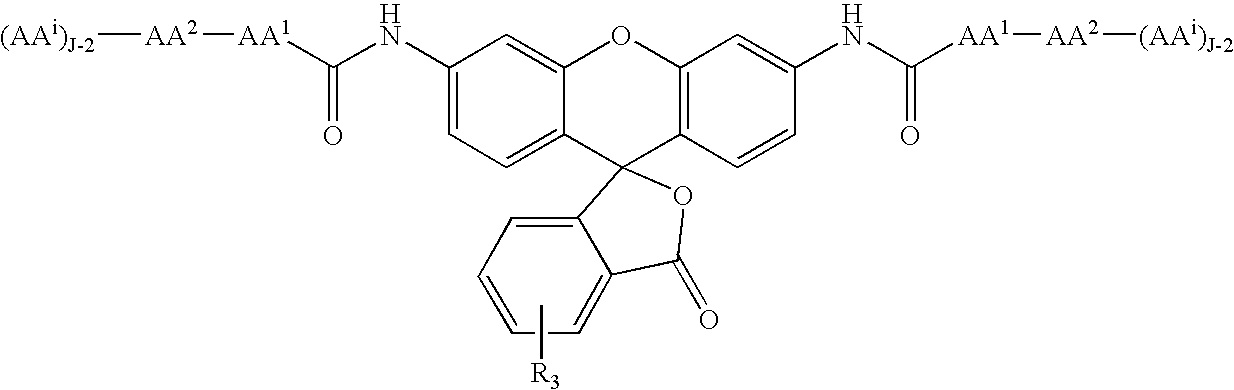
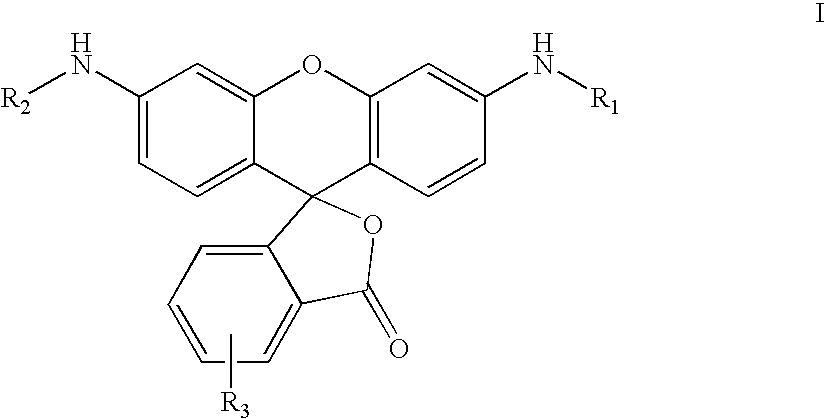
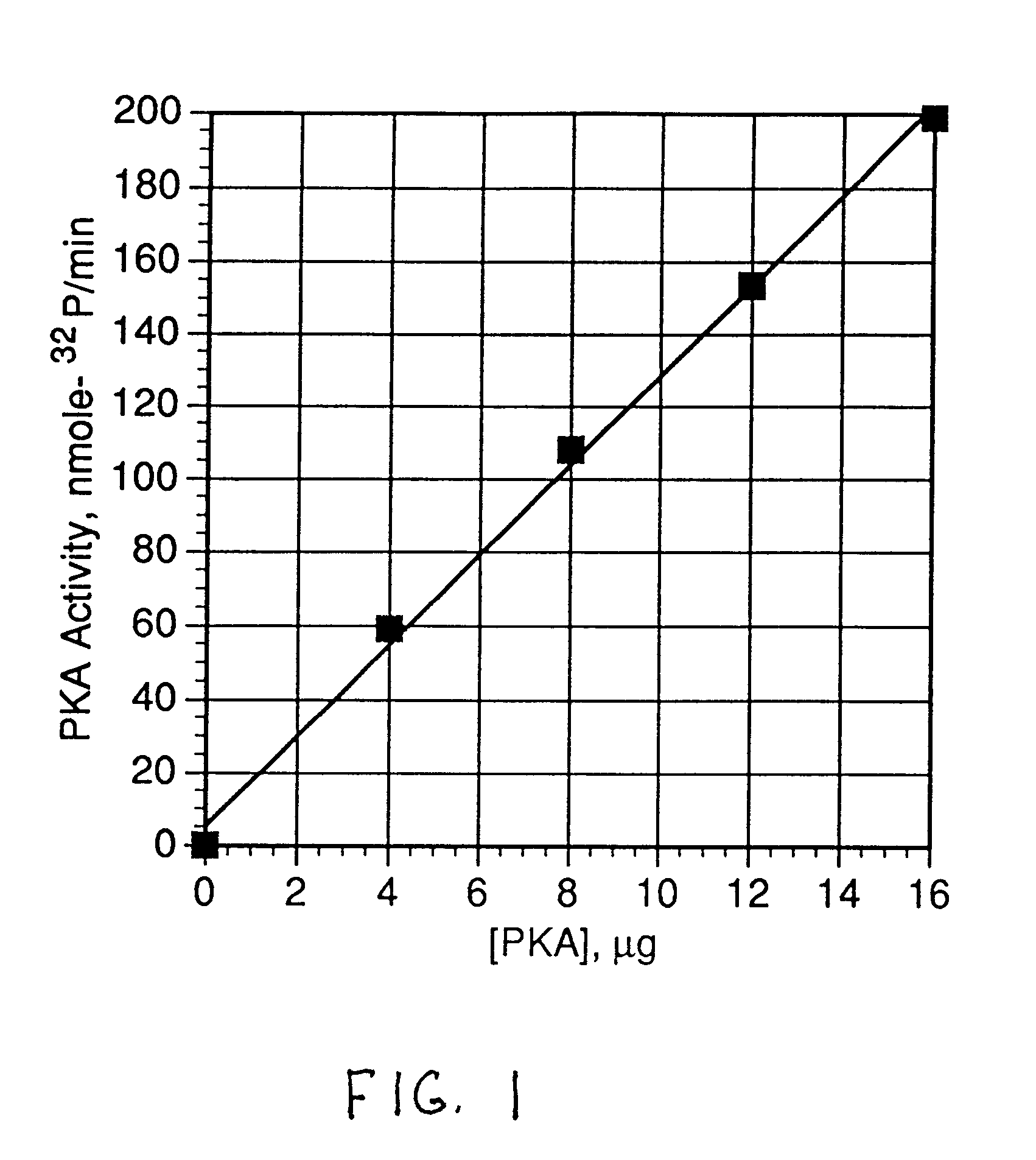
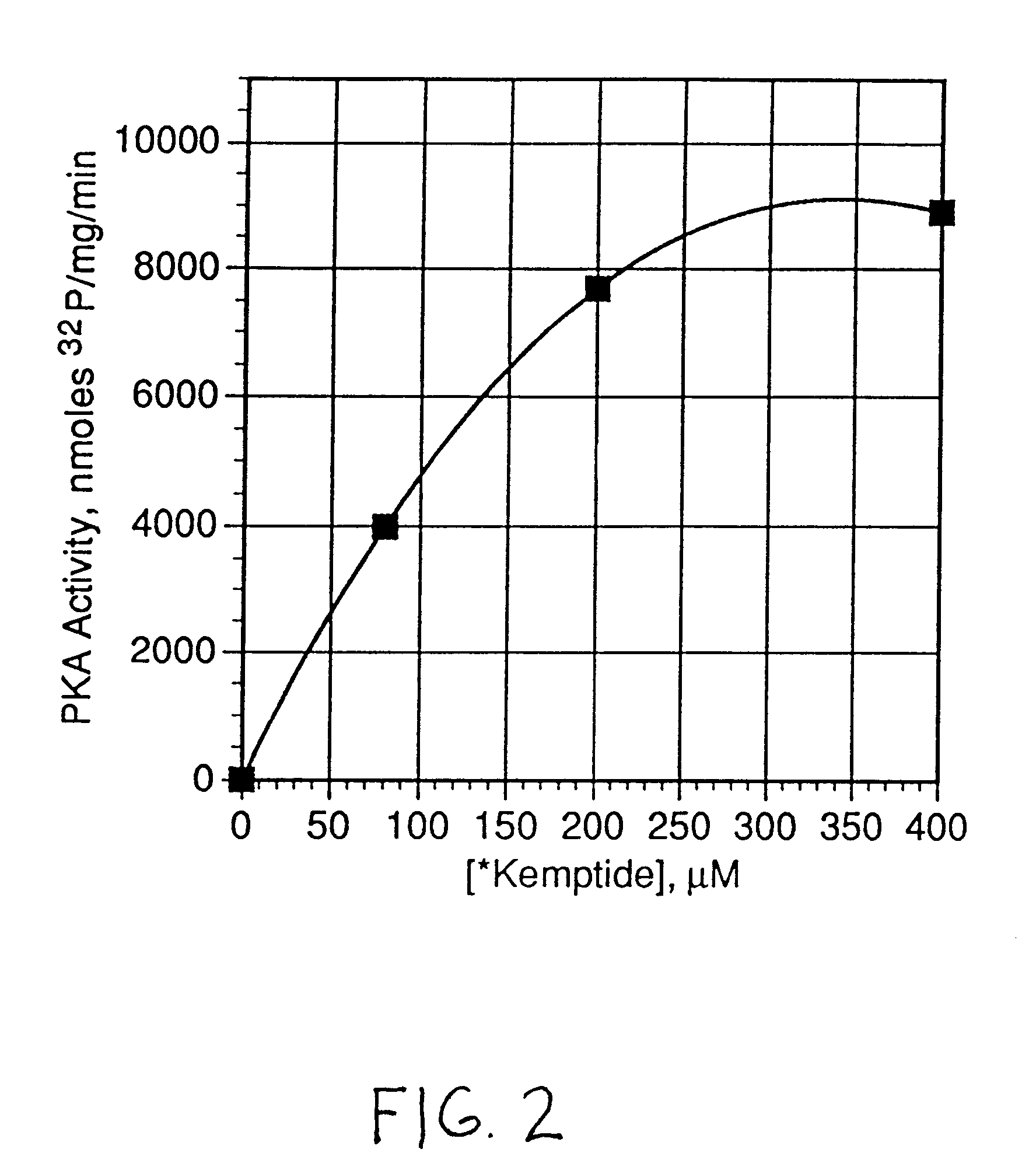
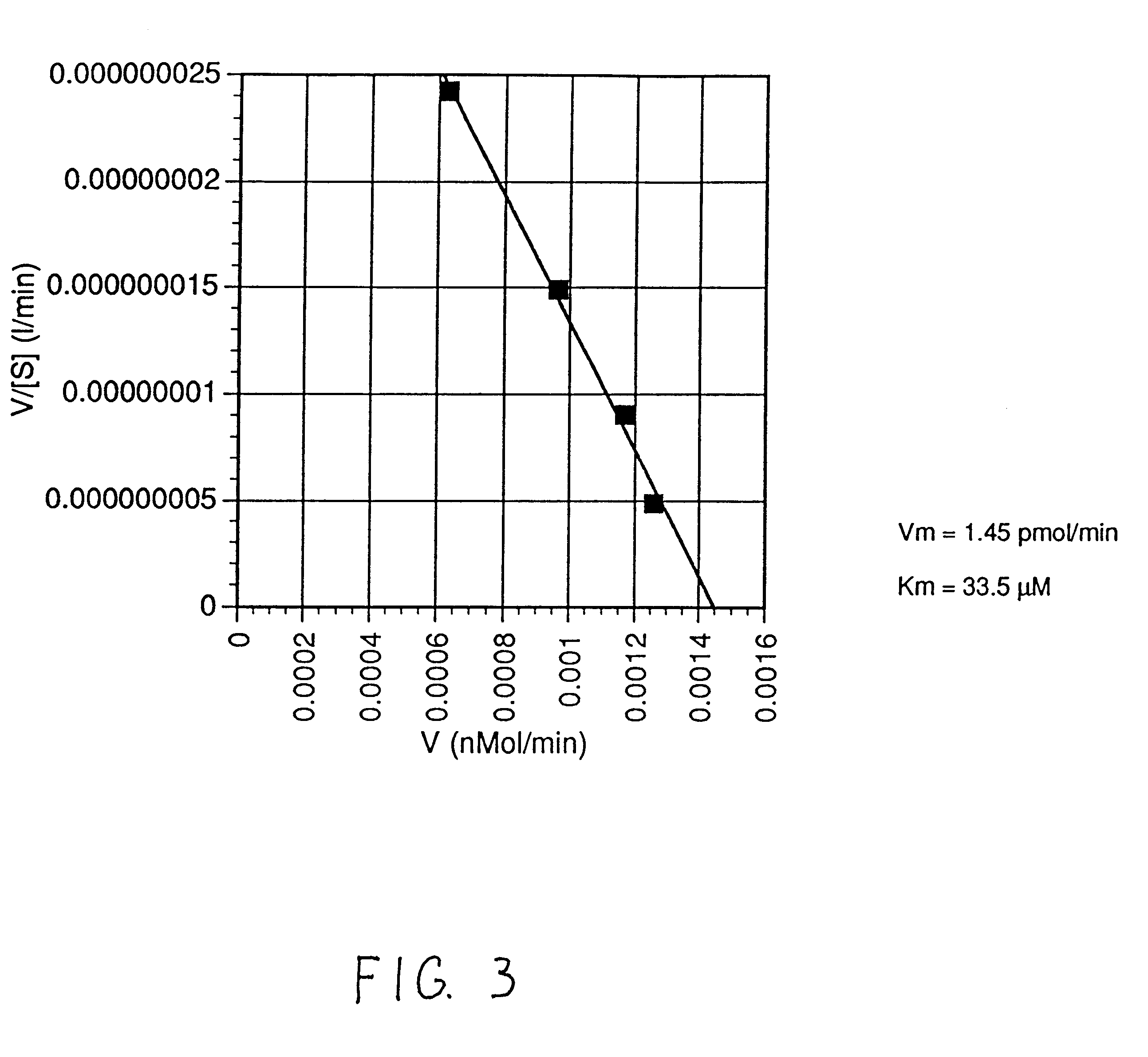
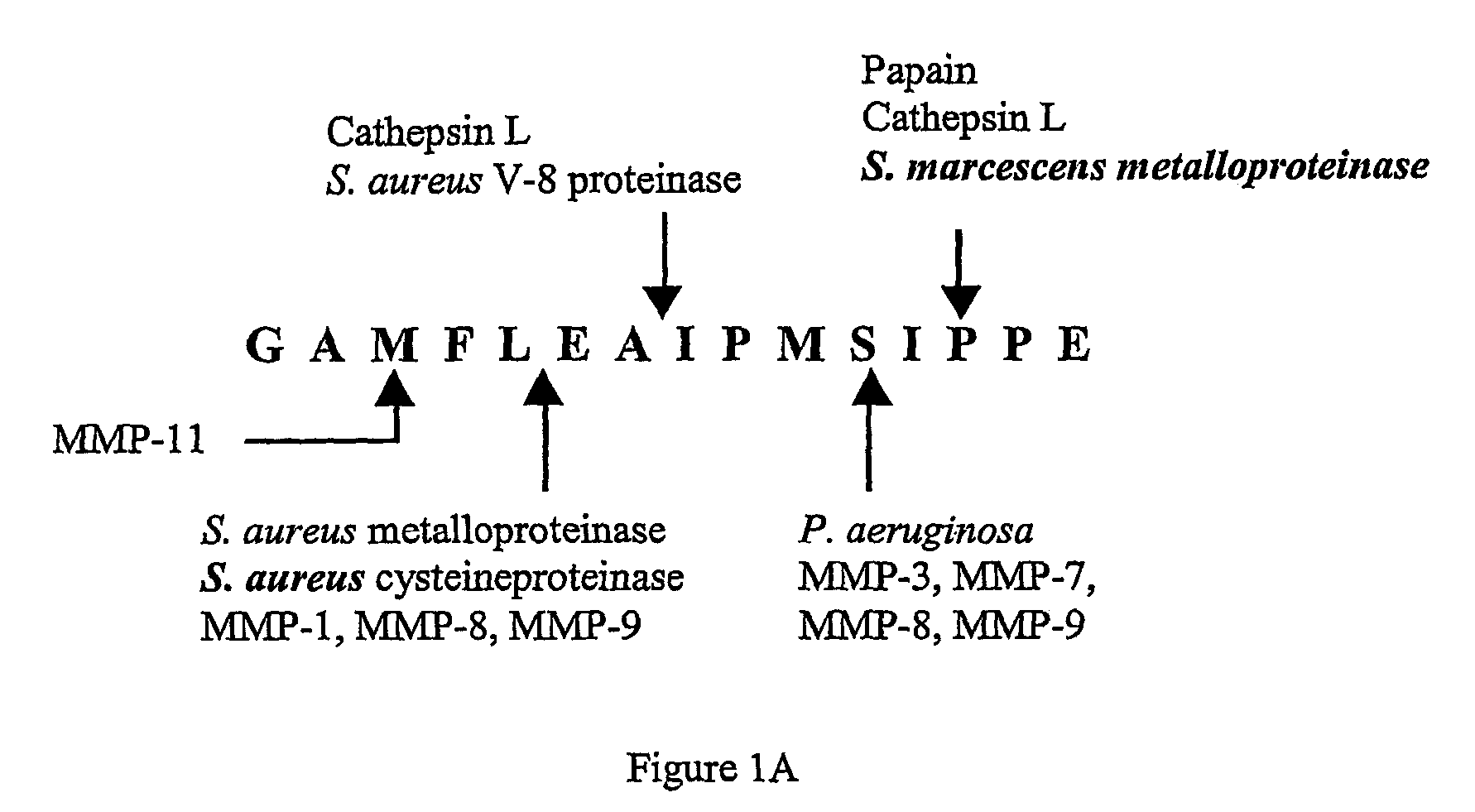
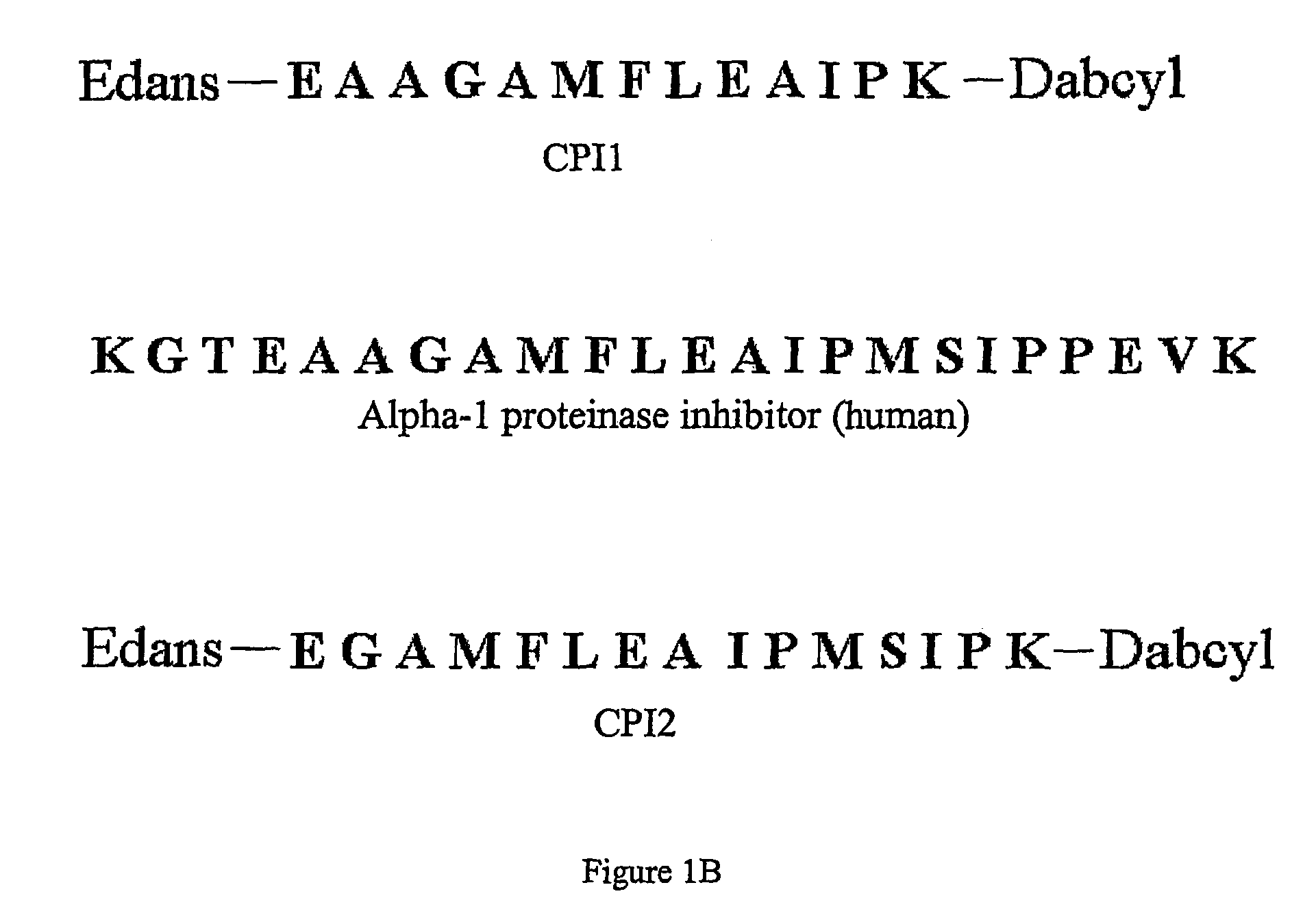
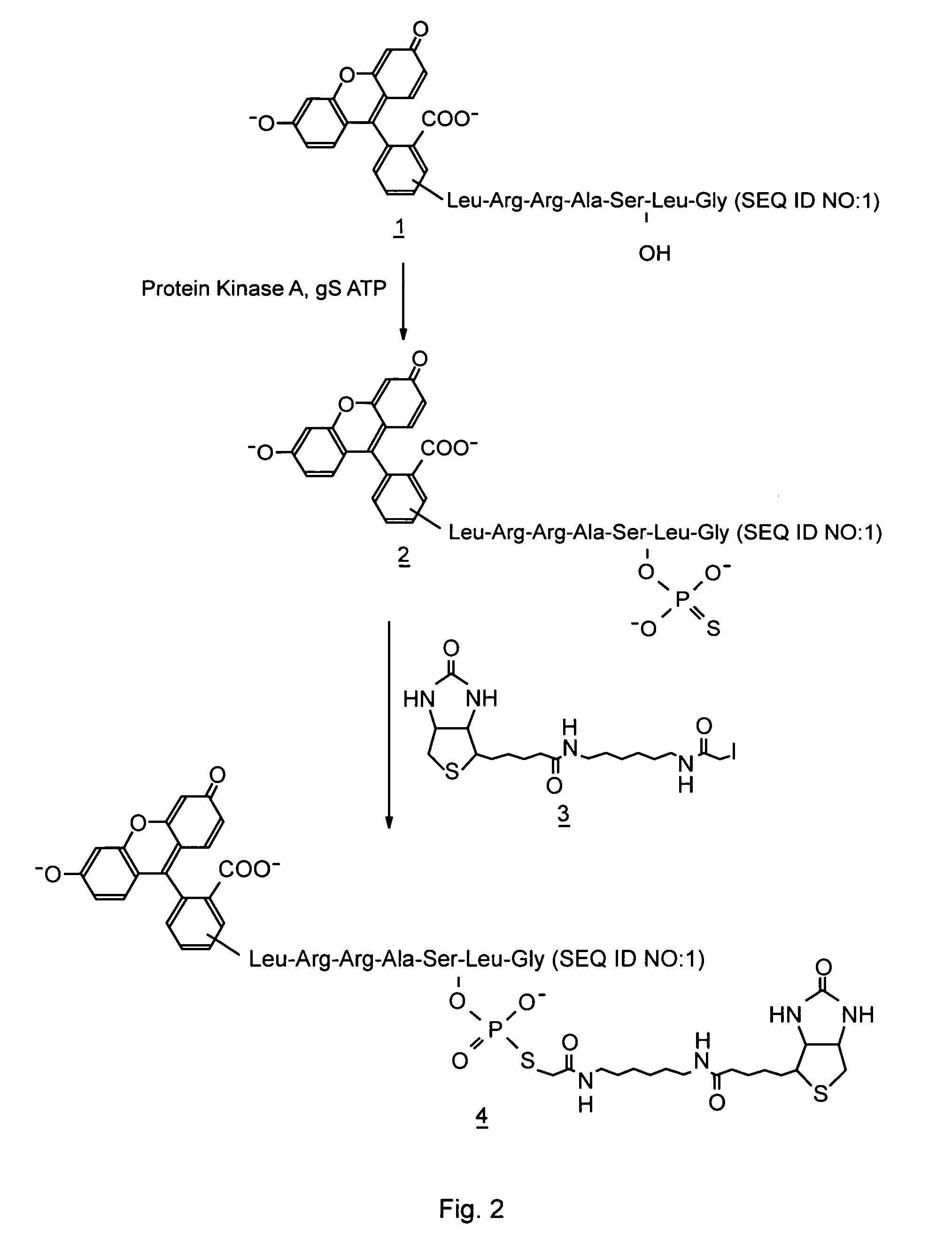
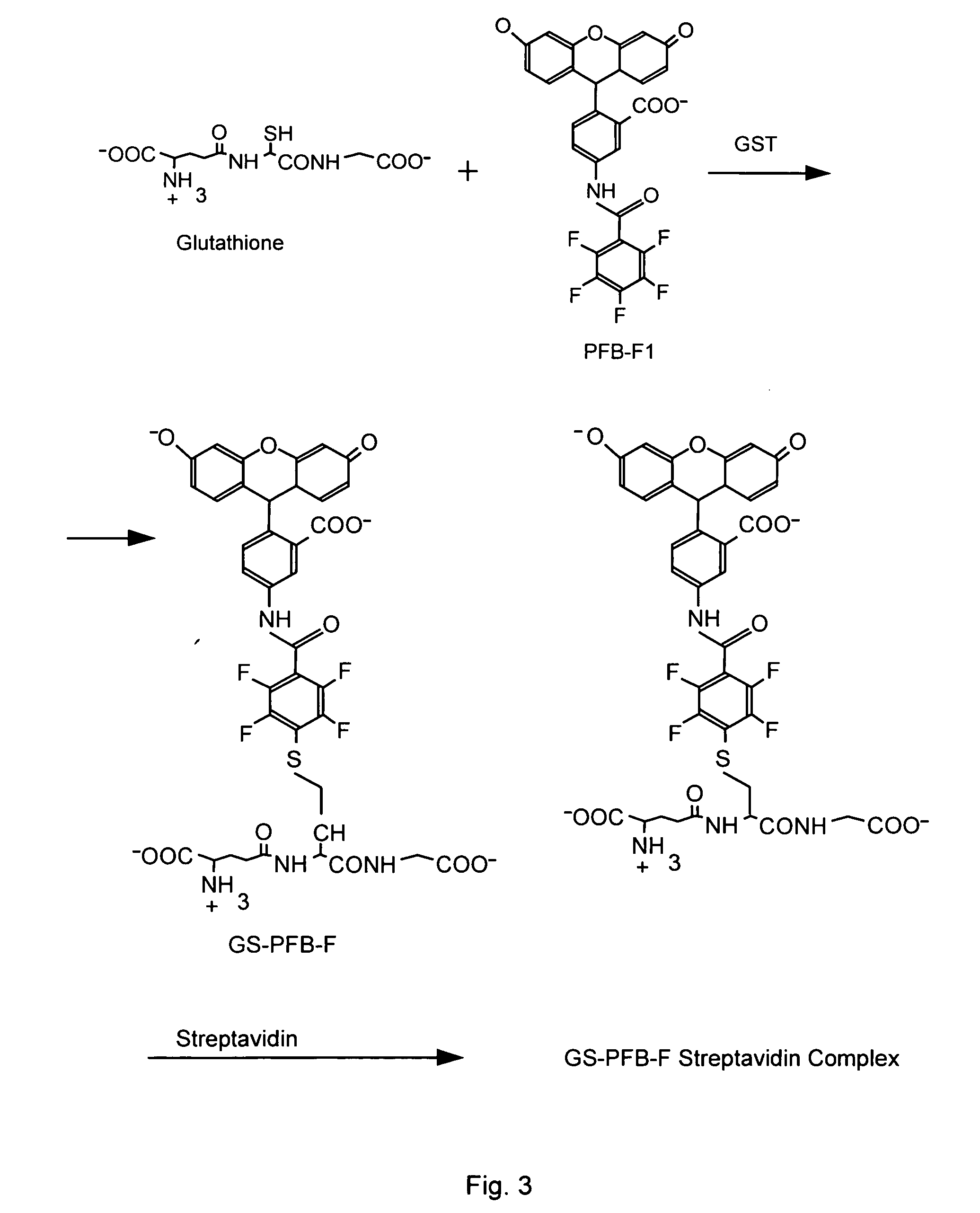
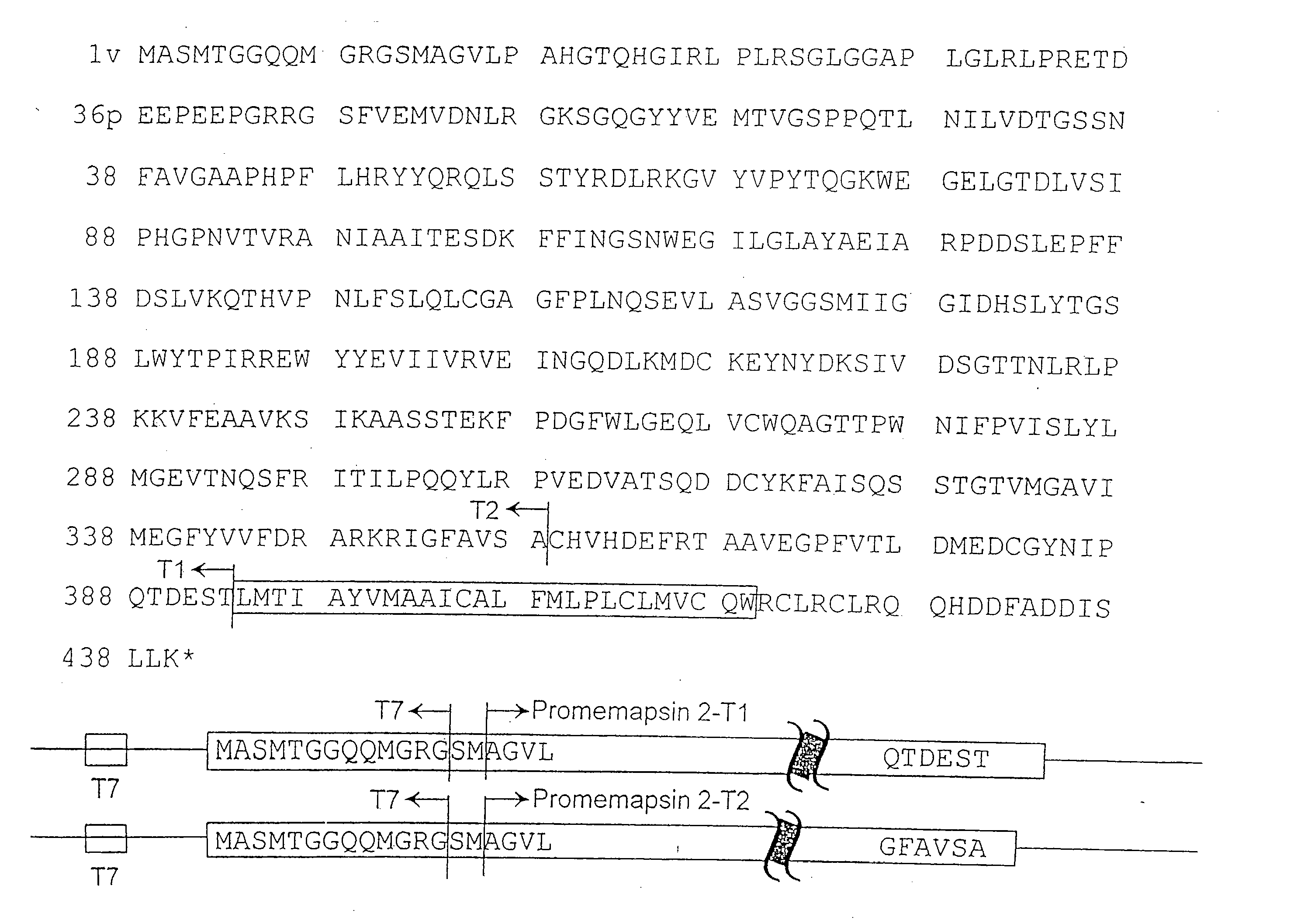
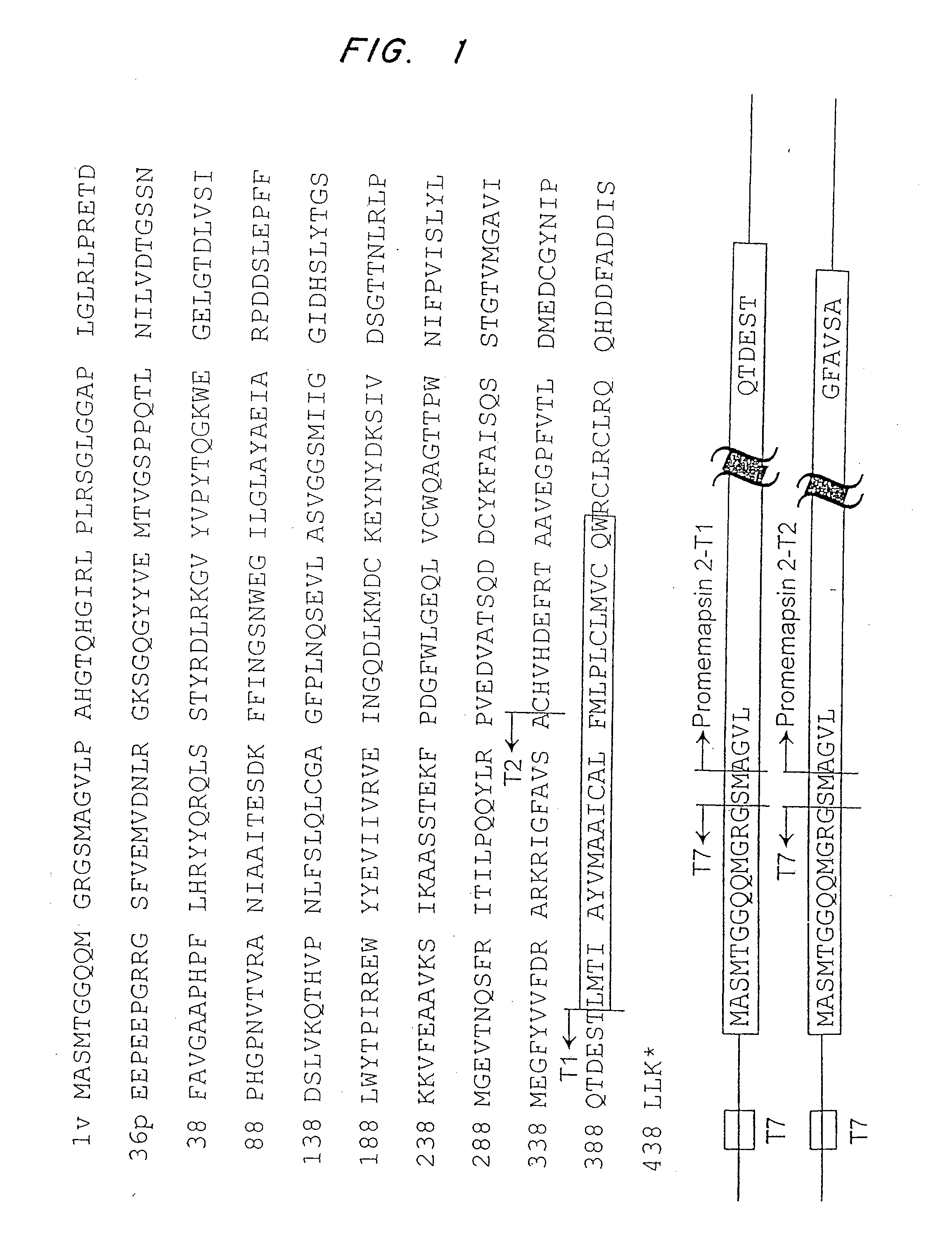
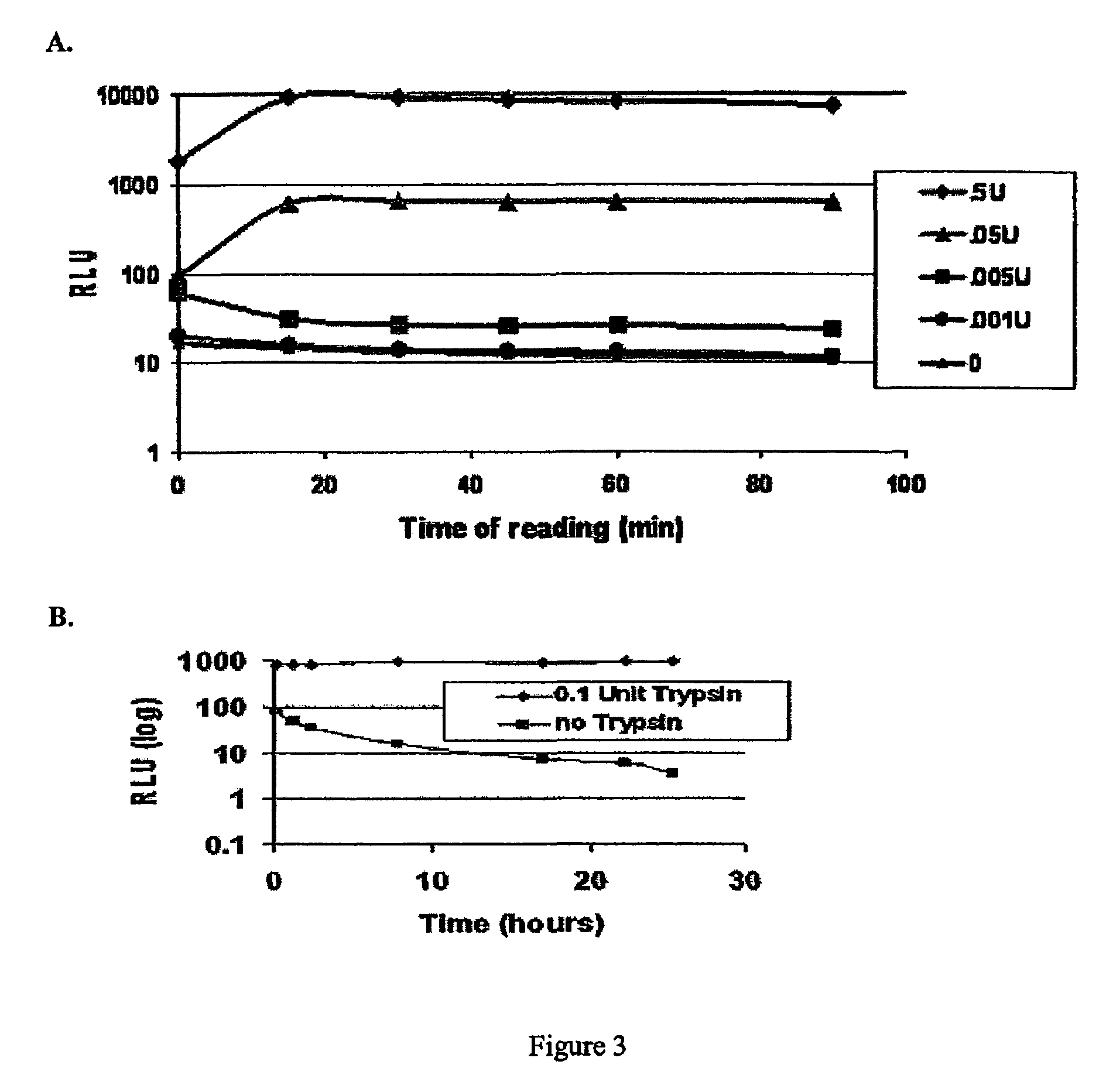
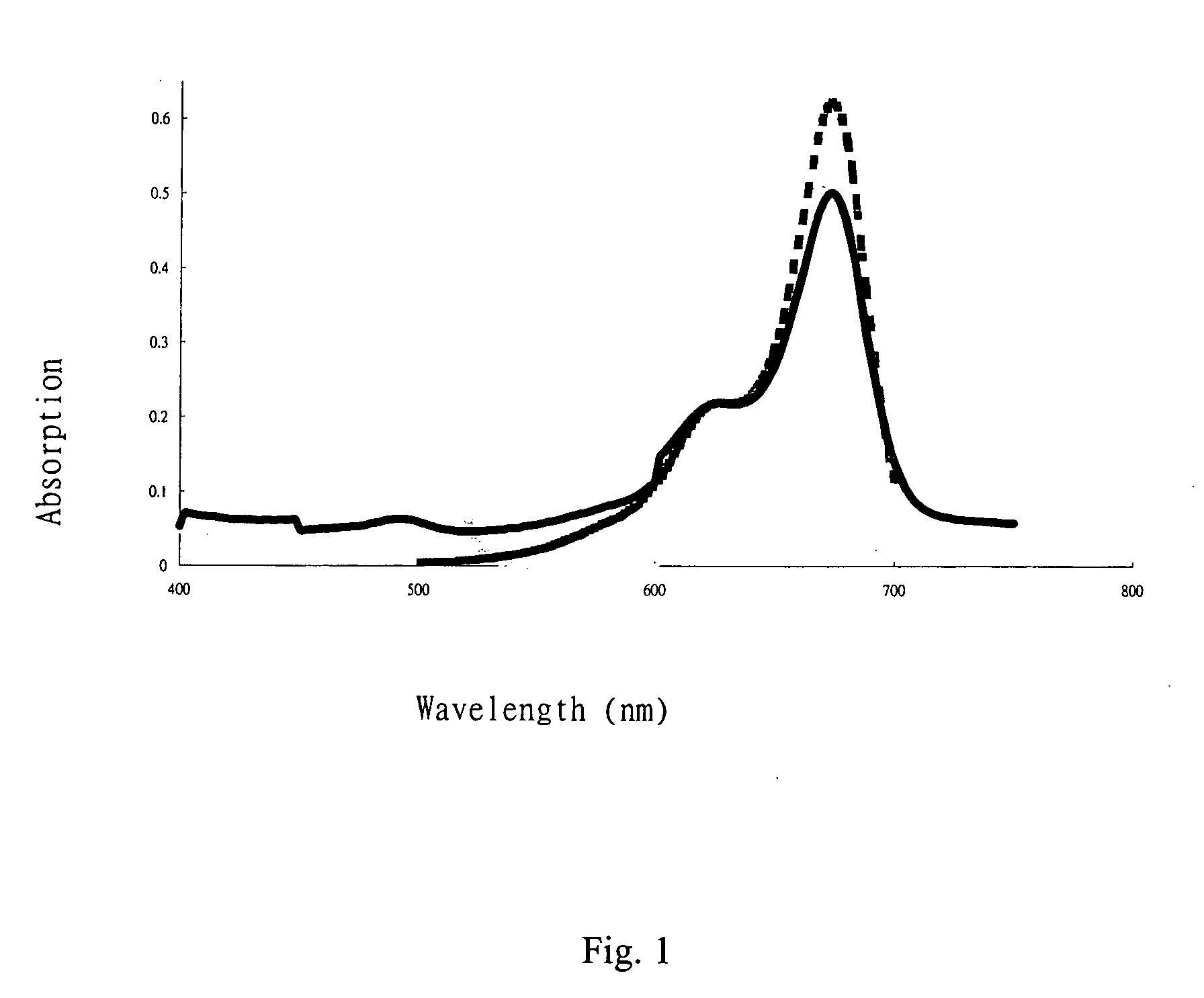
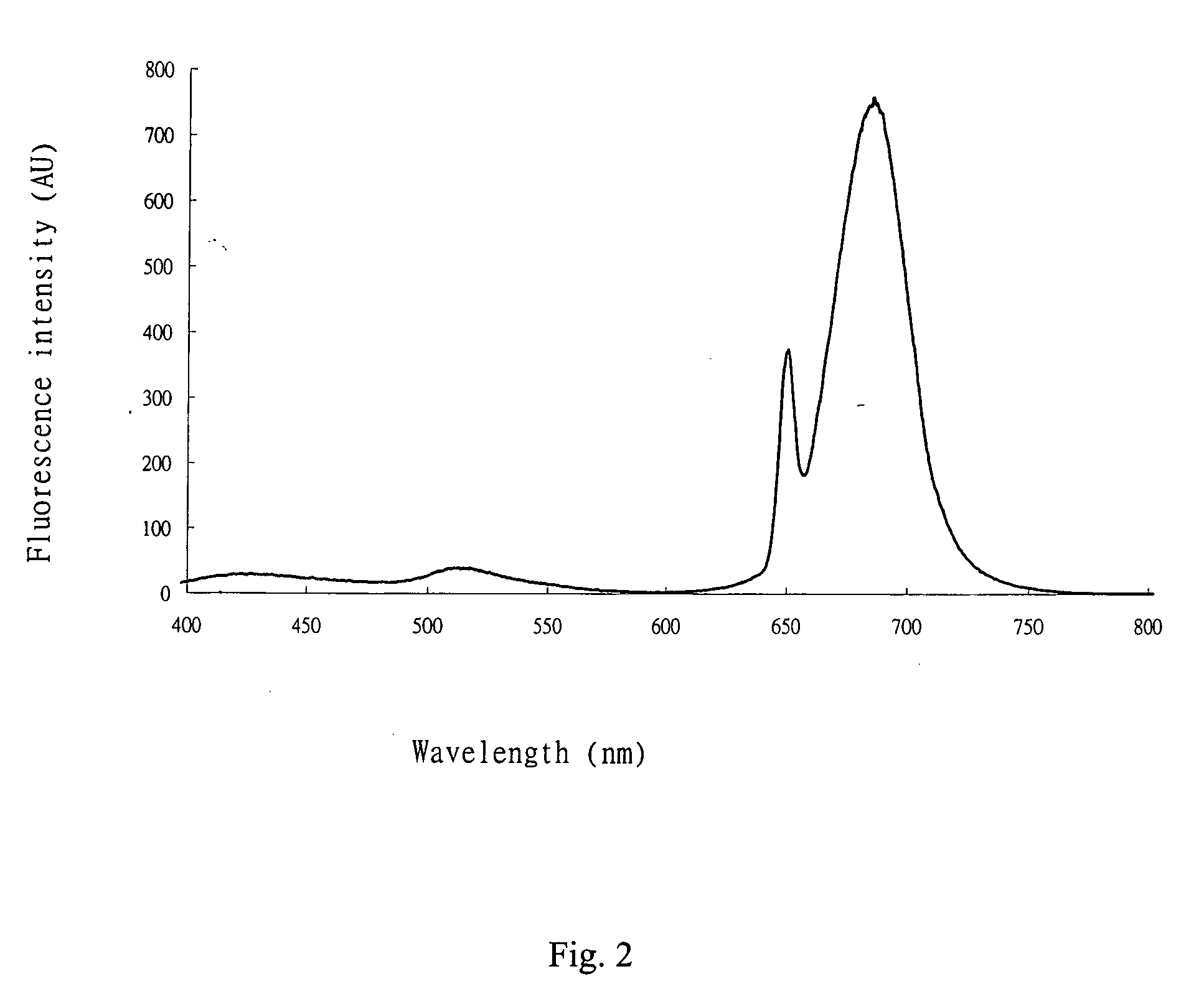
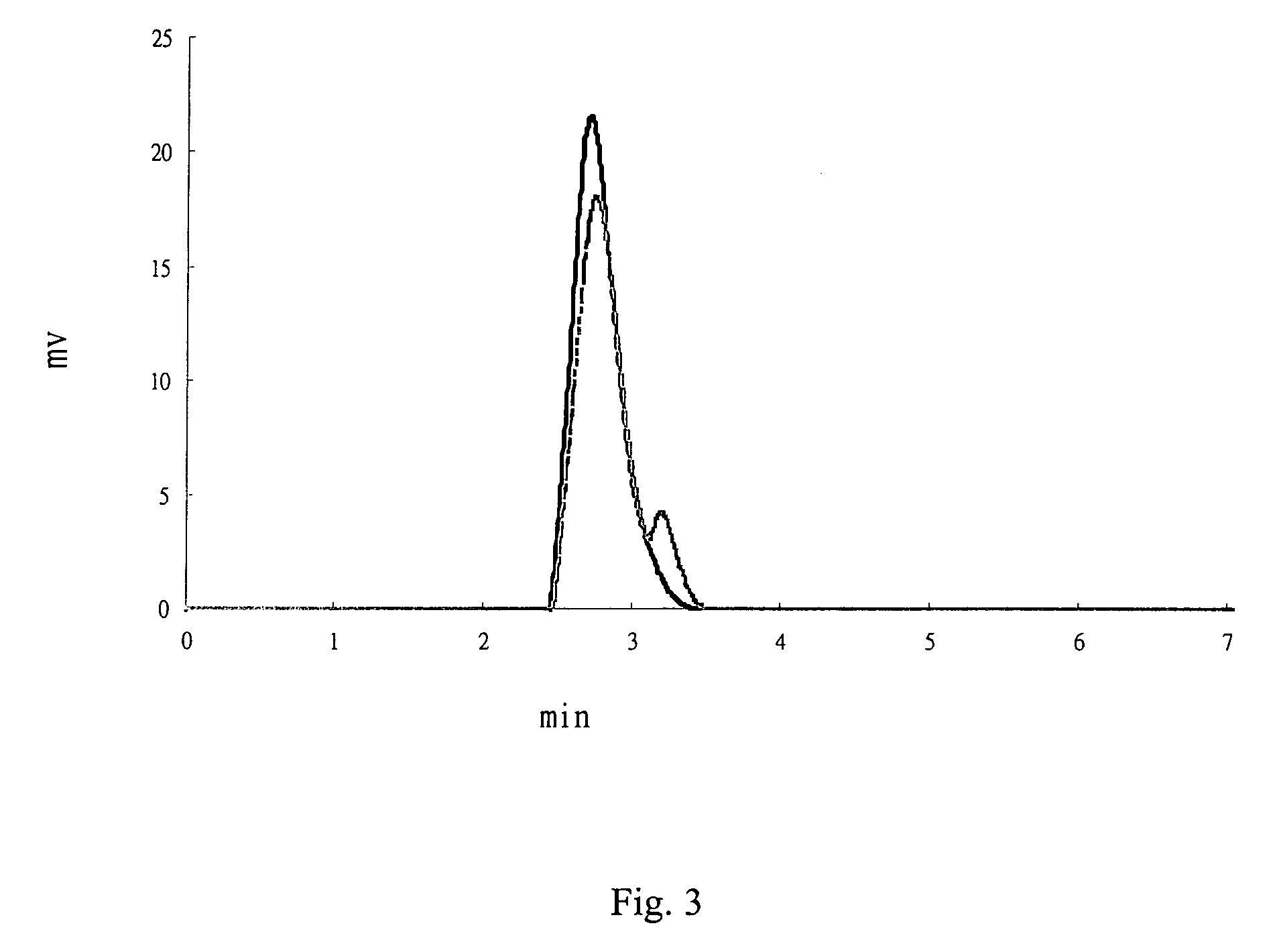
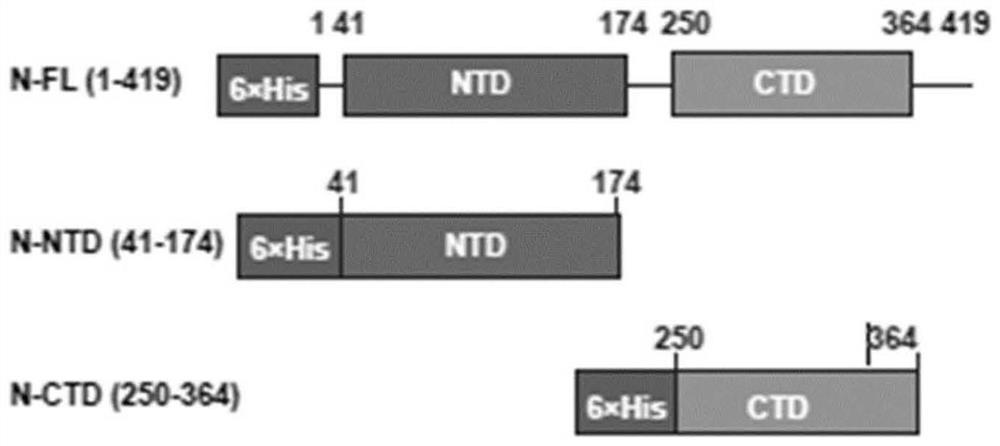
Patents
Literature
120 results about "Peptide substrate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Peptide Substrates are compounds which are acted upon by various enzymes and therefore affect many physiological systems. Peptide Substrates are used in many areas of biological and medical research. Our popular products such as PKC substrate GS, PKC substrate GS, and Bradykinin triacetate are typically available for immediate shipment.
Peptide substrates for HCV NS3 protease assays
InactiveUS6251583B1SsRNA viruses positive-sensePeptide/protein ingredientsPeptide substrateFluorescence
Novel chromogenic, fluorogenic and fluorescence polarization substrates which are useful in HCV NS3 protease and inhibitor assays.
Owner:MERCK SHARP & DOHME CORP
Quantitation of individual protein kinase activity
InactiveUS6066462AMinimal lossAccurate estimateMicrobiological testing/measurementPeptide preparation methodsPeptide substrateSufficient time
A method of quantitating the activity of a selected protein kinase on a peptide substrate is provided. The peptide substrate is conjugated to a binding compound. The modified peptide substrate is then added to a solution containing the selected protein kinase. The protein kinase and the peptide are incubated along with a label for sufficient time to form a modified peptide product having the binding compound and the label. The modified peptide product is then bound to a matrix having a high binding compound affinity. The bound peptide is then washed and the activity of the protein kinase is measured. Also provided is a kit for the stated method.
