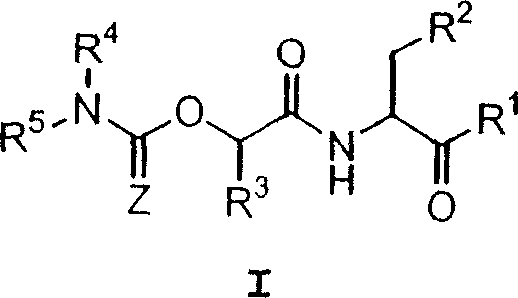
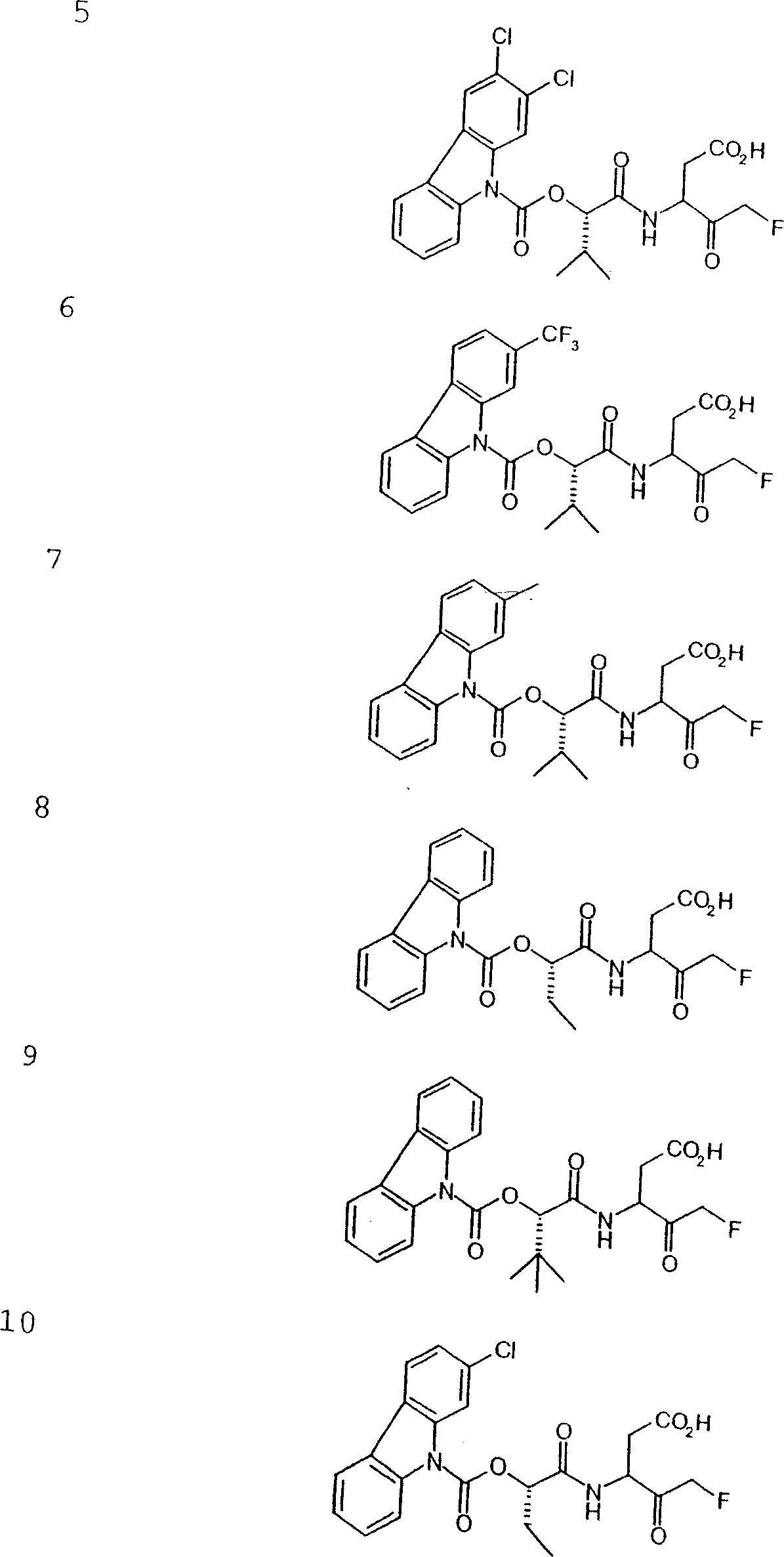
Carbamate aspartic acid specific cysteine proteinase inhibitors and uses thereof
A cysteine protease, specific technology, applied in the direction of non-central analgesics, anti-inflammatory agents, antiviral agents, etc., can solve the problems of poor oral absorption, rapid metabolism, poor stability, etc., and achieve good cell penetration sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
