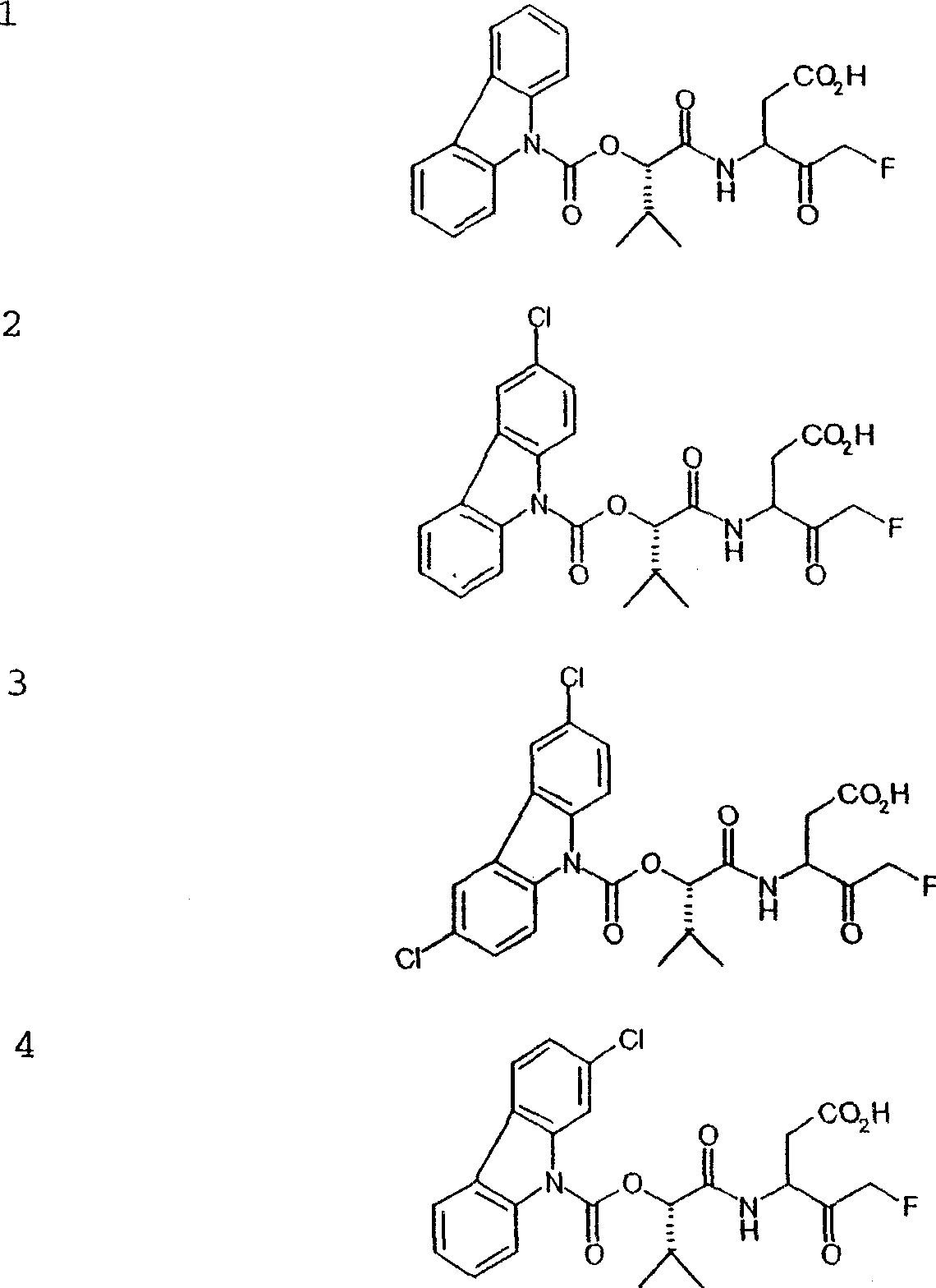
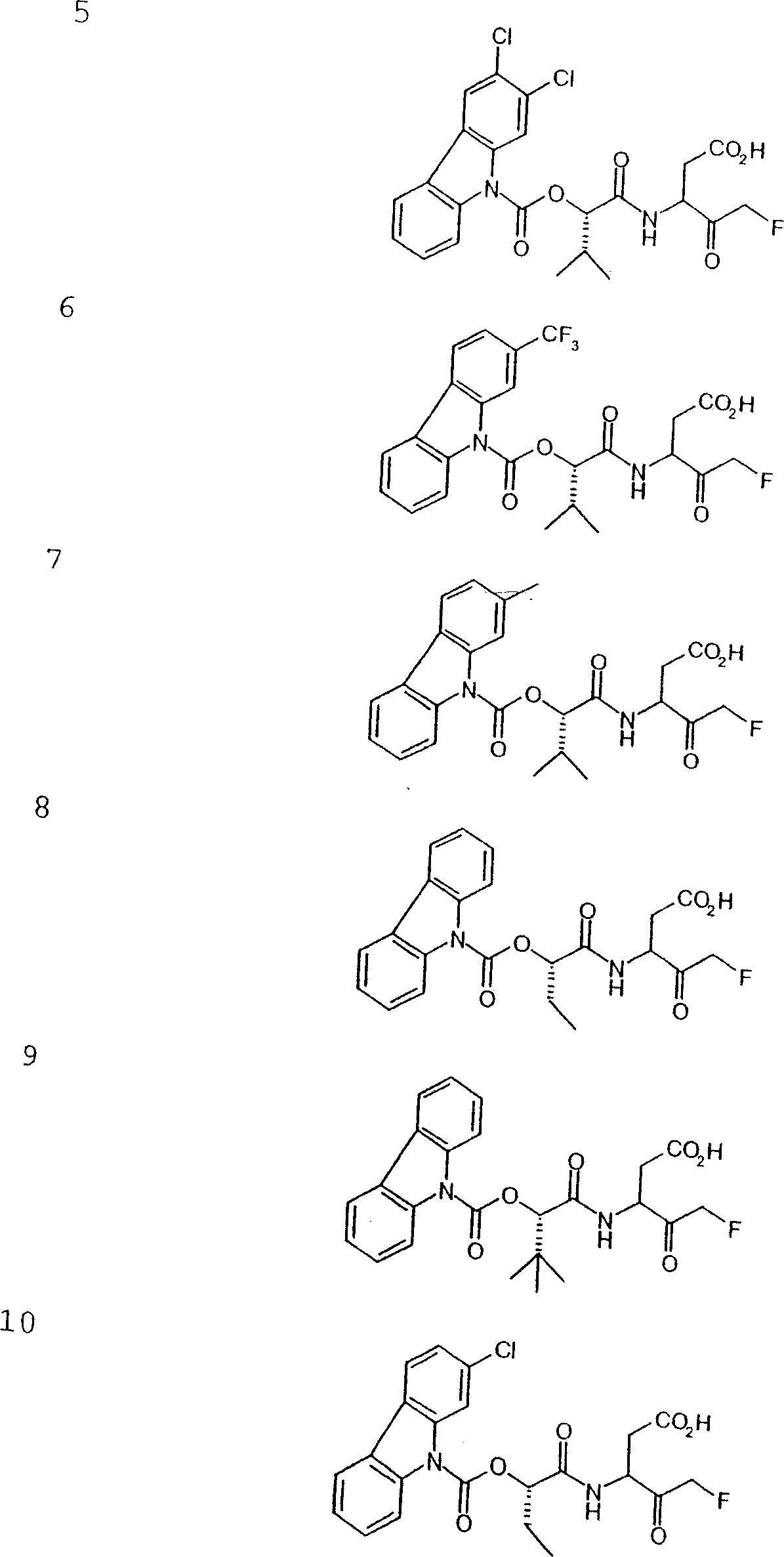
Patents
Literature
44 results about "Isostere" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
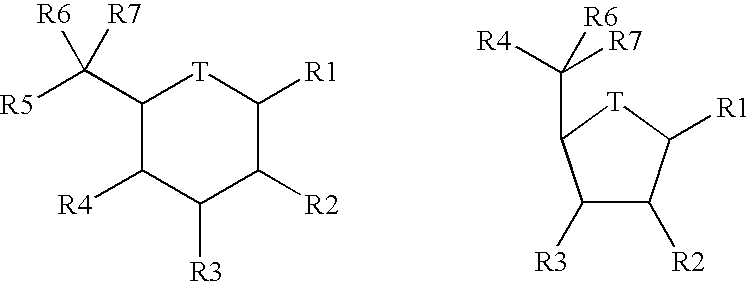
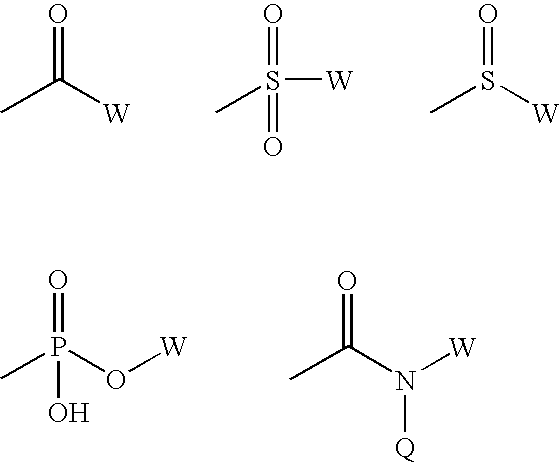
Classical Isosteres are molecules or ions with the similar shape and often electronic properties. Many definitions are available. but the term is usually employed in the context of bioactivity and drug development. Such biologically-active compounds containing an isostere is called a bioisostere. This is frequently used in drug design: the bioisostere will still be recognized and accepted by the body, but its functions there will be altered as compared to the parent molecule.
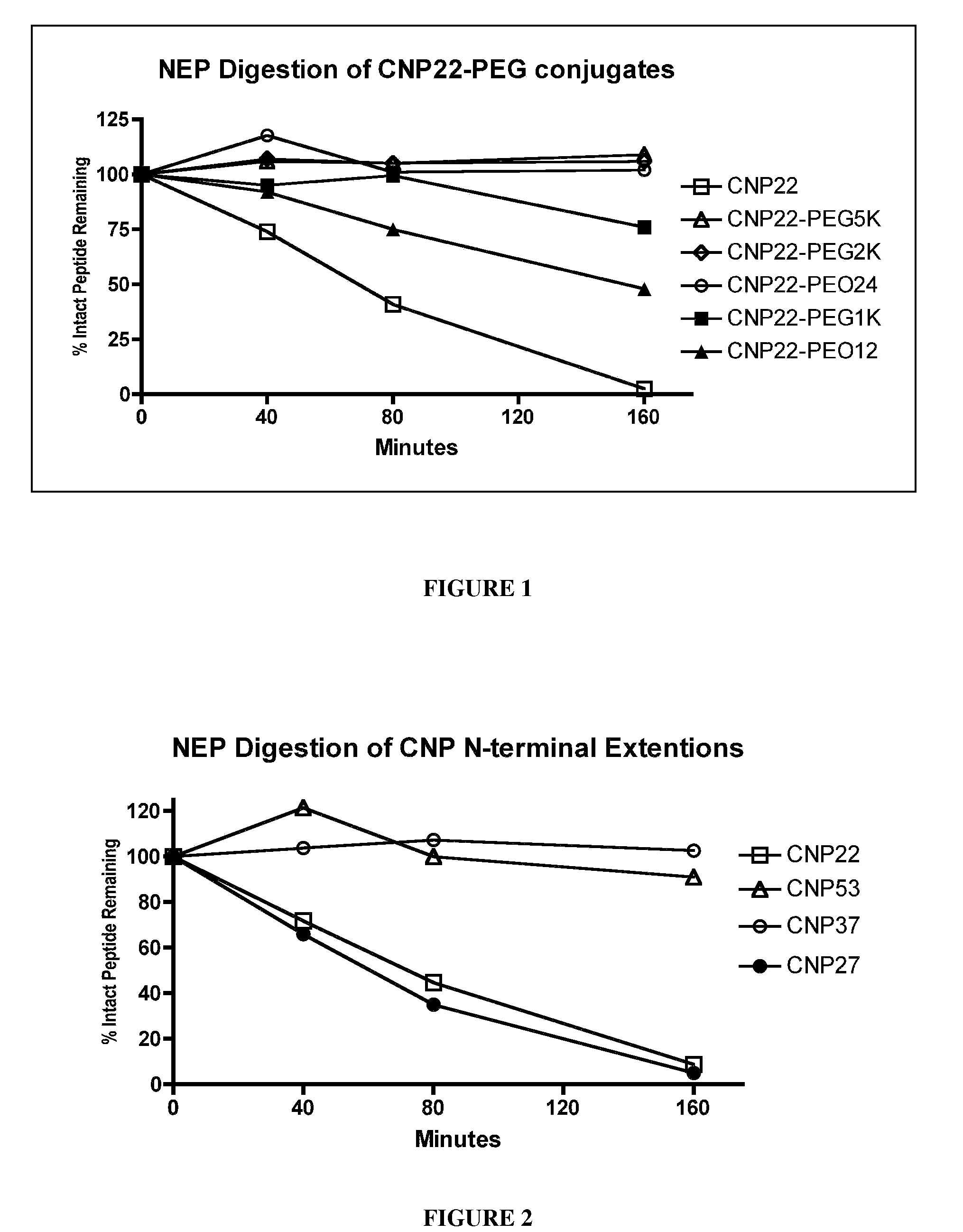
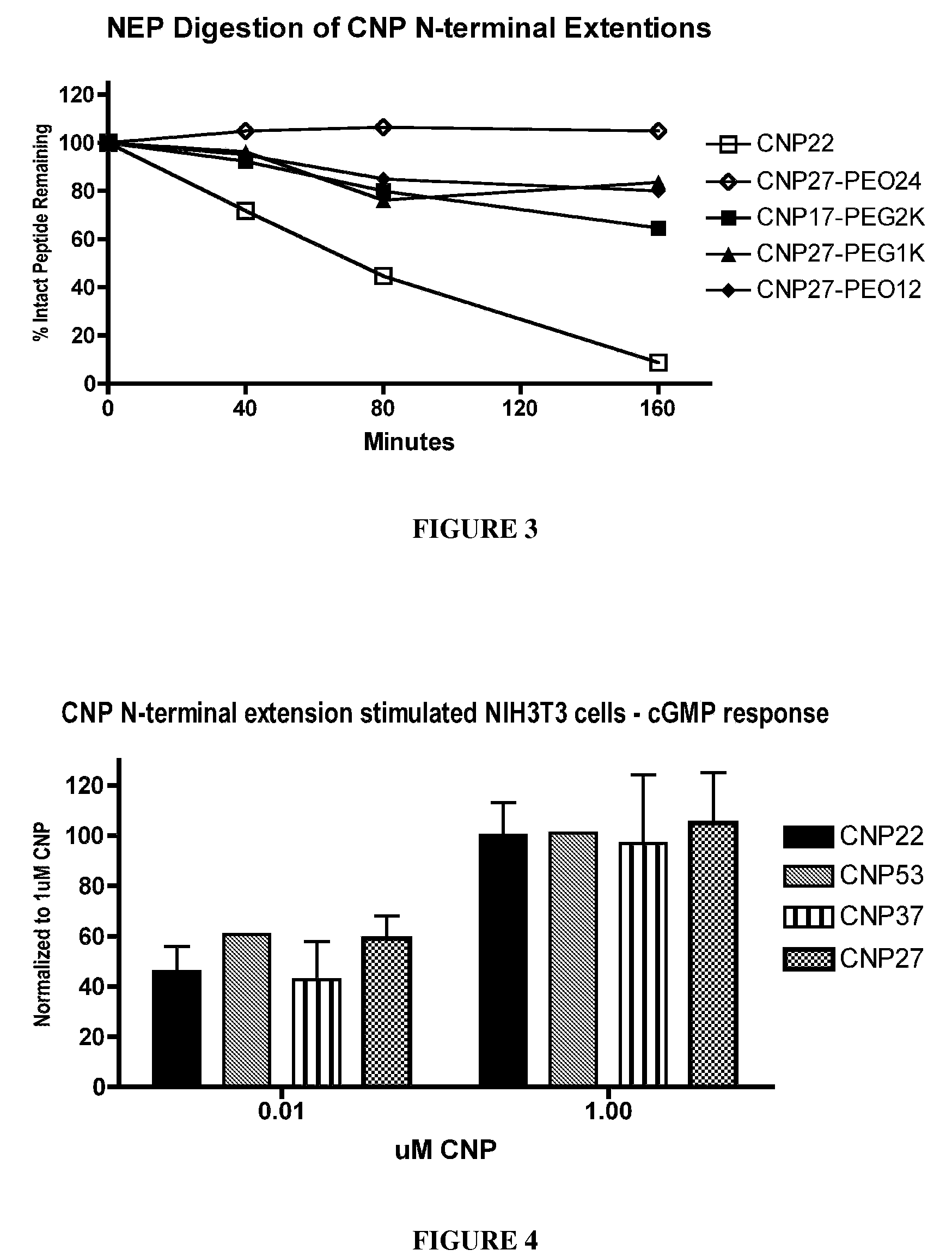
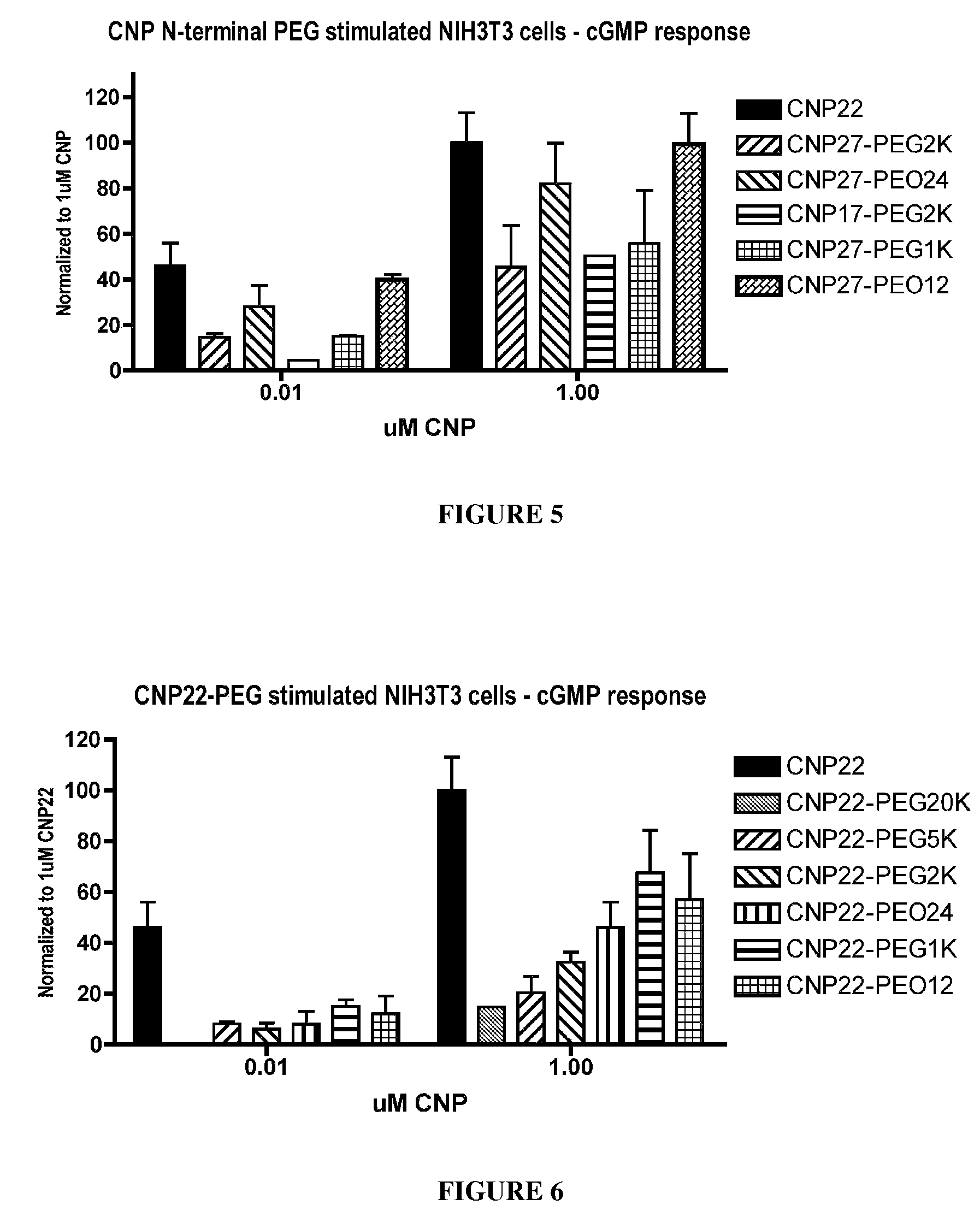
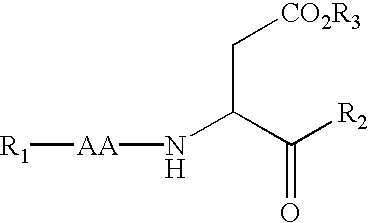
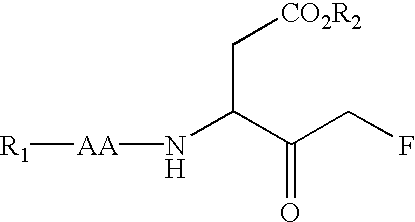
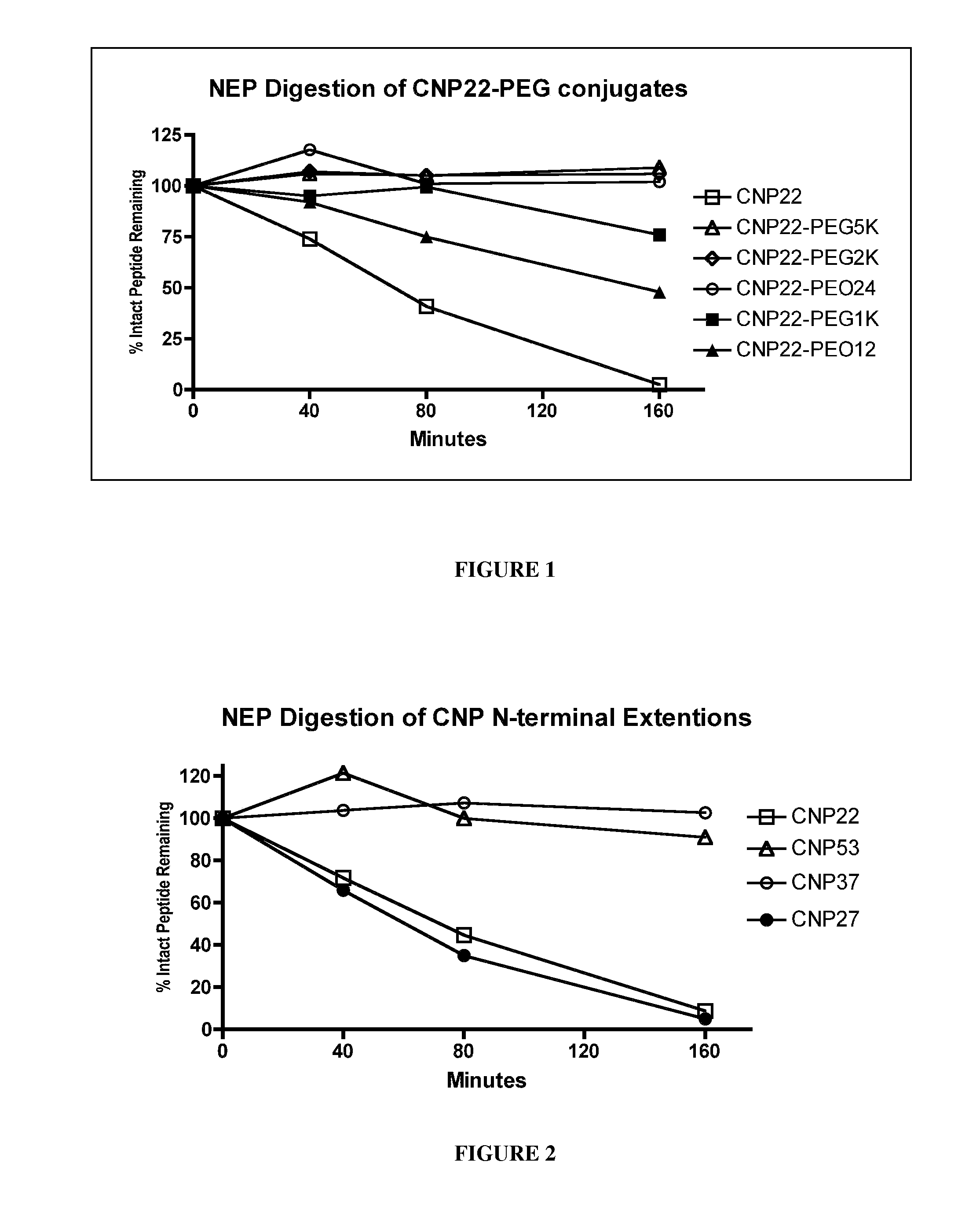
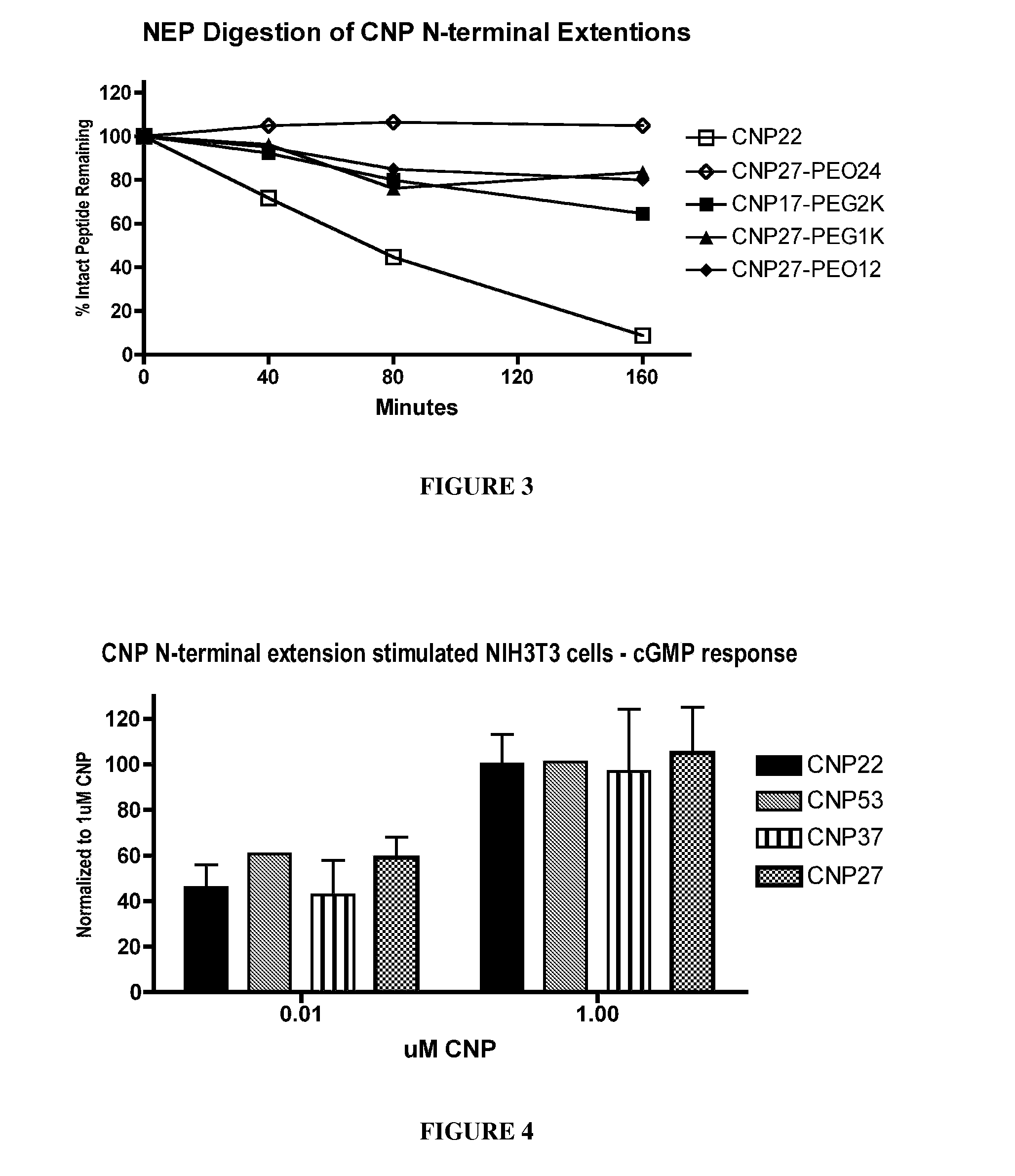
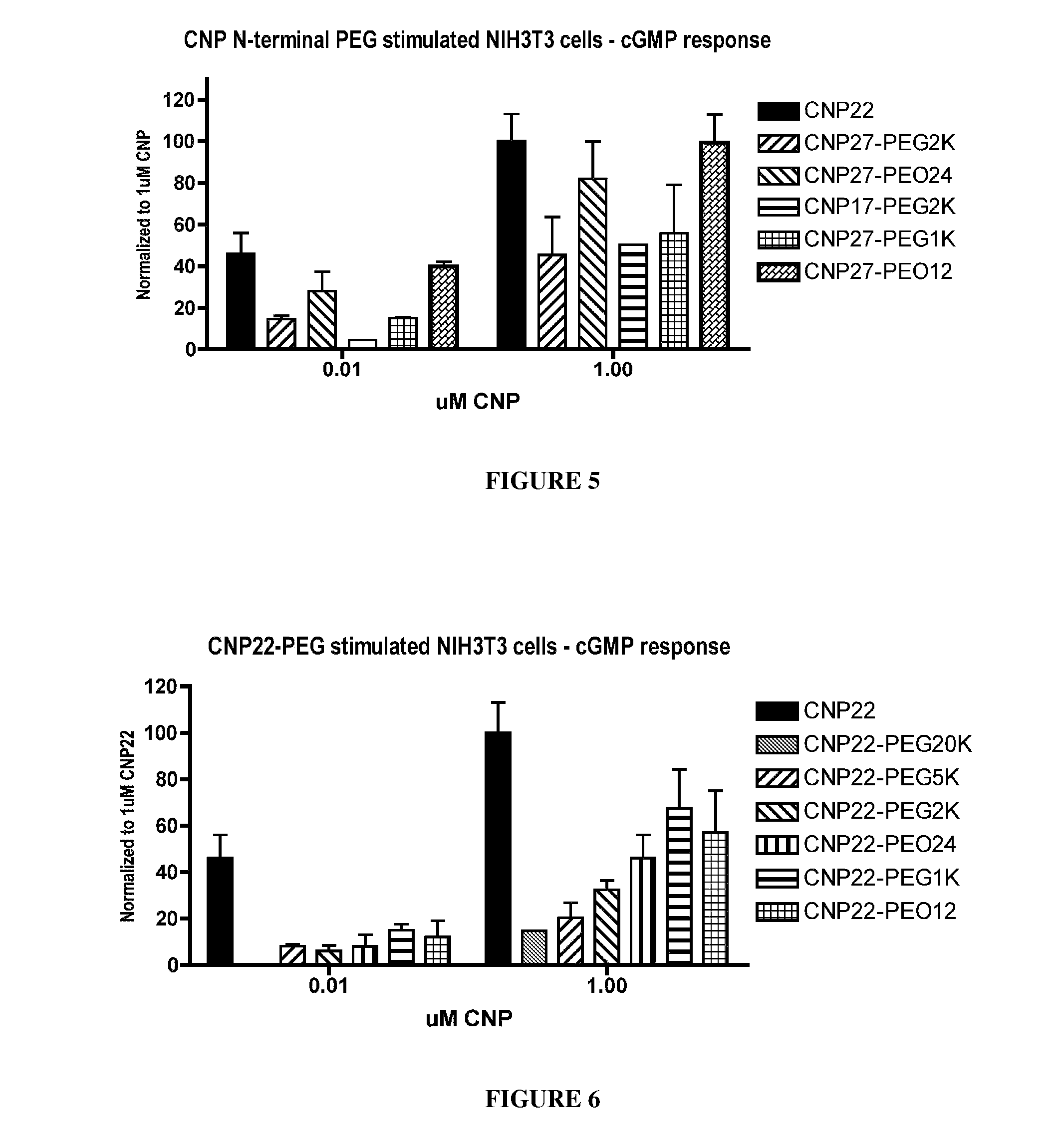
Variants of C-type natriuretic peptides
InactiveUS8377884B2Improve the immunityReduce functionPeptide/protein ingredientsPeptide sourcesChemical MoietyDisease
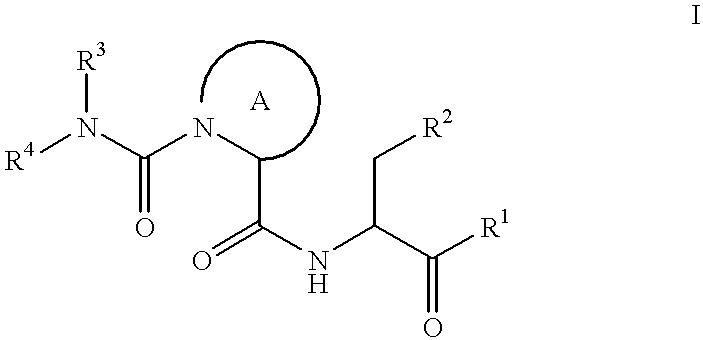
The present invention provides variants of C-type natriuretic peptide (CNP) comprising one or more deletions; additions of and / or substitutions with natural amino acids, unnatural amino acids and / or peptidomimetics (including peptide bond isosteres); amino