Owner:PROMEGA
Prodrugs activated by plasmin and their use in cancer chemotherapy
InactiveUS7402556B2Low toxicityHigh specificity of actionSugar derivativesTetrapeptide ingredientsPeptide substrateOligopeptide
The product of the invention is a modified form of a therapeutic agent and comprises a therapeutic agent, an oligopeptide having a plasmin peptide substrate of 2-4 amino acids and mono- or di-peptide linkage, a stabilizing group and, optionally, a linker group. The prodrug is cleavable by plasmin. Also disclosed are methods of making and using the prodrug compounds.
Owner:MEDAREX LLC
Method for detecting acetyltransferase and deacetylase activities and method for screening inhibitors or enhancers of these enzymes
InactiveUS6884597B1Easy to detectConducive to screeningCompound screeningApoptosis detectionPeptide substrateAcetyltransferase
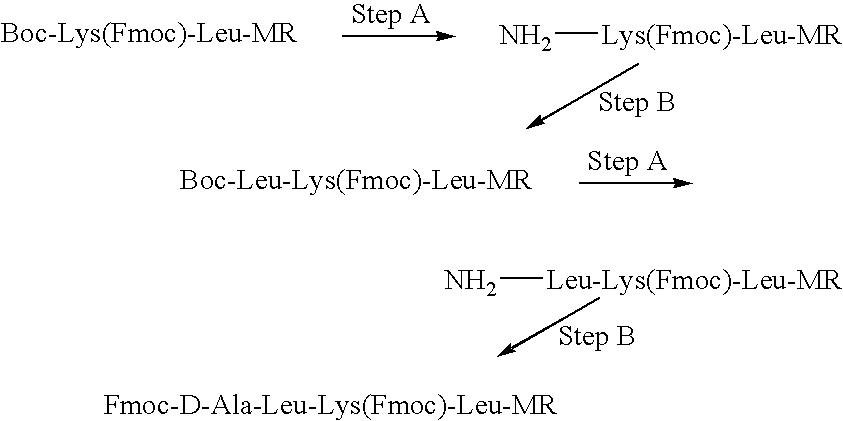
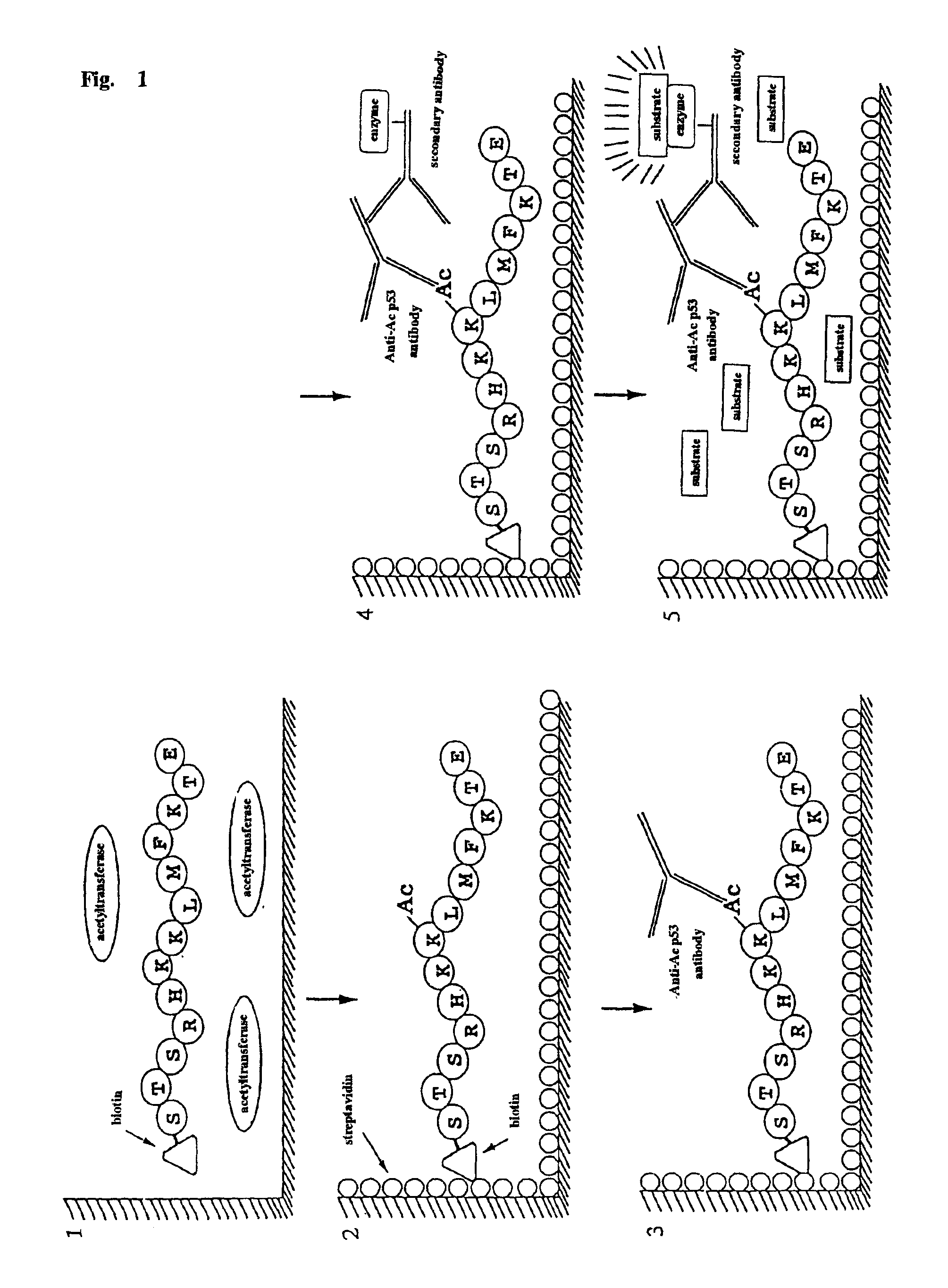
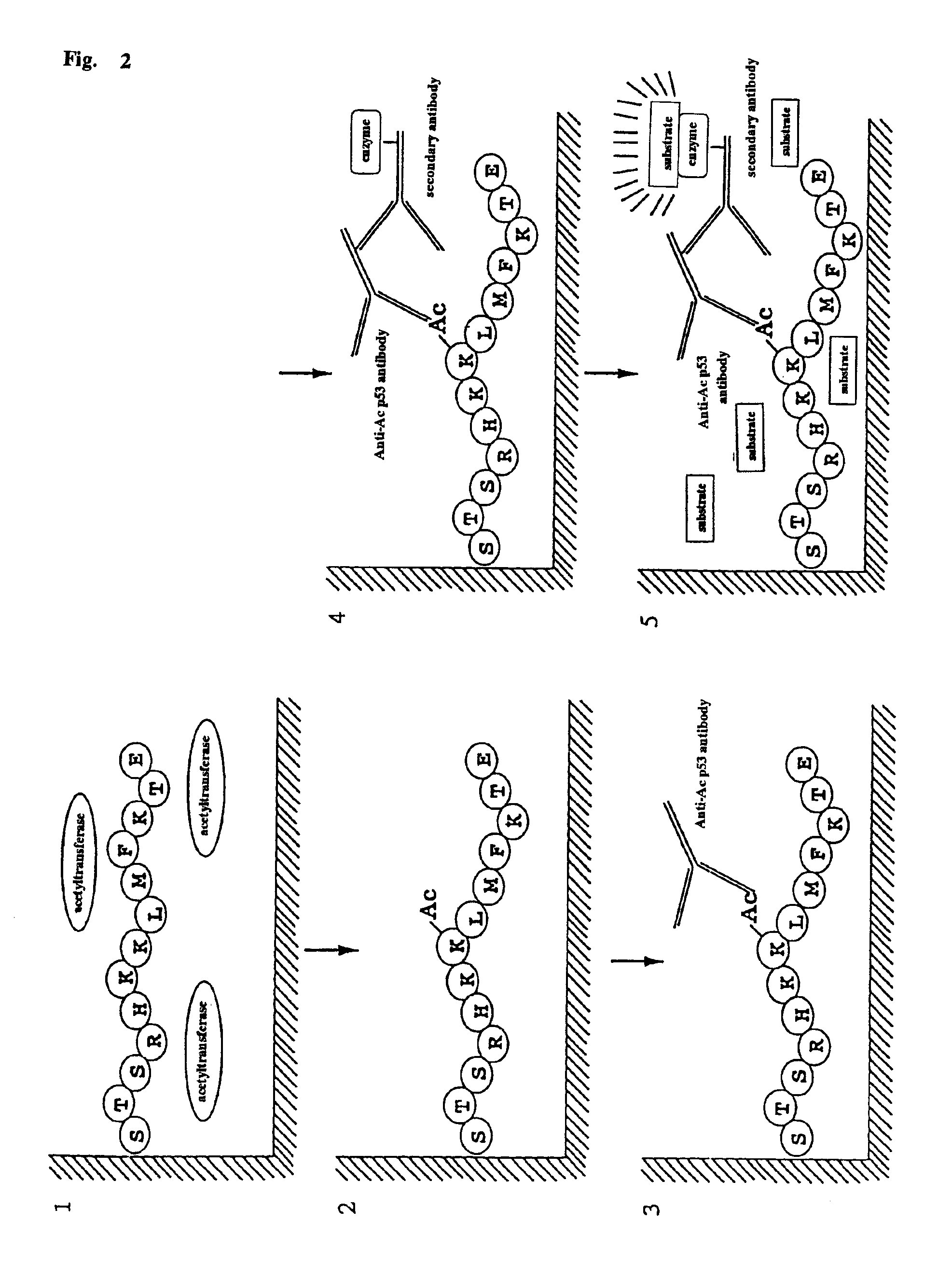
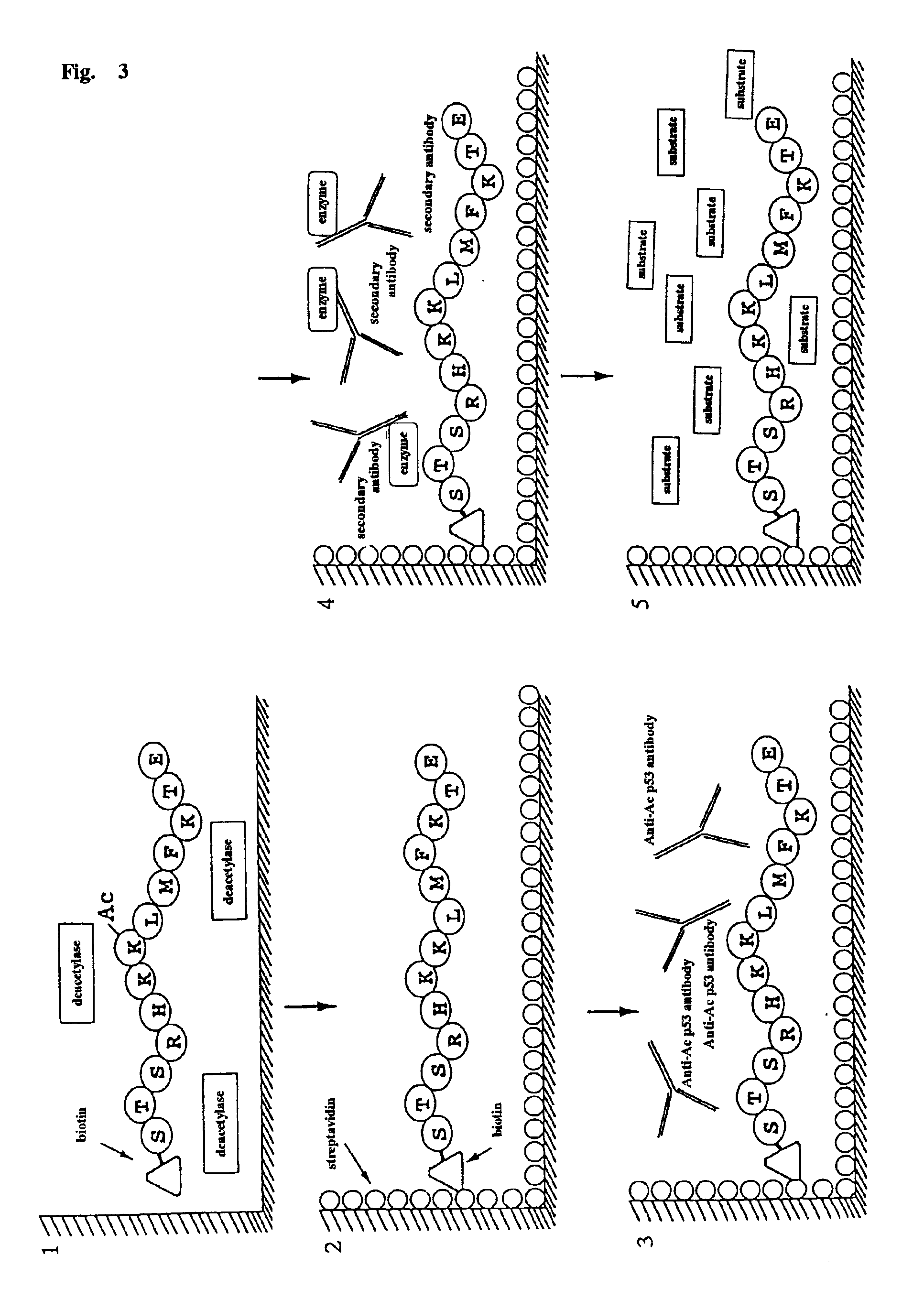
A method for simply and conveniently detecting acetyltransferase and deacetylase activities of proteins by executing an acetylation reaction of a peptide substrate with an acetyltransferase, or a deacetylation reaction of an acetylated peptide substrate with a deacetylase, and after the completion of these reactions, detecting the acetyl group bound to the peptide substrate by using an anti-acetylated peptide antibody. This system for detecting acetyltransferase and deacetylase activities using the anti-acetylated peptide antibody enables screening inhibitors or enhancers of acetyltransferase and deacetylase. A system for screening deacetylase inhibitors or acetyltransferase enhancers using cultured cells is also provided.