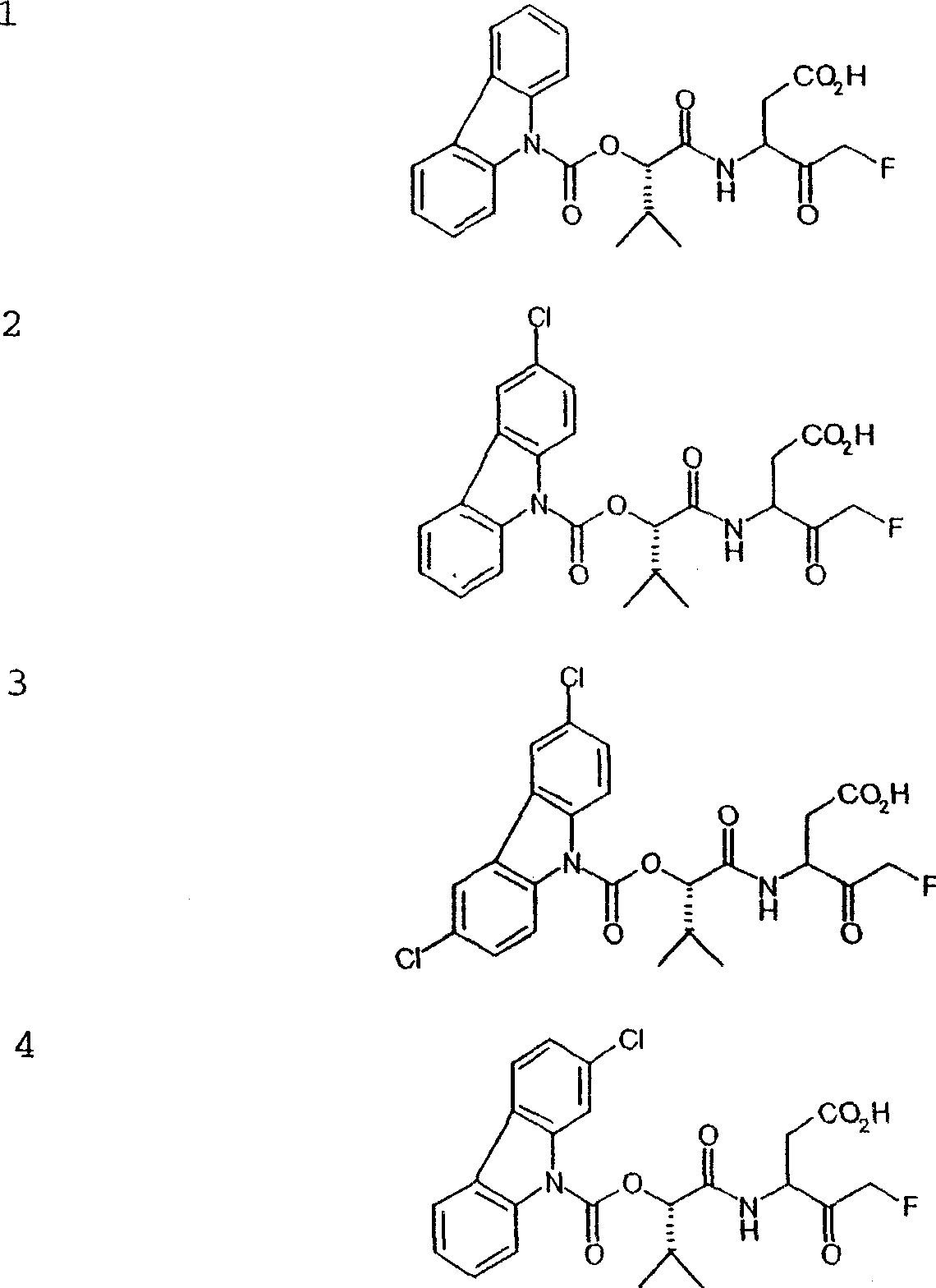
[0138] [3S / R]-5-fluoro-4-oxo-3-[(S)-3-methyl-2-(carbazole-carbamoyloxy-butane Amido)-pentanoic acid
[0139]
[0140] Method A:
[0141] (S)-2-(Chlorocarbamoyloxy)-3-methylbutanoic acid, tert-butyl ester
[0142]
[0143] To a solution of diphosgene (4.55 g) in THF (34 ml) was added dropwise (S)-tert-butyl 2-hydroxy-3-methylbutyrate (for the preparation see Tetrahedron. Lett. (Tetrahedron Letters, (1993), 7409) (4.0 g) and pyridine (1.82 g) in THF (34 ml).