acid extensions; and / or other chemical moieties such as, e.g., poly(ethylene glycol) and hydrophobic acids. The CNP variants are useful as therapeutic agents for the treatment of diseases responsive to CNP, including but not limited to bone-related disorders such as, e.g., skeletal dysplasias and achondroplasia, and vascular smooth muscle disorders such as, e.g., restenosis and arteriosclerosis.
Owner:BIOMARIN PHARMA INC
Caspase inhibitors and uses thereof
InactiveUS20020058630A1Enhanced inhibitory effectGood effectAntibacterial agentsOrganic active ingredientsCaspase inhibitorsAryl
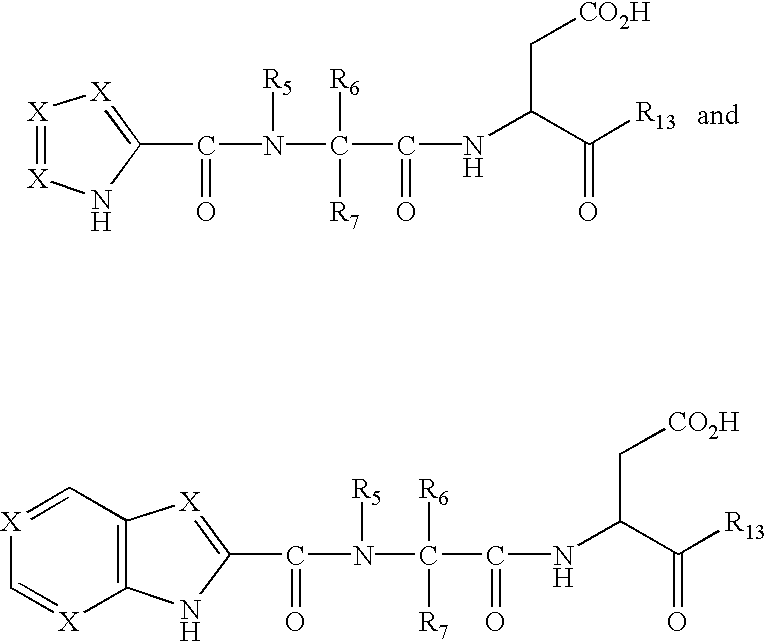
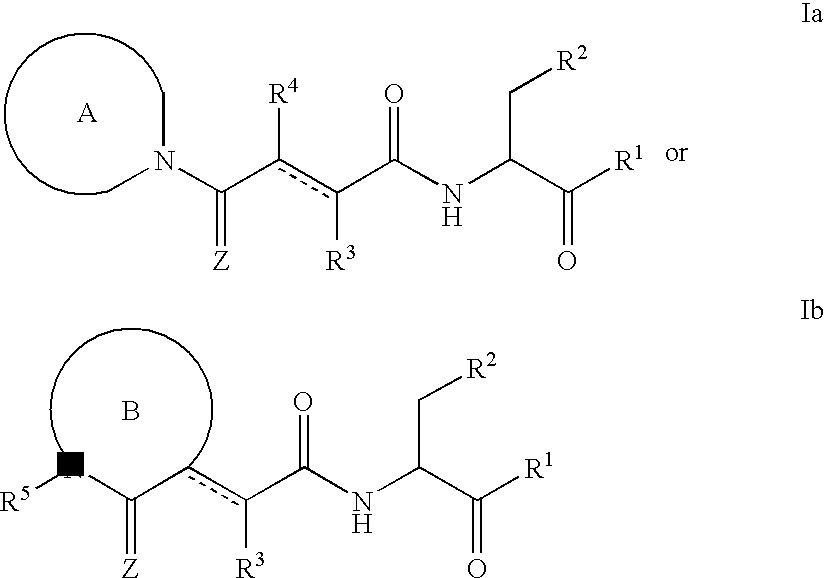
Described herein are compounds that are useful as caspase inhibitors having the formula: wherein Ring A is an optionally substituted piperidine, tetrahydroquinoline or tetrahydroisoquinoline ring; R1 is hydrogen, CN, CHN2, R, or CH2Y; R is an optionally substituted group selected from an aliphatic group, an aryl group, or an aralkyl group; Y is an electronegative leaving group; R2 is CO2H, CH2CO2H, or esters, amides or isosteres thereof; and R3 is hydrogen, an optionally substituted aryl group, an optionally substituted aralkyl group, or an optionally substituted C1-6 aliphatic group, R4 is an optionally substituted group selected from an aryl group or a heterocyclyl group, or R3 and R4 taken together with the nitrogen to which they are attached optionally form a substituted or unsubstituted monocyclic, bicyclic or tricyclic ring.