Owner:MEDICAL & BIOLOGICAL LAB CO LTD
Fluorogenic enzyme substrates and uses thereof
InactiveUS20050153306A1Easy to controlRelieves suppressionSugar derivativesMicrobiological testing/measurementPeptide substrateActive enzyme
Owner:IRM
Quantitation of individual protein kinase activity
InactiveUS6348310B1Minimal lossAccurate estimateMicrobiological testing/measurementTransferasesPeptide substrateSufficient time
A method of quantitating the activity of a selected protein kinase on a peptide substrate is provided. The peptide substrate is conjugated to a binding compound. The modified peptide substrate is then added to a solution containing the selected protein kinase. The protein kinase and the peptide are incubated along with a label for sufficient time to form a modified peptide product having the binding compound and the label. The modified peptide product is then bound to a matrix having a high binding compound affinity. The bound peptide is then washed and the activity of the protein kinase is measured. Also provided is a kit for the stated method.
Owner:PROMEGA CORP
Methods, peptides and biosensors useful for detecting a broad spectrum of bacteria
ActiveUS8609358B2Peptide-nucleic acidsMicrobiological testing/measurementBacteroidesPeptide substrate
Described herein are methods of detecting a wound infection and for detecting the presence or absence of bacteria, for example, wound bacteria in a sample, by contacting a sample with a peptide substrate derived from the modification of the reactive site loop (RSL) domain of the α 1-proteinase inhibitor. In the current invention, we have demonstrated that these peptide substrates without the alpha 1 protein can be efficiently used as peptide substrates. The modification or the absence of modification of this peptide substrate by the enzyme produced and / or secreted by the bacteria, can serve as an indicator for the presence or absence of the bacteria in the sample. The present invention also features a biosensor for detecting the presence or absence of bacteria in a sample.
Owner:WOUNDCHEK LAB US
Luciferyl peptide substrate
InactiveUS20060073529A1Promote localizationPeptide/protein ingredientsMicrobiological testing/measurementLuciferase GeneCell membrane
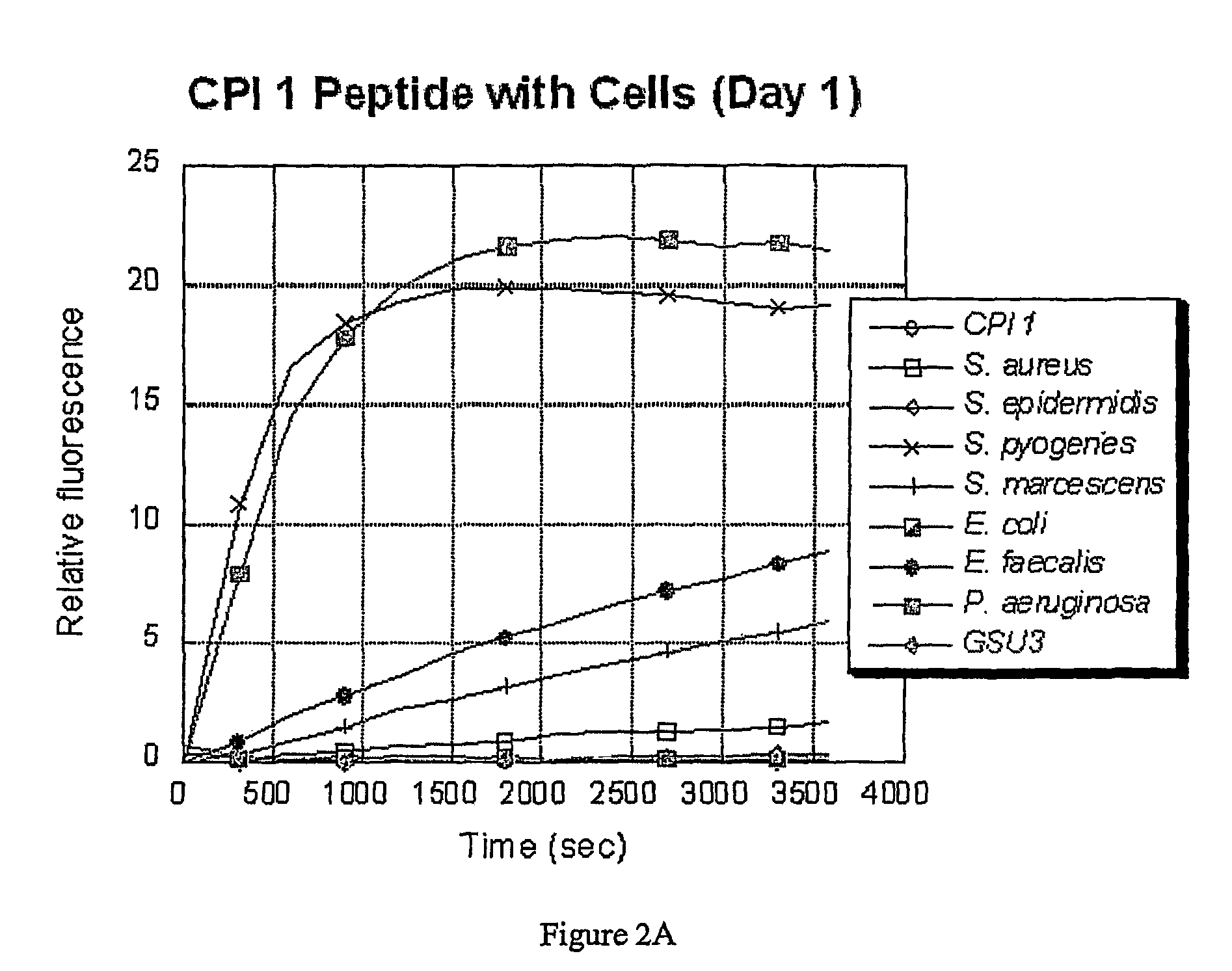
Provided are luciferyl peptide substrates that are produced by attaching specifically prepared peptide conjugates to luciferin, and / or its analogs and derivatives. The luciferyl peptide substrates are incapable of penetrating cell membranes and tissue barriers. Cleavage of the peptide conjugates from the luciferyl peptide substrates releases the luciferin, which upon contact with luciferase emits photons for easy detection. The luciferyl peptide substrates may be used in assays to detect pathogens, test protease inhibitors, probe cell physiology, assess protease activity in oncogenesis, and improve specific and regulated drug delivery.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Antibody locker for the inactivation of protein drug
ActiveUS20160185875A1Sugar derivativesImmunoglobulins against cytokines/lymphokines/interferonsPeptide substrateHeavy chain
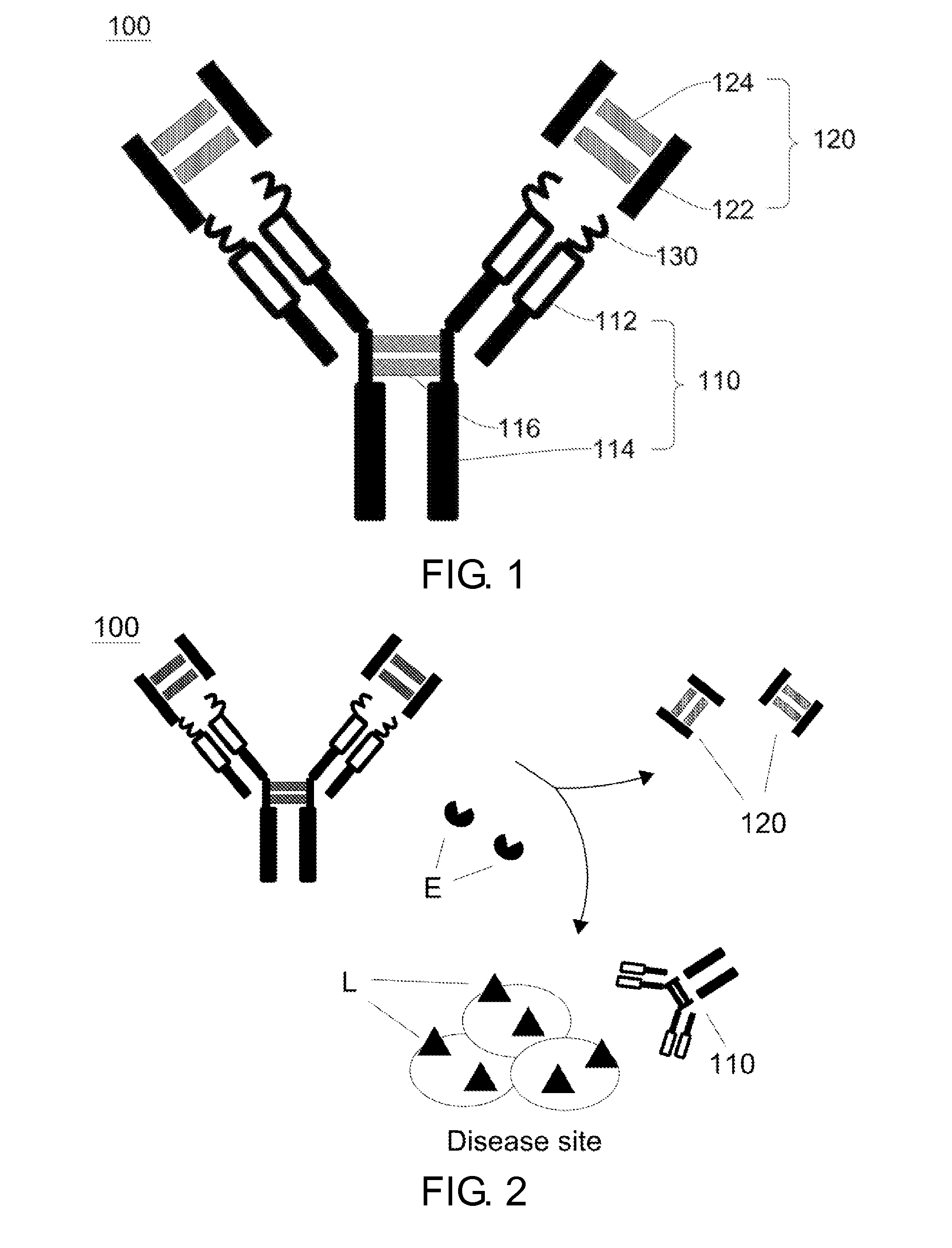
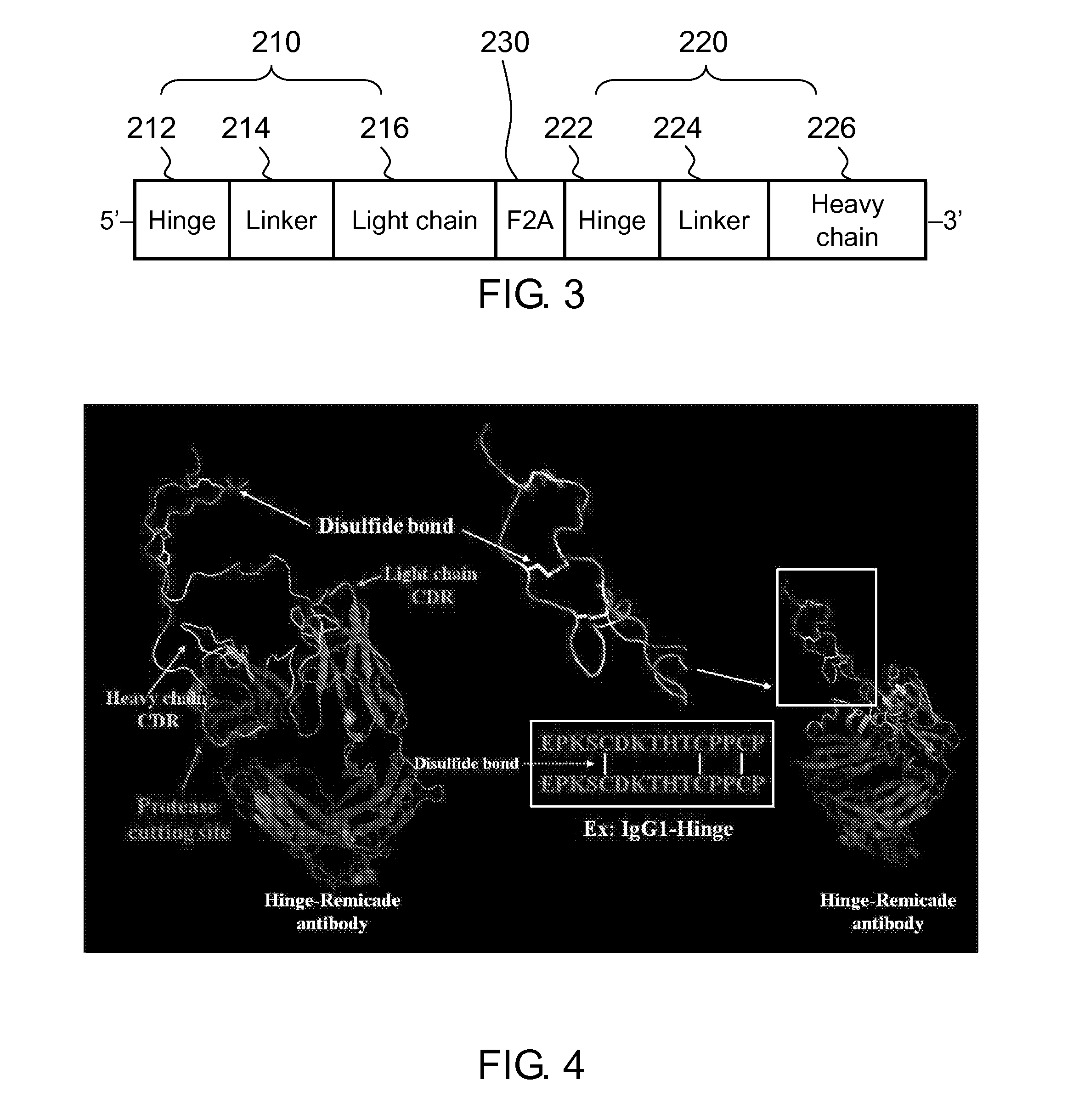
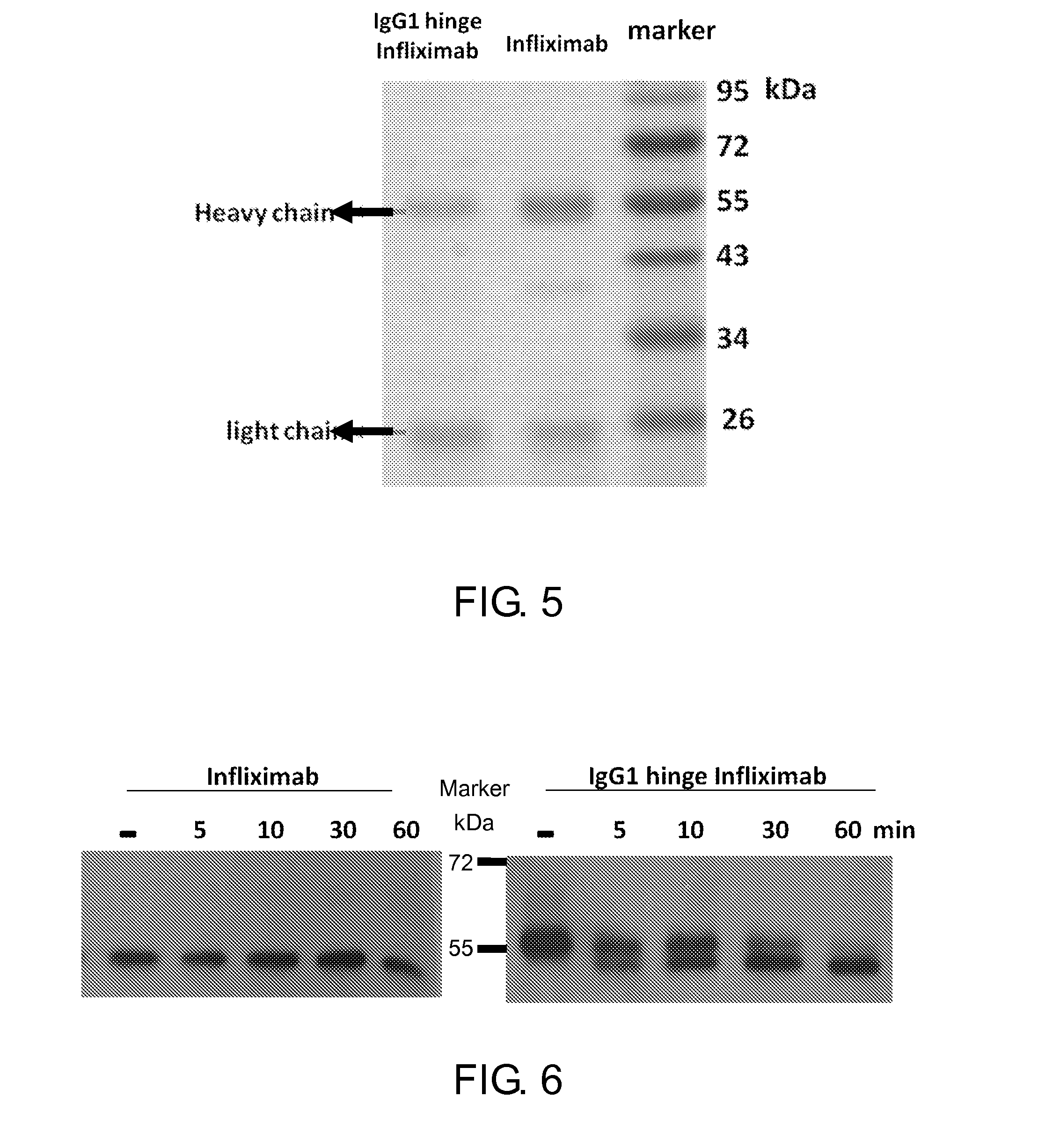
Disclosed herein is a hinge antibody capable of being selectively activated in a target cell or tissue to treat a condition therein. The hinge antibody includes a functional antibody, two inhibitory domains and four cleavable linkers. The functional antibody is capable of treating the condition in an activated state, and has two light chains and two heavy chains. Each inhibitory domain includes a hinge domain of an immunoglobulin and consists of two peptide arms. Each cleavable linker includes a peptide substrate cleavable by an enzyme specifically or highly expressed in the target cell or tissue, and connects one of the peptide arms of the inhibitory domains to the N-terminal of one of the light chains and heavy chains of the functional antibody. Also disclosed herein are methods for preparing and using this hinge antibody.
Owner:DCB USA +1
Methods and compositions for treating dry eye
InactiveUS20090131303A1Reduce capacityGood treatment effectOrganic active ingredientsBiocidePeptide substrateProteinase activity
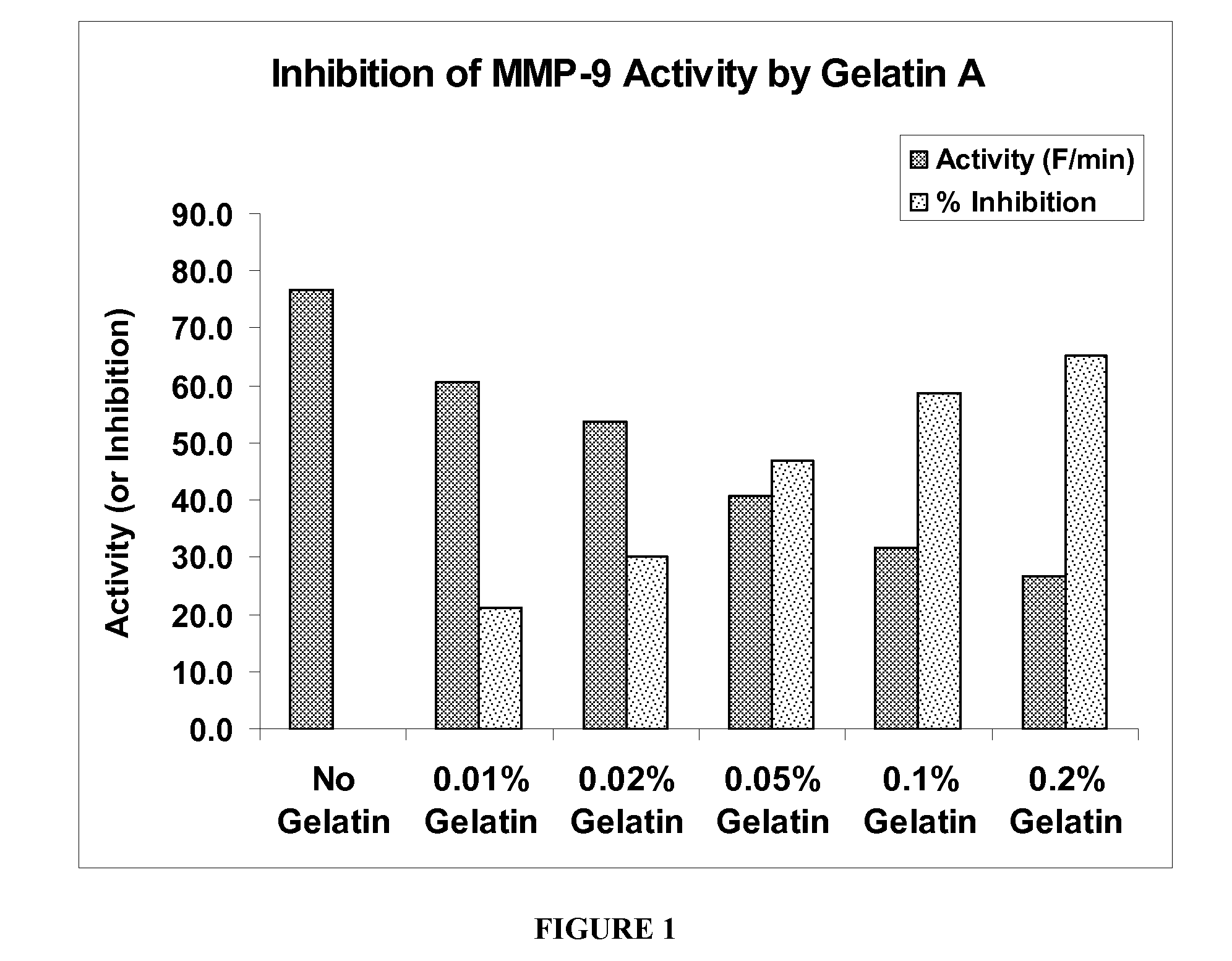
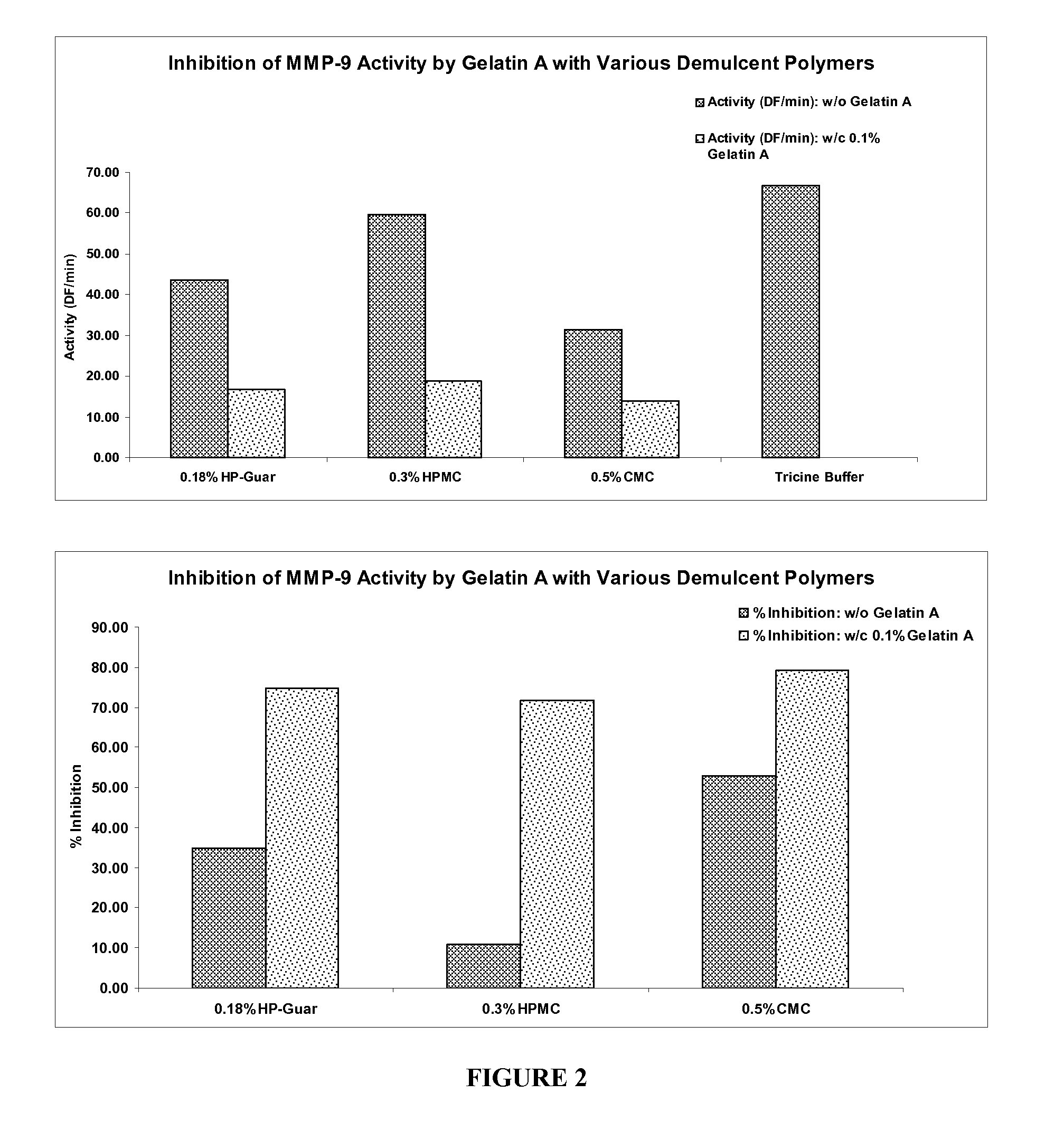
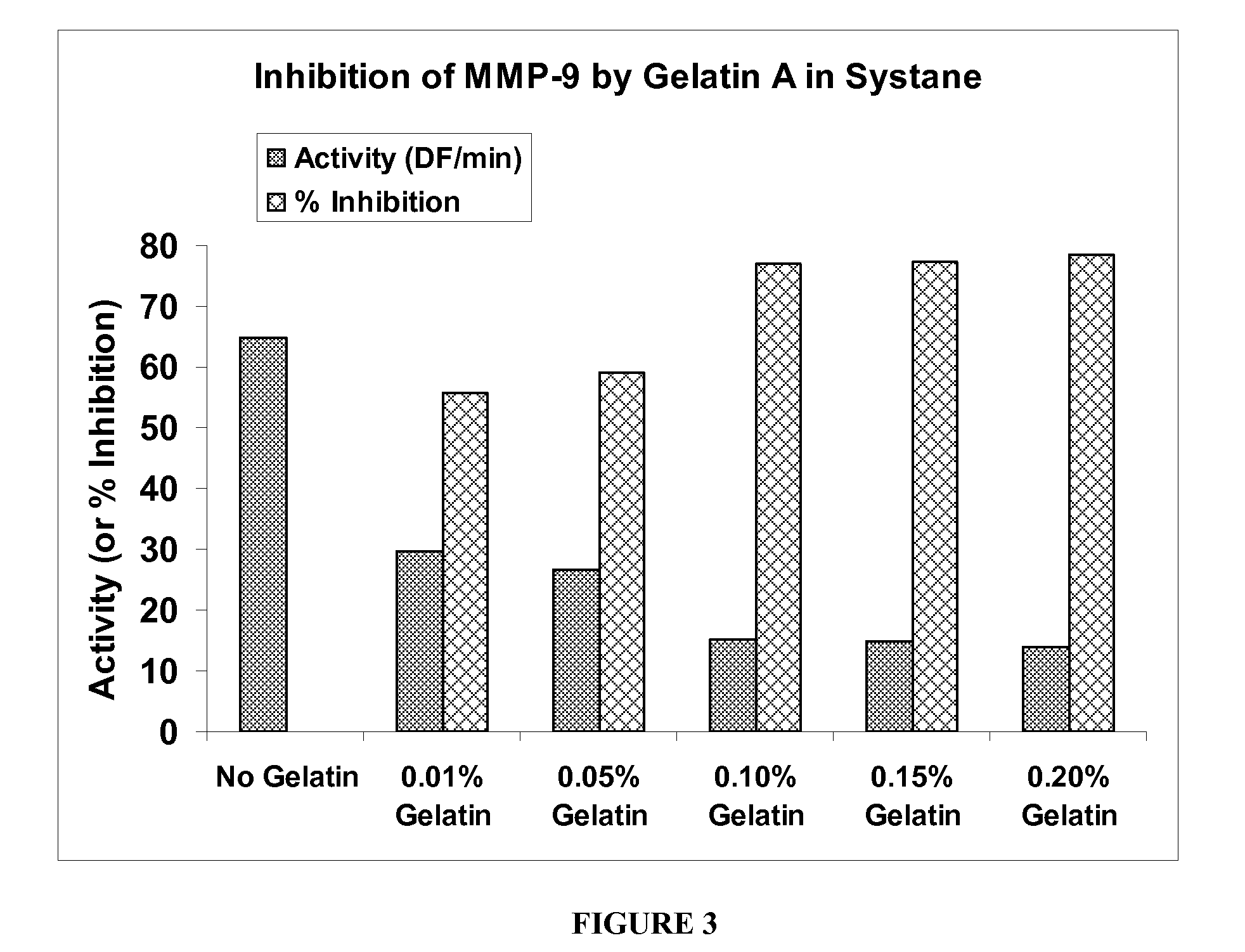
The present invention is directed to ophthalmic compositions containing protease-inhibiting peptide substrates. In a preferred embodiment, the protease-inhibiting peptide substrate is gelatin. The compositions may also contain a galactomannan. In a particularly preferred embodiment, the compositions contain gelatin, a galactomannan and a borate salt. The present invention also describes methods of use of these compositions to inhibit protease MMP-9, and methods of topical administration of the compositions to the eye, particularly for the treatment of dry eye.
Owner:ALCON RES LTD
Microstructure apparatus and method for separating differently charged molecules using an applied electric field
InactiveUS6942778B1Easy constructionSimple device designCellsFatty/oily/floating substances removal devicesPeptide substrateHigh-Throughput Screening Methods
The field of the present invention relates generally to a microstructure apparatus which may be used in a high-throughput screening context to monitor the rate of reaction of an enzyme with its substrate in cases where the product of the reaction has an altered net charge. For example, the systems and methods disclosed herein may be used to detect the activity of phosphatase enzymes, proteases and kinases on charged peptide substrates. The microstructure devices of the present invention comprise a plurality of microstructures, wherein each microstructure comprises a capture matrix located between two electrodes.