[0144] The resulting mixture was allowed to warm to room temperature over 4 hours. The mixture was then filtered through celite and the filtrate was concentrated under reduced pressure. The residue was redissolved in diethyl ether (200ml) and filtered again through celite. The filtrate was concentrated under reduced pressure to afford the subtitle compound as a pale yellow oil (5.27 g): 1 HNMR (400MHz, CDCl 3 )δ0.98-1.10(6H, m), 1.55(9H, s), 2.30(1H, m), 4.83(1H, m).
[0145] Method B:
[0146] (S...
Embodiment 1A
[0162] [3S / R]-5-fluoro-4-oxo-3-[(S)-3-methyl-2-(carbazole)-carbamoyloxy- Butyrylamino]-valeric acid
[0163]
[0164] It was prepared using a method similar to Method C above. The product was isolated as a white solid (88% last step): IR (solid) 1721.2, 1695.6, 1664.9, 1449.8, 1378.1, 1198.9, 1040.1, 758.5 cm -1 ; 1 H NMR (400MHz, d 6 -DMSO) δ1.10 (6H, brm), 2.41 (1H, m), 2.54-3.04 (2H, m), 4.31-4.82 (1.6H, m, CH2F), 5.10-5.41 (2.4H, m), 7.45(2H, m), 7.57(2H, m), 8.22(2H, m), 8.30(2H, m), 8.51-8.99(1H, brm), 12.60(1H, brs); 13 C NMR (100MHz, d 6 -DMSO) δ19.0, 19.1, 19.3, 30.4, 30.5, 30.6, 32.9, 34.5, 34.7, 47.3, 47.4, 52.0, 52.3, 80.4, 80.8, 83.2, 83.4, 83.4, 85.1, 85.2, 116.2, 116.3, 124.1 , 125.7, 125.9, 137.9, 151.7, 151.9, 152.0, 168.8, 169.0, 169.2, 172.0, 172.1, 173.1, 173.2, 202.2, 202.4, 202.5, 202.6; 19 F NMR (376MHz, d 6 -DMSO) -226.6(t), -226.8(t), -230.5(t), -230.9(t), -232.9(t), -233.0(t); MS(ESI+ve) 443(M+H ).
Embodiment 2
[0166] [3S / R]-5-fluoro-4-oxo-3-[(S)-3-methyl-2-(3-chlorocarbazole)-carbamoyloxy yl-butyrylamino]-pentanoic acid
[0167]
[0168]It was prepared using methods similar to Methods A-E above. The product was isolated as a white solid (99% last step): IR (solid) 1721.2, 1690.5, 1664.9, 1444.7, 1367.9, 1209.1, 1040.1 cm -1 ; 1 H NMR (400MHz, d 6 -DMSO) δ1.02-1.13 (6H, m), 2.40 (1H, m), 2.50-2.99 (2H, m), 4.30-4.85 (1.6H, m), 5.09-5.48 (2.4H, m), 7.48(1H, m), 7.56-7.66(2H, m), 8.20-8.32(3H, m), 8.39(1H, m), 8.55-8.99(1H, brm), 12.5(1H, br); 13 CNMR (100MHz, d 6 -DMSO) δ18.1, 18.9, 19.1, 30.4, 30.5, 33.0, 34.5, 34.7, 47.4, 52.0, 52.3, 80.6, 80.9, 81.1, 83.4, 83.43, 85.1, 85.2, 103.8, 104.0, 117.0, 119.3, 121.3 ( ); 19 F NMR (376MHz, d 6 -DMSO) -226.6(t), -226.8(t) -230.4(t), -230.9(t), -231.0(t), -232.8(t), -232.84(t), -232.9(t); MS(ESI+ve)477(M+H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com