Owner:VERTEX PHARMA INC
N-linked sulfonamides of N-heterocyclic carboxylic acids or carboxylic acid isosteres
InactiveUS7078424B2Promotes hair growthPromote regenerationCosmetic preparationsBiocideHair growthCarboxylic acid
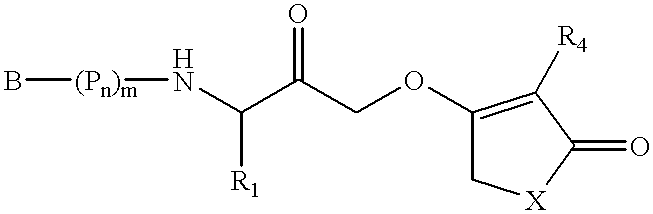
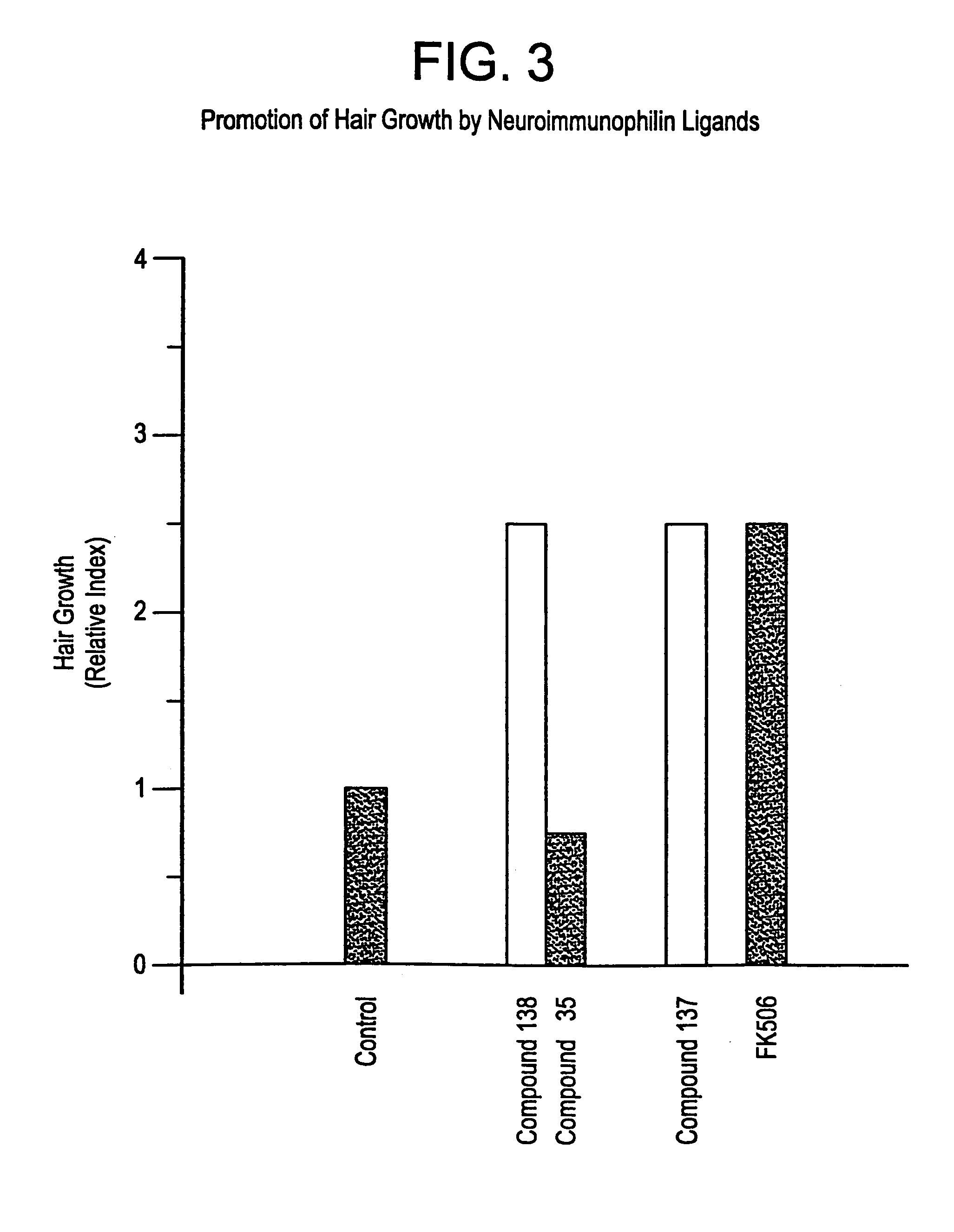
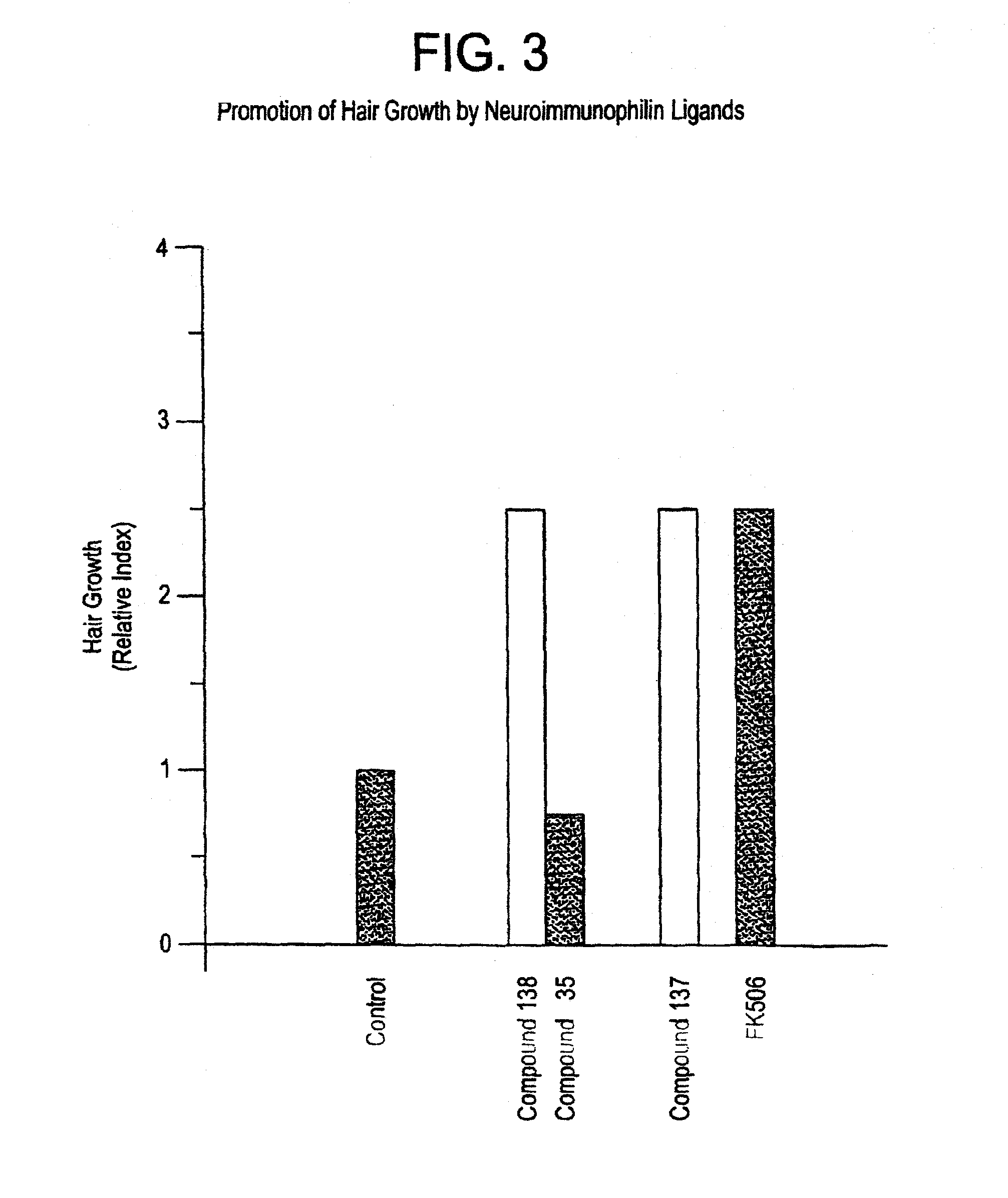
This invention relates to novel N-linked sulfonamides of N-heterocyclic carboxylic acid and carboxylic acid isosteres, their preparation, and use for treating neurological disorders including physically damaged nerves and neurodegenerative diseases, and for treating alopecia and promoting hair growth.
Owner:GLIAMED
Caspase inhibitors and uses thereof
InactiveUS7053057B2Enhanced inhibitory effectGood effectAntibacterial agentsOrganic active ingredientsDiseaseAryl
This invention provides caspase inhibitors having the formula:wherein Ring A is an optionally substituted piperidine, tetrahydroquinoline or tetrahydroisoquinoline ring; R1 is hydrogen, CHN2, R, or —CH2Y; R is an optionally substituted group selected from an aliphatic group, an aryl group, an aralkyl group, a heterocyclic group, or an heterocyclylalkyl group; Y is an electronegative leaving group; R2 is CO2H, CH2CO2H, or esters, amides or isosteres thereof; Ar is an optionally substituted aryl group; and R3 is hydrogen, an optionally substituted C1-6 alkyl, F2, CN, aryl or R3 is attached to Ar to form an unsaturated or partially saturated five or six membered fused ring having 0–2 heteroatoms. The compounds are useful for treating caspase-mediated diseases in mammals.
Owner:VERTEX PHARMA INC
Variants of C-Type Natriuretic Peptides
InactiveUS20100331256A1Improve the immunityReduce functionPeptide/protein ingredientsPeptide sourcesChemical MoietyDisease
The present invention provides variants of C-type natriuretic peptide (CNP) comprising one or more deletions; additions of and / or substitutions with natural amino acids, unnatural amino acids and / or peptidomimetics (including peptide bond isosteres); amino acid extensions; and / or other chemical moieties such as, e.g., poly(ethylene glycol) and hydrophobic acids. The CNP variants are useful as therapeutic agents for the treatment of diseases responsive to CNP, including but not limited to bone-related disorders such as, e.g., skeletal dysplasias and achondroplasia, and vascular smooth muscle disorders such as, e.g., restenosis and arteriosclerosis.