Owner:NANOGEN INC
Peptide substrate libraries
InactiveUS20040210036A1Reduce in quantityImprove throughputCompound screeningApoptosis detectionPeptide substrateMicrotiter plate
Peptide libraries containing peptides having the same net charge or same like charge are described, which may be used to screen for substrates of kinases and phosphatases and any other enzyme that causes a difference in net charge in a suitable substrate. The libraries are designed using representative amino acids for one or more classes of amino acids, thereby reducing the number of members in the peptide library. Reducing the number of peptides in the library to a manageable size permits the peptides to be segregated into individual wells of a microtiter plate, where the sequences of suitable substrates are immediately ascertainable upon exposure to enzyme by detecting the position of charge inverted peptide substrates in the plate.
Owner:NANOGEN INC
Gold nanoparticle based protease imaging probes and use thereof
InactiveUS20100124757A1Good biocompatibilityRapidly screen activationPowder deliveryNanostructure manufactureHuman bodyDisease
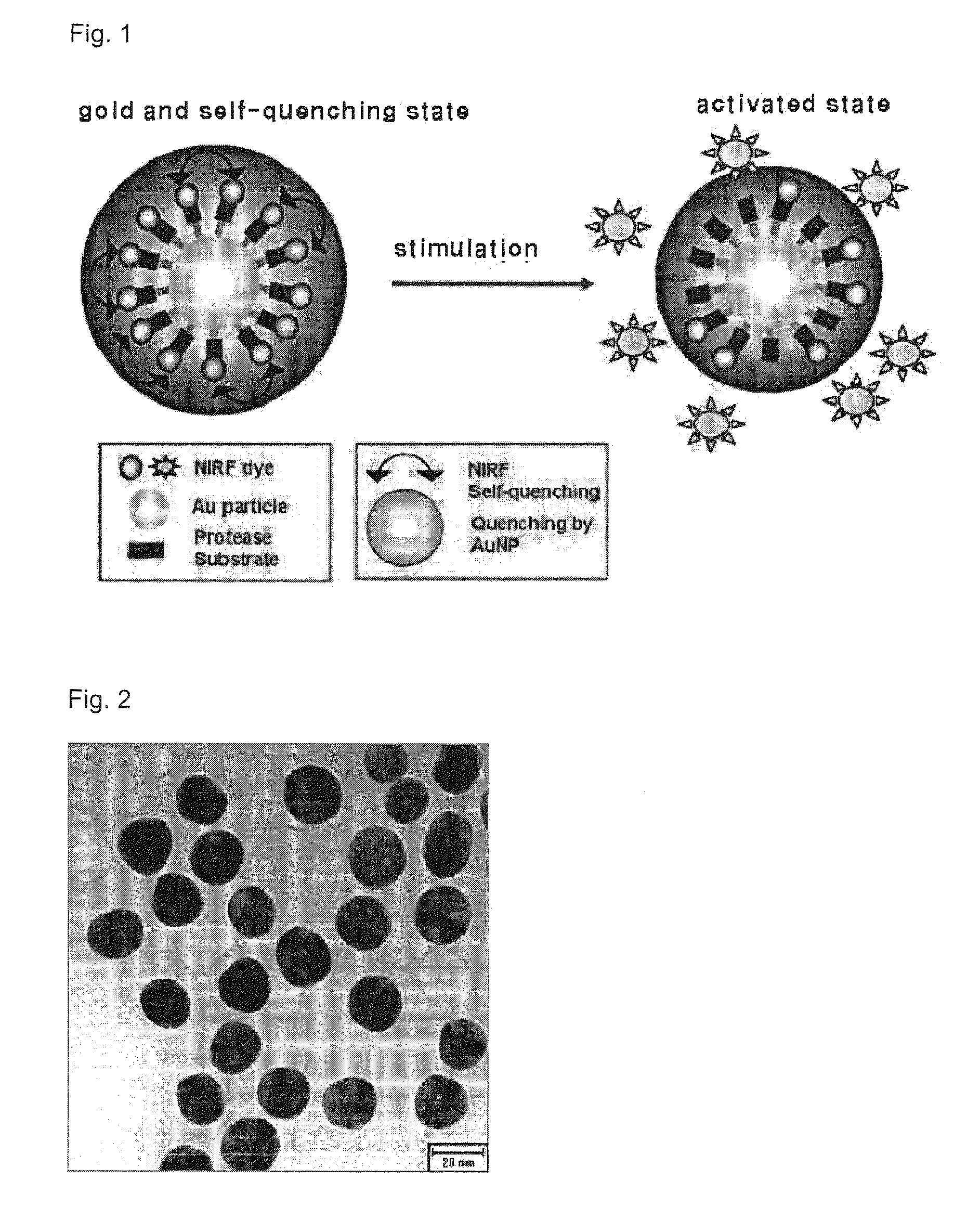
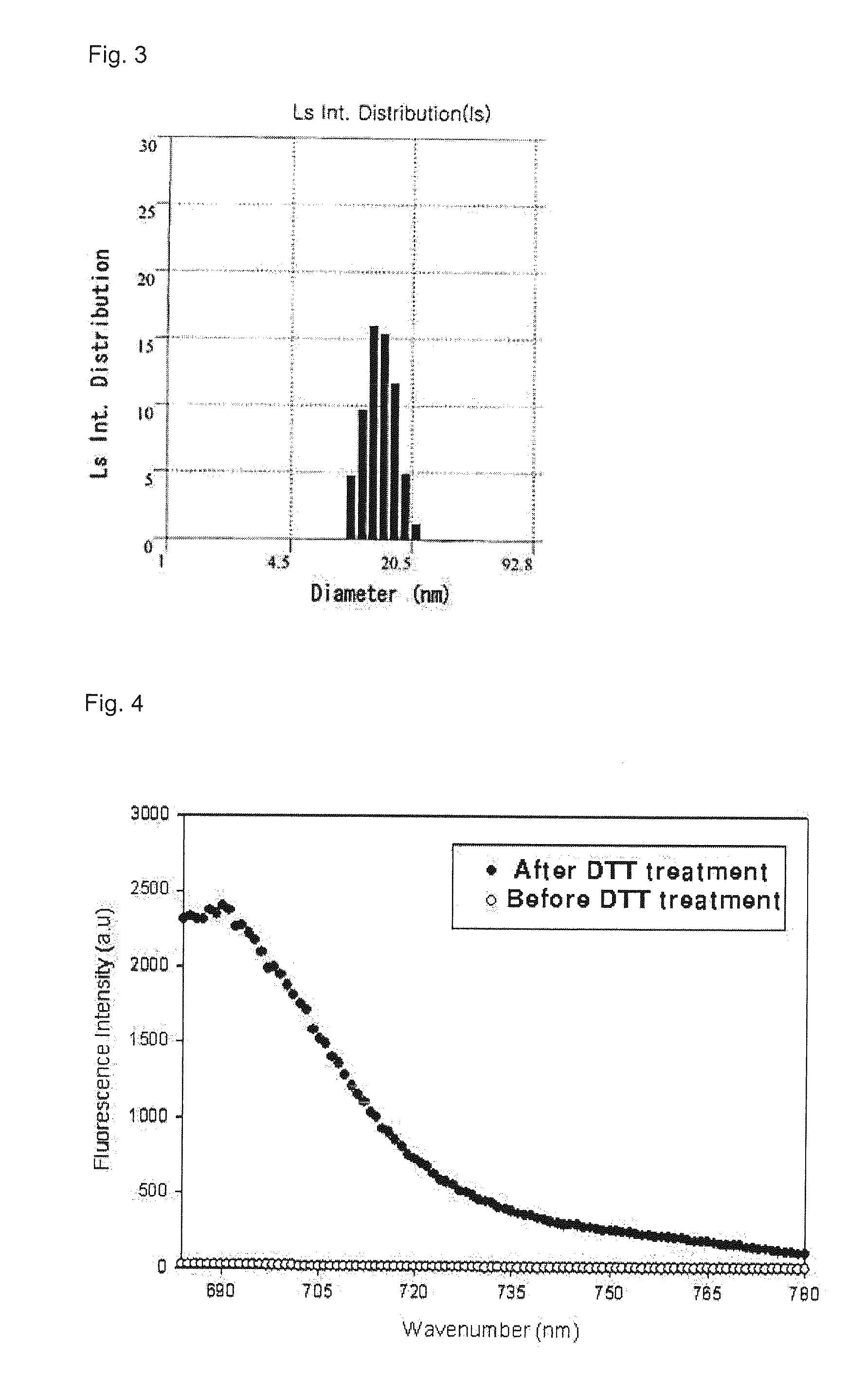
Disclosed are a metal nanoparticle onto which a peptide substrate specifically degraded by protease and fluorophore are chemically modified for selectively imaging protease expressed in cell and in tissue in a human body, and the use thereof. Also, a quantitative analysis method of protease using the metal nanoparticle, a cell imaging method and a drug screening method of inhibiting a protease overexpression are provided. In detail, the present invention is directed to a metal nanoparticle having a peptide substrate and fluorophore coupled thereto, the peptide substrate and the fluorophore being specifically degraded by due to a protease activated in various ways in cell and in a human body to exhibit fluorescence. Hence, the metal nanoparticle can be used to rapidly screen activation and inhibition of the protease in the imaging manner. Also, the metal nanoparticle is capable of being selectively absorbed into a cell and a tissue so as to be possibly used as a sensor for real-time cell imaging and early diagnosis of non-invasive diseases.
Owner:KOREA INST OF SCI & TECH
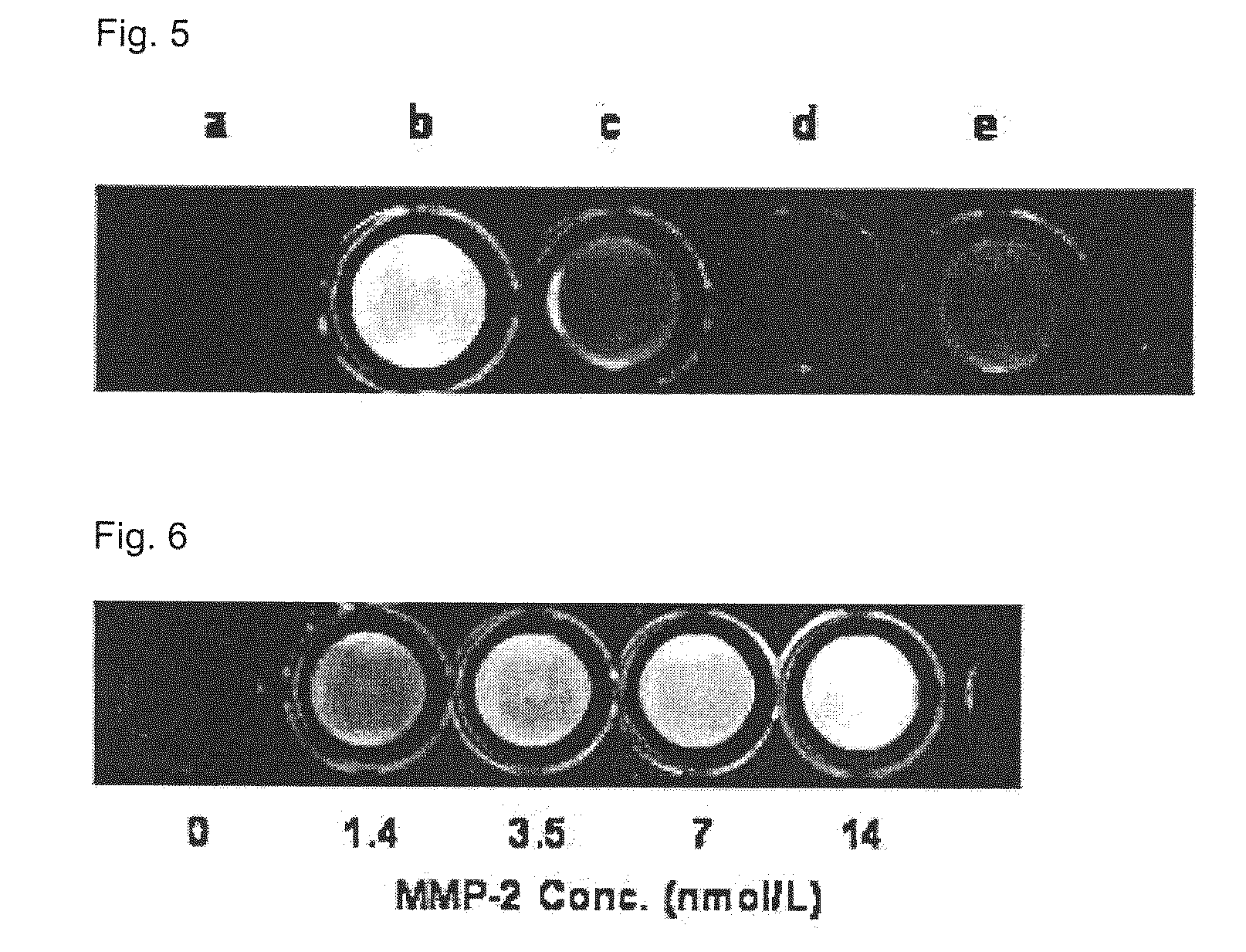
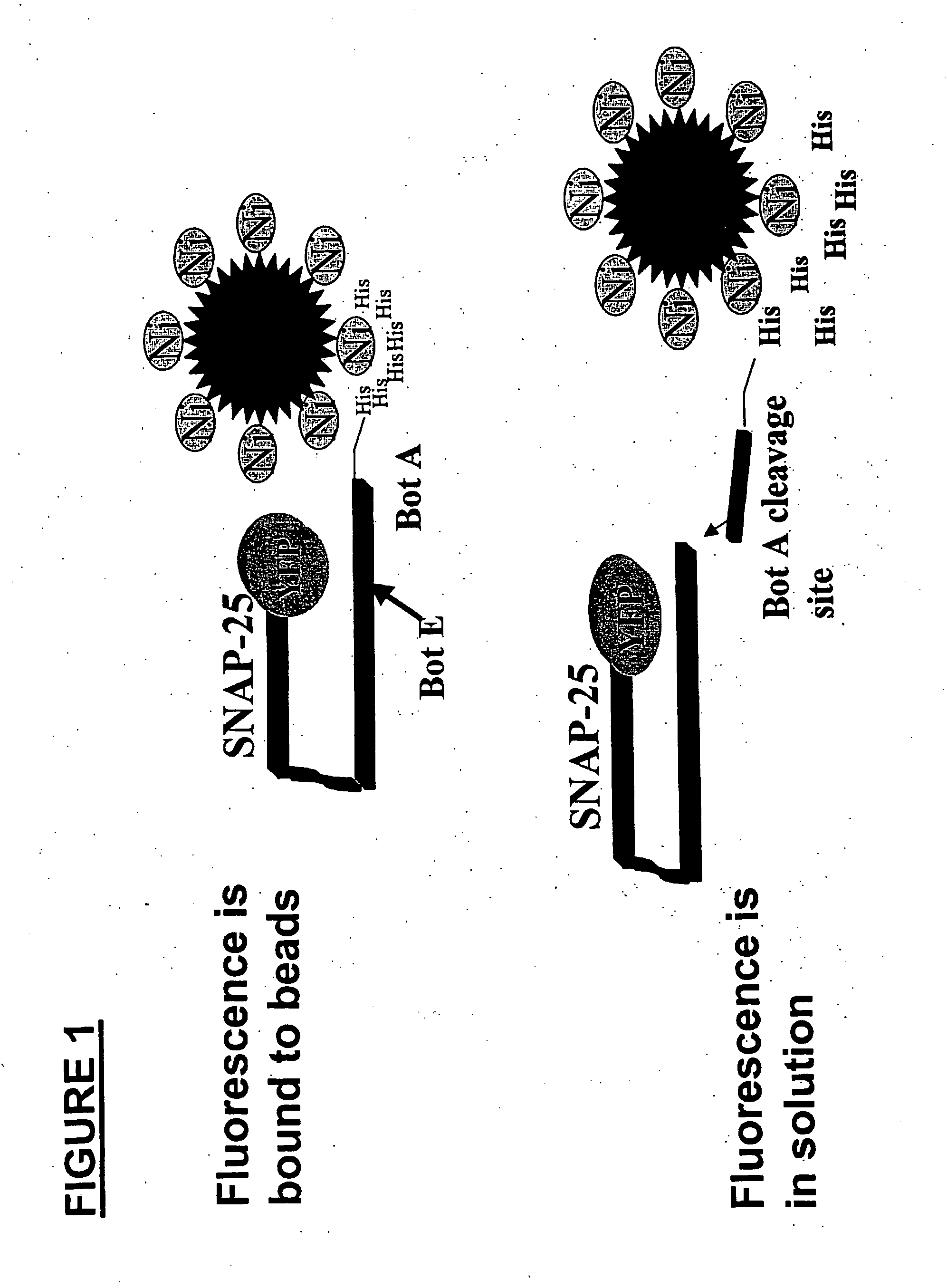
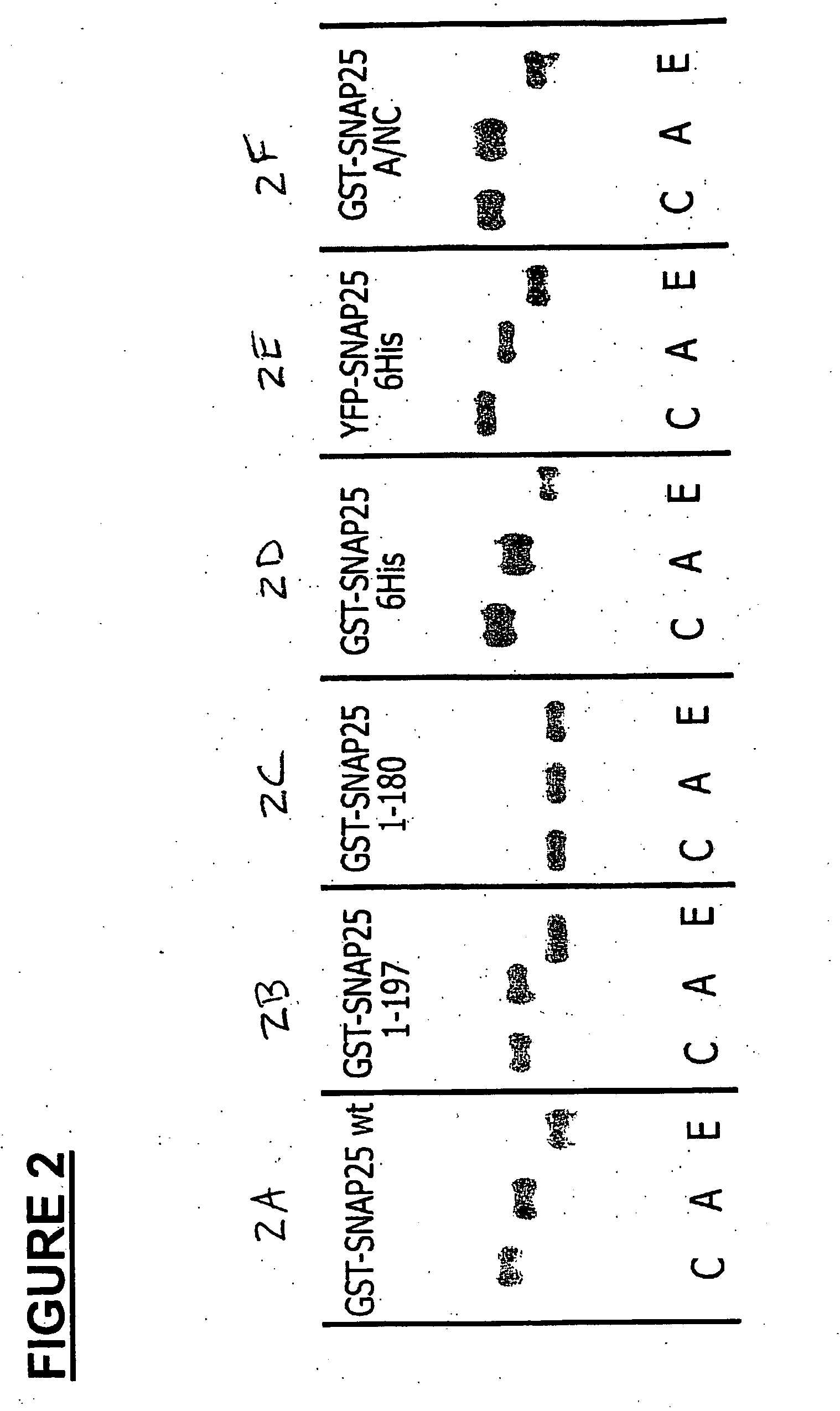
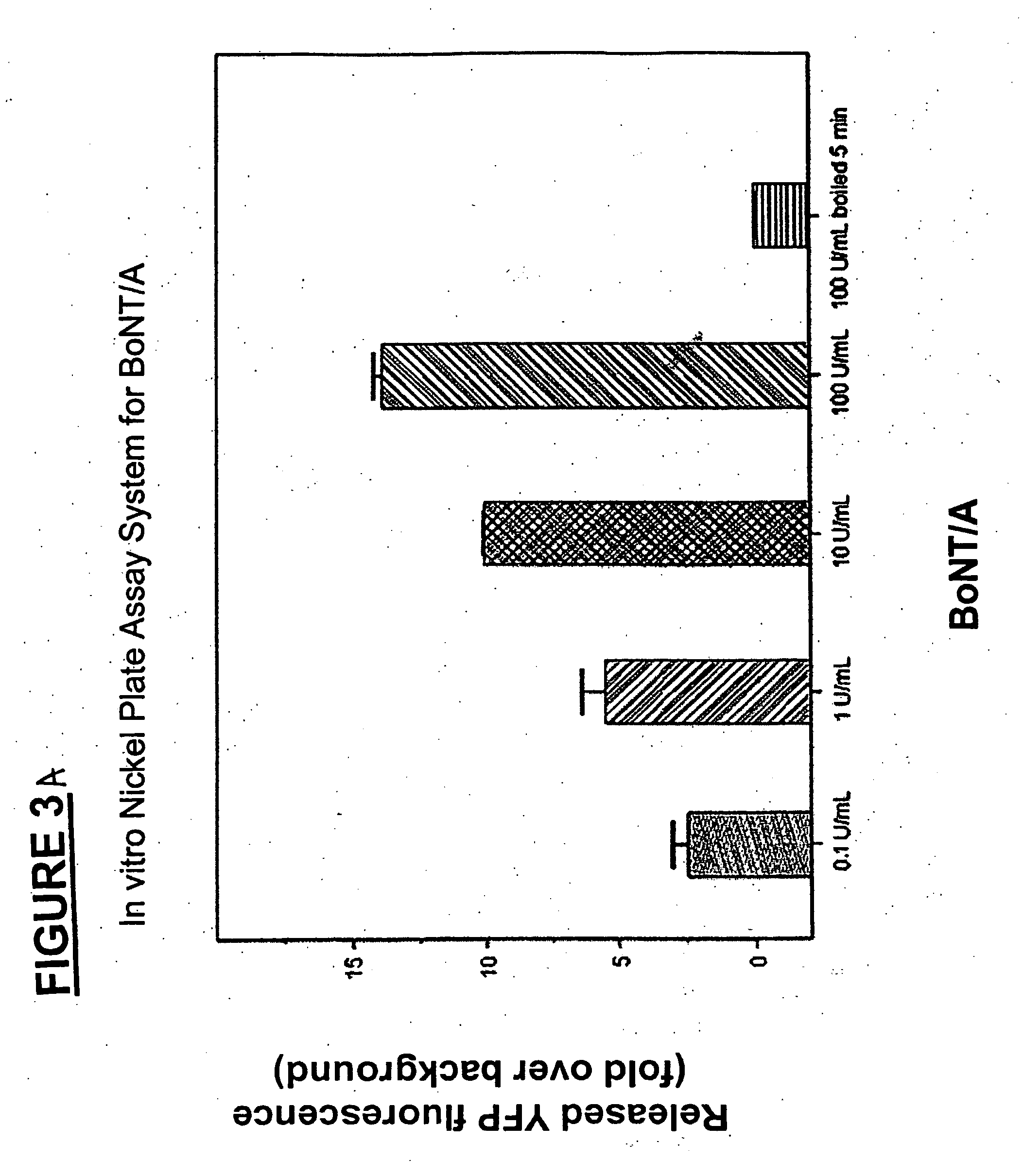
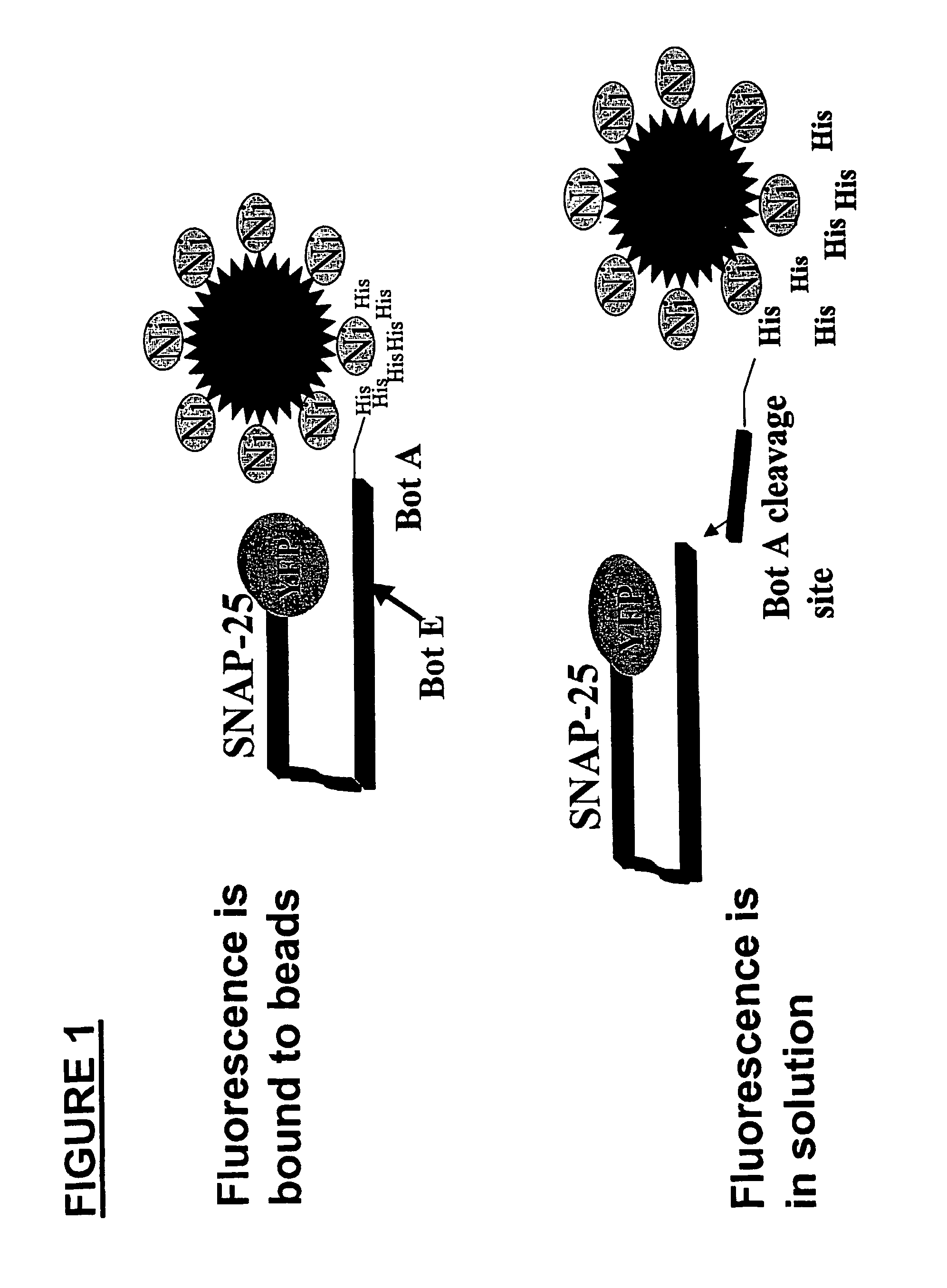
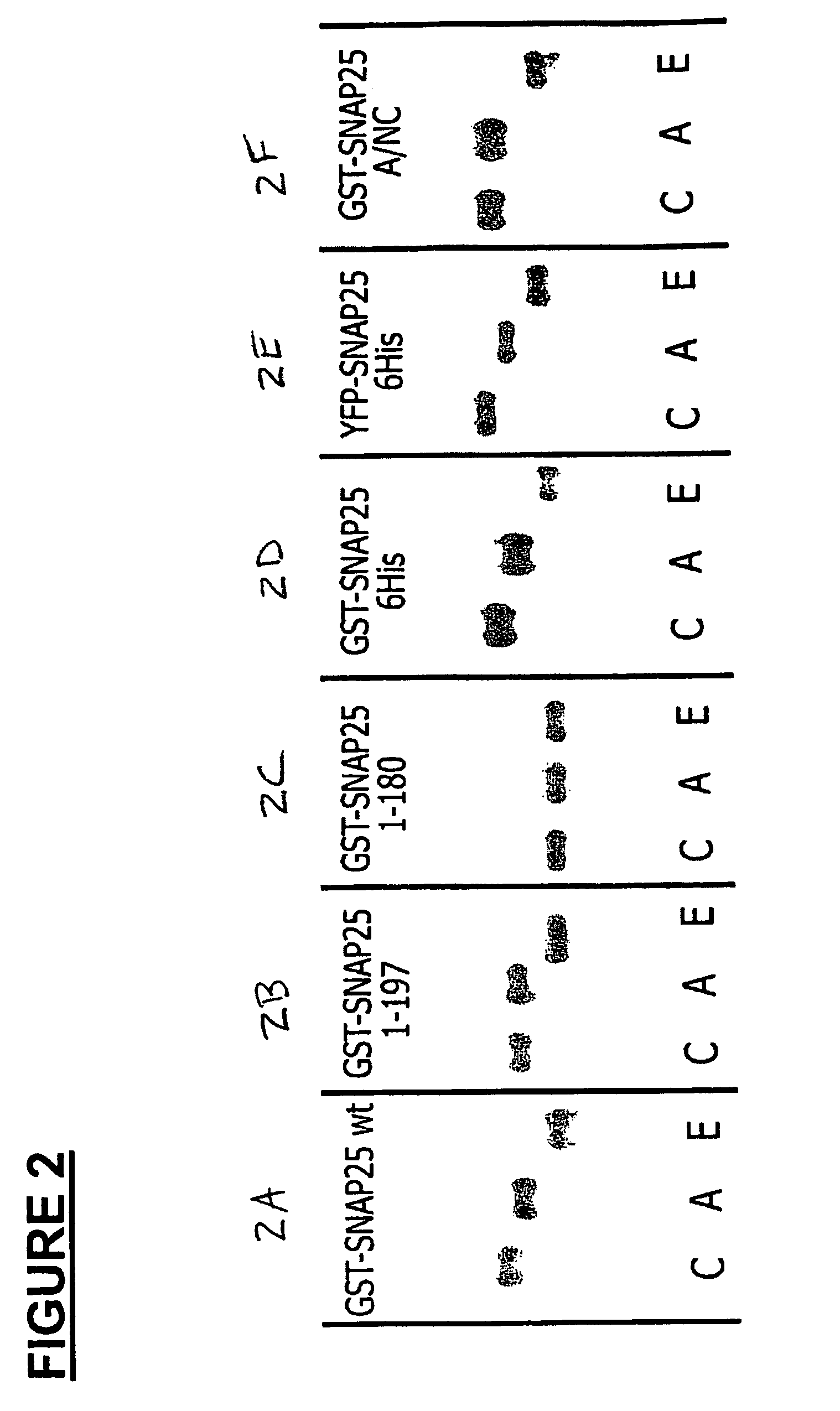
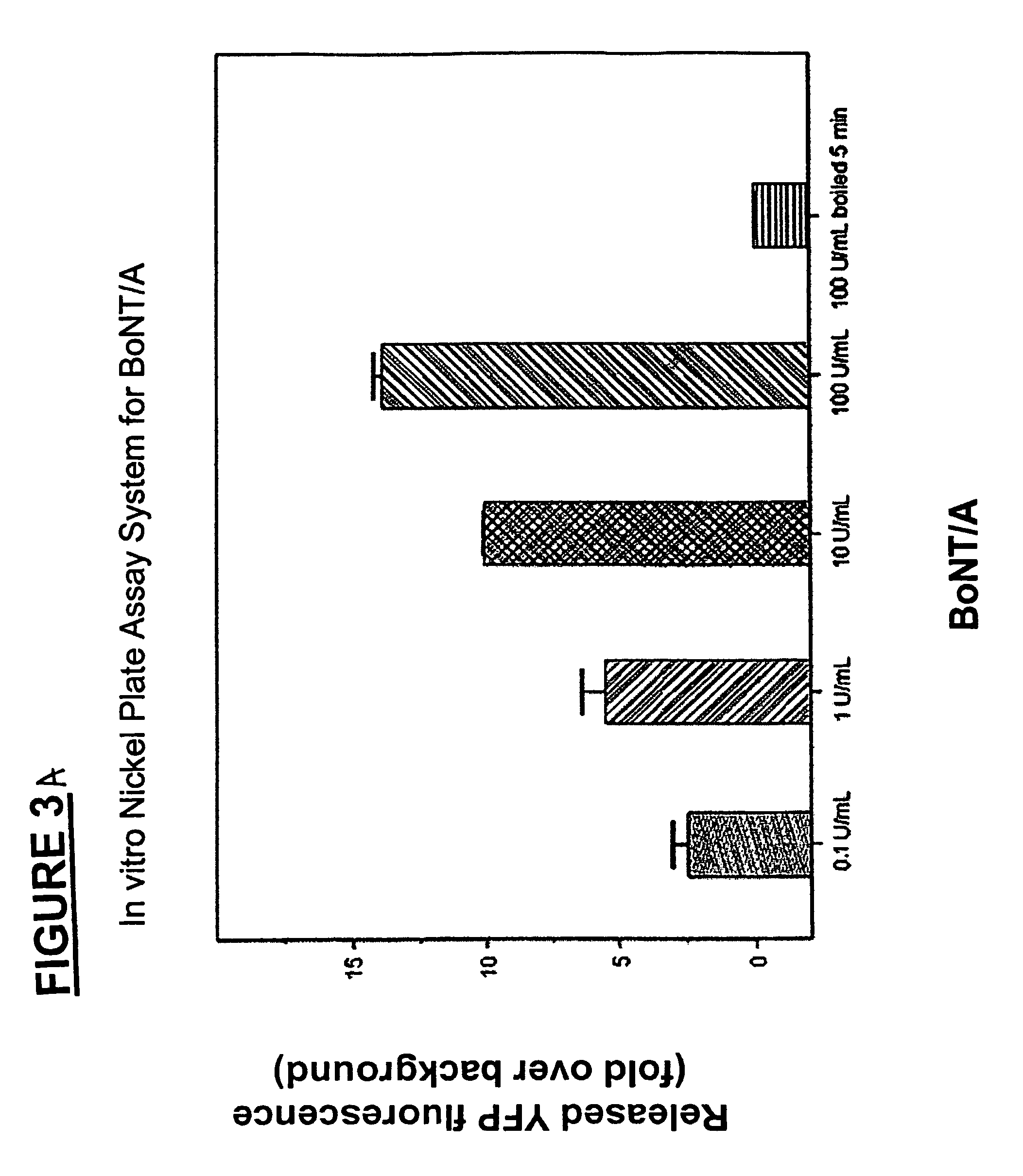
Methods for identifying inhibitors of botulinum neurotoxins
InactiveUS20060233836A1Compound screeningBacterial antigen ingredientsPeptide substrateStable cell line
A system and method for identifying a botulinum neurotoxin inhibitor employing a botulinum neurotoxin substrate complex having a peptide substrate, preferably SNAP-25, a reporter domain on one side of said peptide substrate and an immobilization domain on the opposite side of said peptide substrate. The botulinum neurotoxin inhibitor is identified by its ability to decrease the relative amount of cleaved complex, detected through measuring a decrease in complex bound to a solid support. The method of the present invention also utilizes novel cells that express a botulinum neurotoxin substrate complex. The methods of the present invention are adapted for cell based screening to monitor the catalytic activity of a BoNT in living cells and to identify molecules that inhibit the catalytic activity of a BoNT in living cells. Also provided are novel stable cell lines that express the botulinum toxin substrate complex and viral vectors capable of efficiently expressing an active light chain of the BoNT within mammalian cells.