Owner:BIOMARIN PHARMA INC
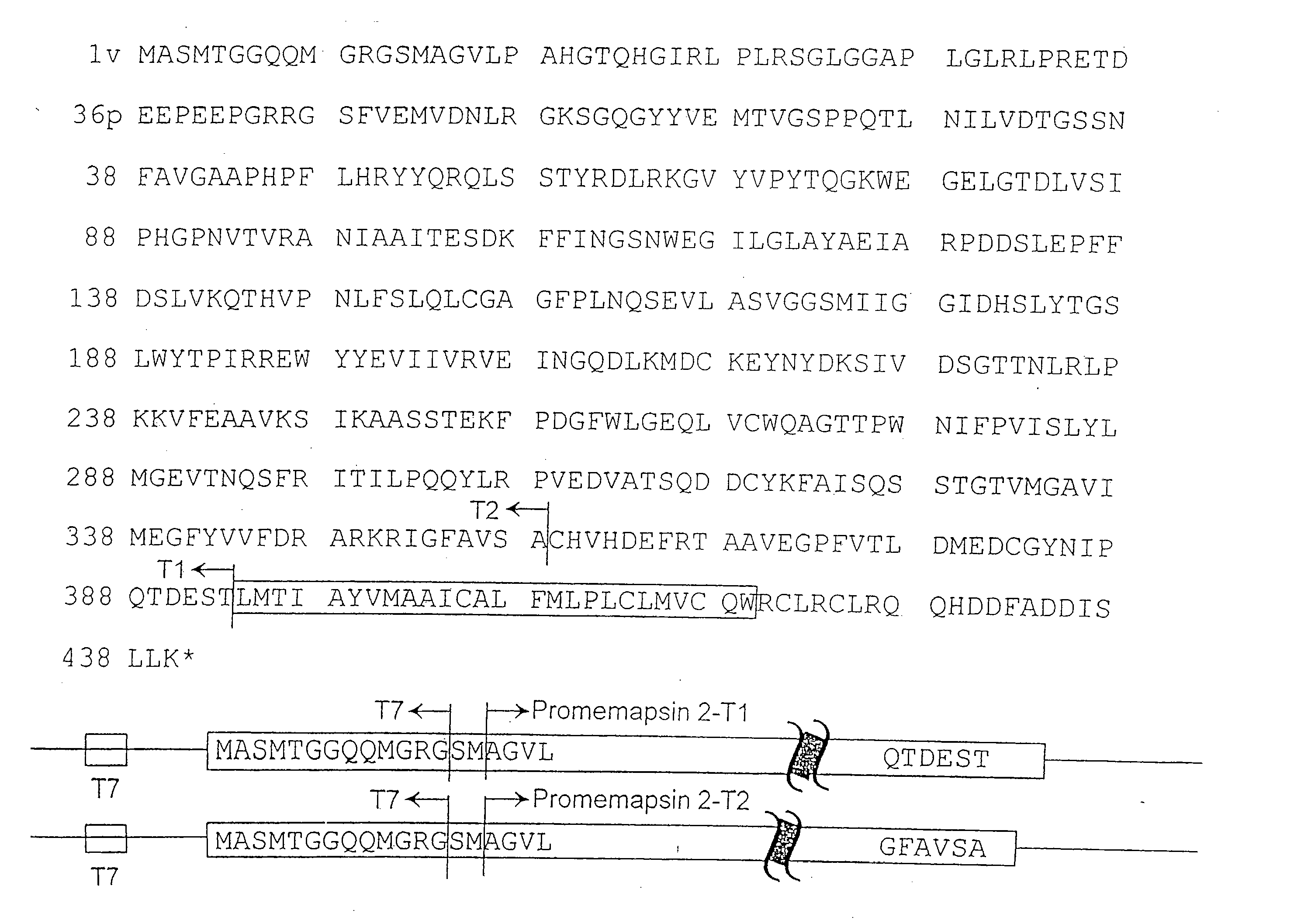
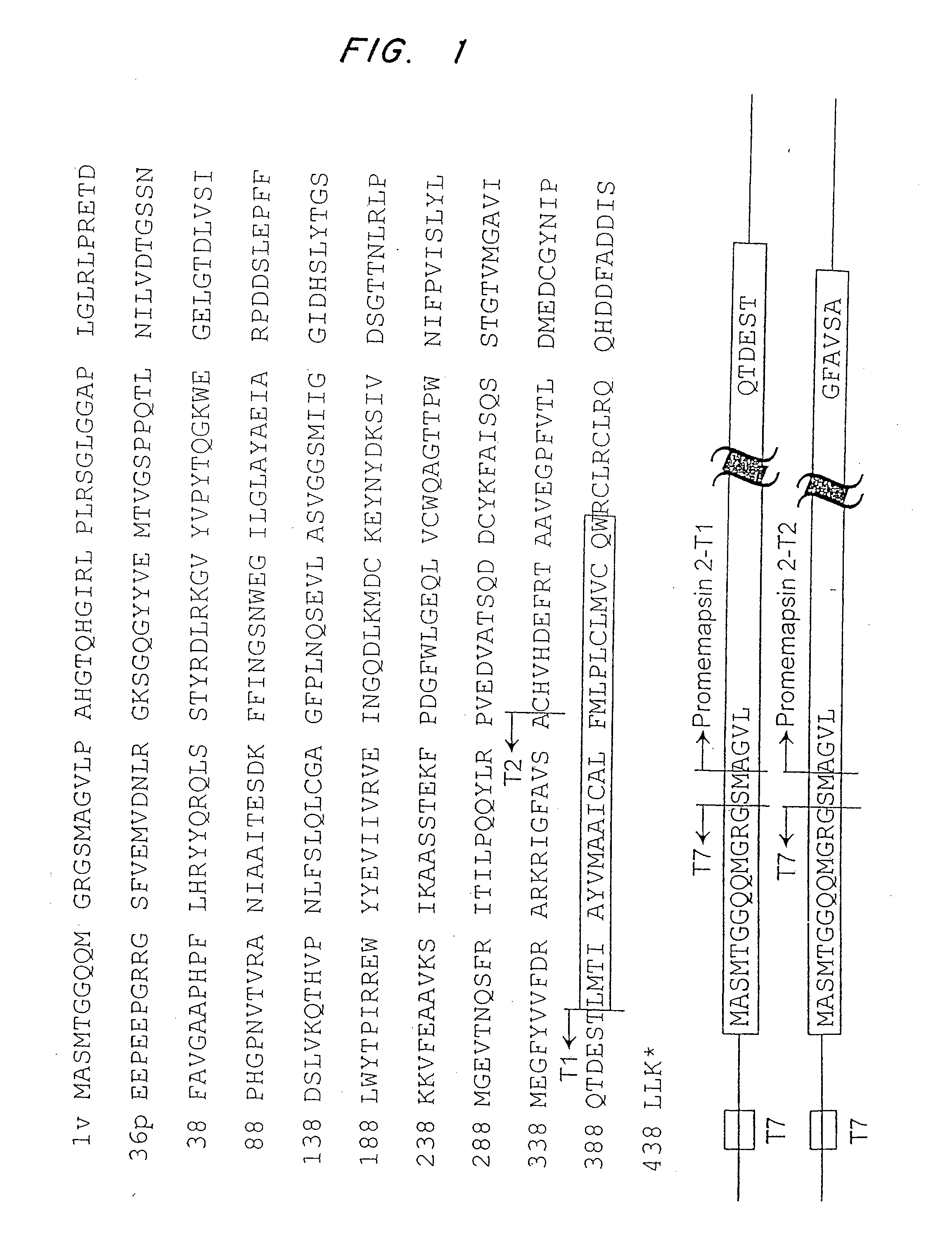
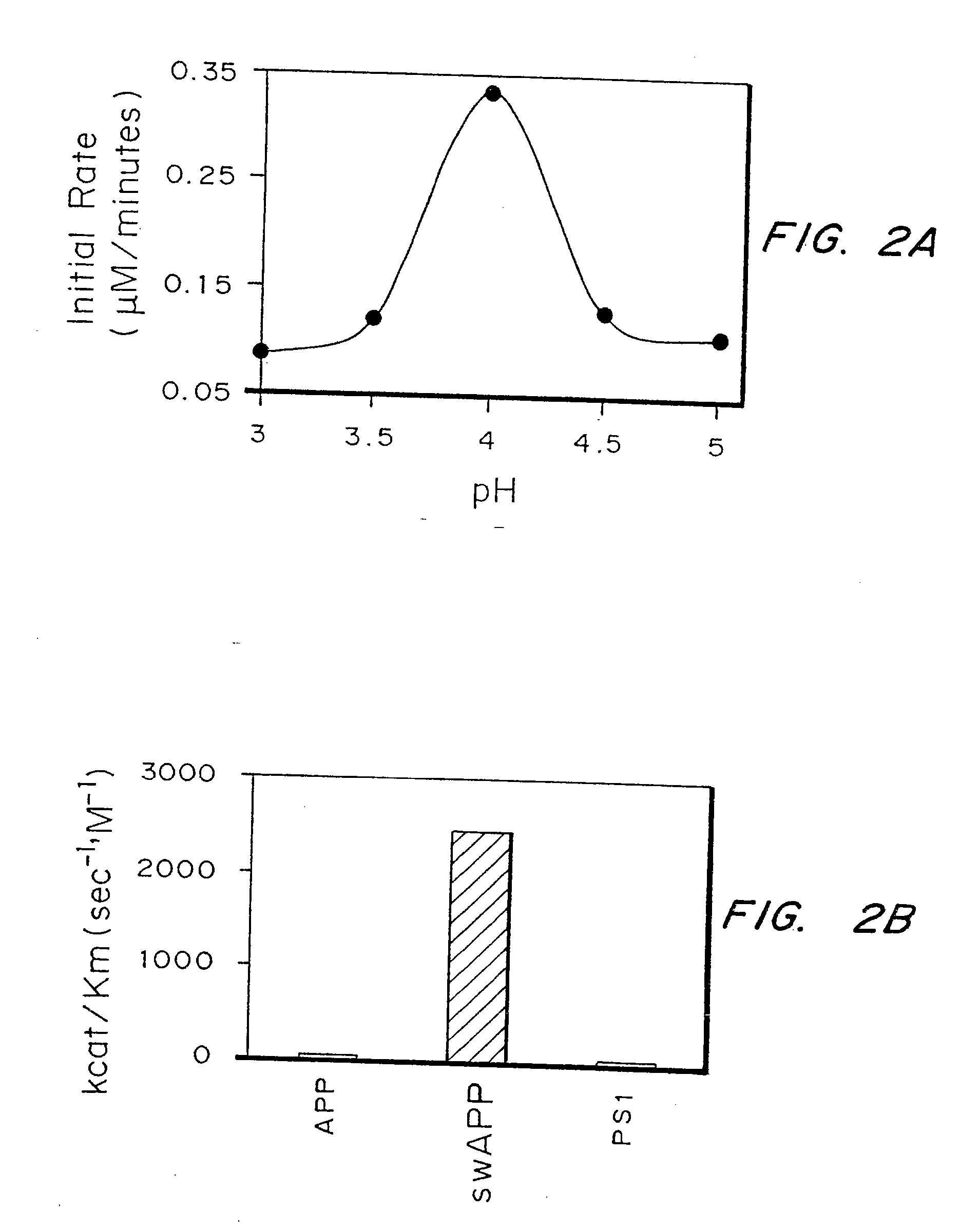
Inhibitors of memapsin 2 and use thereof
InactiveUS7244708B2Reduce molecular weightGrowth inhibitionNervous disorderHydrolasesDiseaseSubstrate analog
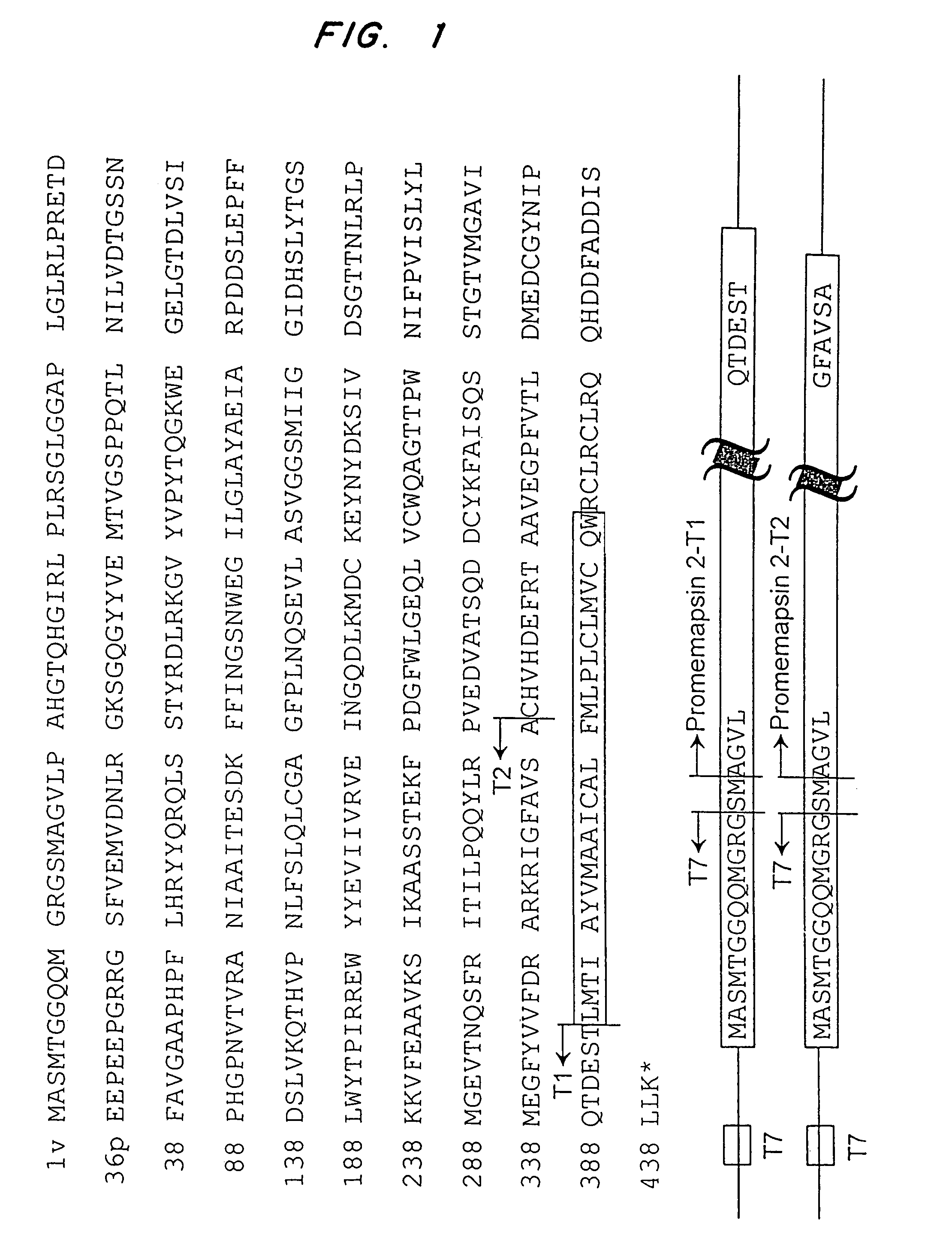
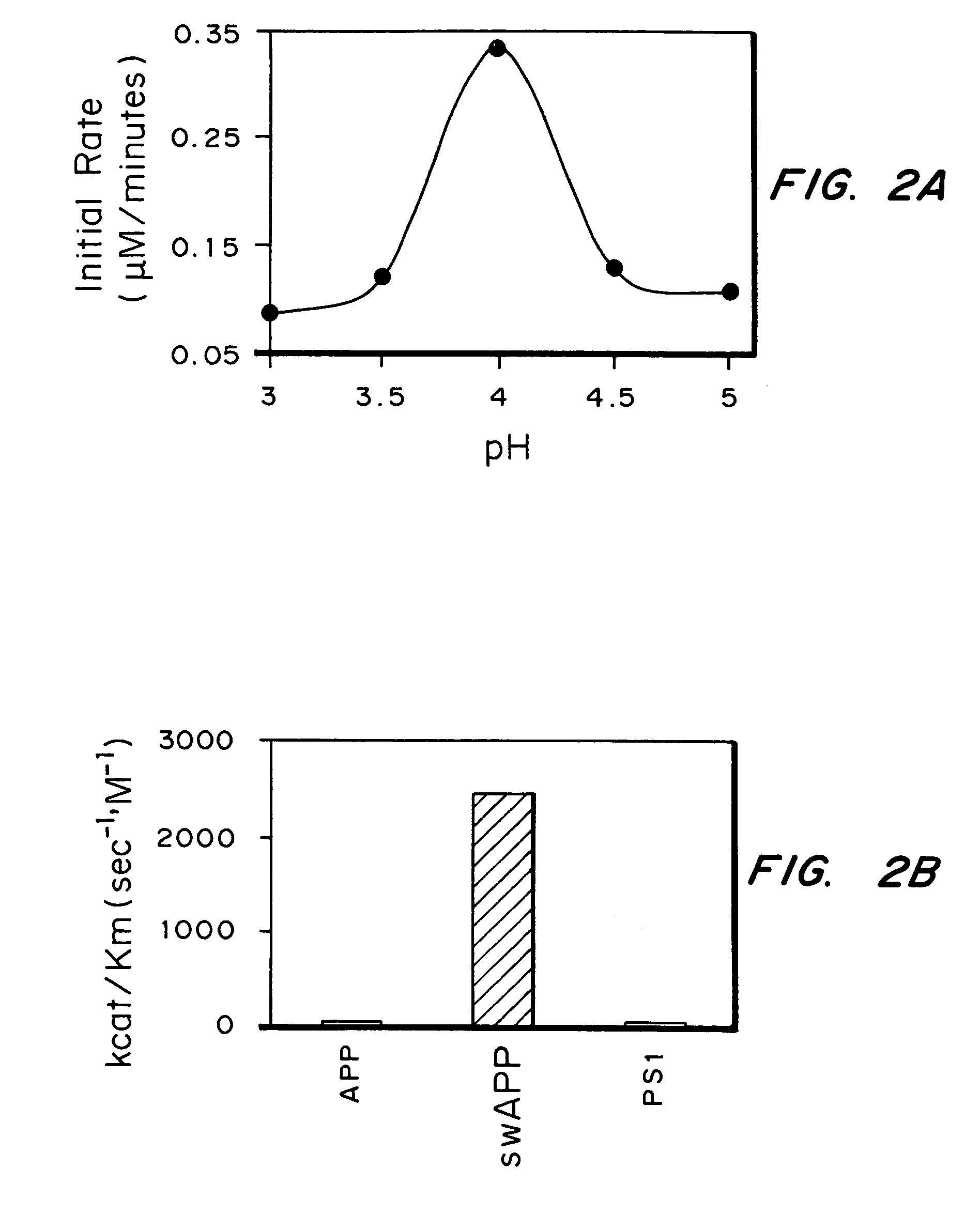
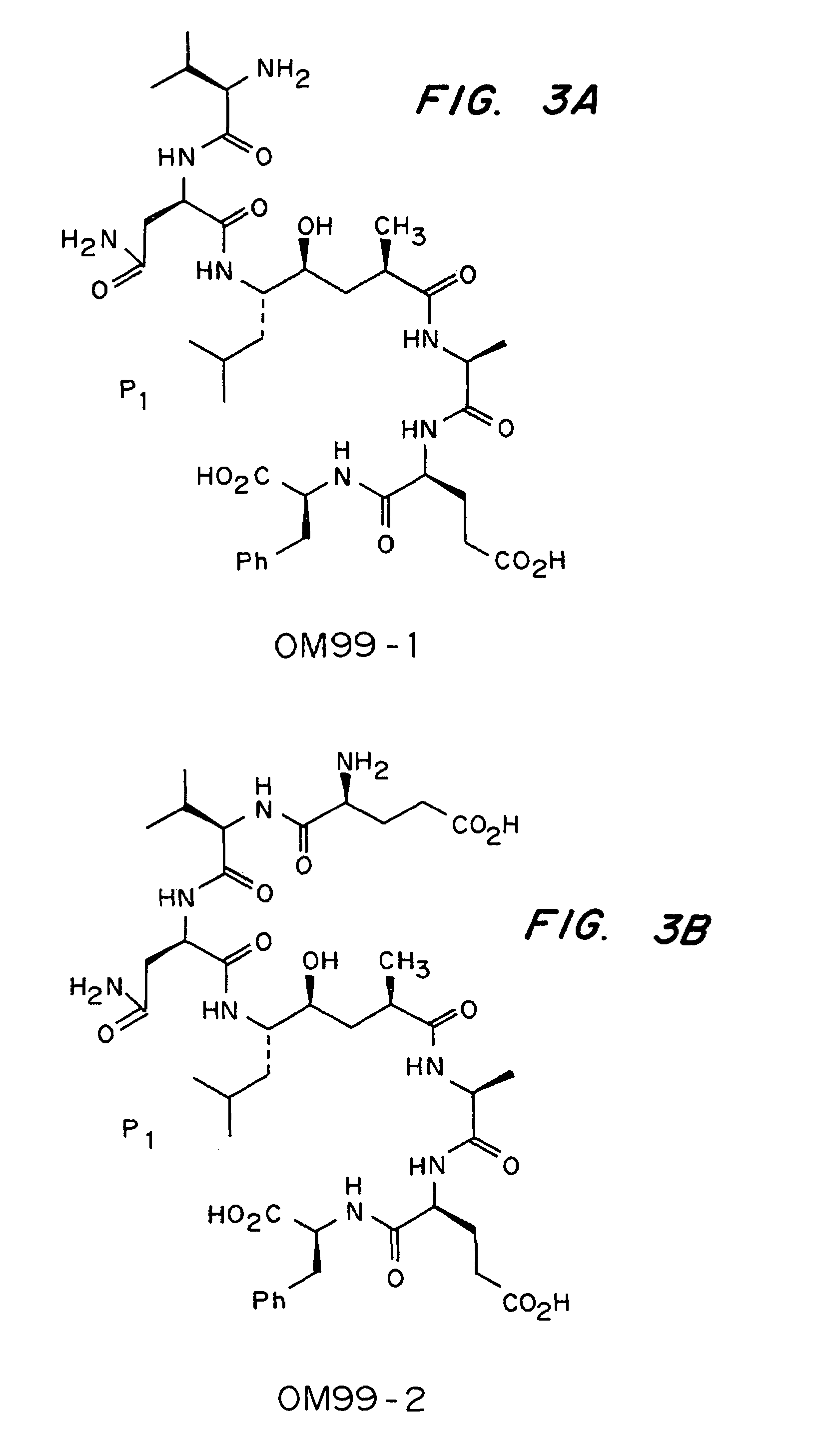
Methods for the production of purified, catalytically active, recombinant memapsin 2 have been developed. The substrate and subsite specificity of the catalytically active enzyme have been determined and were used to design substrate analogs of the natural -2 substrate that can inhibit the function of memapsin 2. Processes for the synthesis of two substrate analogues including isosteres at the sites of the critical amino acid residues were developed and the substrate analogues, OMR99-1 and OM99-2, were synthesized. The inhibition constant of OM99-2 is 1.6×10−9 M against recombinant pro-memapsin 2. Crystallography of memapsin 2 bound to this inhibitor was used to determine the three dimensional structure of the protein, and the importance of the various residues in binding. This information is useful for designing new inhibitors to memapsin 2, for diagnosing and treating and / or preventing Alzheimer's disease.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS +1
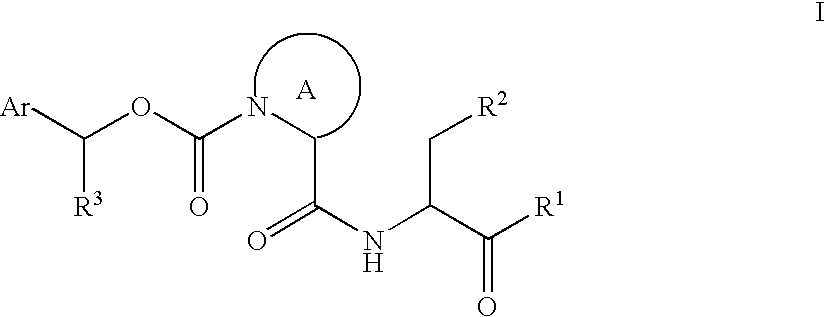
Carbamate caspase inhibitors and uses thereof
InactiveUS7074782B2Good effectGood cell penetrationBiocideSenses disorderCaspase inhibitorsCarbamate
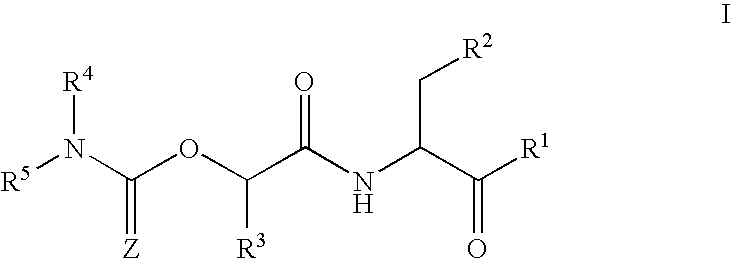
This invention provides caspase inhibitors of formula I:wherein Z is oxygen or sulfur; R1is hydrogen, —CHN2, R, CH2OR, CH2SR, or —CH2Y; Y is an electronegative leaving group; R2 is CO2H, CH2CO2H, or esters, amides or isosteres thereof; R3 is a group capable of fitting into the S2 subsite of a caspase enzyme; R4 and R5 are taken together with the intervening nitrogen to form heterocyclic ring and R is as described in the specification. The compounds are effective inhibitors of apoptosis and IL-1β secretion.