Owner:BOTX TECH LLC
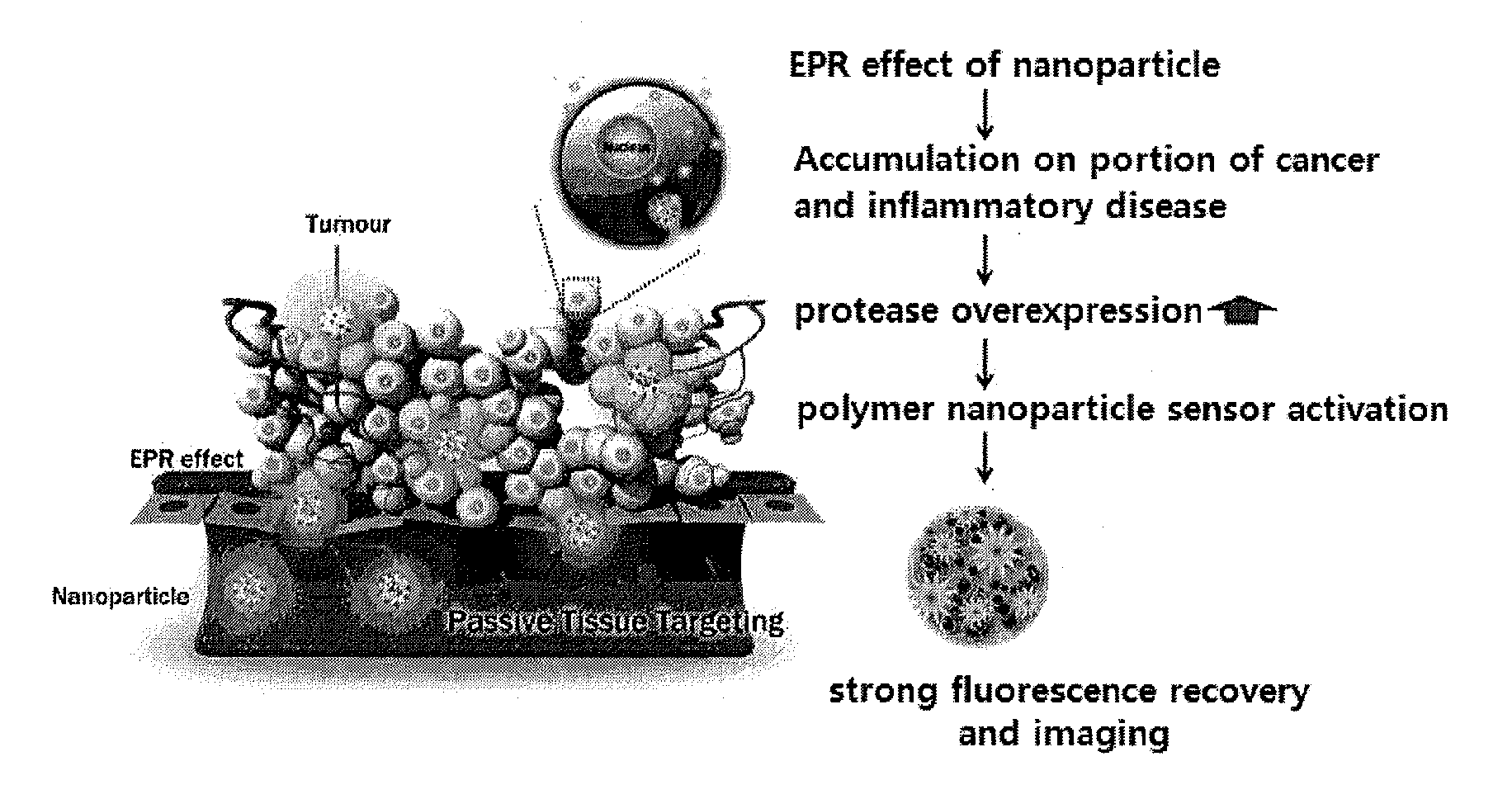
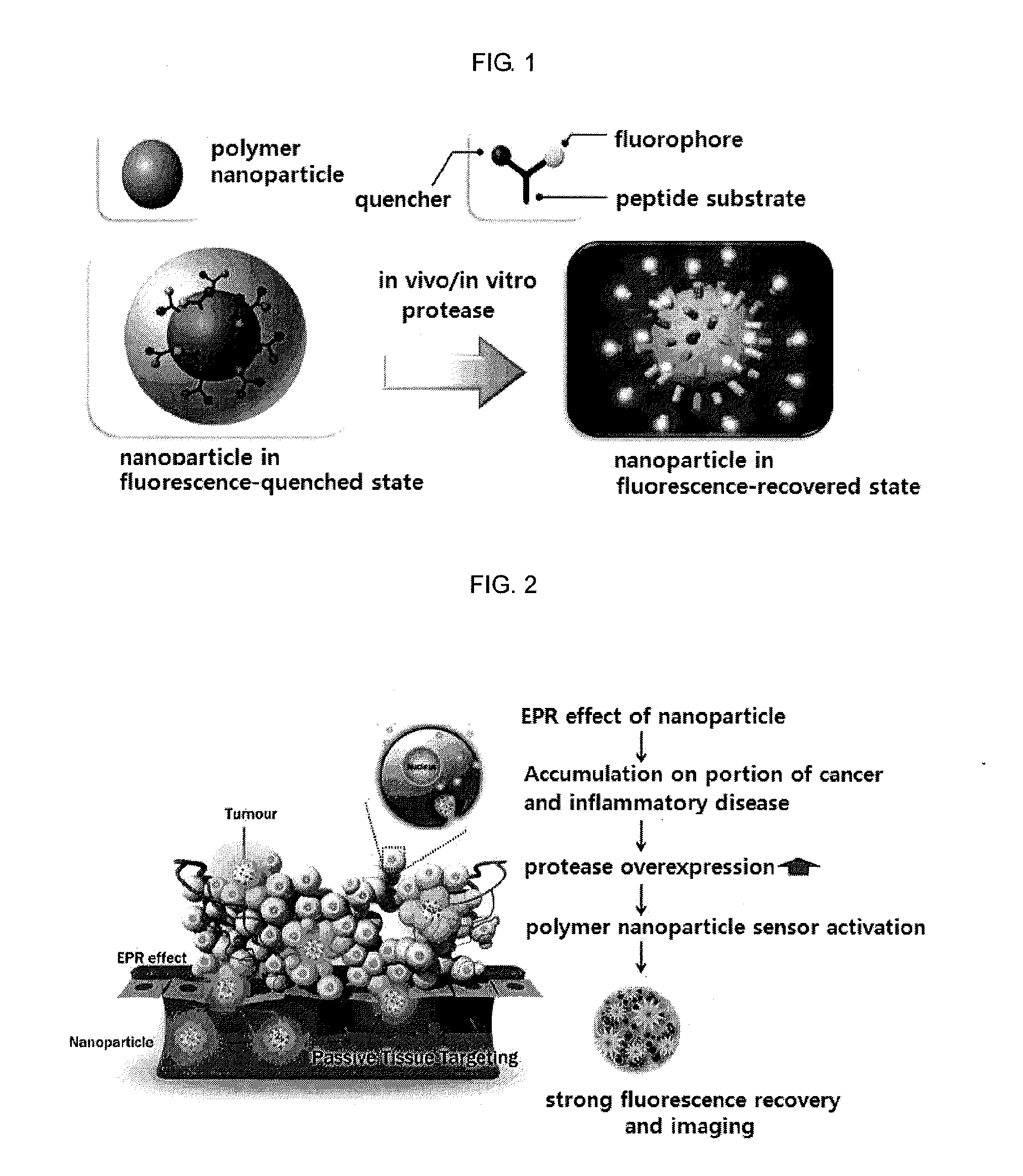
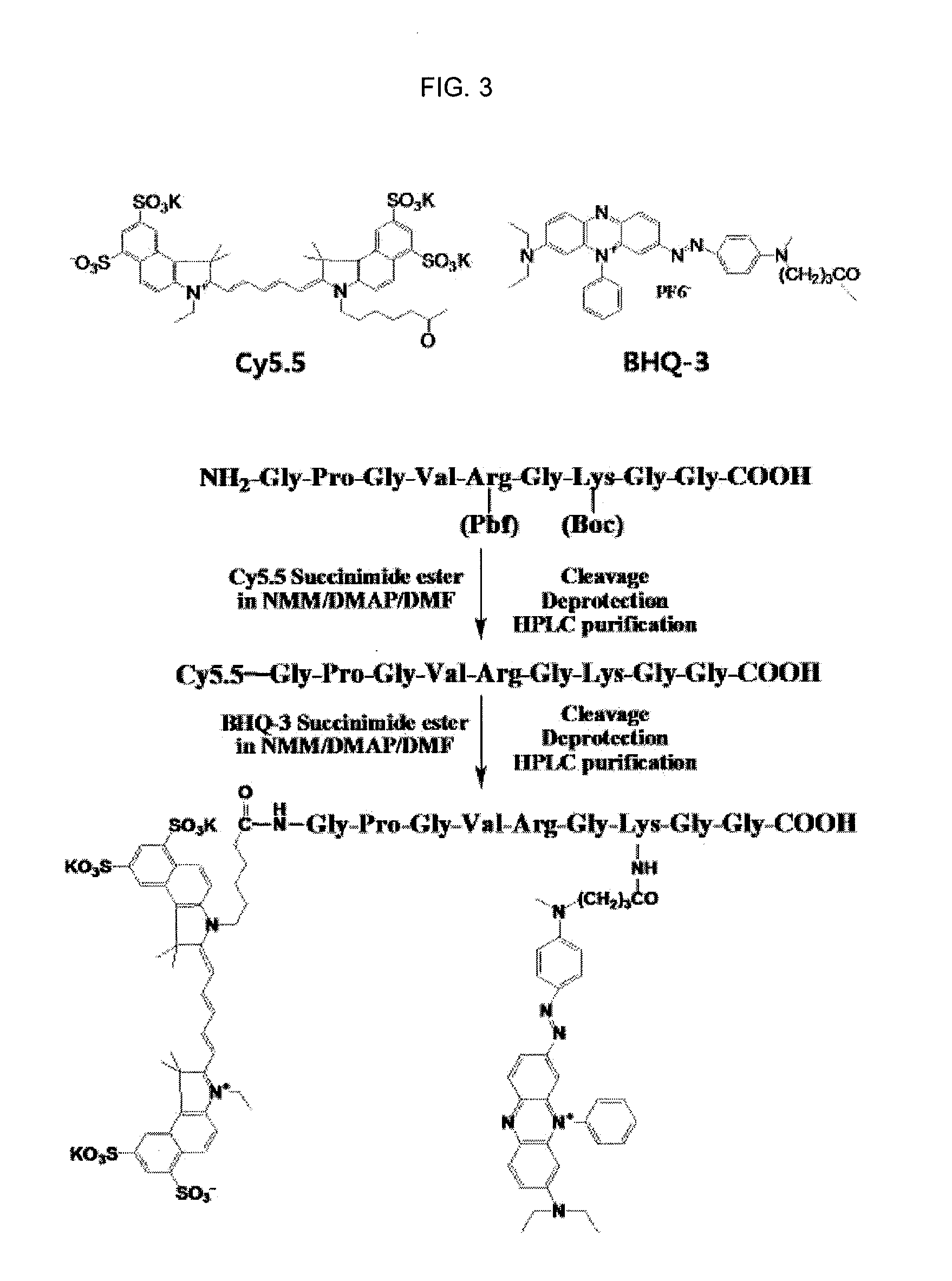
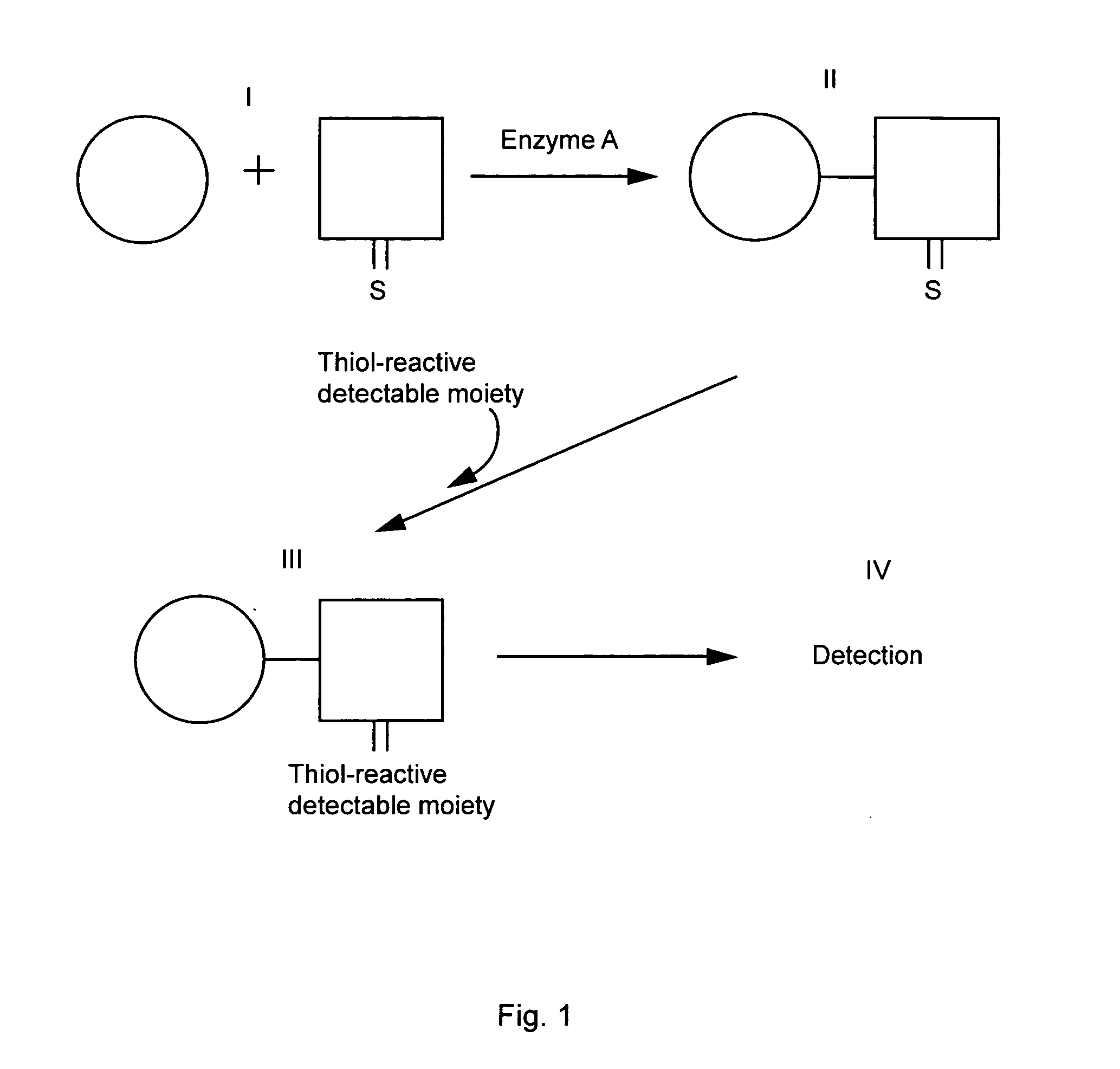
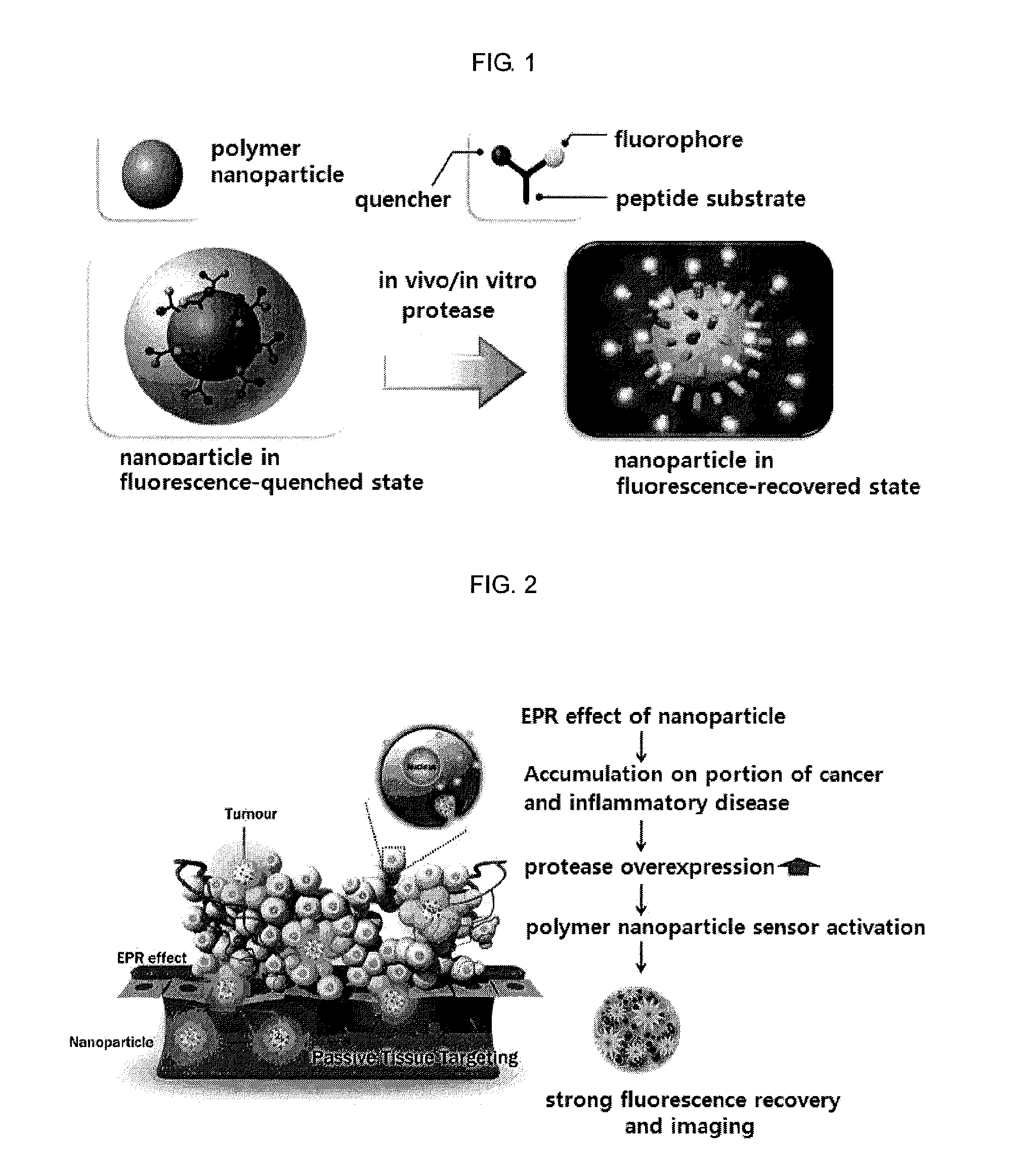
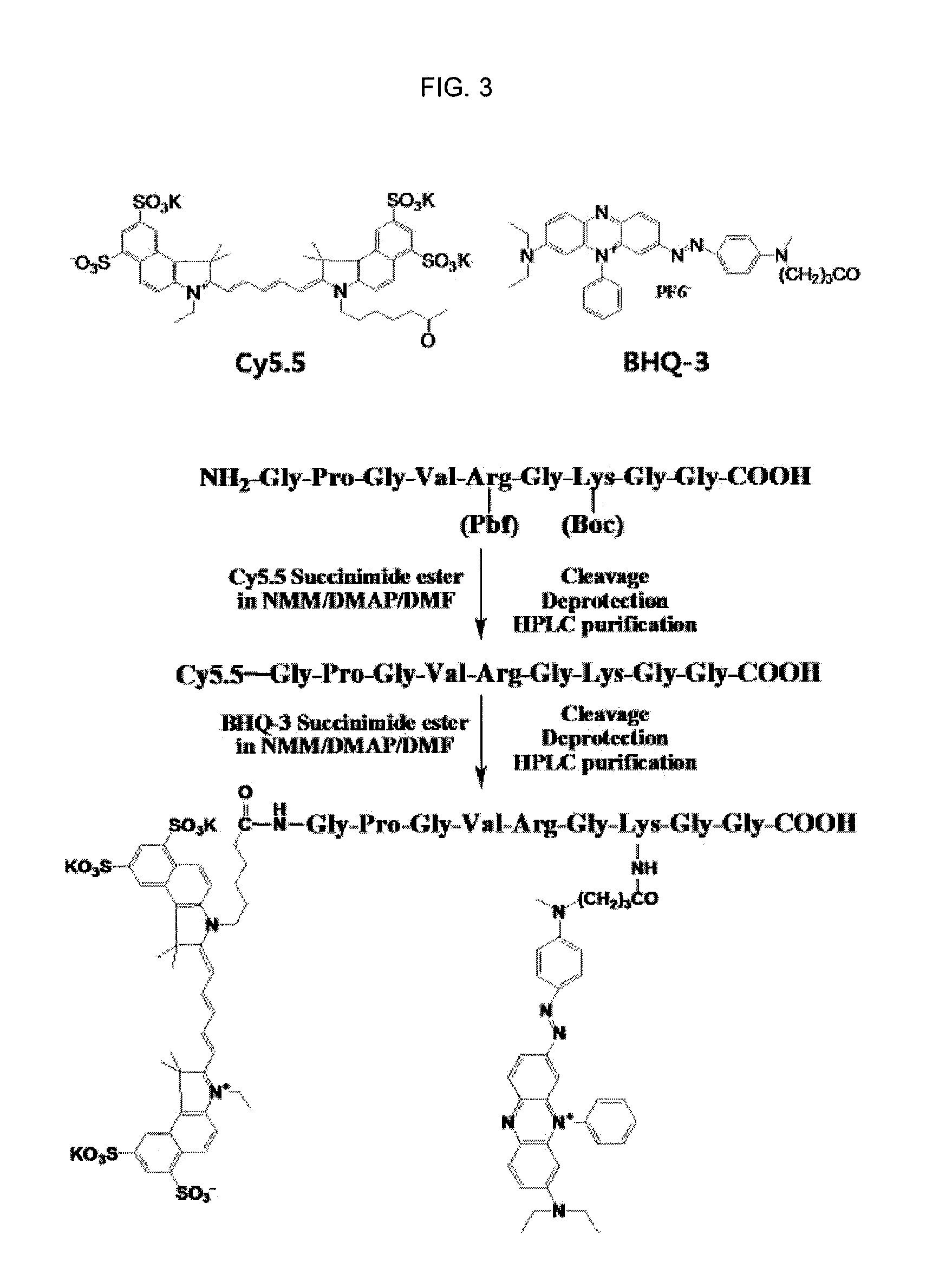
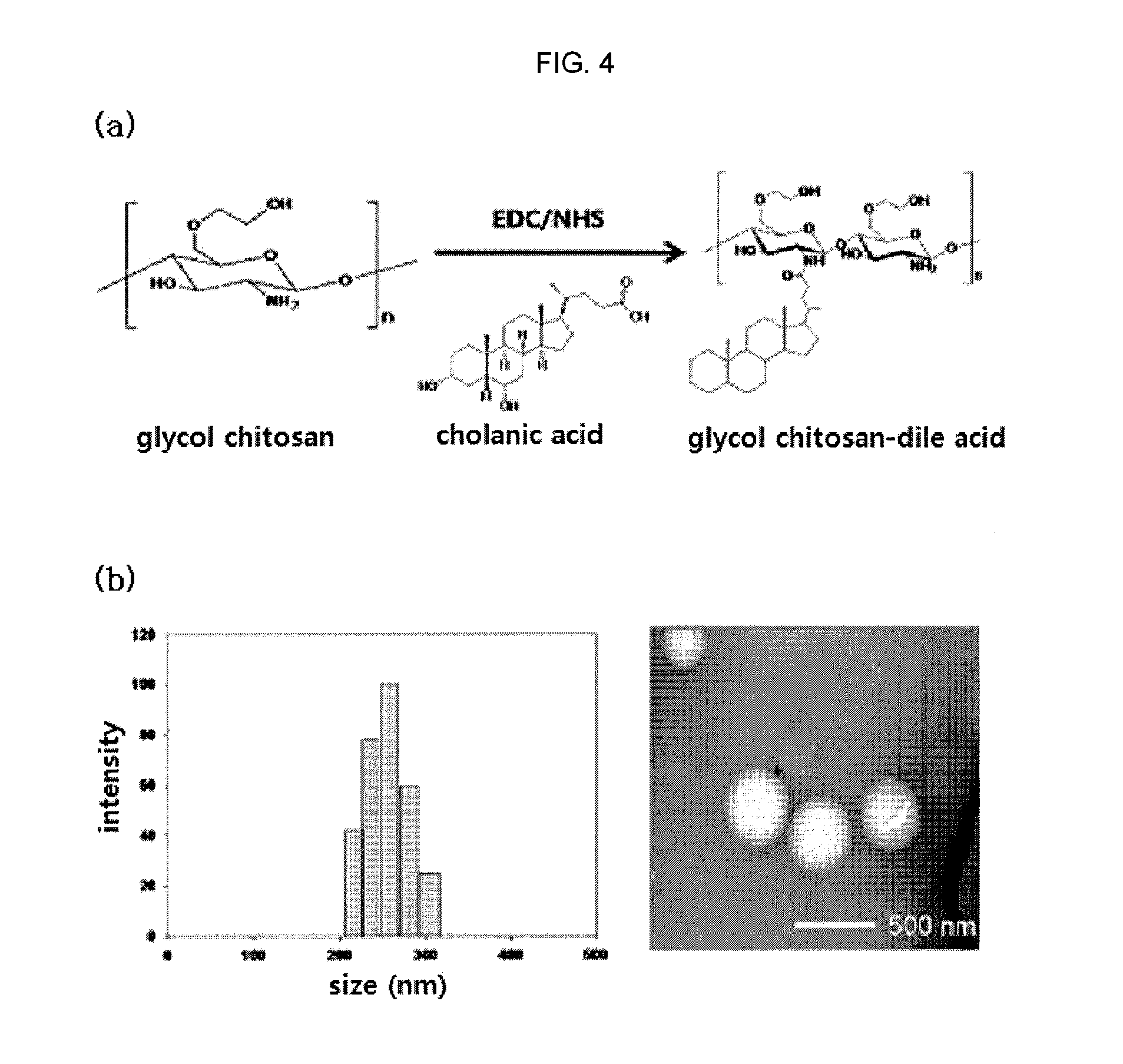
Nanoparticle sensor for measuring protease activity and method for manufacturing the same
ActiveUS20110213121A1Microbiological testing/measurementNanomedicineIncurable diseasesAutoimmune disease
Disclosed are a nanoparticle sensor for measuring protease activity, for protease imaging, and a method for preparing the same. More specifically, the present invention relates to a nanoparticle sensor for measuring protease activity in which a fluorophore- and a quencher-conjugated peptide substrate is conjugated to a biocompatible polymer nanoparticle. The peptide substrate is specifically lysed by a protease. The sensor according to the present invention is capable of inhibiting emission of fluorescence with high extinctive activity of the quencher on a fluorescent material. But strong fluorescence is specifically emitted only if the peptide substrate is lysed by a specific protease. Therefore, the sensor is especially useful as a method for screening a novel drug such as a protease overexpression inhibitor, and early diagnosis of incurable diseases and various diseases such as autoimmune diseases including cancer, osteoarthritis, rheumatoid arthritis and dementia.
Owner:KOREA INST OF SCI & TECH +2
Homogeneous assay methods
InactiveUS20050176071A1Microbiological testing/measurementBiological testingPeptide substrateKinase activity
The present invention provides a method of assaying for kinase activity, comprising contacting a fluorescently labeled phosphorylatable peptide substrate with an ATP analog in the presence of a kinase enzyme to yield a first product; contacting the first product with a reactant that comprises a biotin derivative to yield a second product; contacting the second product with a biotin-binding protein; and detecting a difference in a fluorescence polarization level from the second product as compared to a fluorescence polarization of the peptide substrate.