Owner:VERTEX PHARMA INC
Inhibitors of memapsin 2 and use thereof
InactiveUS20080021196A1Reduce molecular weightGrowth inhibitionNervous disorderHydrolasesDiseaseActive enzyme
Methods for the production of purified, catalytically active, recombinant memapsin 2 have been developed. The substrate and subsite specificity of the catalytically active enzyme have been determined. The substrate and subsite specificity information was used to design substrate analogs of the natural memapsin 2 substrate that can inhibit the function of memapsin 2. The substrate analogs are based on peptide sequences, shown to be related to the natural peptide substrates for memapsin 2. The substrate analogs contain at least one analog of an amide bond which is not capable of being cleaved by memapsin 2. Processes for the synthesis of two substrate analogues including isosteres at the sites of the critical amino acid residues were developed and the substrate analogues, OMR99-1 and OM99-2, were synthesized. OM99-2 is based on an octapeptide Glu-Val-Asn-Leu-Ala-Ala-Glu-Phe (SEQ ID NO:28) with the Leu-Ala peptide bond substituted by a transition-state isostere hydroxyethylene group (FIG. 1). The inhibition constant of OM99-2 is 1.6×10−9 M against recombinant pro-memapsin 2. Crystallography of memapsin 2 bound to this inhibitor was used to determine the three dimensional structure of the protein, as well as the importance of the various residues in binding. This information can be used by those skilled in the art to design new inhibitors, using commercially available software programs and techniques familiar to those in organic chemistry and enzymology, to design new inhibitors to memapsin 2, useful in diagnostics and for the treatment and / or prevention of Alzheimer's disease.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS +1
Imidazole and benzimidazole caspase inhibitors and uses thereof
InactiveUS7205327B2Enhanced inhibitory effectGood effectBiocideSenses disorderCaspase inhibitorsMedicinal chemistry
This invention provides caspase inhibitors having the formula:wherein R1 is CO2H, CH2CO2H, or esters, amides or isosteres thereof; R2 and R3 are each independently selected from hydrogen or an optionally substituted C1–C6 aliphatic group; and R4 and R5 are each independently selected from hydrogen, an optionally substituted C1–C6 aliphatic group, or R4 and R5 taken together with the ring to which they are attached form an optionally substituted bicyclic ring. The caspase inhibitors are useful for treating a number of diseases such as cancer, acute inflammatory and autoimmune disorders, ischemic diseases and certain neurodegenerative disorders.
Owner:VERTEX PHARMA INC
HUD (Head Up Display) front windshield of automobile
ActiveCN102135663ASimple designNo ghosting and other bad defectsWindowsWindscreensHead-up displayLiquid-crystal display
The invention provides an HUD (Head Up Display) front windshield of an automobile, which relates to the filed of sandwich glass. The HUD front windshield provided by the invention comprises two layers of glass, two high polymer layers, a first substrate layer, a second substrate layer and a mixing layer between the first substrate layer and the second substrate layer; the structure layers of the front windshield are sequentially the first layer of glass, a high polymer layer, the first substrate layer, the mixing layer, the second substrate layer, another high polymer layer and the second layer of glass; the mixing layer comprises a smectic liquid crystal display isostere region and a peripheral polymer cementing layer; the thickness of the polymer cementing layer is the same as the thickness of the smectic liquid crystal display isostere; the smectic liquid crystal display isostere region is formed by mixing smectic liquid crystals and additives; and the spatial geometrical position of the smectic liquid crystal display isostere between the two substrate layers is an HUD display region. In the liquid crystal HUD provided by the invention, the information of the automobile is converted into electric signals through a display controller to control the liquid crystal arrangement state of each dot in a liquid crystal dot matrix on the front windshield so that the display of word and graph information is realized, and projection display equipment does not need to be additionally arranged.
Owner:FUYAO GLASS IND GROUP CO LTD
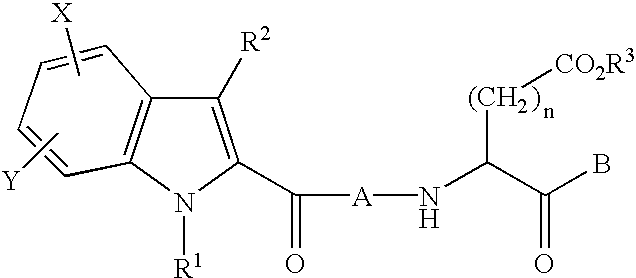
Carboxylic acids and carboxylic acid isosteres of N-heterocyclic compounds for vision and memory disorders
InactiveUS7265150B1Improve eyesightImprove memory performanceBiocideSenses disorderMemory disorderCarboxylic acid
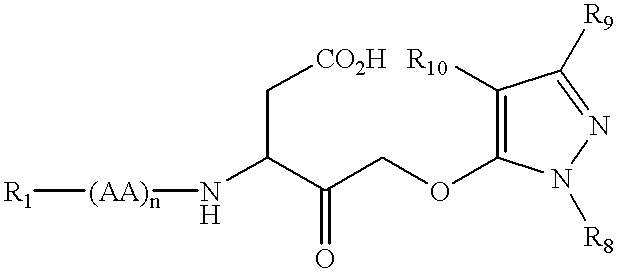
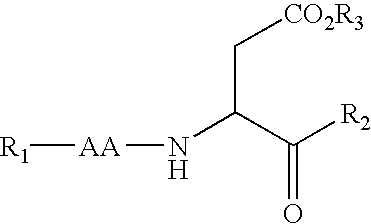
This invention relates to novel compositions and uses of N-heterocyclic carboxylic acids and carboxylic acid isosteres for treating a vision disorder, improving vision, treating memory impairment or enhancing memory performance in an animal.
Owner:GLIAMED +1
Carboxylic acids and carboxylic acid isosteres of N-heterocyclic compounds
InactiveUS7153883B2Promotes hair growthImprove eyesightBiocideSenses disorderCarboxylic acidNeuro-degenerative disease
This invention relates to novel N-heterocyclic carboxylic acids and carboxylic acid isosteres, their preparation and use for treating neurological disorders including physically damaged nerves and neurodegenerative diseases, for treating alopecia and promoting hair growth, for treating vision disorders and / or improving vision, and for treating memory impairment and / or enhancing memory performance by administering such compounds.
Owner:GPI NIL HLDG INC +1
Caspase inhibitors and uses thereof
InactiveUS20080015172A1Good effectGood cell penetrationBiocideSenses disorderCaspase inhibitorsLeaving group
This invention provides caspase inhibitors of formula I: wherein Z is oxygen or sulfur; R1 is hydrogen, —CHN2, R, CH2OR, CH2SR, or —CH2Y; between R3 and R4 represents a single or double bond; Y is an electronegative leaving group; R2 is CO2H, CH2CO2H, or esters, amides or isosteres thereof; R3is a group capable of fitting into the S2 subsite of a caspase enzyme; R4 is a hydrogen or C1-6 alkyl or R3 and R4 taken together form a ring; Ring A and Ring B are each heterocyclic rings, and R and R5 are as described in the specification. The compounds are effective inhibitors of apoptosis and IL-1β secretion.
Owner:VERTEX PHARMA INC
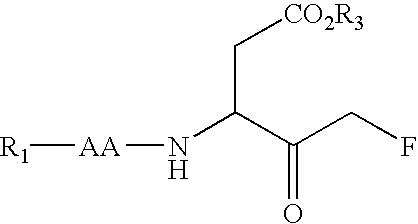
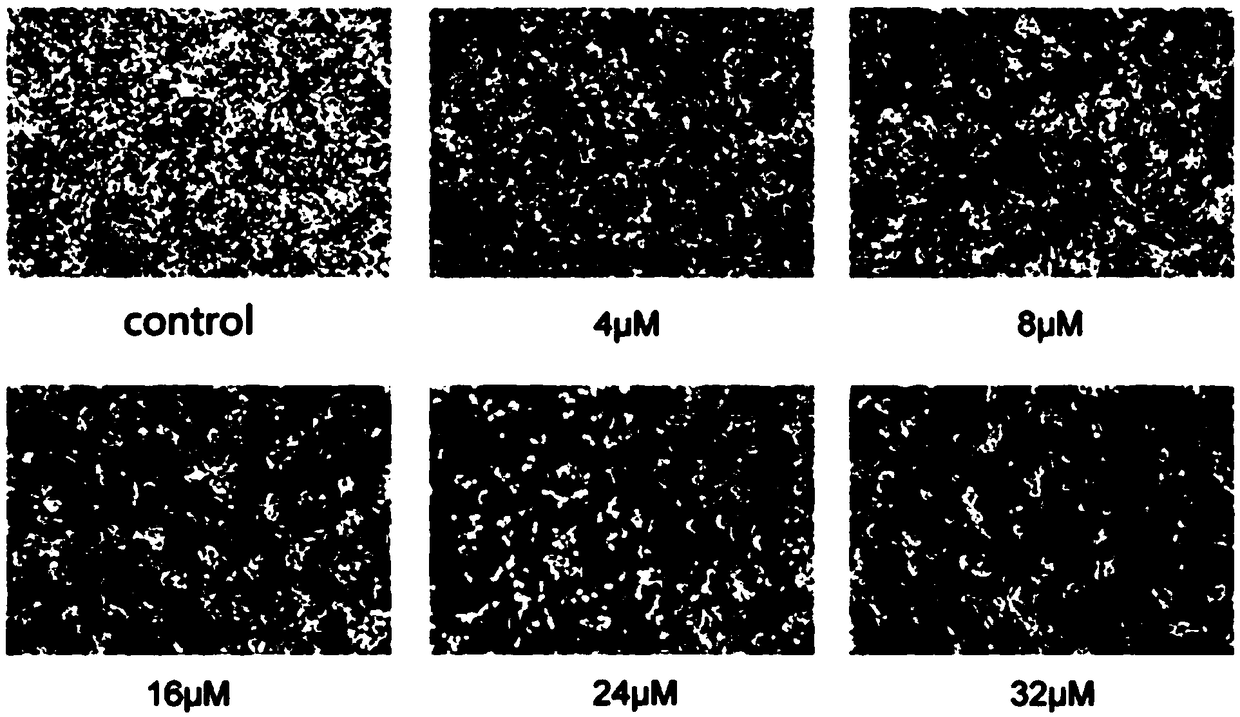
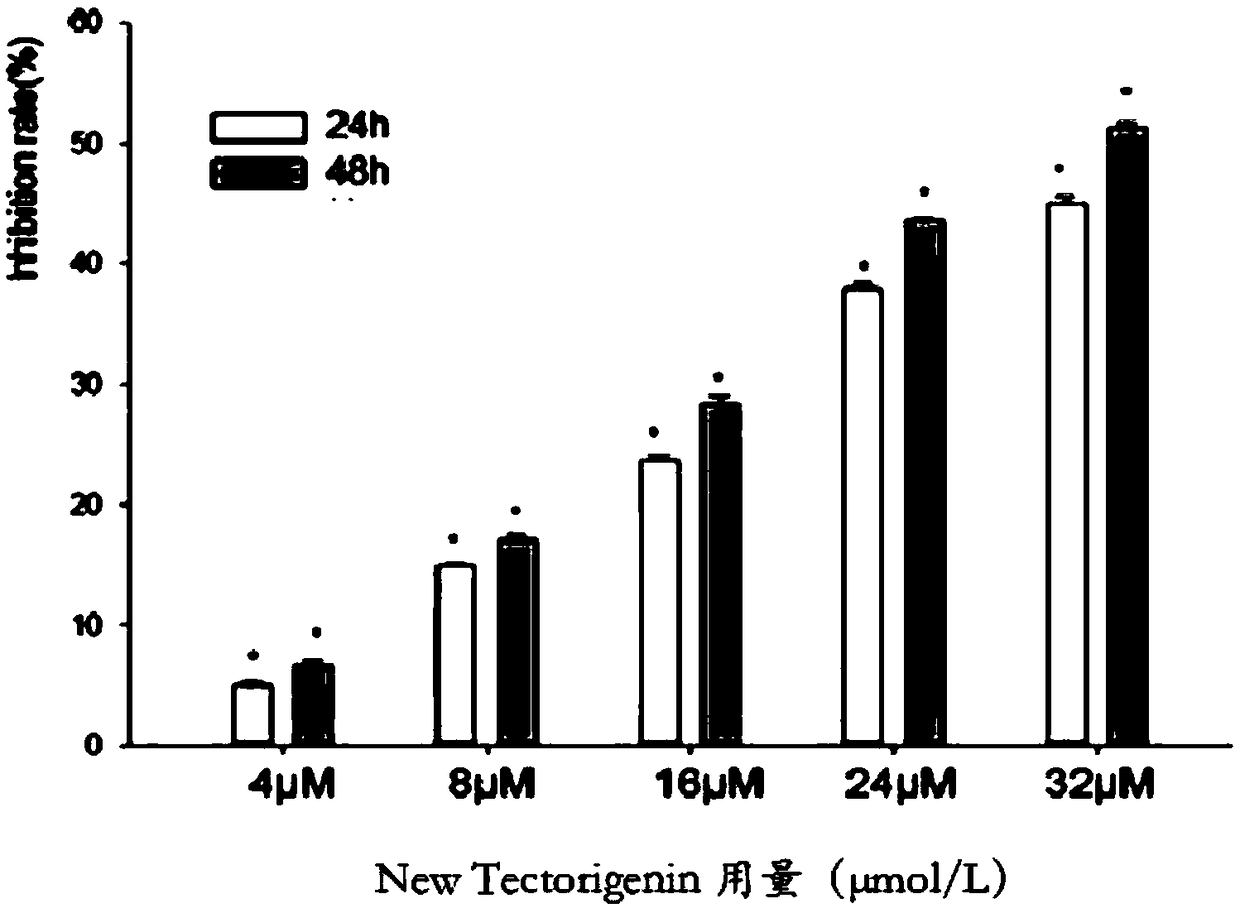
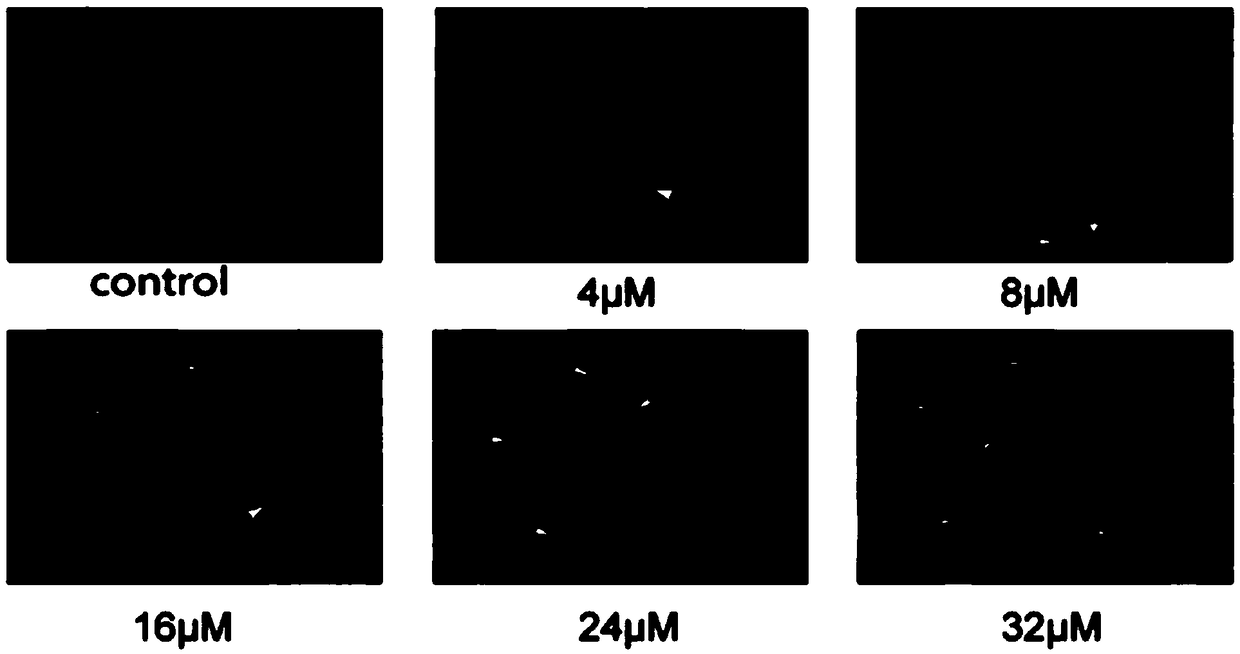
Application of quinoline derivative of N isostere tectoridin in anti-hepatoma drugs
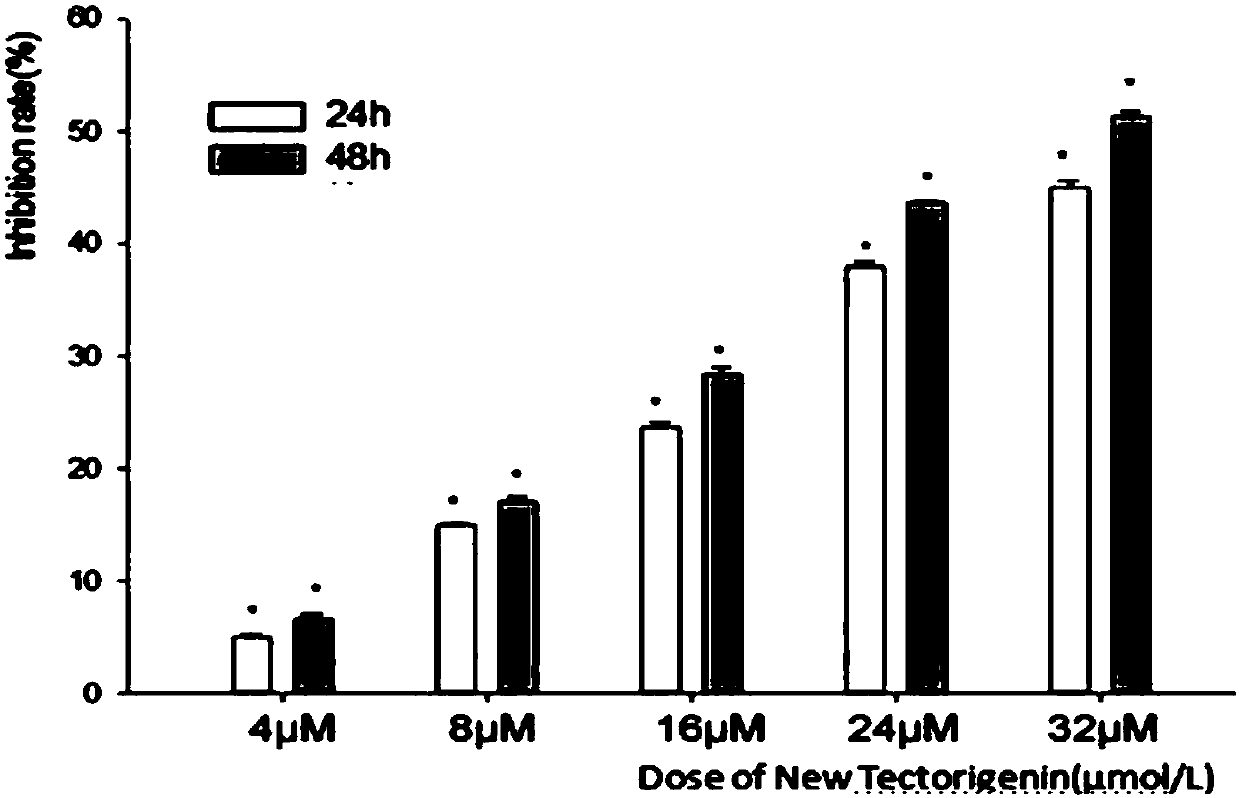
InactiveCN107669676AGood treatment effectLess likely to develop drug resistanceOrganic chemistryAntineoplastic agentsIridoid GlucosidesTreatment effect
The invention discloses an application of a quinoline derivative of N isostere tectoridin in anti-hepatoma drugs. The chemical structural formula of the quinoline derivative of the N isostere tectoridin is shown as Formula (as shown in the description); and the invention also provides an application of the quinoline derivative, namely New Tectorigenin, of the N isostere tectoridin in preparing drugs for inhibiting hepatic carcinoma HepG2 cells. According to the invention, the proliferation of the hepatic carcinoma HepG2 cells can be inhibited by inhibiting an RAF / MEK / ERK pathway and a JAK / STATpathway. The anti-hepatoma drugs provided by the invention are obvious in therapeutic effect, and cannot cause drug resistance and adverse reactions easily.
Owner:GUANGZHOU UNIVERSITY
Beta-substituted beta-amino acids and analogs as chemotherapeutic agents and uses thereof
ActiveCN108026026AHigh uptake selectivityLow nonspecific uptakeNervous disorderOrganic chemistry methodsCytotoxicityIn vivo
Beta-Substituted Beta-amino acids, Beta-substituted Beta-amino acid derivatives, and Beta-substituted Beta-amino acid analogs and (bio)isosteres and their use as chemotherapeutic agents are disclosed.The Beta-substituted Beta-amino acid derivatives and Beta -substituted Beta-amino acid analogs and (bio)isosteres are selective LAT1 / 4F2hc substrates and exhibit rapid uptake and retention in tumorsexpressing the LAT1 / 4F2hc transporter. Methods of synthesizing the Beta-substituted Beta-amino acid derivatives and Beta-substituted Beta-amino acid analogs and methods of using the compounds for treating cancer are also disclosed. The Beta-substituted Beta-amino acid derivatives and Beta-substituted Beta-amino acid analogs exhibit selective uptake in tumor cells expressing the LAT1 / 4F2hc transporter and accumulate in cancerous cells when administered to a subject in vivo. The Beta-substituted Beta-amino acid derivatives and Beta-substituted Beta-amino acid analogs and (bio)isosteres exhibit cytotoxicity toward several tumor types.
Owner:QUADRIGA BIOSCI
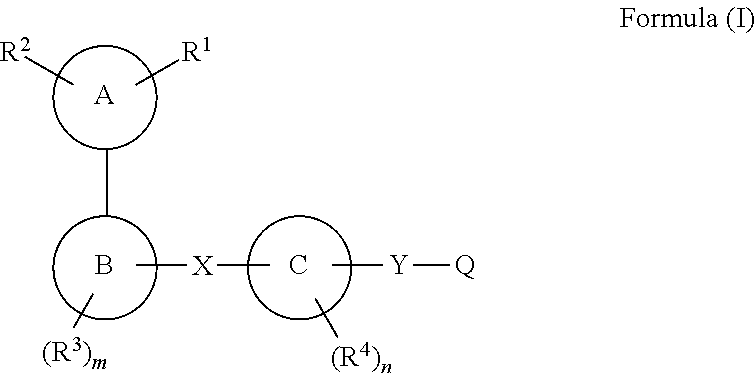
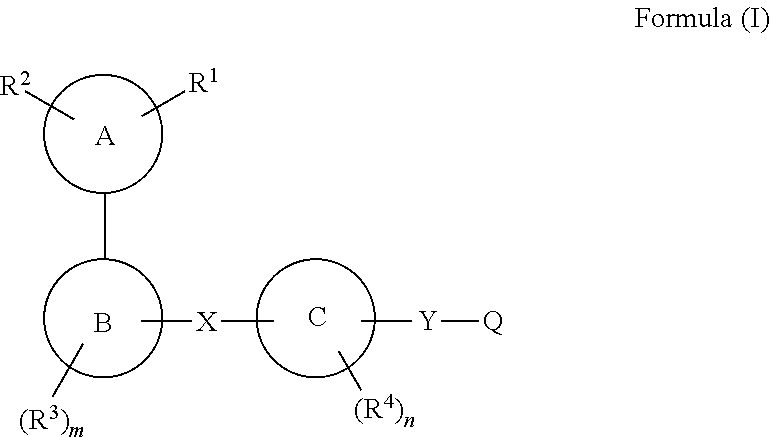
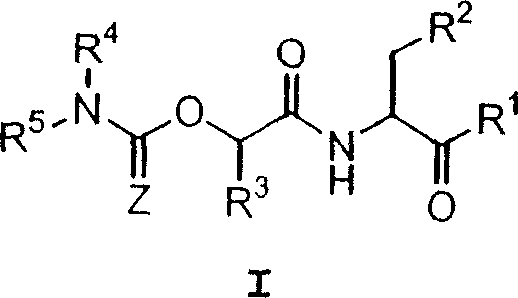
Compounds for use as gpr120 agonists
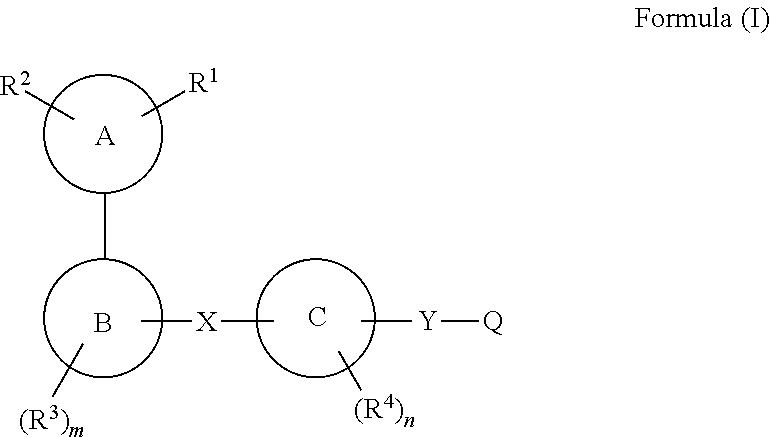
The present invention relates to a compound of formula (I), or a tautomer, stereoisomer, geometrical isomer, prodrug, carboxylic acid isostere, solvate, polymorph, N-oxide, S-oxide or pharmaceutically acceptable salt thereof, which are GPR120 agonists. The present invention also relates to a pharmaceutical composition of a compound of formula (I) for the treatment of metabolic disorders, particularly Type 2 diabetes and associated diseases.
Owner:PIRAMAL ENTERPRISES LTD
Nonpeptide insulin receptor agonists
InactiveUSRE38915E1Lowering blood-glucoseBiocideHydrocarbon active ingredientsDiabetes mellitusProton
Modulation of the activity of the insulin receptor, enhancement of glucose uptake by cells, and other effects significant in the control and management of diabetes are accomplished using compounds of the formula whereineach A is independently a proton-accepting substituent;each R is independently a noninterfering substituent;n is 0, 1, or 2; andeach linker is independently an isostere of —NHCONH— or of —N═N— or of —NHCO—.Compounds in the genus of Formula (1) can also be used for structure activity studies to identify features responsible for the relevant activities.
Owner:TELIK INC
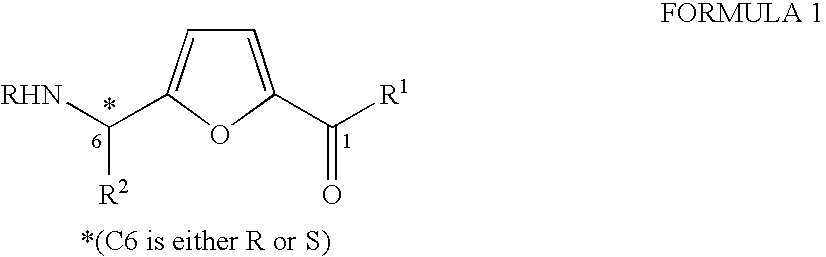
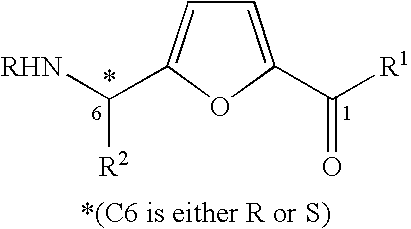
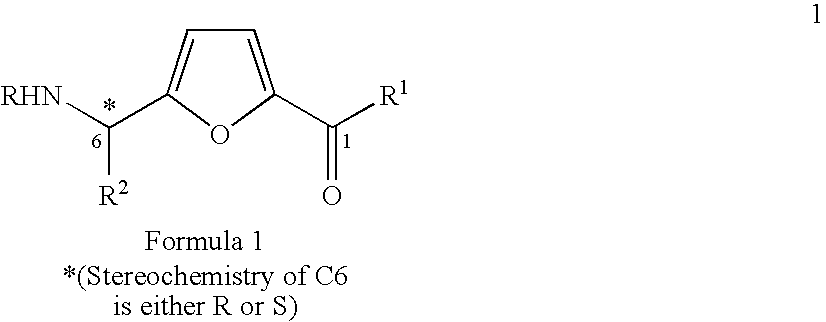
Synthesis of chiral furan amino acids as novel peptide building blocks
The present invention provides a chiral furan amino acids, in enantiomerically pure forms, either R or S. The starting materials are being used chiral N-terminal-protected amino aldehydes derived from the corresponding N-terminal-protected protected L- or D-amino acids. The present invention also relates to a process for preparing these chirally substituted furan amino acids constitute an important class of conformationally constrained peptide based molecules that can be used as dipeptide isosteres in peptidomimetic studies.
Owner:COUNCIL OF SCI & IND RES
Mucarinic Agonists and Methods of Use Thereof
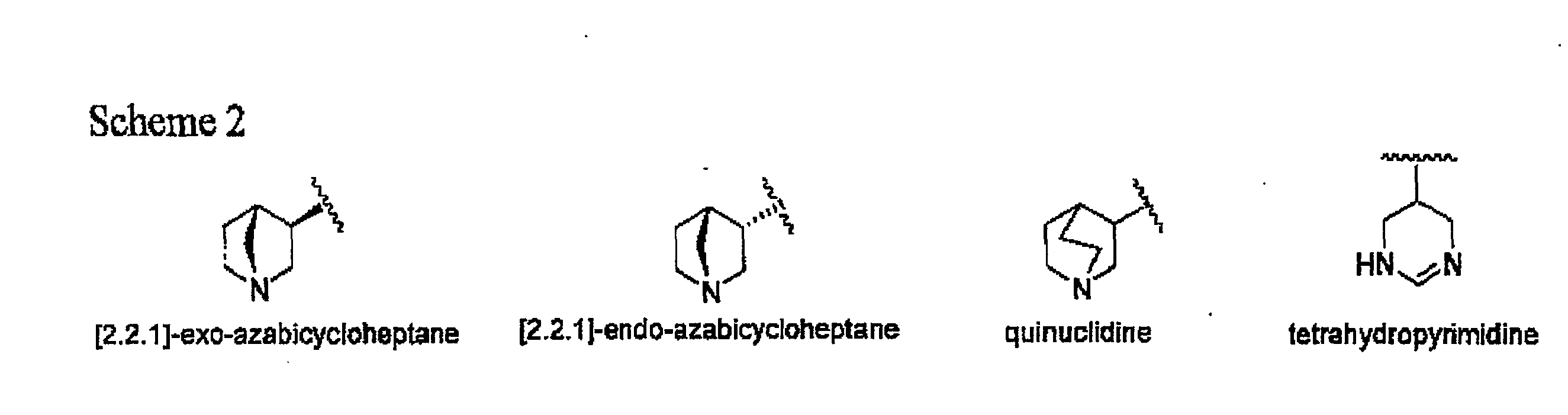
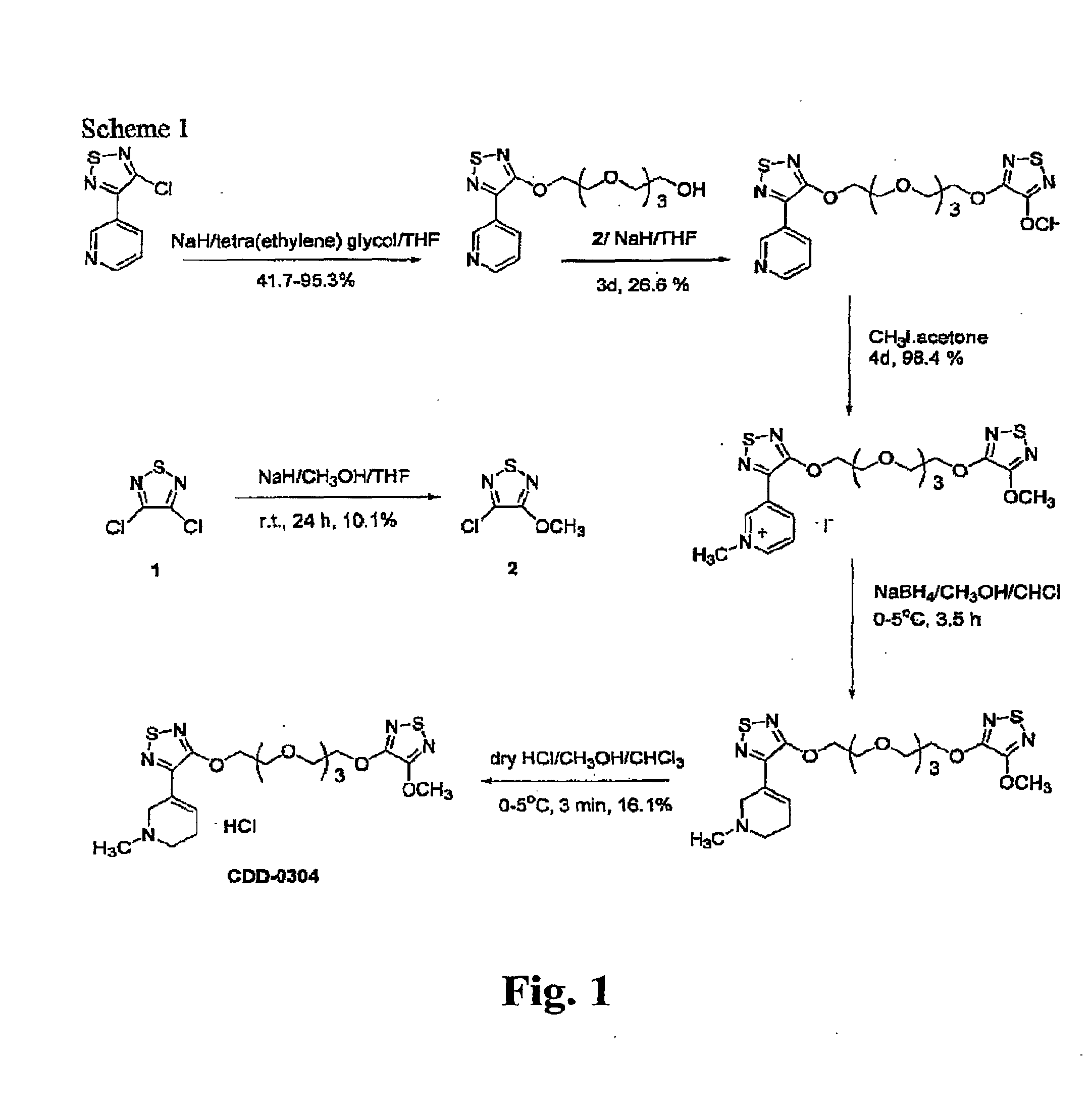
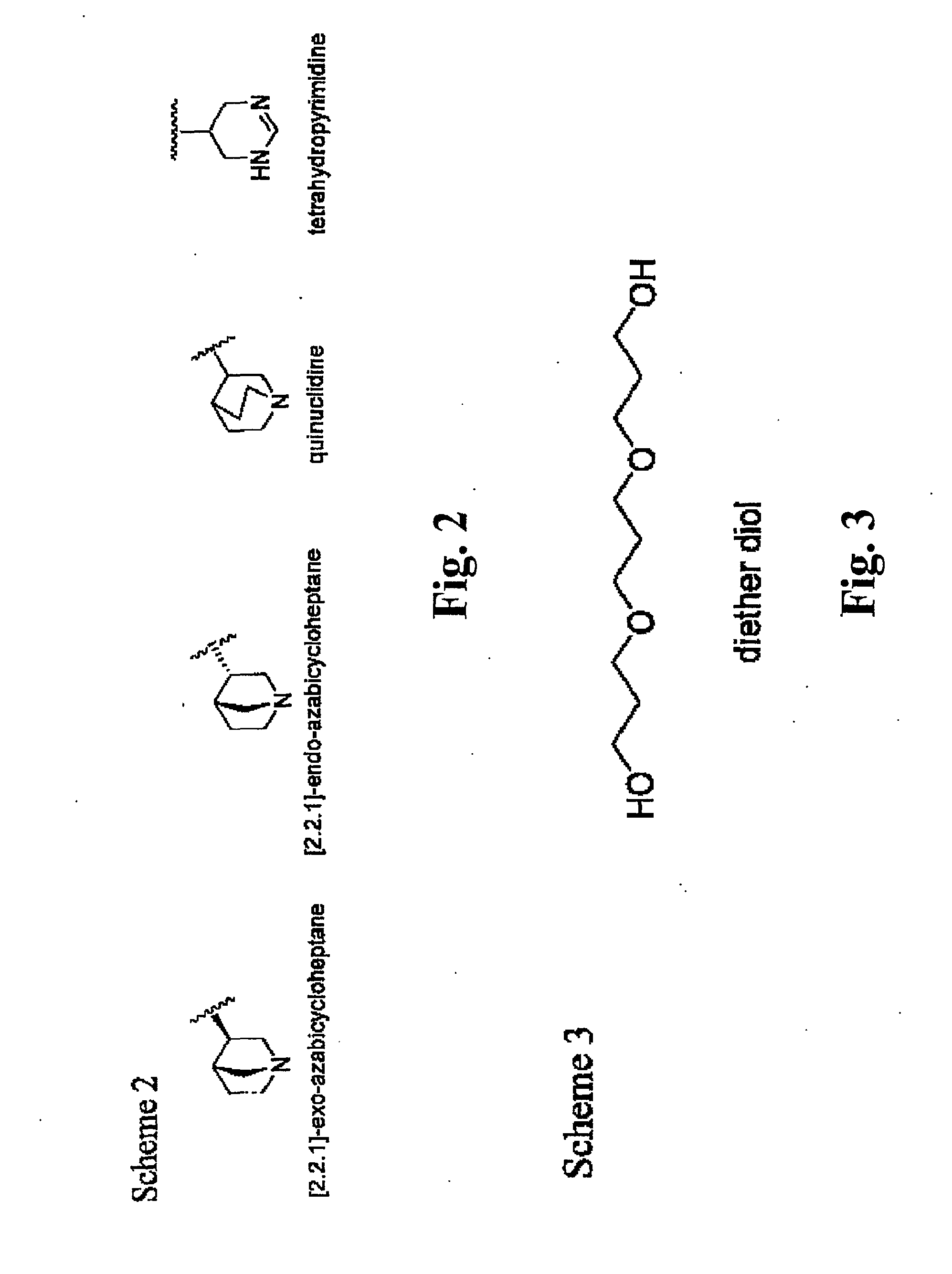
A method of forming analogs of CDD-0304, i.e., tetra(ethyleneglycol) (4-methoxy-1,2,5-thiadiazol-3-yl)[3-(1-methyl-1,2,4,5-tetrahydropyrid-3-yl)-1,2,5-thiadiazol-4-yl]ether hydrochloride includes one or more of the following steps: a) replacing the tetrahydropyridine moiety with one of the following heterocyclic rings, including quinuclidine, [2.2.1]-exo-azabicy-cloheptane, [2.2.1]-endo-azabicycloheptane and terahydropyrimidine; b) varying the length of the linking group by replacing the tetra(ethylene) glycol moiety with one of: ethylene glycol, di(ethylene) glycol, penta(ethylene) glycol, or diether diol; and / or, c) replacing the 1,2,5-thiadiazole moiety with an ester isostere. Also, a method for an asymmetric analog CCD-0304 includes replacing at least one moiety with an ester isostere and at least a second moiety with an ammonium isostere. Also, such analogs compounds and their uses are disclosed.