Owner:CAPLIPER LIFE SCI INC
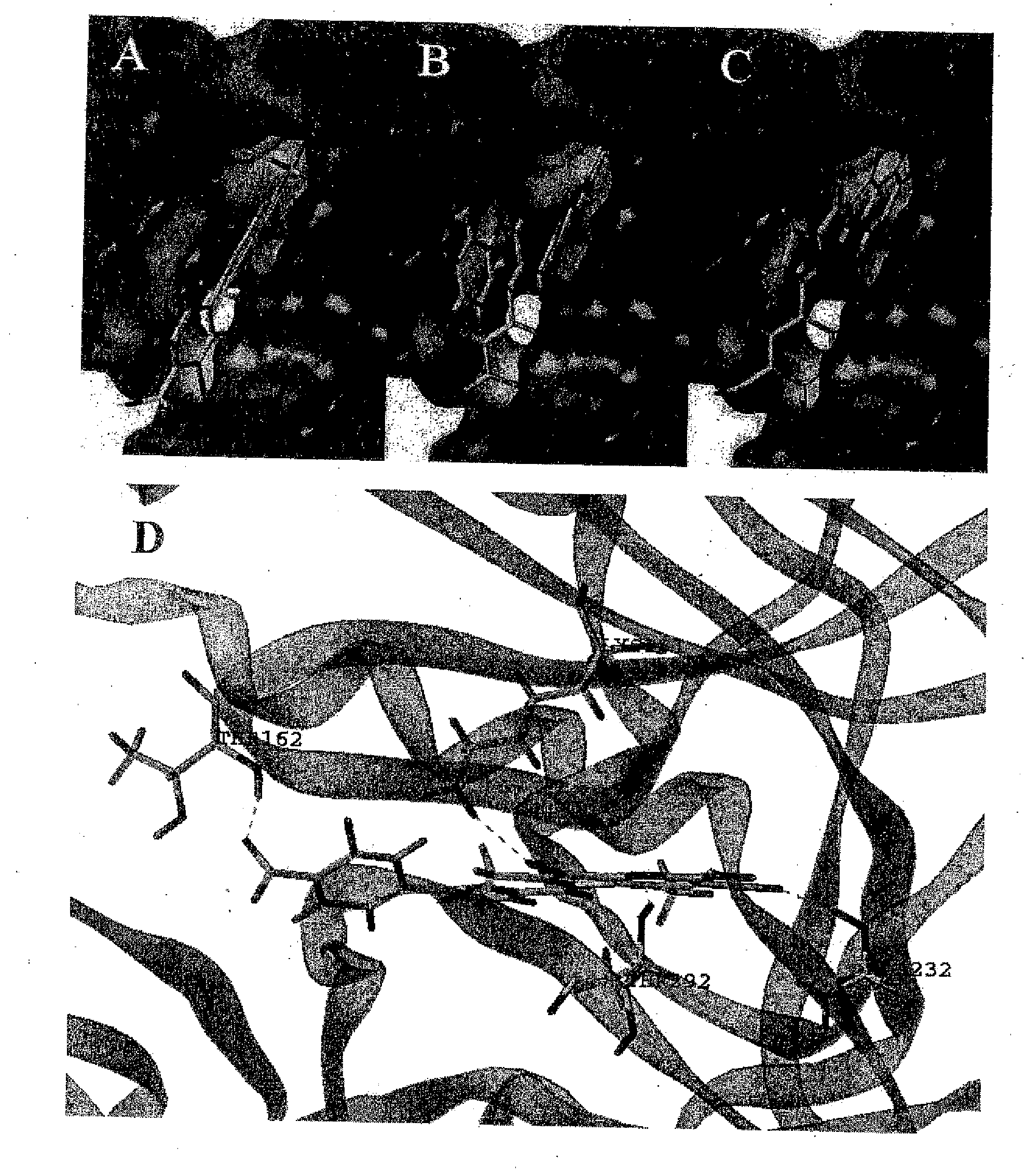
Inhibitors of memapsin 2 and use thereof
InactiveUS20080021196A1Reduce molecular weightGrowth inhibitionNervous disorderHydrolasesDiseaseActive enzyme
Methods for the production of purified, catalytically active, recombinant memapsin 2 have been developed. The substrate and subsite specificity of the catalytically active enzyme have been determined. The substrate and subsite specificity information was used to design substrate analogs of the natural memapsin 2 substrate that can inhibit the function of memapsin 2. The substrate analogs are based on peptide sequences, shown to be related to the natural peptide substrates for memapsin 2. The substrate analogs contain at least one analog of an amide bond which is not capable of being cleaved by memapsin 2. Processes for the synthesis of two substrate analogues including isosteres at the sites of the critical amino acid residues were developed and the substrate analogues, OMR99-1 and OM99-2, were synthesized. OM99-2 is based on an octapeptide Glu-Val-Asn-Leu-Ala-Ala-Glu-Phe (SEQ ID NO:28) with the Leu-Ala peptide bond substituted by a transition-state isostere hydroxyethylene group (FIG. 1). The inhibition constant of OM99-2 is 1.6×10−9 M against recombinant pro-memapsin 2. Crystallography of memapsin 2 bound to this inhibitor was used to determine the three dimensional structure of the protein, as well as the importance of the various residues in binding. This information can be used by those skilled in the art to design new inhibitors, using commercially available software programs and techniques familiar to those in organic chemistry and enzymology, to design new inhibitors to memapsin 2, useful in diagnostics and for the treatment and / or prevention of Alzheimer's disease.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS +1
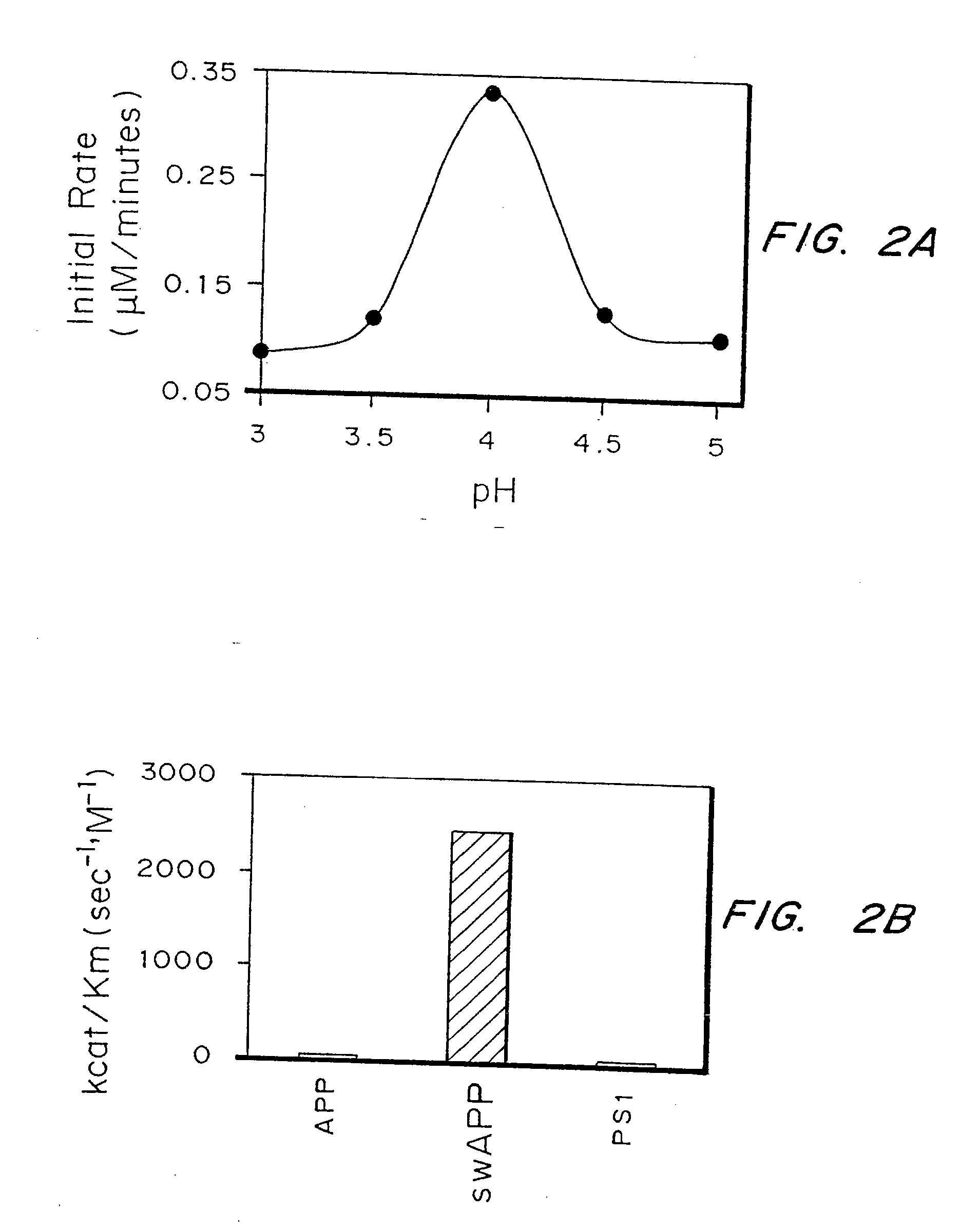
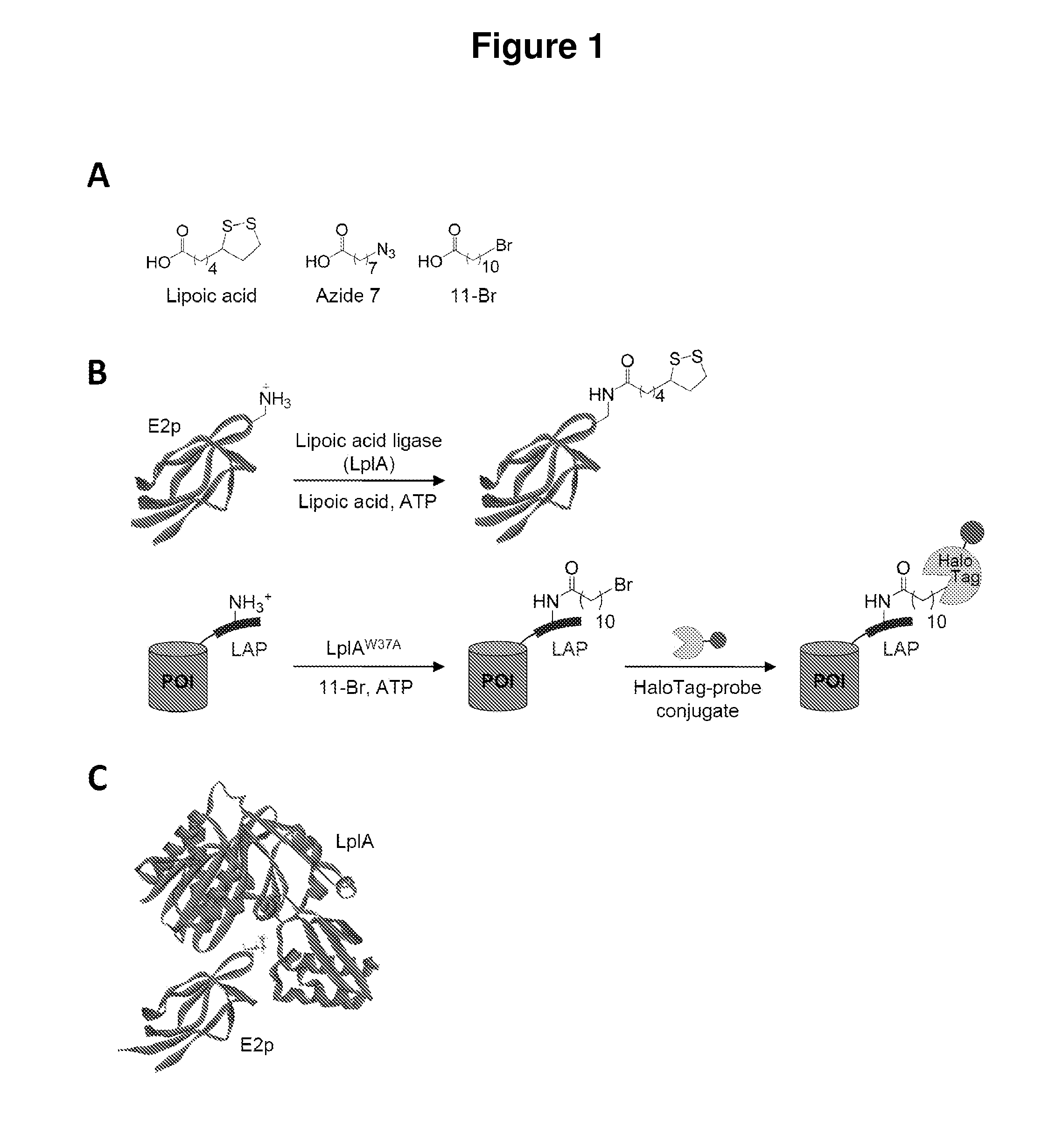
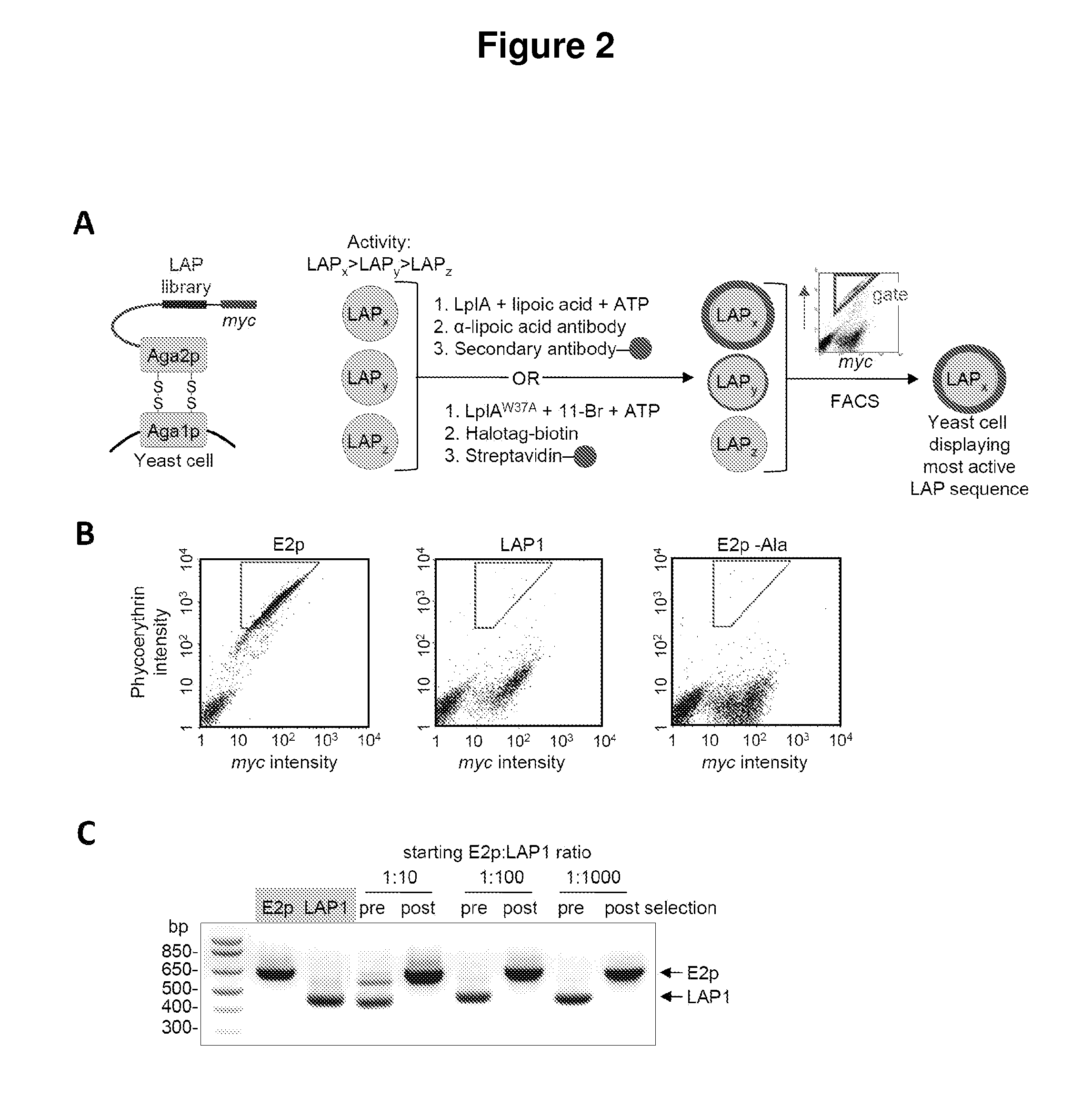
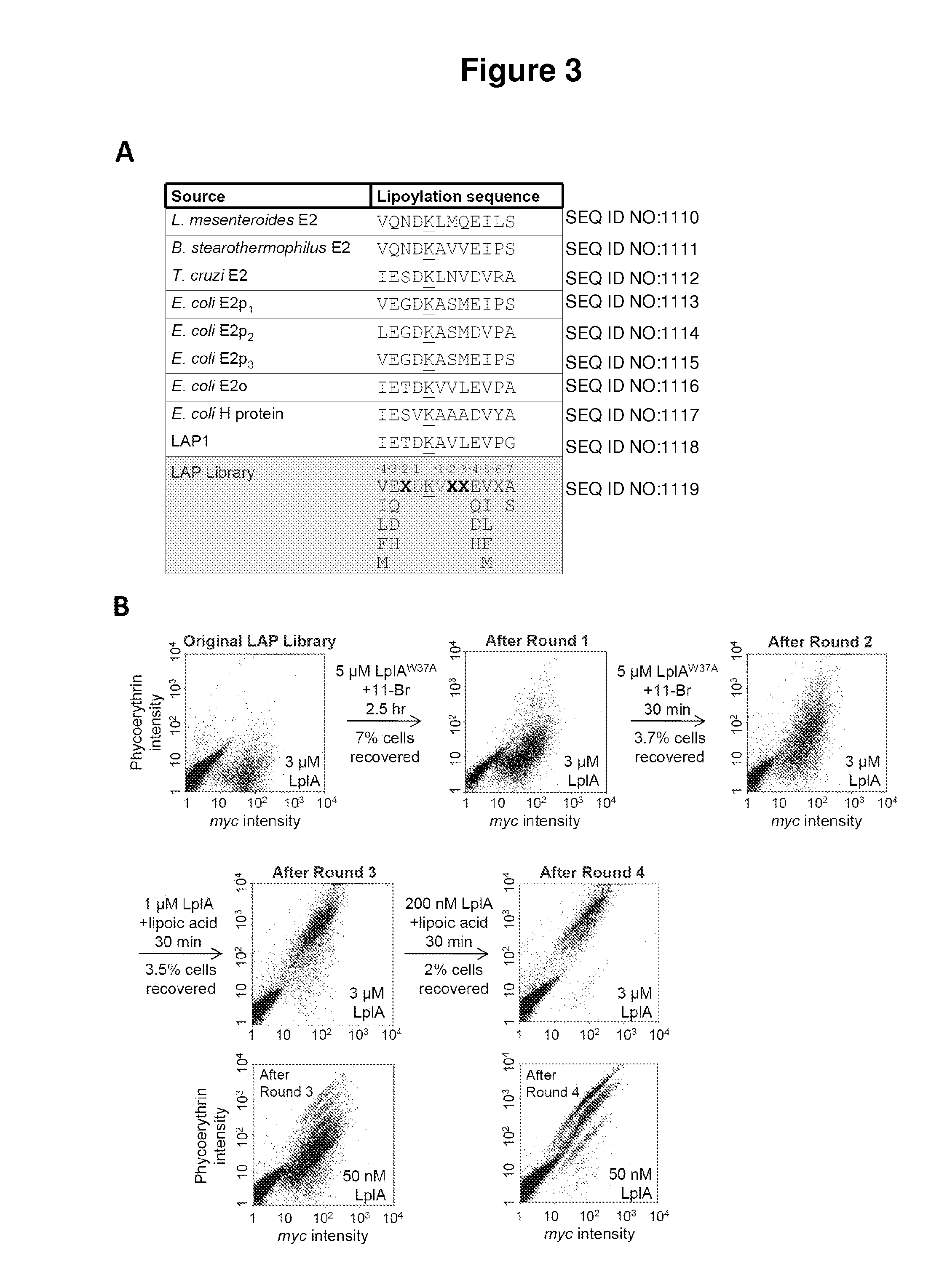
Kinetically efficient substrate for lipoic acid ligase
The invention provides methods for identifying and optimizing peptide substrates for enzymes such as lipoic acid ligase (Lp1A).
Owner:MASSACHUSETTS INST OF TECH
Methods and kits for measuring ADAMTS13/FXI complexes
InactiveUS20060073535A1Peptide/protein ingredientsMicrobiological testing/measurementPeptide substrateAdamts13 activity
Provided are assays and kits to detect ADAMTS13 activity using peptide substrates and ADAMTS13 / Factor XI complexes using ELISA. These assays and kits can be used for diagnostic applications and to evaluate treatment of thrombotic thrombocytopenic purpura (TTP). Also provided is a novel form of ADAMTS13 found on platelets.
Owner:AMERICAN DIAGNOSTICA
Methods and kits for detecting and measuring ADAMTS13/FXI complexes
InactiveUS20080138837A1Inhibit and enhance formationMicrobiological testing/measurementDisease diagnosisHemostatic DisordersPeptide substrate
Provided are assays and kits to detect ADAMTS13 activity using peptide substrates and ADAMTS13 / Factor XI complexes using ELISA. These assays and kits can be used for diagnostic applications and to evaluate treatment of thrombotic or hemostatic disorders, for example, thrombotic thrombocytopenic purpura (TTP). Also provided is a novel form of ADAMTS13 found on platelets, and anti-ADAMTS13 antibodies.
Owner:AMERICAN DIAGNOSTICA
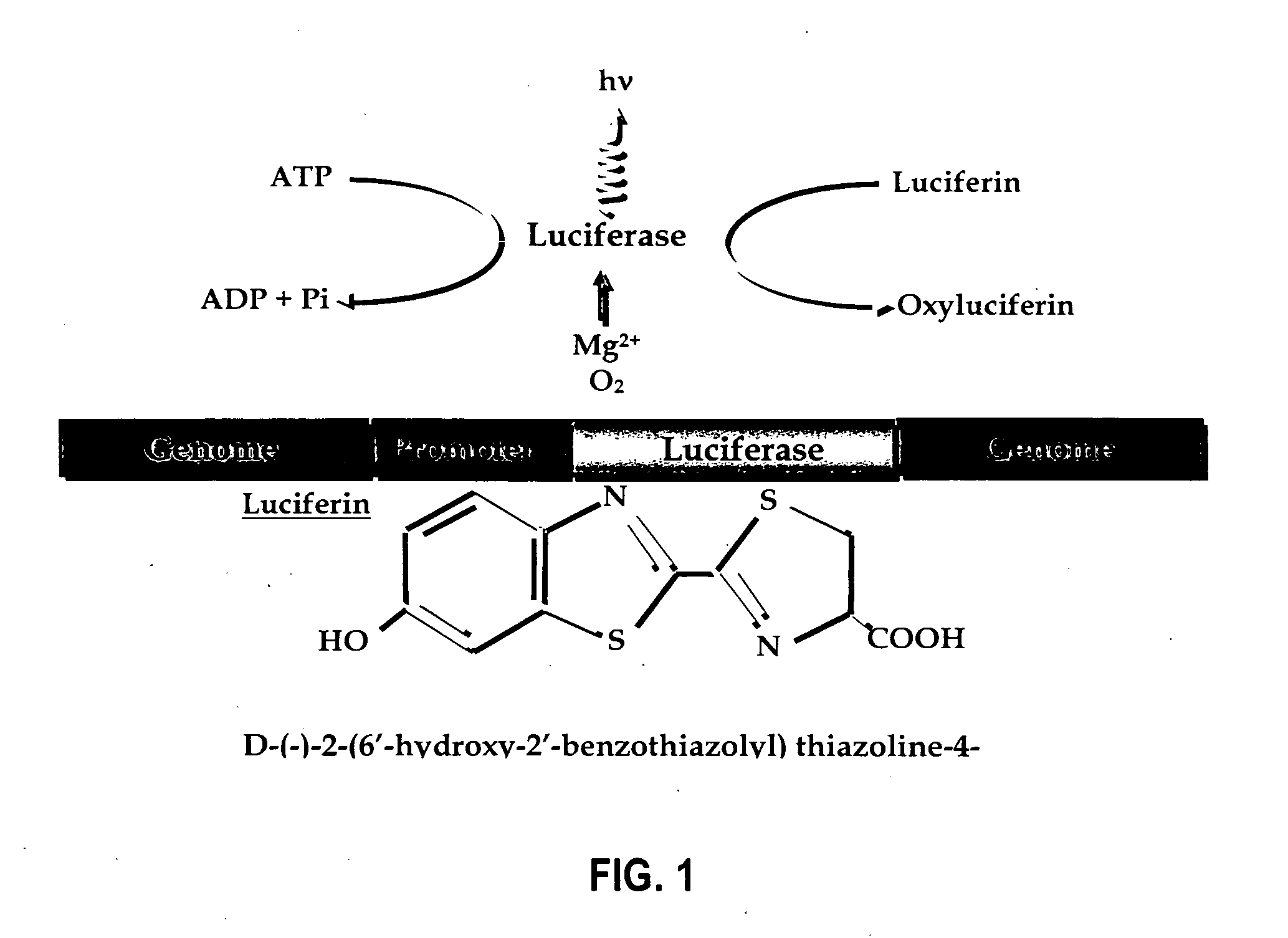
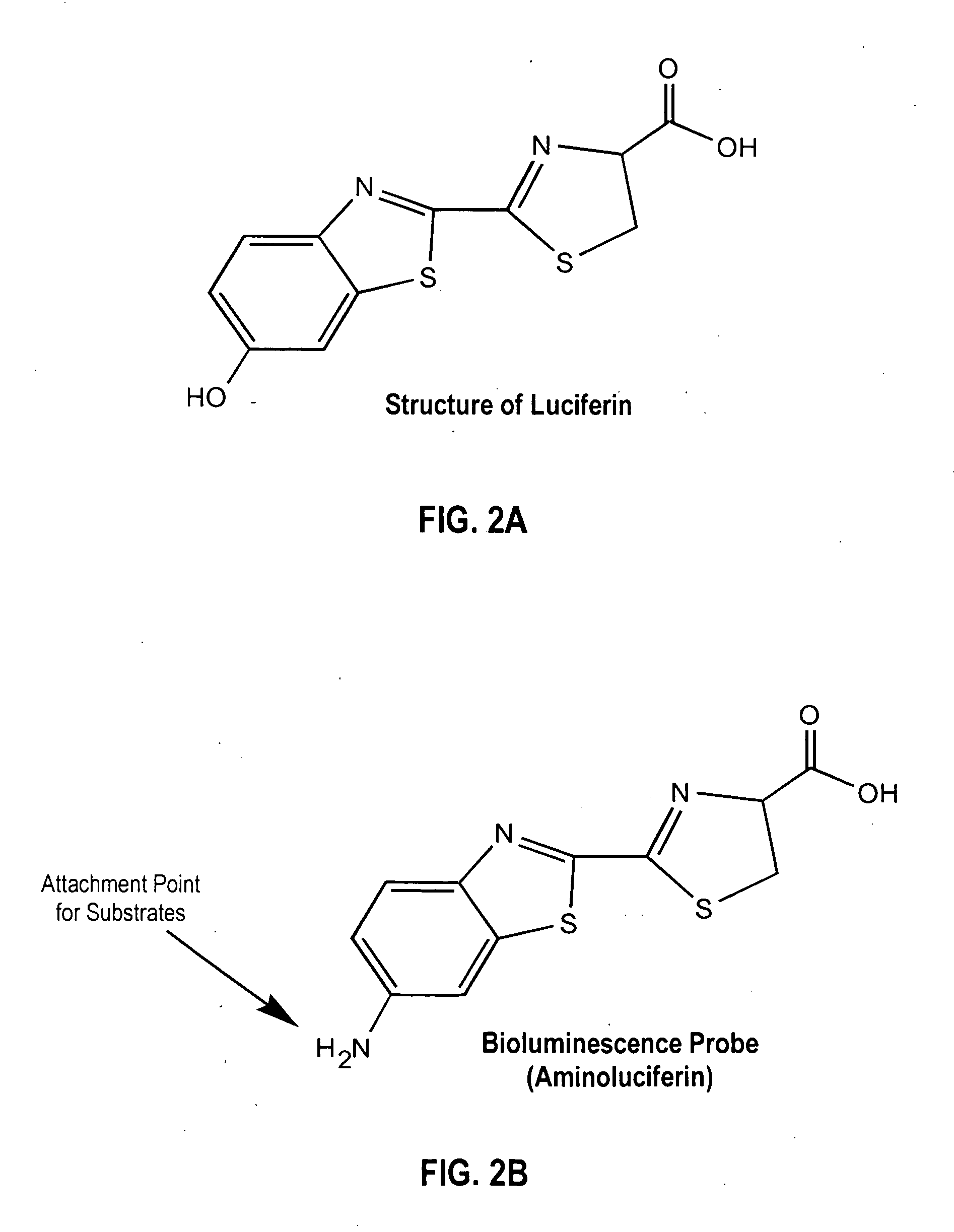
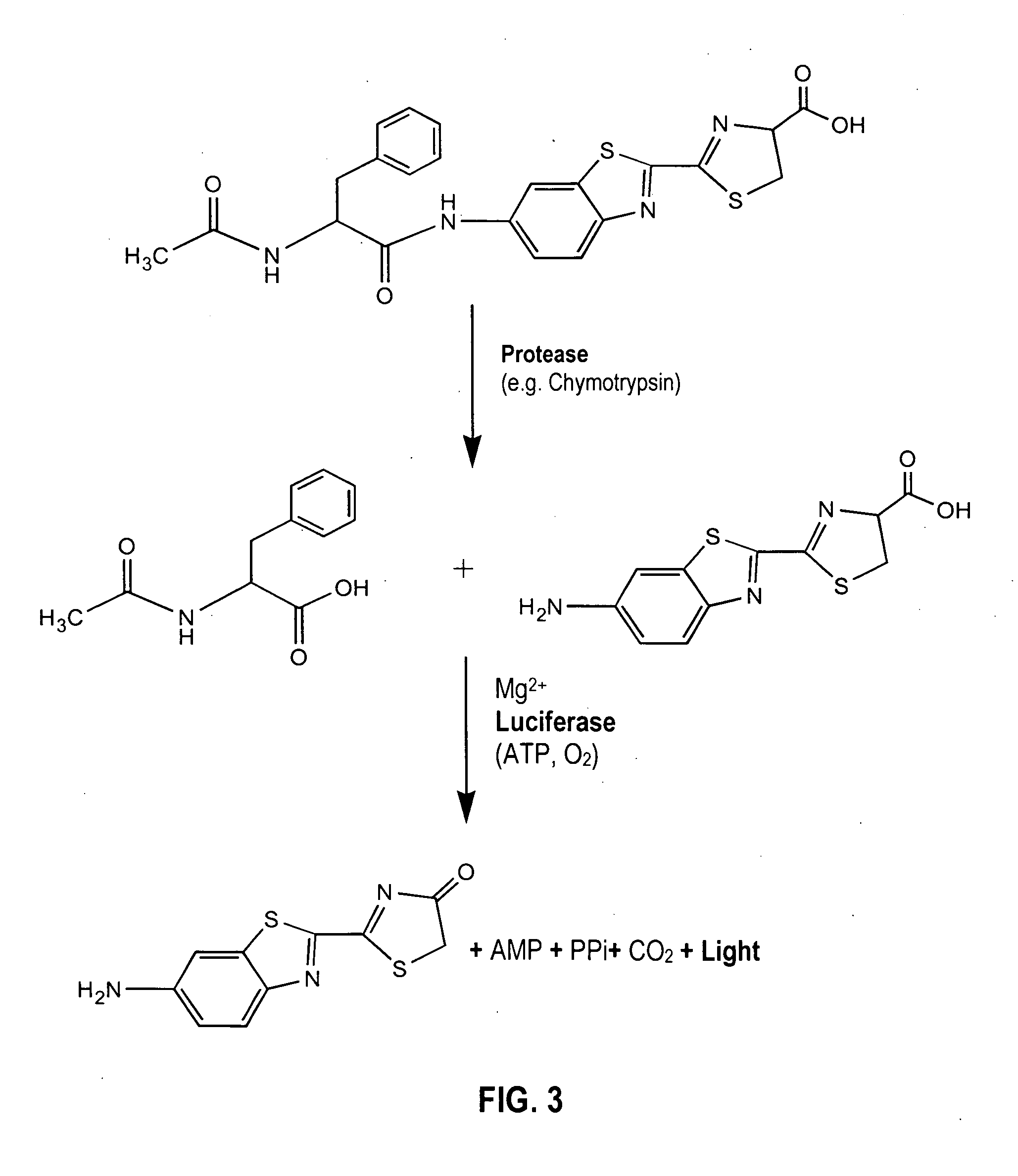
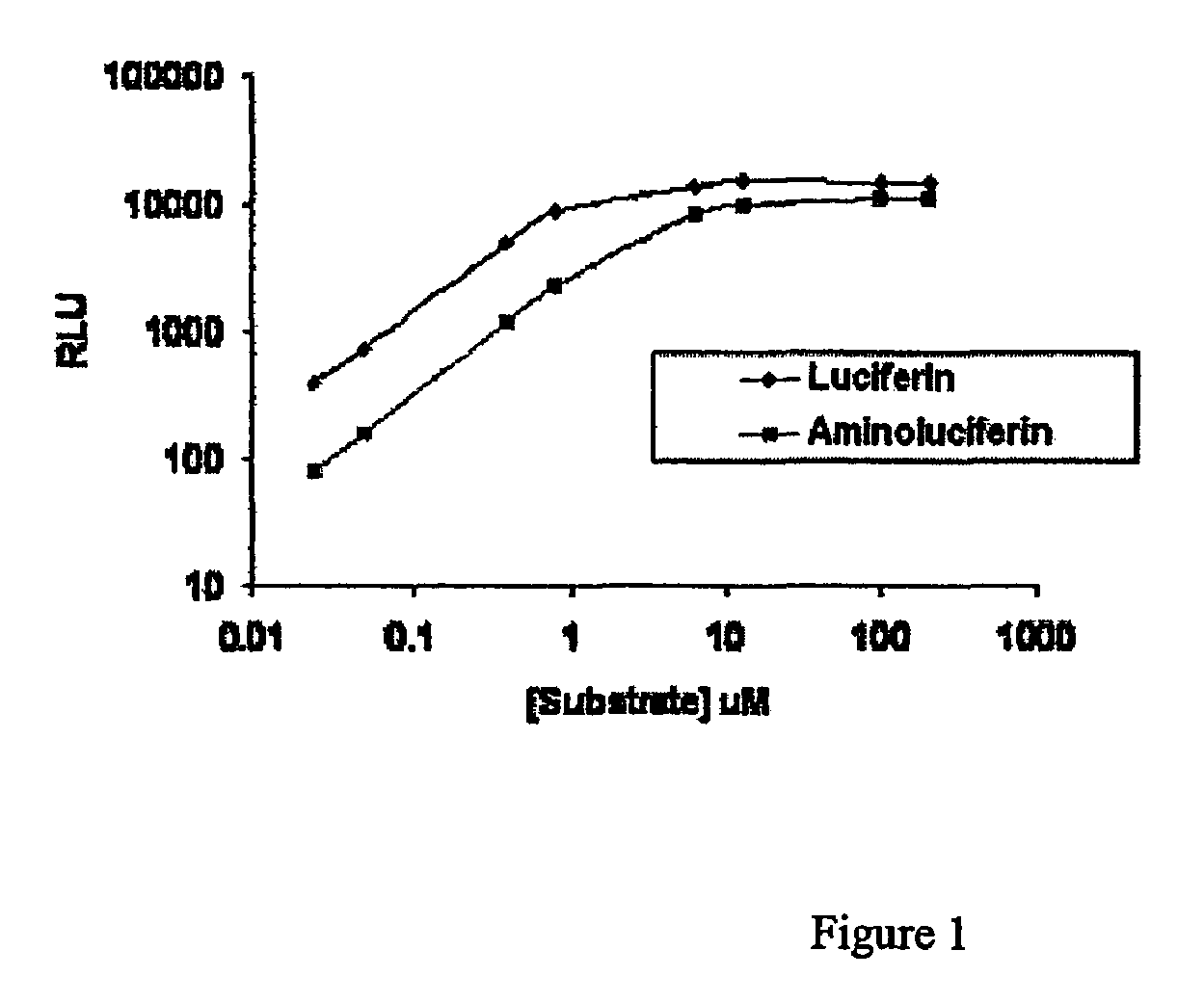
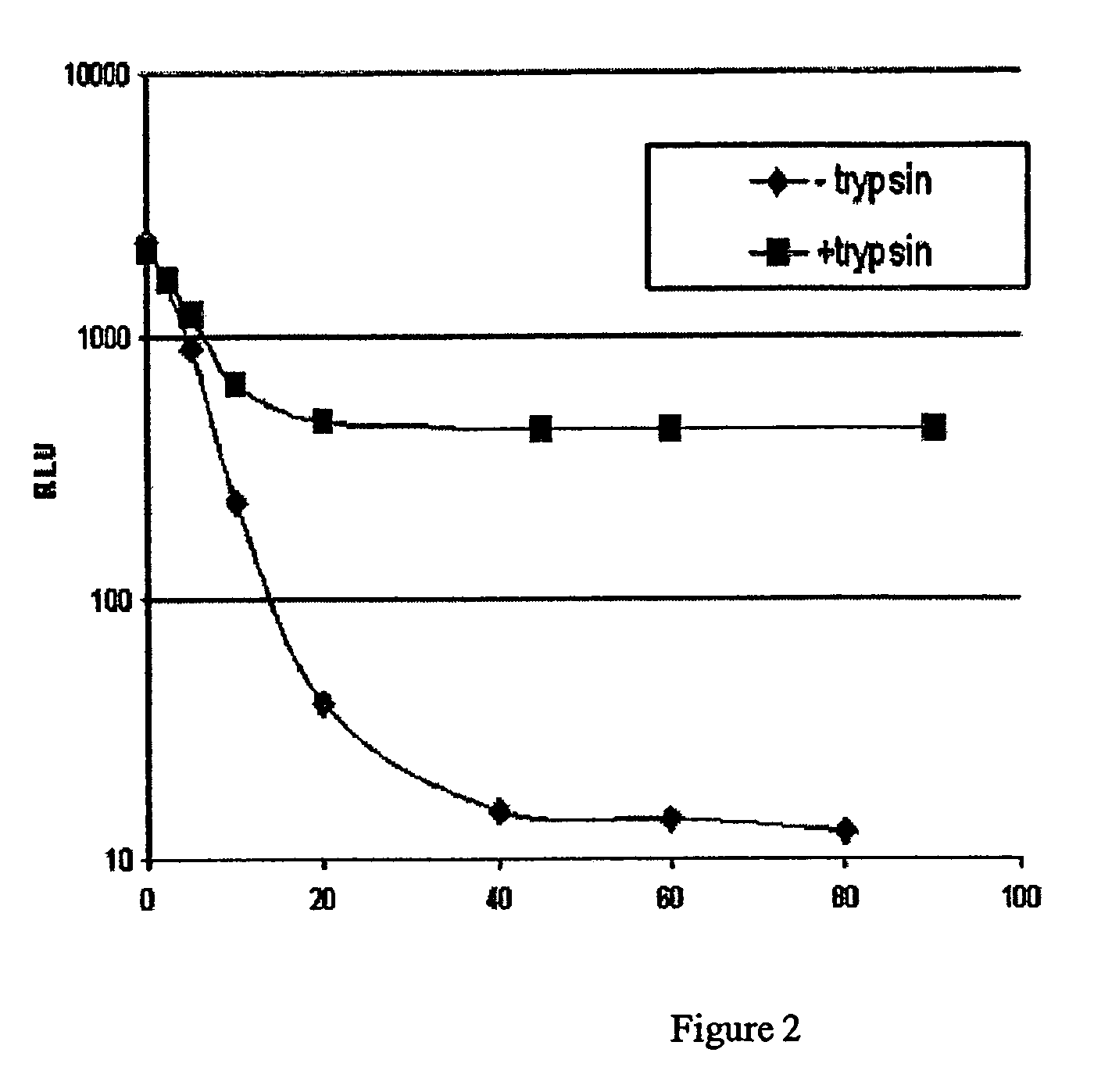
Bioluminescent protease assay with modified aminoluciferin or derivatives thereof
InactiveUS7384758B2High detection sensitivityLeveling precisionMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementPeptide substrateProteinase activity
A sensitive bioluminescent assay to detect proteases, including caspases and proteases that specifically cleave a peptide substrate having aspartate, is provided. In one embodiment, the assay employs an aminoluciferin or a carboxy-terminal protected derivative thereof covalently linked via a peptide bond to a substrate for a caspase or an aminoluciferin or a carboxy-terminal protected derivative thereof covalently linked via a peptide bond to a peptide substrate comprising aspartate that is specifically cleaved by a protease specific for the substrate.
Owner:PROMEGA CORP
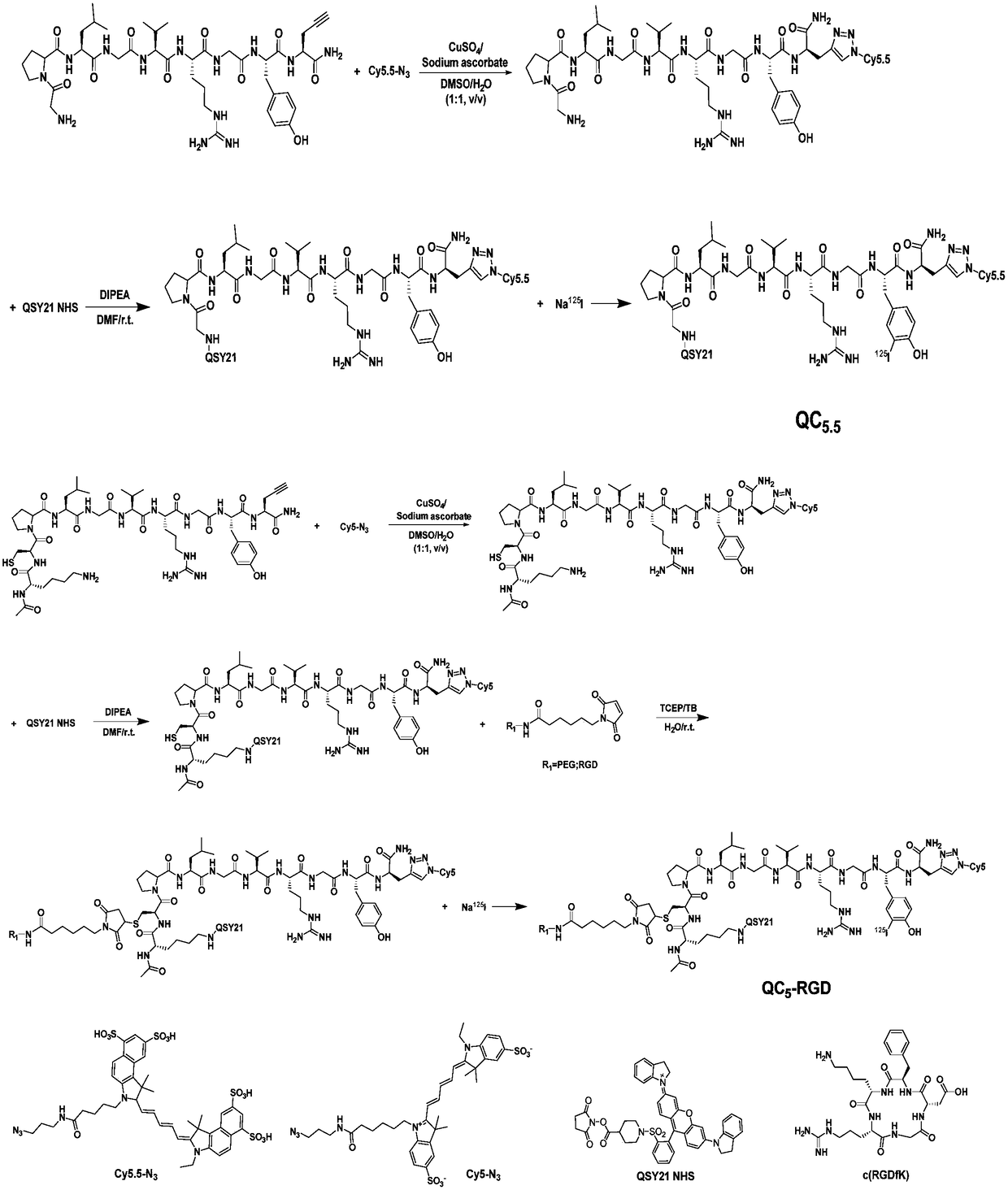
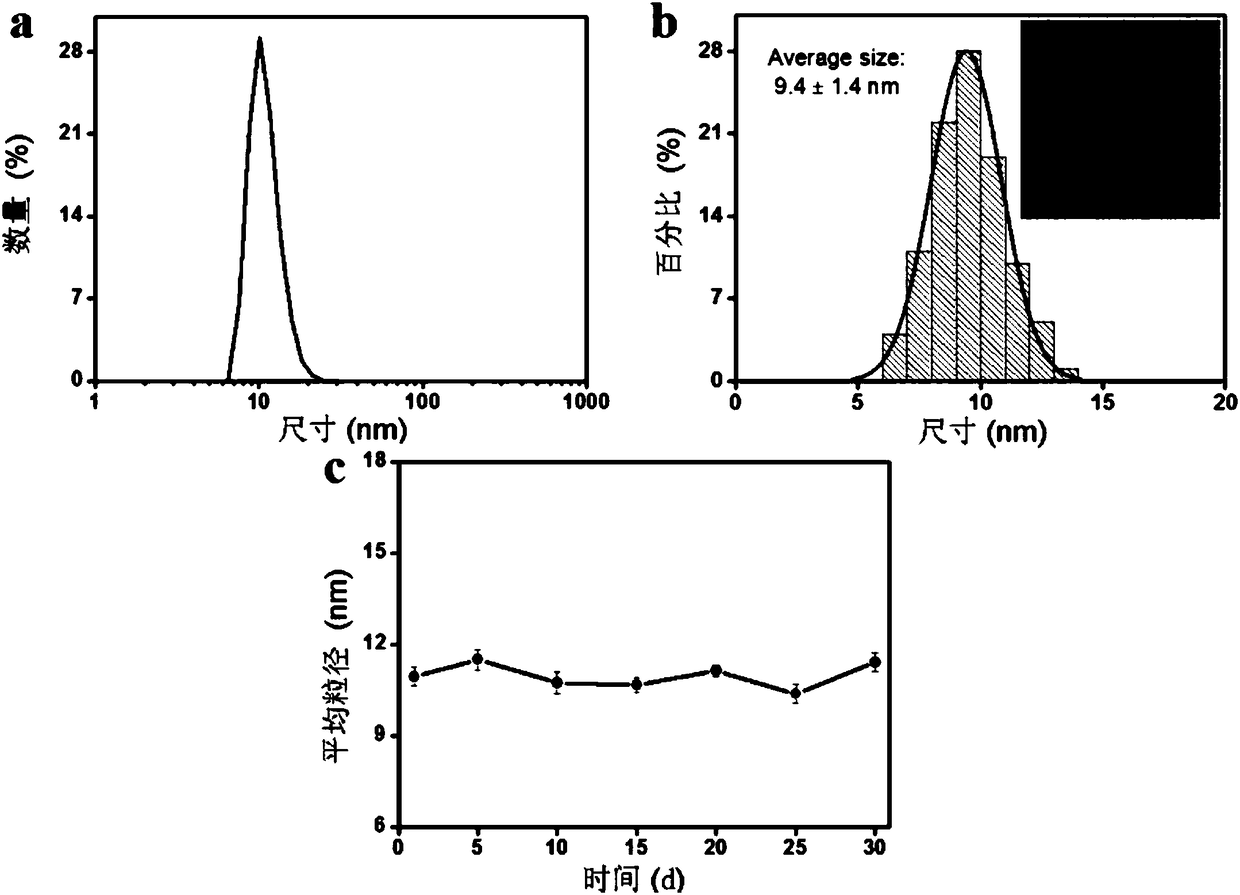
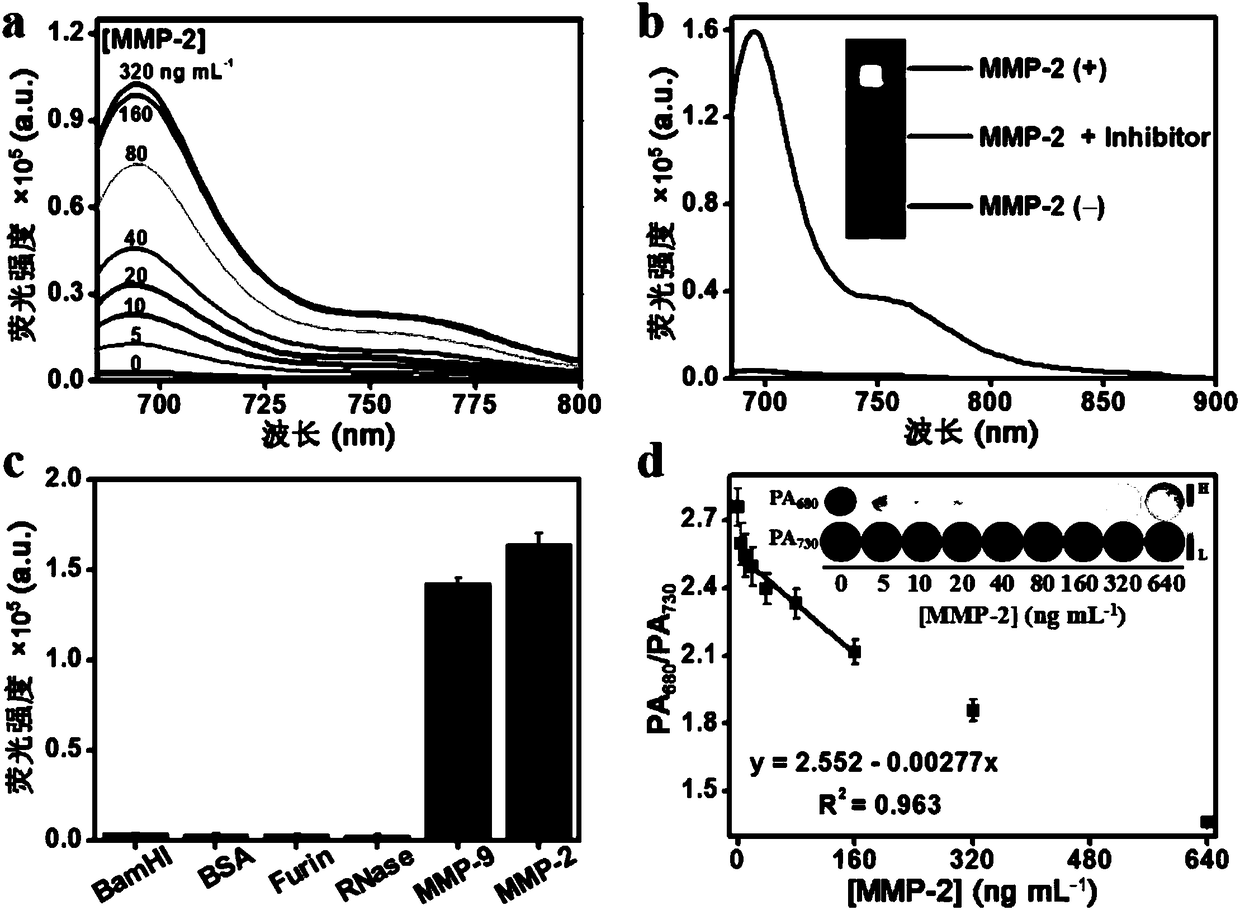
Matrix metalloproteinase-2 specific multi-modality molecular image probe and preparation method and application in preparation of tumor imaging agent thereof
ActiveCN108514647AImprove targetingImprove featuresGeneral/multifunctional contrast agentsEchographic/ultrasound-imaging preparationsSide chainTumor targeting
The invention discloses a matrix metalloproteinase-2 specific multi-modality molecular image probe preparation method and application thereof. The preparation method comprises the following steps: 1)preparing peptide substrates for matrix metalloproteinase-2(MMP-2) specific identification; 2) modifying near infrared fluorescent dye on peptide substrate; 3) modifying fluorescent quenching group onpeptide substrate; 4) connecting different molecular weight PEG or tumor targeting group RGD on peptide substrate modified with near infrared fluorescent dye and fluorescent quenching group; 5) labeling the nuclide on the above modified peptide substrate side chain tyrosine. Compared with the prior art, the matrix metalloproteinase-2 specific multi-modality molecular image probe preparation method has the advantages that: 1) by utilizing the specificity and responsiveness of the MMP-2 protease by the probe, so that the probe is selectively enriched at the tumor site so as to improve the targeting property of the probe to the tumor; 2) performing multi-modality imaging in a living body tumor by using advanced molecular imaging technology such as optical, opto-acoustic, SPECT and the like,improving the sensitivity and accuracy of tumor imaging, and finally achieving accurate positioning of the tumor; 3) providing a new train of thought and method for early diagnosis, process research and prognosis evaluation of tumor.
Owner:苏州智影特生物医药技术有限公司
Electrochemical detection of proteases using ac voltammetry on nanoelectrode arrays
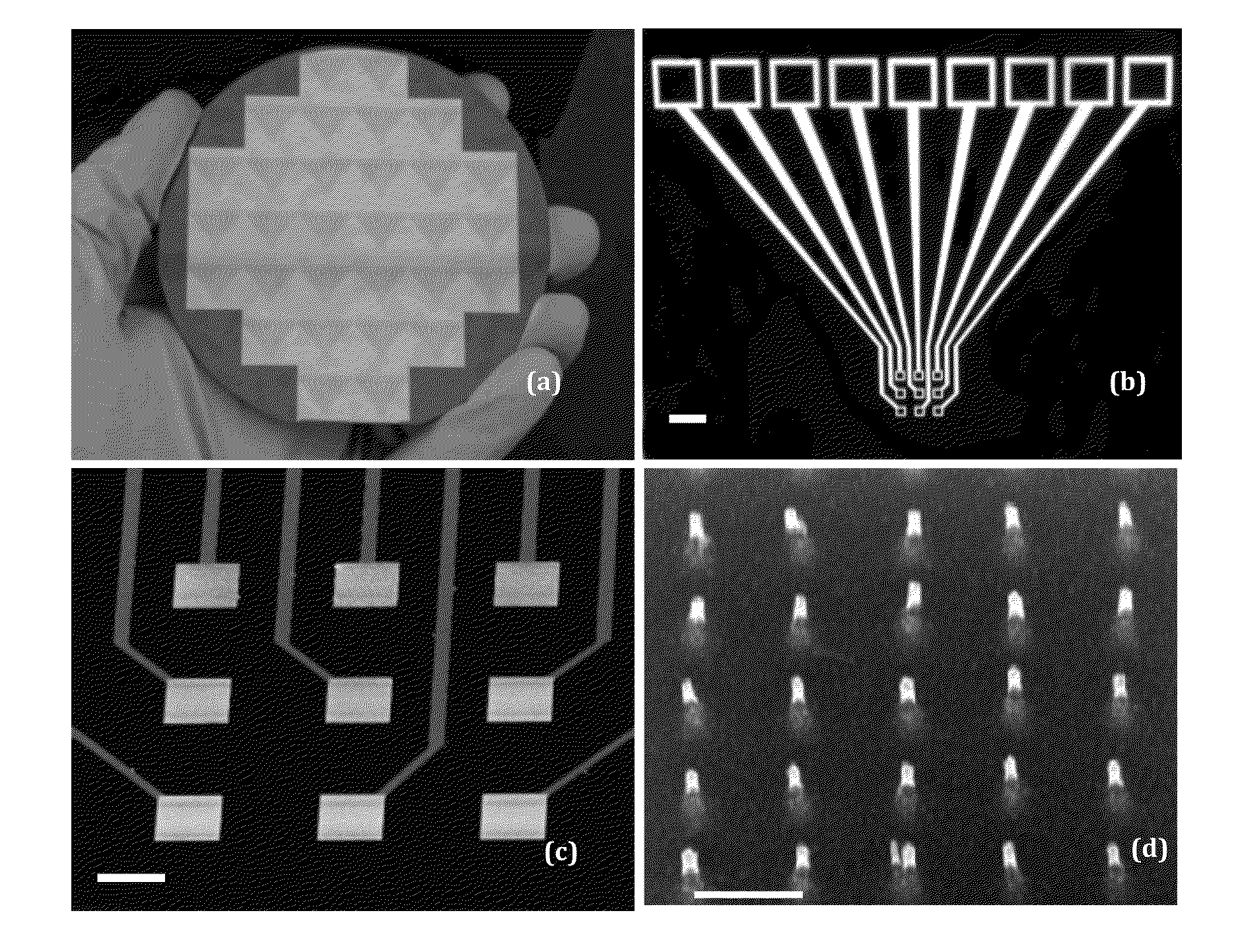
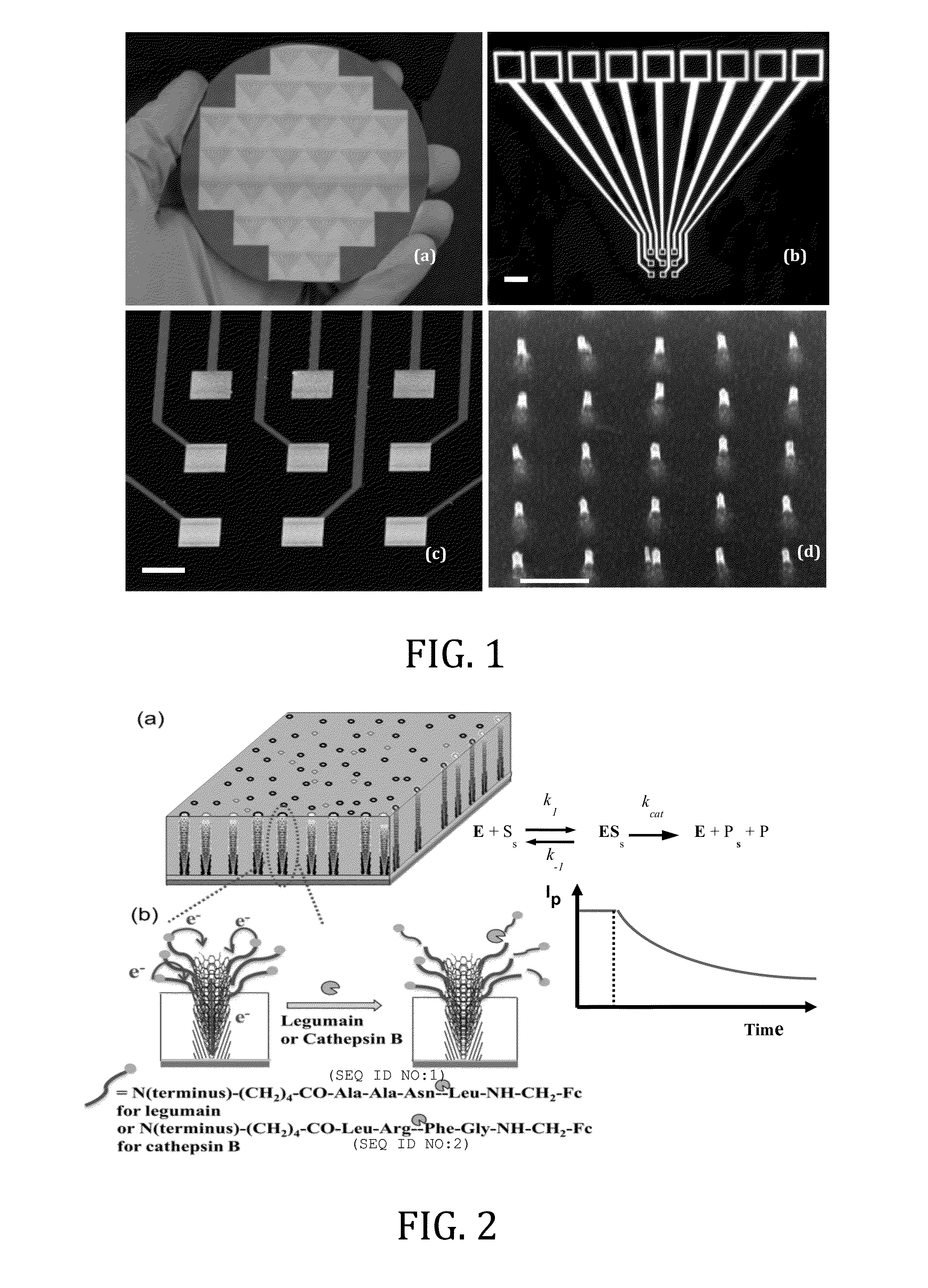
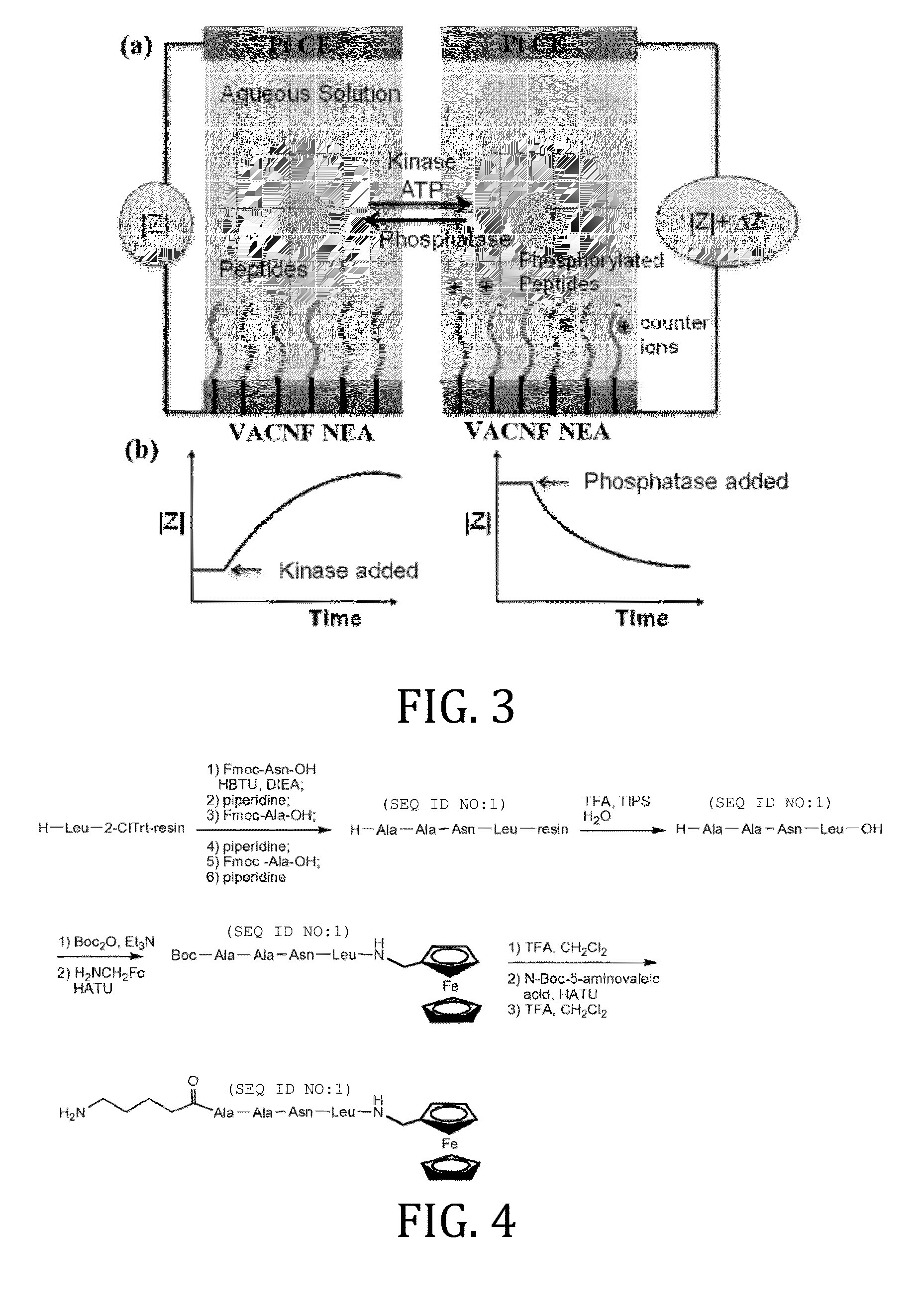
ActiveUS20150011421A1Quick testPeptide librariesSequential/parallel process reactionsPeptide substrateProteinase activity
An electrochemical method for measuring the activity of enzymes using nanoelectrode arrays fabricated with vertically aligned carbon nanofibers. Short peptide substrates specific to disease-related enzymes are covalently attached to the exposed nanofiber tips. A redox moiety, such as ferrocene, can be linked at the distal end of the nanofibers. Contact of the arrays with a biological sample containing one or more target enzymes results in cleavage of the peptides and changes the redox signal of the redox moiety indicating the presence of the target enzymes.