Owner:UNIVERSITY OF TOLEDO
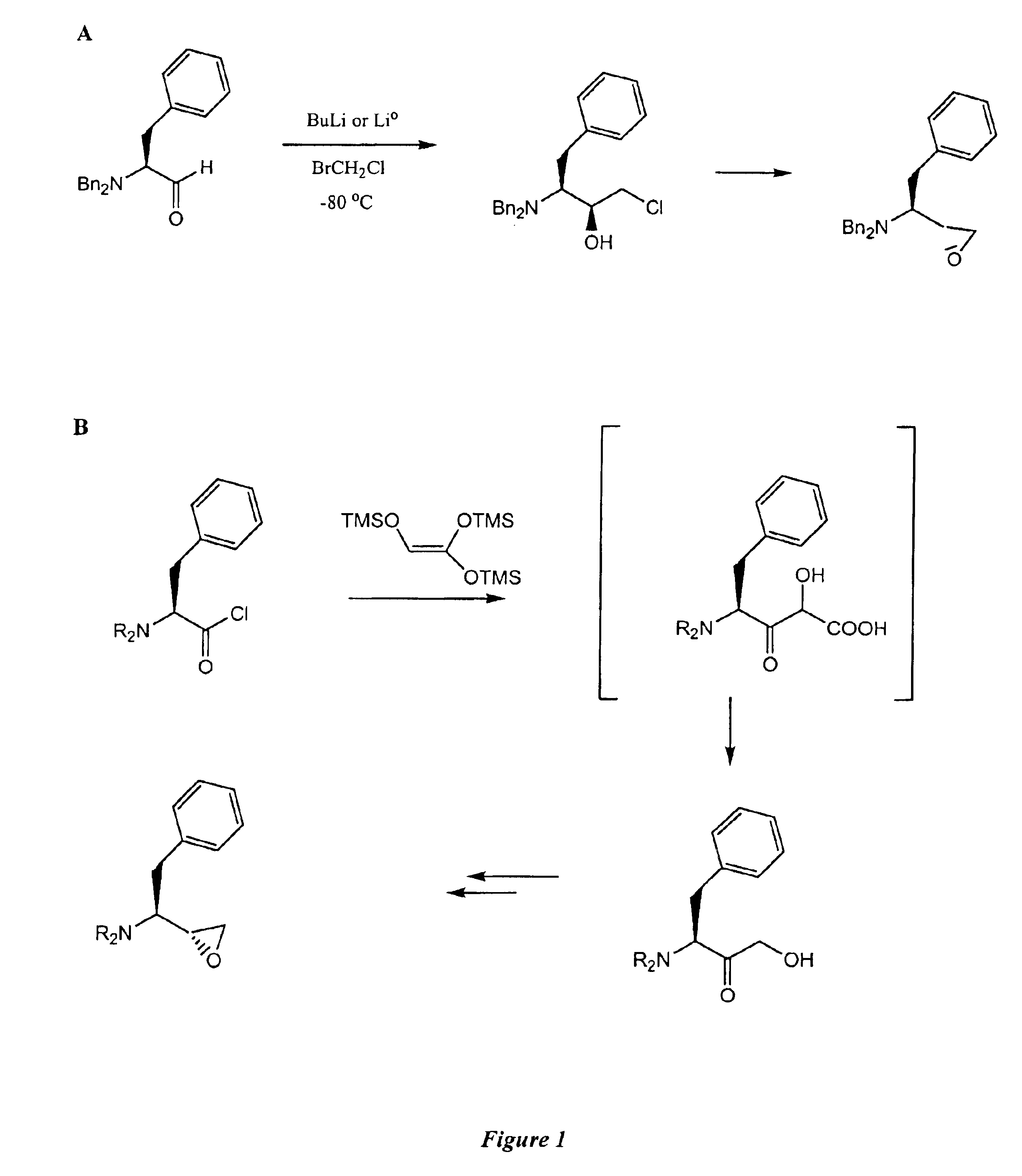
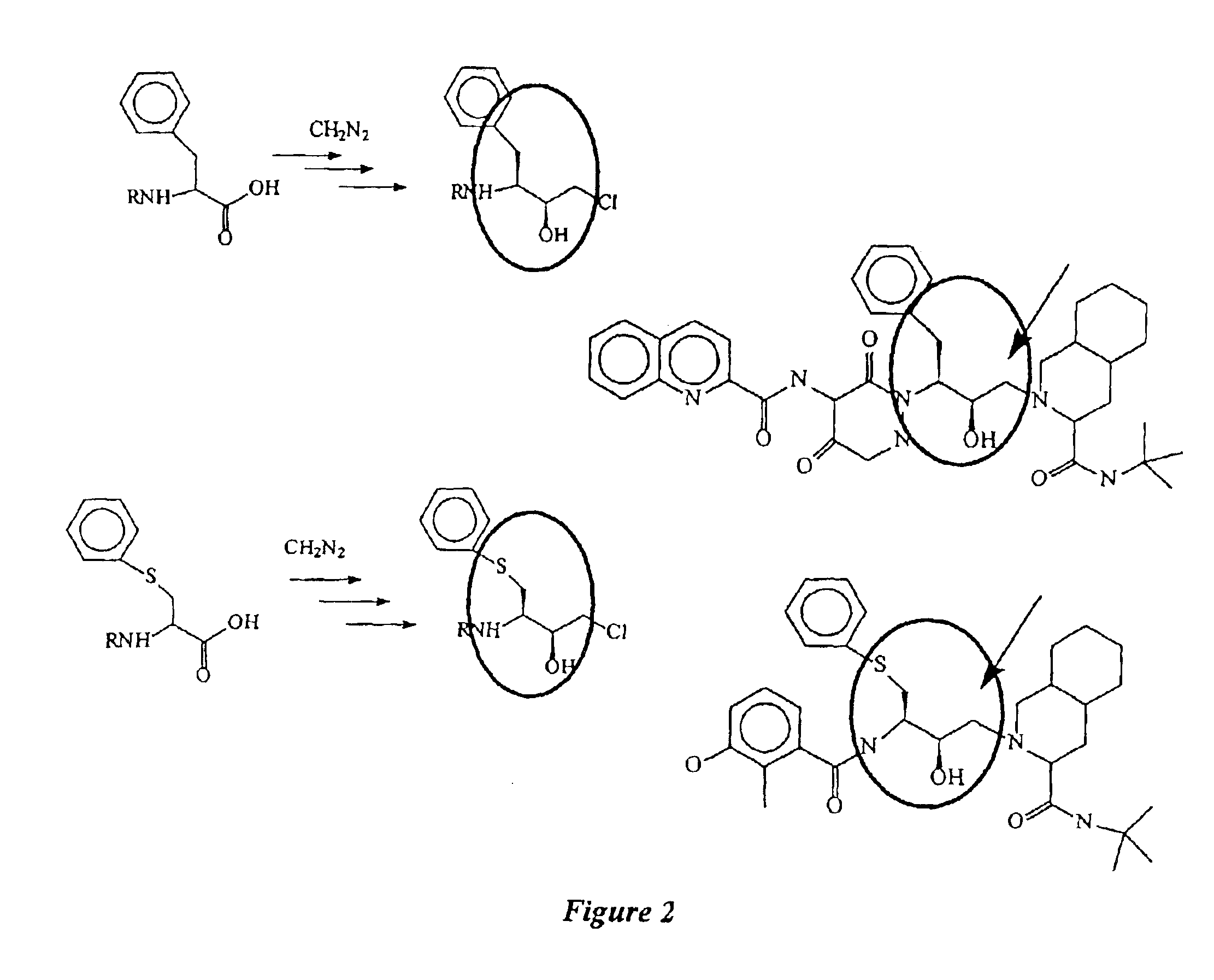
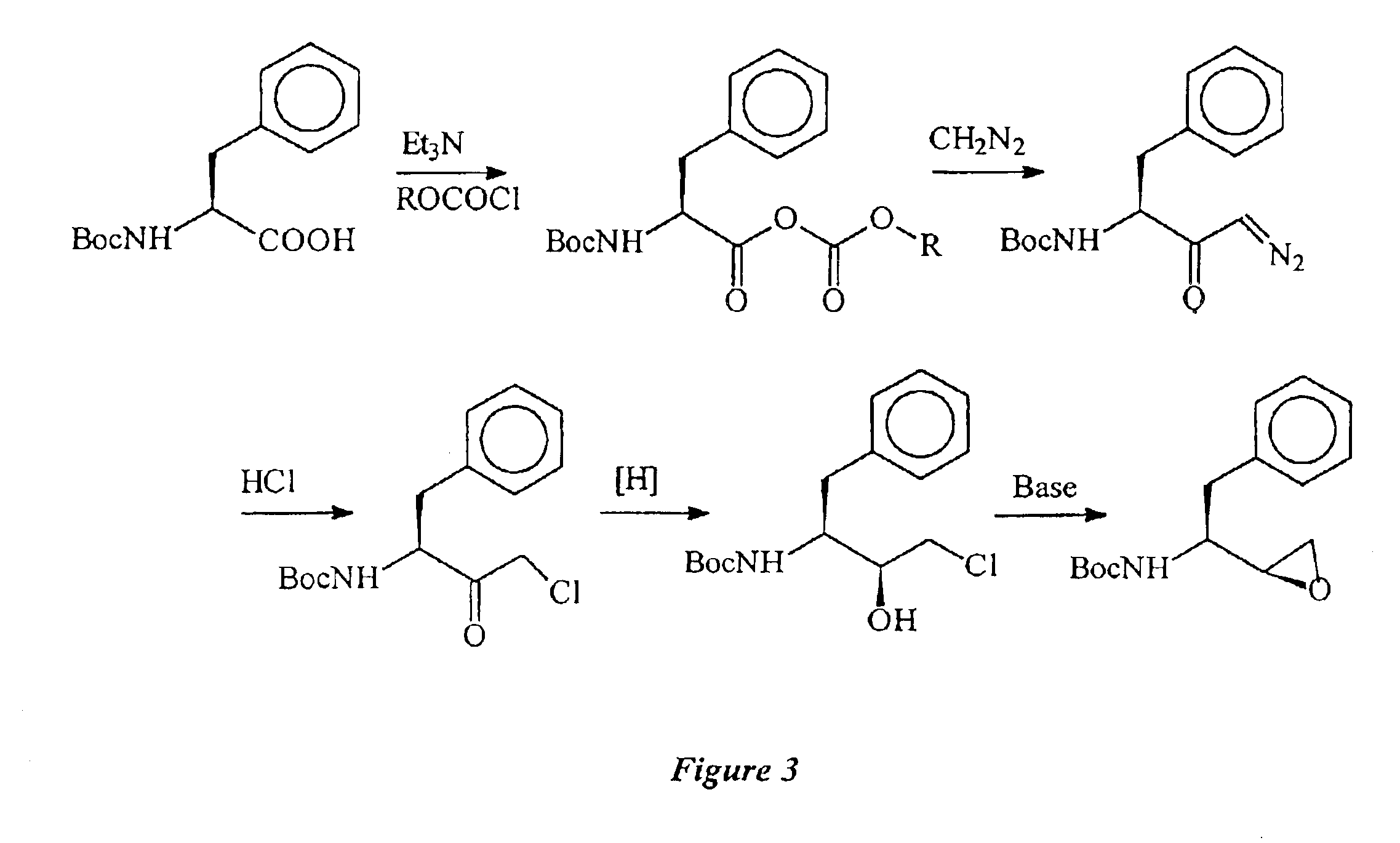
Clean, high-yield preparation of S,S and R,S amino acid isosteres
InactiveUS6867311B2High yieldHigh purityOrganic compound preparationOrganic chemistry methodsKetoneDiastereomer
The present invention provides compounds and methods that can be used to convert the intermediate halomethyl ketones (HMKs), e.g., chloromethyl ketones, to the corresponding S,S- and R,S-diastereomers. More particularly, the present invention provides: (1) reduction methods; (2) inversion methods; and (3) methods involving the epoxidation of alkenes. Using the various methods of the present invention, the R,S-epoxide and the intermediary compounds can be prepared reliably, in high yields and in high purity.
Owner:AMPAC FINE CHEM
11-Beta-Hydroxysteroid Dehydrogenase Inhibitors
InactiveUS20090042929A1Relieve effectIncrease insulin sensitivityBiocideSenses disorderHalogen11-beta-Hydroxysteroid Dehydrogenases
There is provided a compound having Formula (I) R1-Z-R2 wherein R1 is a group selected from optionally substituted fused polycyclic groups, substituted alkyl groups, branched alkyl groups, and optionally substituted cycloalkyl groups Z is a linker which is or comprises a carbonyl group or a isostere of a carbonyl group R2 is selected from optionally substituted aromatic rings and optionally substituted heterocyclic rings wherein (a) R2 is a 2-substituted thiophene group, and / or (b) Z is a group of the formula —C(═O)—CR3R4—X—(CR5R6)n-, wherein X is selected from NR7, S, O, S═O, and S(═O)2, wherein n is 0 or 1 and / or (c) R1 is an adamantyl group and Z is or comprises an amide group, and / or (d) R1 is an adamantyl group and Z is or comprises a group of the formula —(CR8R9)p-NR10—S(═O)2—(CR11R12)q-, wherein p is 0 or 1 and q is 0 or 1 and / or (e) R1 is an adamantyl group and Z is or comprises a group of the formula —(CR13R14)V—Y—(CR15R16)W— where Y is a heteroaryl group in which a bond in the heteroaryl ring is a isostere of a carbonyl group, wherein v is o or 1 and w is 0 or 1; wherein each of R3, R4, R5, R6, R8, R9, R11, R12, R13, R14, R15 and R16, are independently selected from H, hydrocarbyl and halogen, wherein each of R7 and R10 are independently selected from H and hydrocarbyl.
Owner:STRIX LTD
Method of measuring information for adsorption isostere creation, adsorption isostere creation method, adsorption heat calculation method, computer program, and measurement system
InactiveUS20110120301A1Improve accuracyDispersed particle filtrationIsotope separationEngineeringCalculation methods
Information used for creating an adsorption isostere of a substance to be measured in a measuring system (container) is obtained by repeating alternately a first control step of changing temperature or pressure of the measuring system by a fixed amount by adjusting a gas amount supplied to / discharged from the measuring system, and a second control step of changing pressure or temperature of the measuring system by adjusting the gas amount supplied to / discharged from the measuring system until a gas adsorption amount of the substance to be measured becomes the same as before execution of the first control step.
Owner:SHINSHU UNIVERSITY +1
Application of quinoline derivatives of N-isostere tectorigenin in anti-hepatocarcinoma drugs
ActiveCN108685921APrevent proliferationImprove permeabilityOrganic chemistryAntineoplastic agentsIridoid GlucosidesQuinoline
The invention discloses application of quinoline derivatives of N-isostere tectorigenin in anti-hepatocarcinoma drugs. The invention relates to the quinoline derivatives of the N-isostere tectorigenin, and the chemical structural formula of the quinoline derivatives is described in the description. The invention provides the application of the quinoline derivatives (new tectorigenin) of the N-isostere tectorigenin in preparation of drugs for inhibiting hepatocelular carcinoma HepG2 cells. The the quinoline derivatives of the N-isostere tectorigenin inhibit the proliferation of the hepatocelular carcinoma HepG2 cells by inhibiting RAF / MEK / ERK pathway and JAK / STAT pathway. The anti-hepatocarcinoma drugs are remarkable in therapeutic effects, and do not easily produce drug resistance and adverse effects. Furthermore, the invention also discloses a higher-yield preparation method of the quinoline derivatives of the N-isostere tectorigenin; the New Tectorigenin is remarkable in effect of inhibiting transplantation tumors.
Owner:GUANGZHOU UNIVERSITY
N-linked sulfonamides of N-heterocyclic carboxylic acids or carboxylic acid isosteres
This invention relates to novel N-linked sulfonamides of N-heterocyclic carboxylic acid and carboxylic acid isosteres, their preparation, and use for treating neurological disorders including physically damaged nerves and neurodegenerative diseases, and for treating alopecia and promoting hair growth.
Owner:AMGEN INC +1
Disaccharides for drug discovery
Methods are described for the preparation of combinatorial libraries of potentially biologically active disaccharide compounds. These compounds are variously functionalized, with a view to varying lipid solubility size, function an other properties, with the particular aim of discovering novel drug or drug-like compounds, or compounds with useful properties. The invention provides intermediates, processes and synthetic strategies for the solution or solid phase synthesis of disaccharides, variously functionalized about the sugar ring, including the addition of aromaticity and charge, and the placement of pharmaceutically useful groups and isosteres.
Owner:VAST BIOSCI PTY
Method of measuring information for adsorption isostere creation, adsorption isostere creation method, adsorption heat calculation method, computer program, and measurement system
InactiveUS8480786B2Improve accuracyDispersed particle filtrationIsotope separationEngineeringCalculation methods
Owner:SHINSHU UNIVERSITY +1
Compounds for use as GPR120 agonists
The present invention relates to a compound of formula (I), or a tautomer, stereoisomer, geometrical isomer, prodrug, carboxylic acid isostere, solvate, polymorph, N-oxide, S-oxide or pharmaceutically acceptable salt thereof, which are GPR120 agonists. The present invention also relates to a pharmaceutical composition of a compound of formula (I) for the treatment of metabolic disorders, particularly Type 2 diabetes and associated diseases.
Owner:PIRAMAL ENTERPRISES LTD
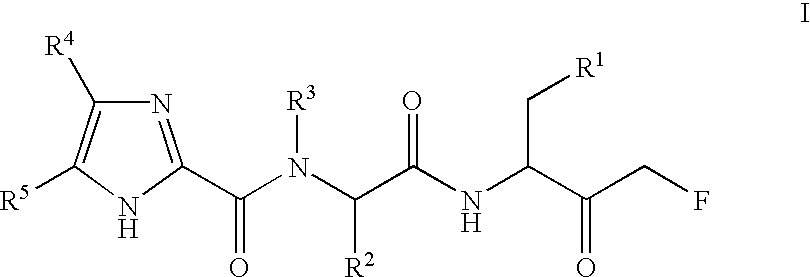
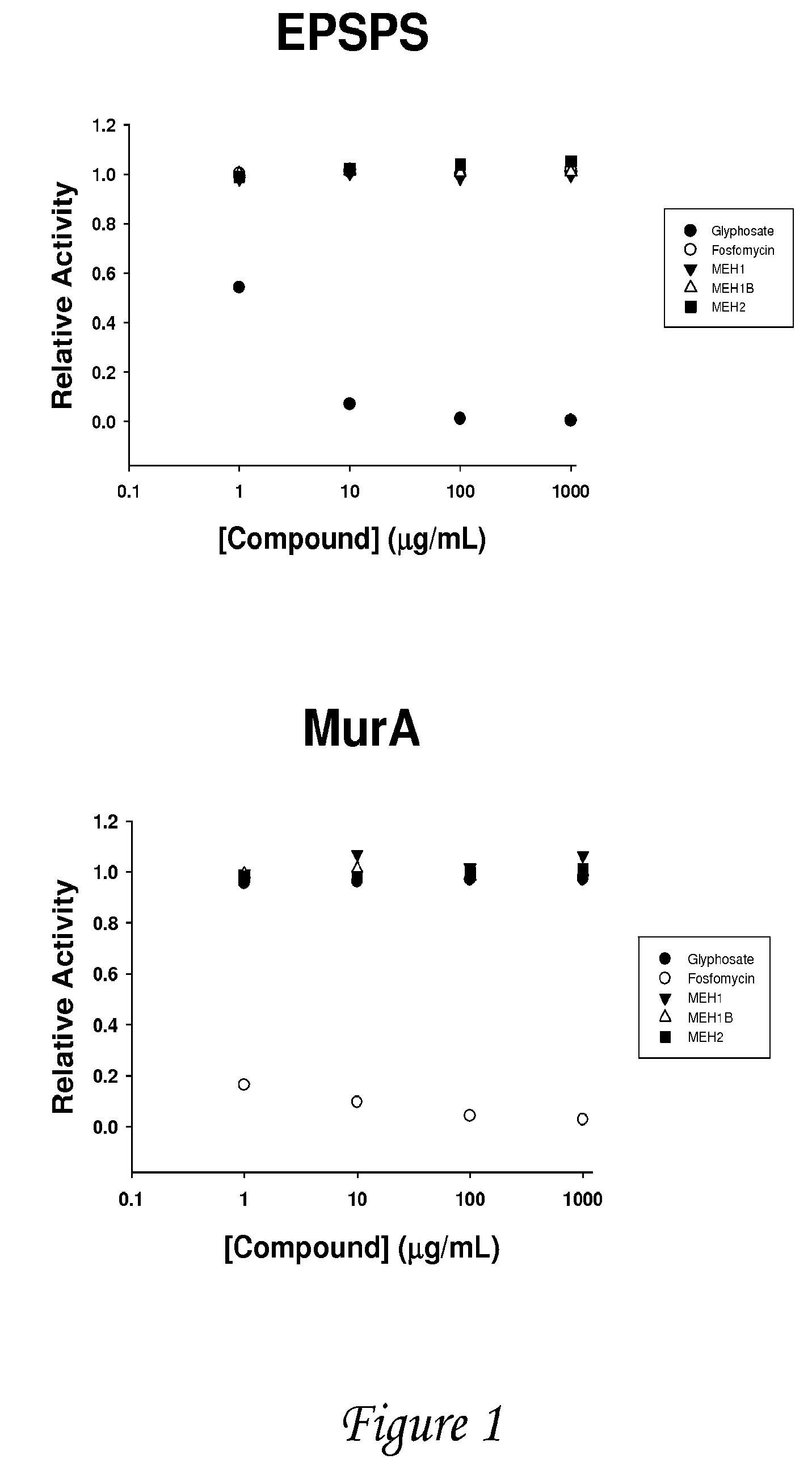
Multiple substituted fluoromethanes as selective and bioactive isosteres
Disclosed herein are substituted fluoromethanes; pharmaceutical compositions comprising a therapeutically effective amount of the same; processes for preparing these fluoromethanes; and methods of using the same in alleviating infection and parasitism. Also disclosed are methods for identifying substituted fluoromethanes for modulating the activity of receptors and enzymes that bind and / or modify phosphate containing ligands and substrates.
Owner:MEH ASSOC
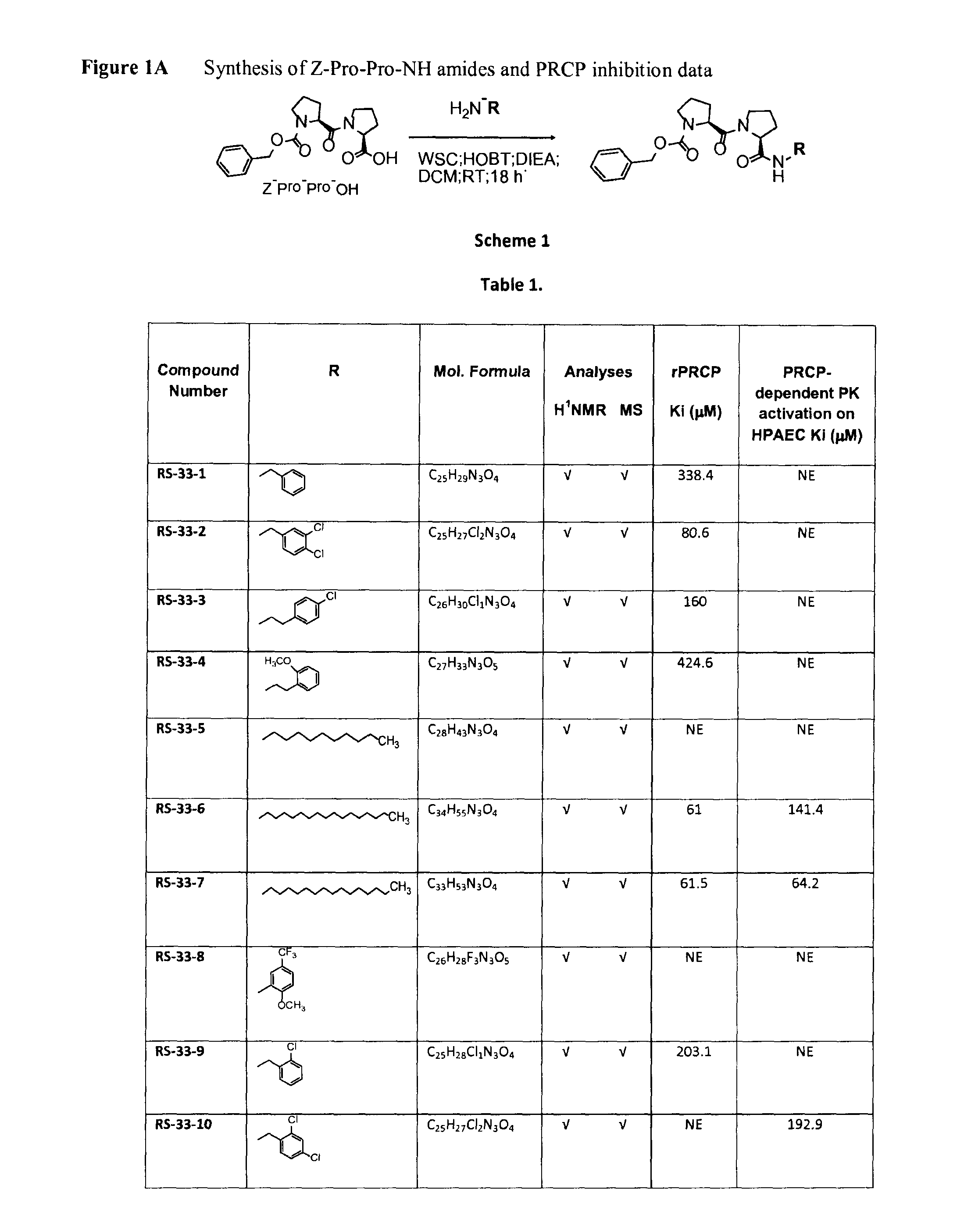
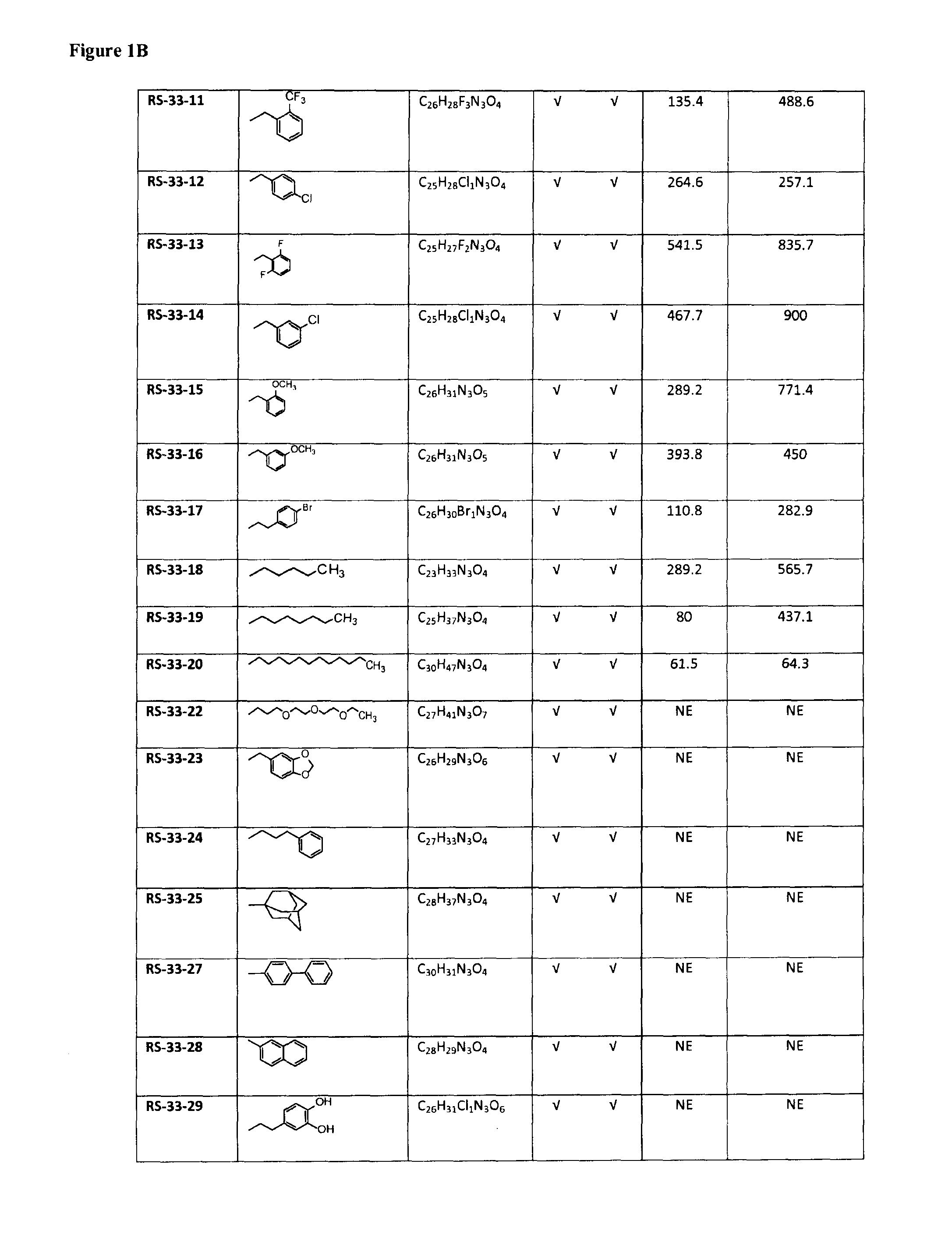
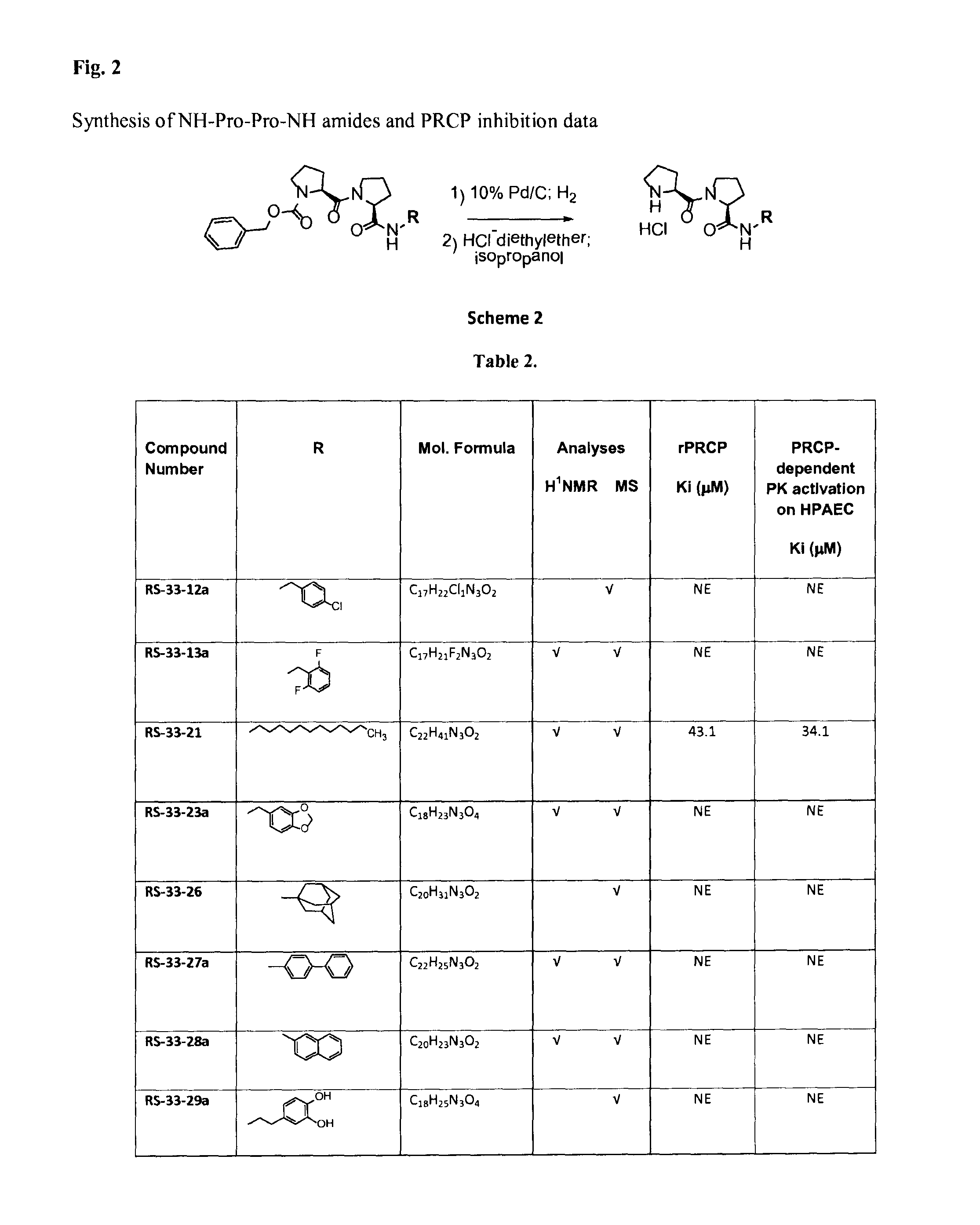
Selective inhibitors of prolylcarboxypeptidase
ActiveUS9193762B2Good choiceMaintain weightDipeptide ingredientsAntipyreticProlyl carboxypeptidaseChemistry
The present invention relates to compounds of the formulae:in which R is C5-C16 alkyl, R1 isand isosteres and salts thereof.
Owner:UNIVERSITY OF MISSISSIPPI
Carbamate aspartic acid specific cysteine proteinase inhibitors and uses thereof
InactiveCN1994298AGood cell penetrationImprove pharmacokinetic propertiesAntibacterial agentsSenses disorderLeaving groupInhibitor of apoptosis
This invention provides caspase inhibitors of formula (I): wherein Z is oxygen or sulfur; R<1> is hydrogen, -CHN2,R, CH2OR, CH2SR, or -CH2Y; Y is an electronegative leaving group; R<2> is CO2H, CH2CO2H, or esters, amides or isosteres thereof; R<3> is a group capable of fitting into the S2 subsite of a caspase enzyme; R<4> and R<5> are taken together with the intervening nitrogen to form heterocyclic ring and R is as described in the specification. The compounds are effective inhibitors of apoptosis and IL-1 beta secretion.
Owner:VERTEX PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com