Owner:KANSAS STATE UNIV RES FOUND
Tumor-targeting novel polypeptide
InactiveCN105294831AGood treatment effectGood effectCyclic peptide ingredientsMacromolecular non-active ingredientsTumor targetTumor targeting
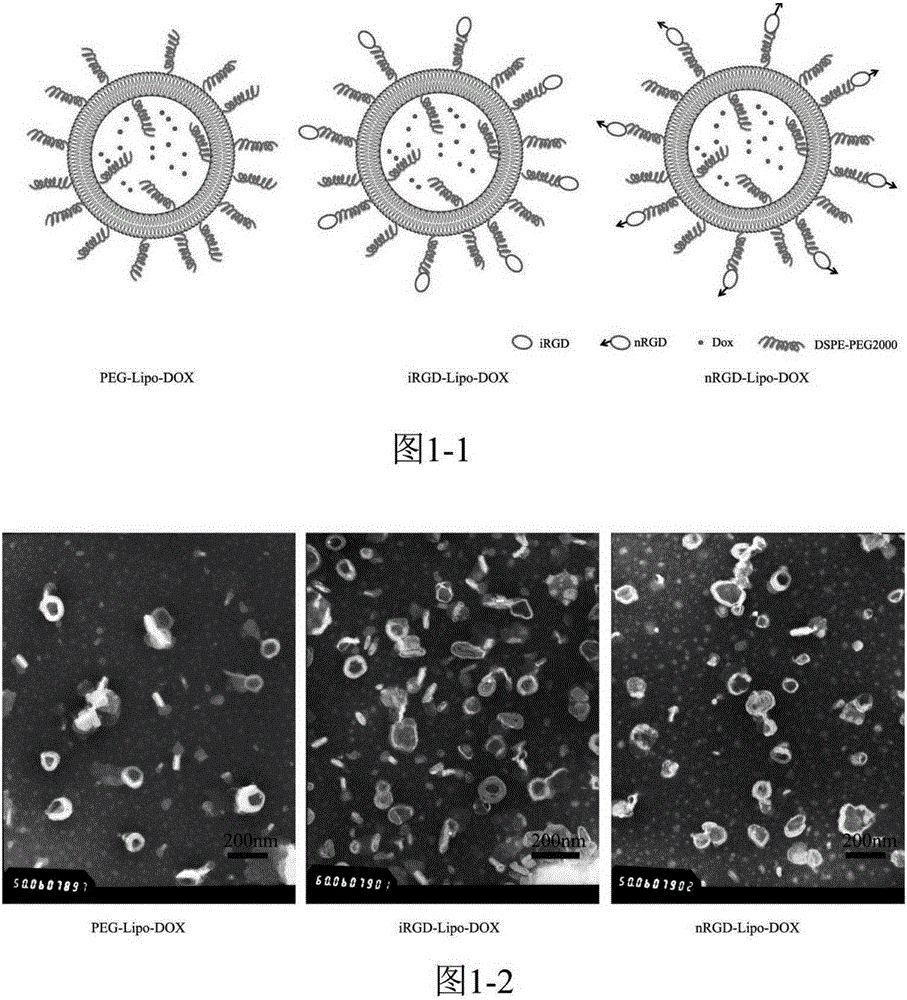
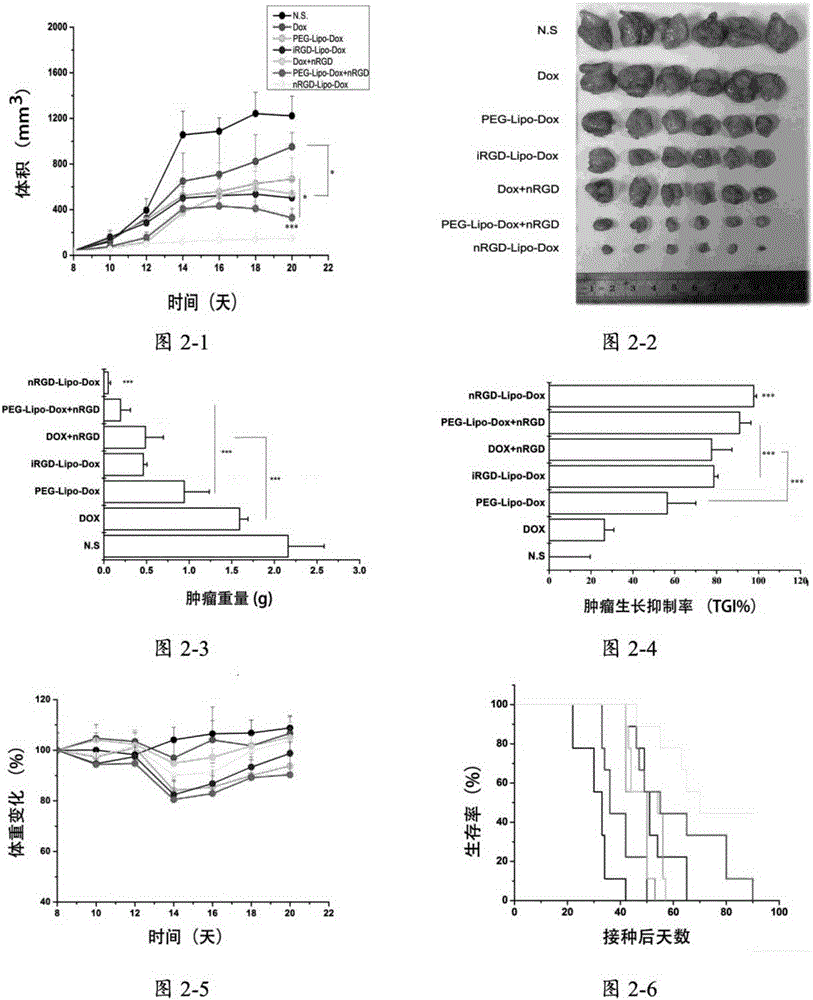
The invention provides a tumor-targeting novel polypeptide, which is a tandem polypeptide that can target not only tumor cells and blood vessels but also tumor-related macrophages to adjust the tumor microenvironment and enhance the anti-tumor effect. Peptide substrates (AAN) which target tumor blood vessels and tumor cells, contain RGD polypeptides and targeted tumor-related macrophages and are sensitive to aspartic acid endoproteinase are connected to form the polypeptide (nRGD). The polypeptide (nRGD) not only can be applied to be directly used along with drugs or vectors, but also can modify the prodrugs of anti-tumor drugs or modify drug delivery vectors, and can effectively mediate tumor-targeting delivery, thus greatly enhancing curative effect.
Owner:SICHUAN UNIV +1
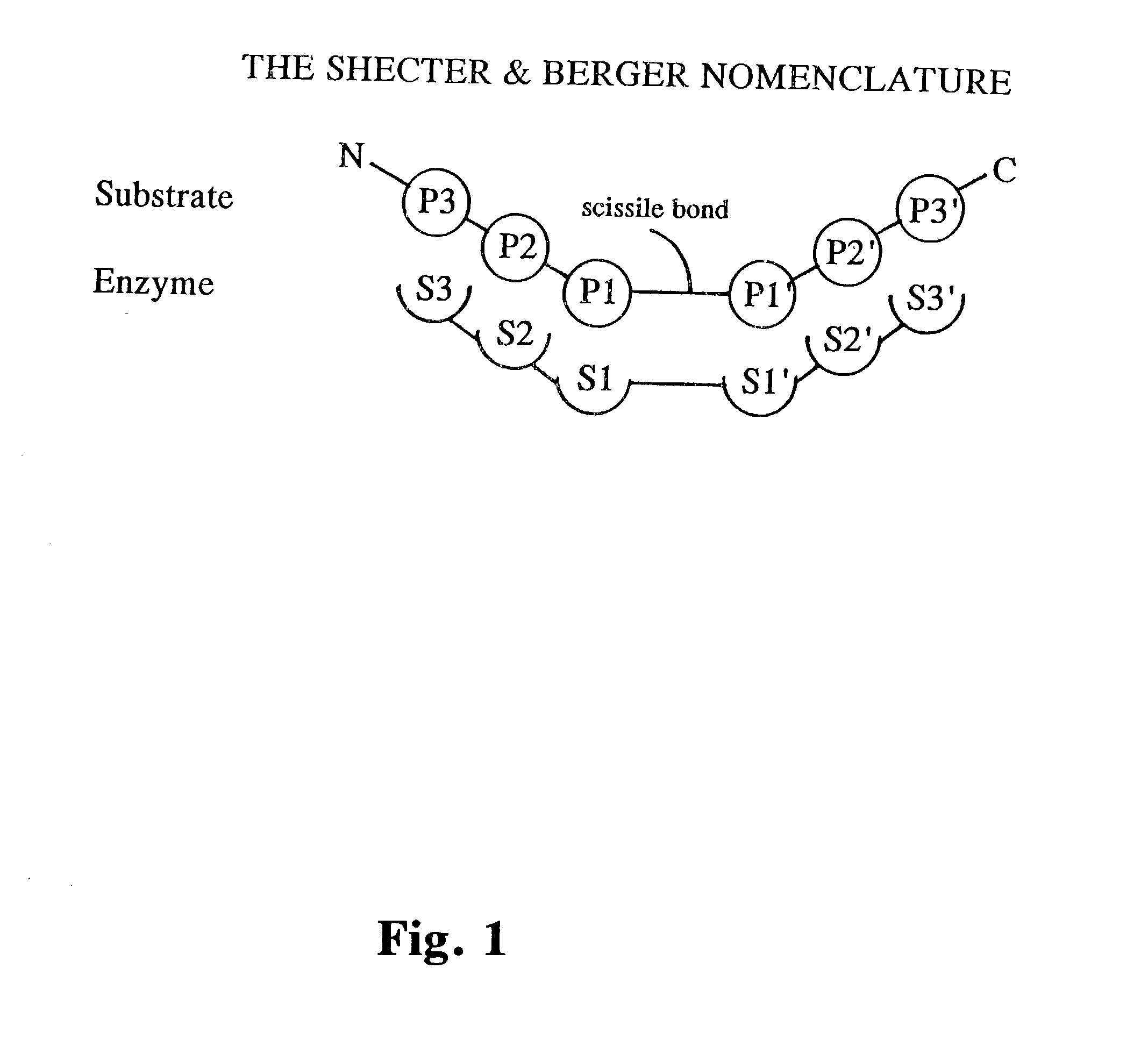
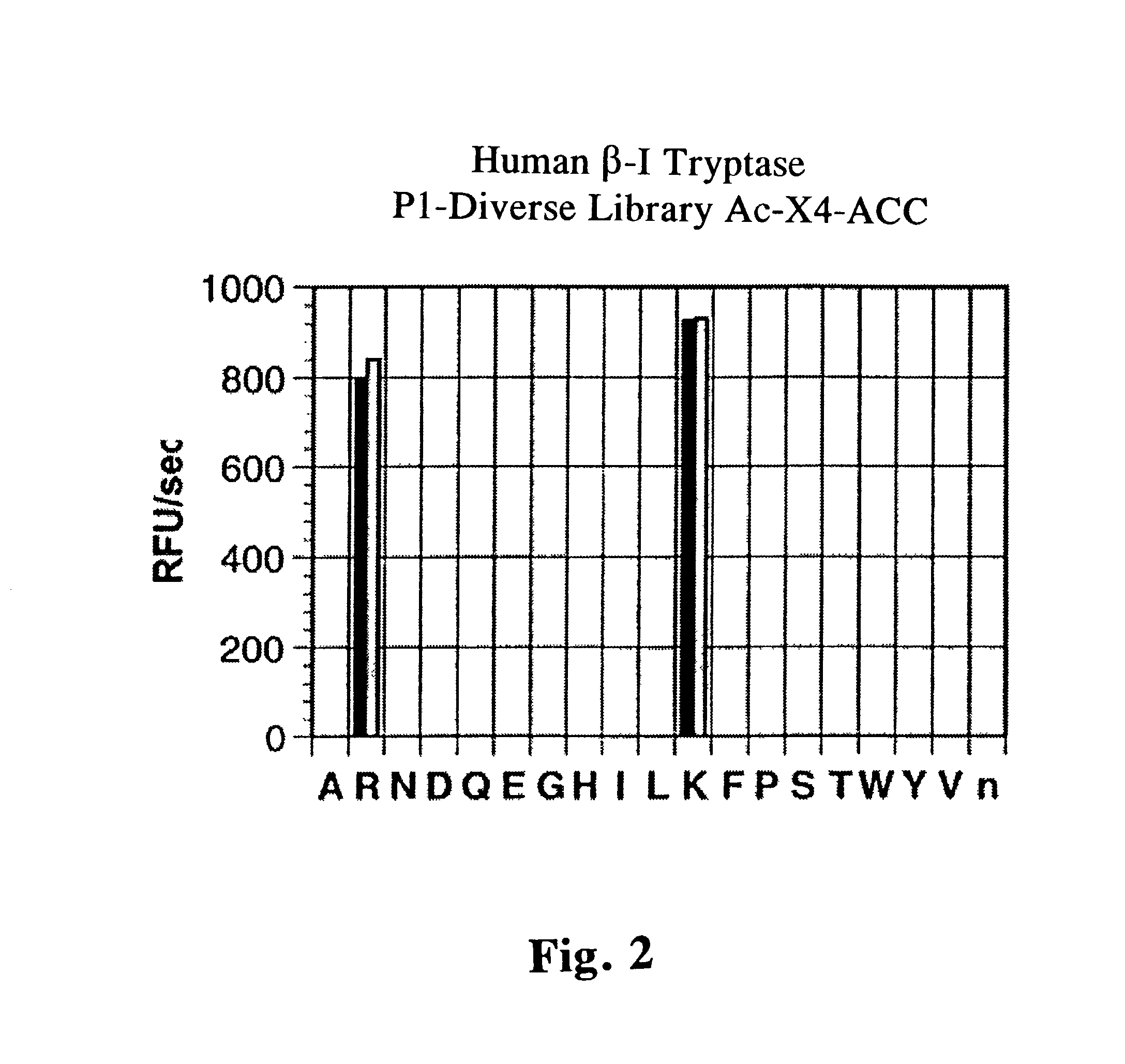
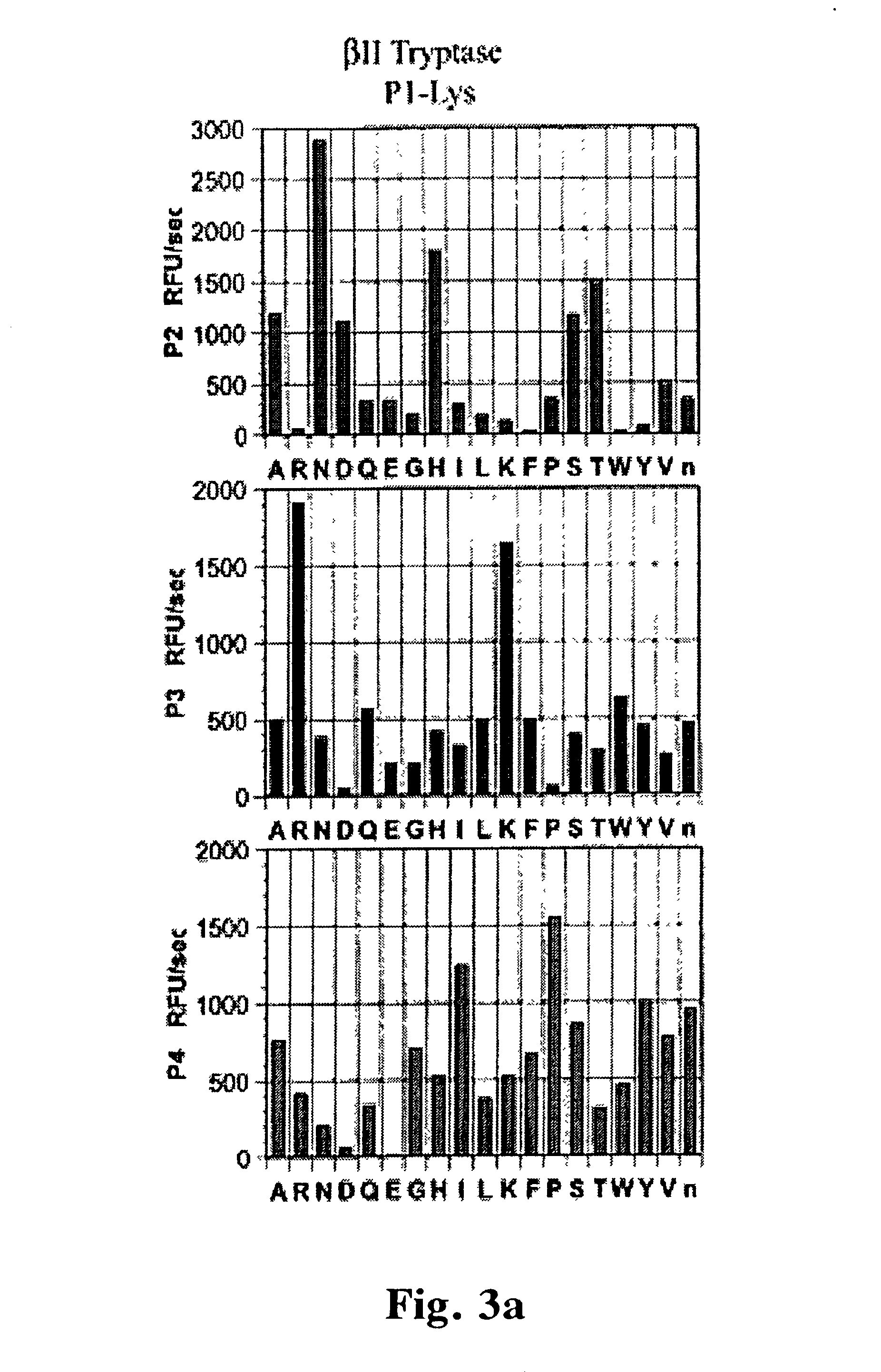
Tryptase substrates and assay for tryptase activity using same
InactiveUS20020197661A1Peptide/protein ingredientsMicrobiological testing/measurementPeptide substrateAssay
The invention is directed to synthetic polypeptide substrates for tryptase enzymes and assays for tryptase activity that utilize the synthetic polypeptide substrates. The preferred synthetic polypeptide substrates are tetramers of the formula P4-P3-P2-P1, wherein the substrate is selected from the group consisting of P-R-N-K (SEQ. ID. NO: 2), P-K-N-K (SEQ. ID. NO: 3), P-R-N-R (SEQ. ID. NO: 4), P-K-N-R (SEQ. ID. NO: 5), P-A-N-K (SEQ. ID. NO: 6), and P-R-T-K (SEQ. ID. NO: 7).
Owner:PROMEGA CORP
Nanoparticle sensor for measuring protease activity and method for manufacturing the same
Disclosed are a nanoparticle sensor for measuring protease activity, for protease imaging, and a method for preparing the same. More specifically, the present invention relates to a nanoparticle sensor for measuring protease activity in which a fluorophore- and a quencher-conjugated peptide substrate is conjugated to a biocompatible polymer nanoparticle. The peptide substrate is specifically lysed by a protease. The sensor according to the present invention is capable of inhibiting emission of fluorescence with high extinctive activity of the quencher on a fluorescent material. But strong fluorescence is specifically emitted only if the peptide substrate is lysed by a specific protease. Therefore, the sensor is especially useful as a method for screening a novel drug such as a protease overexpression inhibitor, and early diagnosis of incurable diseases and various diseases such as autoimmune diseases including cancer, osteoarthritis, rheumatoid arthritis and dementia.
Owner:KOREA INST OF SCI & TECH +2
Prostate-specific antigen probes for optical imaging
ActiveUS20060154324A1Antibody mimetics/scaffoldsMicrobiological testing/measurementAntigenPeptide substrate
This invention provides a novel probe, which contains a prostate peptide substrate. The probe can be used as a prostate diagnostic agent for optical imaging. An 11 amino acid peptide substrate is synthesized for specific prostate-specific antigen (PSA). This protected graft copolymer (PGC) consists of a poly-L-lysine (PL) backbone and methoxypoly(ethylene glycol) (MPEG). Two terminals of peptide substrate are bound to poly-L-lysine and Cy5.5 fluorochrome. This probe will be used as a NIR (near-infrared fluorescence) probe for optical imaging.
Owner:KAOHSIUNG MEDICAL UNIVERSITY
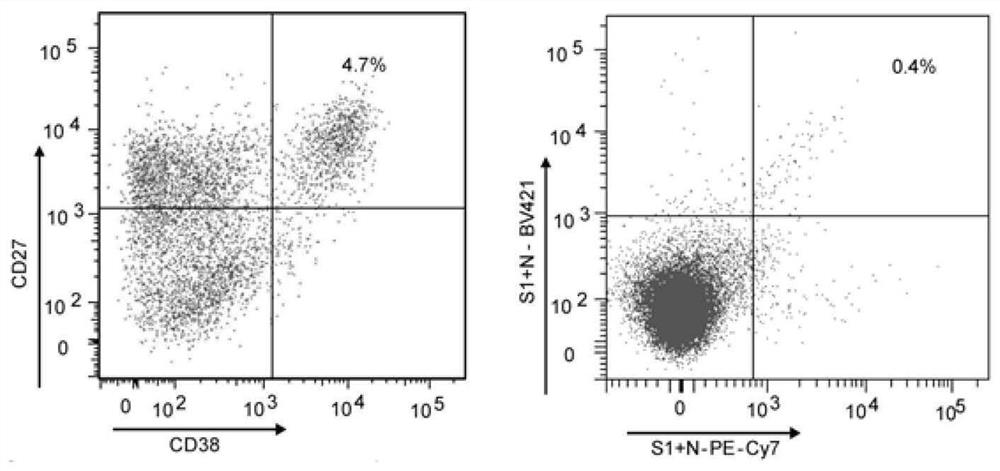
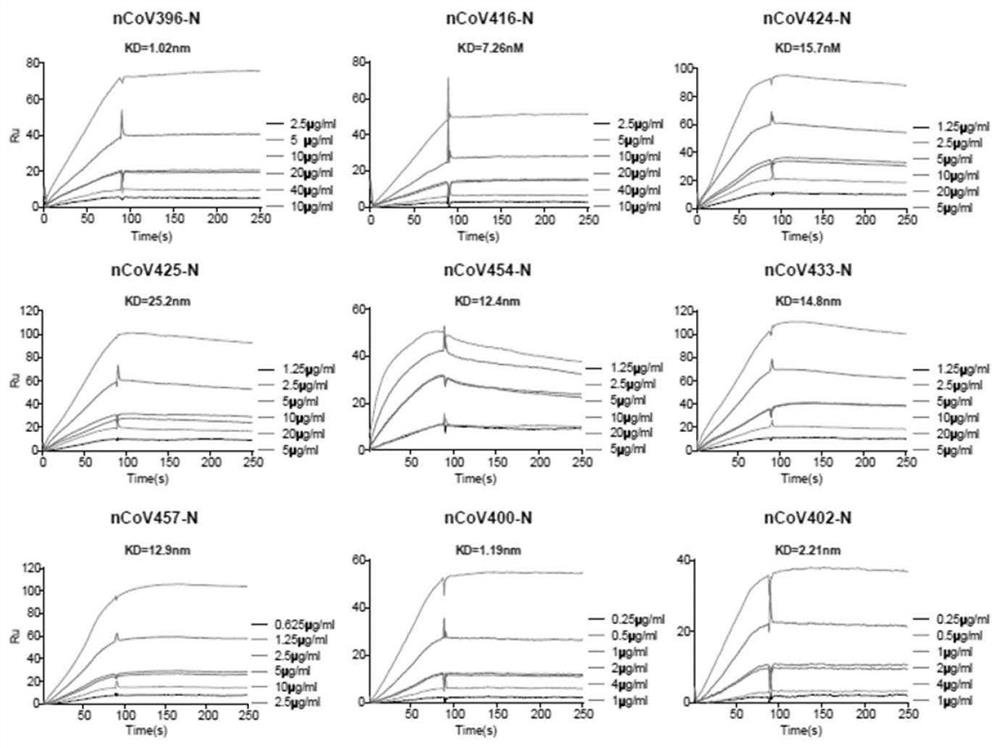
Antibody specifically binding to novel coronavirus
InactiveCN113234145ASsRNA viruses positive-senseVirus peptidesARDs - Acute respiratory distress syndromeCapsid
The complement activation is a risk factor for the morbidity and mortality of novel coronavirus-19 (COVID-19) patients, and is mediated by a highly immunopathogenic nucleocapsid protein (N) in which severe acute respiratory distress syndrome-related coronavirus 2 (SARS-CoV-2) binds to serine protease MASP-2 in the agglutinin pathway of complement activation. According to the invention, a dominant antibody with anti-SARS-CoV-2 virus N protein is separated and identified from a rapidly recovered COVID-19 convalescent person, and the antibody has high binding affinity. In addition, based on the cleavage activity of the complement protease MASP-2 on a specific fluorescence quenching peptide substrate, the invention further develops a virus-free complement hyper-activation analysis method.
Owner:THE FIFTH AFFILIATED HOSPITAL SUN YAT SEN UNIV +1
Methods for identifying inhibitors of botulinum neurotoxins
A system and method for identifying a botulinum neurotoxin inhibitor employing a botulinum neurotoxin substrate complex having a peptide substrate, preferably SNAP-25, a reporter domain on one side of said peptide substrate and an immobilization domain on the opposite side of said peptide substrate. The botulinum neurotoxin inhibitor is identified by its ability to decrease the relative amount of cleaved complex, detected through measuring a decrease in complex bound to a solid support. The method of the present invention also utilizes novel cells that express a botulinum neurotoxin substrate complex. The methods of the present invention are adapted for cell based screening to monitor the catalytic activity of a BoNT in living cells and to identify molecules that inhibit the catalytic activity of a BoNT in living cells. Also provided are novel stable cell lines that express the botulinum toxin substrate complex and viral vectors capable of efficiently expressing an active light chain of the BoNT within mammalian cells.
Owner:BOTX TECH LLC
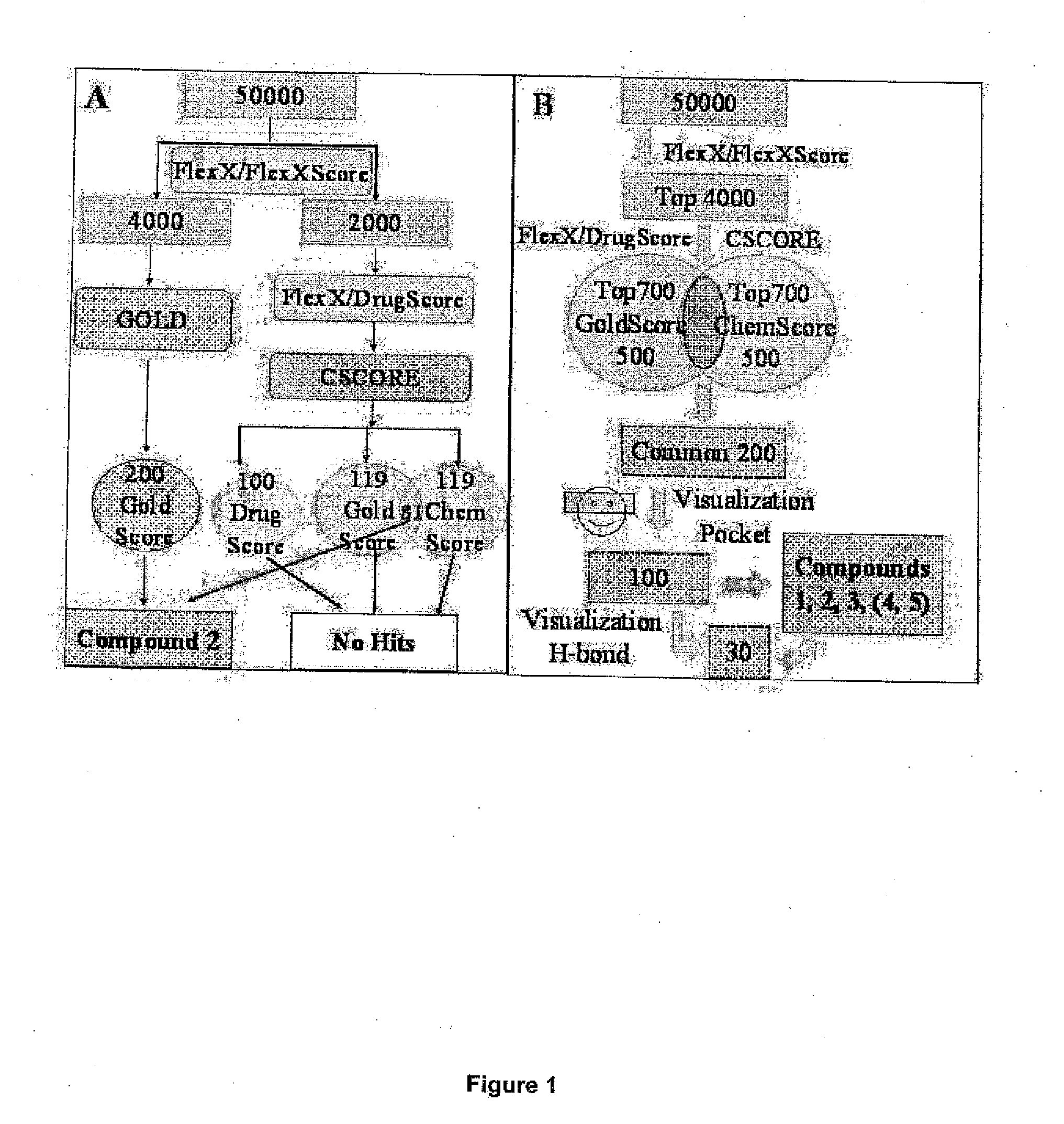
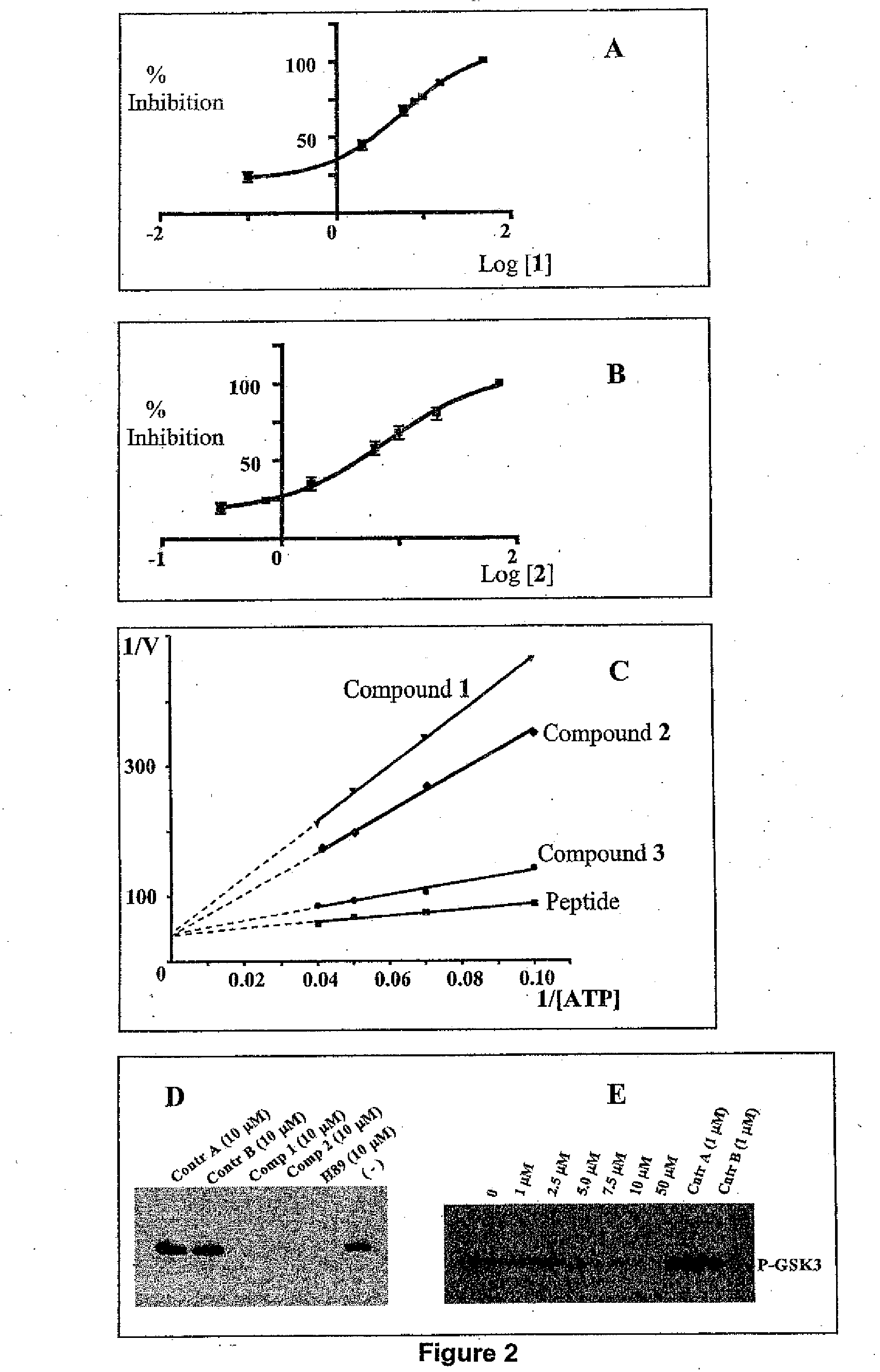
Screening methods for protein kinase b inhibitors employing virtual docking approaches and compounds and compositions discovered thereby
The present invention describes an improved method for screening compounds for activity in inhibiting the enzymatic activity of Akt1 protein kinase, also known as Protein Kinase B, an enzyme that is believed to play a key role in the inhibition of apoptosis and thus in the etiology of cancer and other conditions, including neurodegenerative diseases. In general, the method comprises: (1) providing a plurality of compounds suspected of having Akt1 kinase inhibitory activity; (2) modeling the docking of each of the plurality of the compounds with a target binding site derived from the crystal structure of a ternary complex involving Akt1, a nonhydrolyzable ATP analogue, and a peptide substrate derived from a physiological AKT substrate such that the protein active site is defined including those residues within a defined distance from the nonhydrolyzable ATP analogue; (3) ranking the docked compounds by goodness of fit; (4) further selecting compounds from compounds high ranked by goodness of fit in docking by using one or more screening criteria; (5) optionally, visually analyzing structures of compounds selected in step (4) to remove any compounds with improbable docking geometry; and (6) experimentally testing the selected compounds from step (4) or step (5), if step (5) is performed, to determine their inhibitory activity against Akt1 in order to select compounds with Akt1 inhibitory activity. The invention also encompasses pharmaceutical compositions including compounds whose inhibitory activity against Akt1 is discovered by the screening method, as well as methods of use of the pharmaceutical compositions to treat cancer and other conditions.
Owner:BURNHAM INST FOR MEDICAL RES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com