Fluorescent switch system, preparation method and application
A fluorescent switch and system technology, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of cumbersome preparation of fluorescent switch systems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
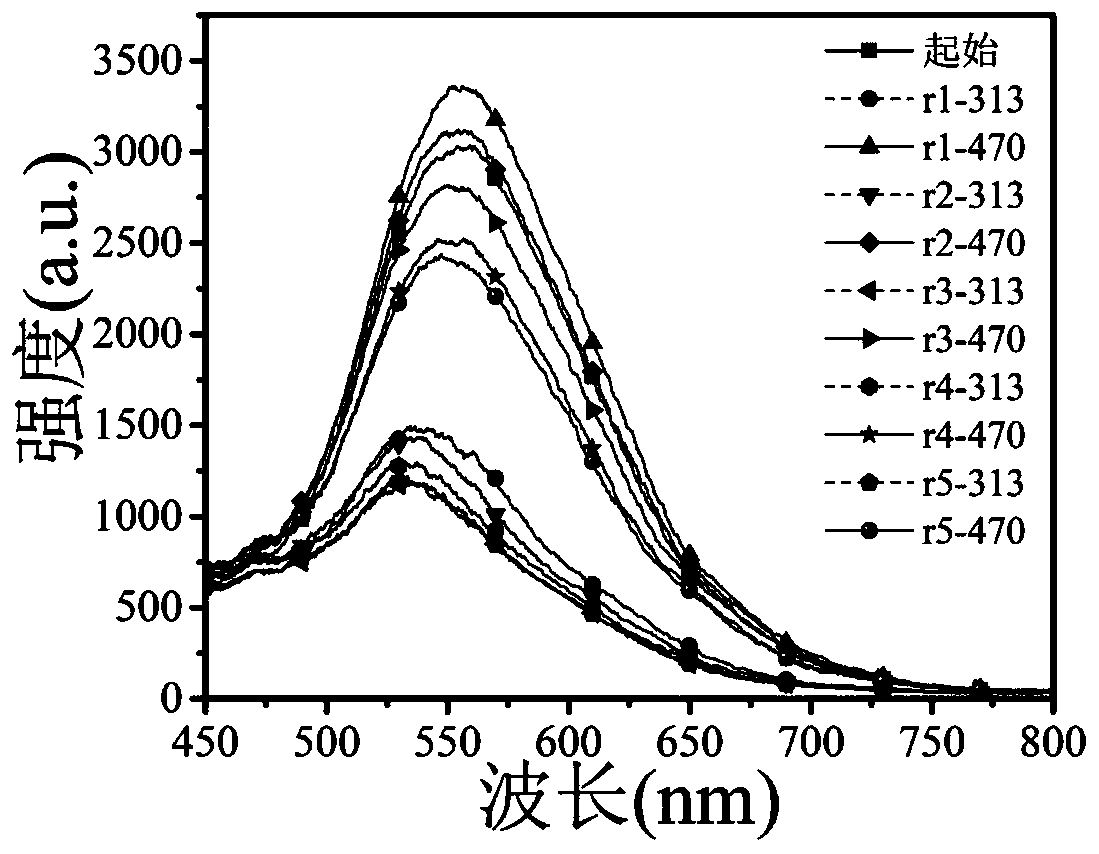
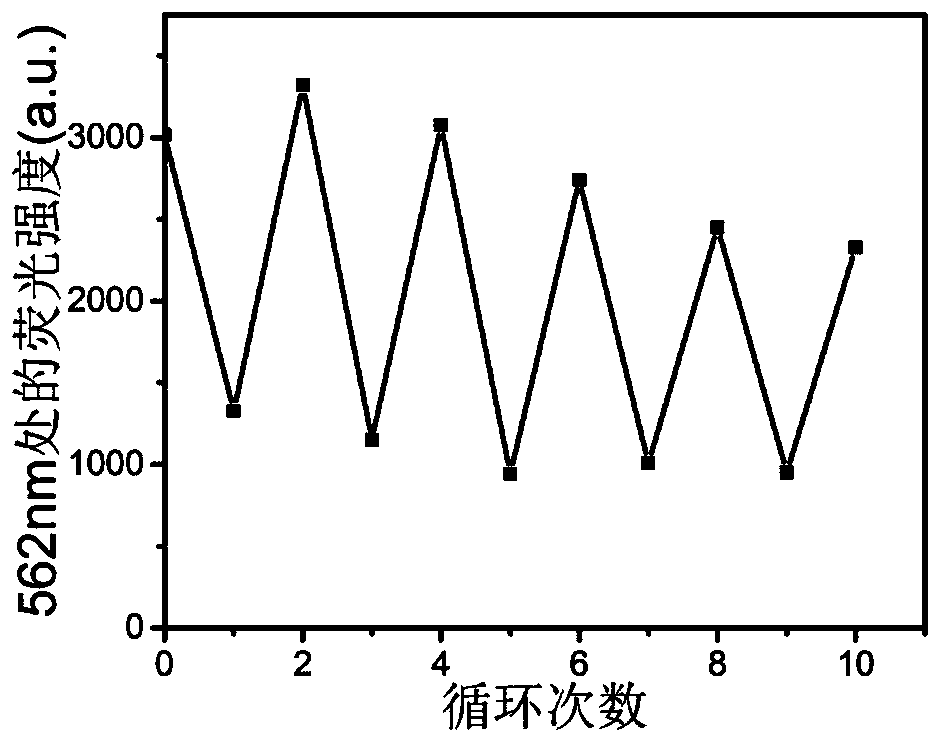
[0062] A formula (I 1 ) / (II) poly 3-(2-(6-bromotrimethylaminohexyloxy)ethyl)thiophene / 1,2-bis(5-p-carboxyphenyl-2-methylthiophene- 3-yl) sodium perfluorocyclopentene fluorescent switch system.
[0063] Its preparation method comprises the following steps:
[0064] (1) poly 3-(2-(6-bromotrimethylaminohexyloxy) ethyl) thiophene (structural formula such as formula (Ⅰ) 1 ) Synthesis shown in ).
[0065] m is 100
[0066] 3-Hydroxyethanethiophene (663μL, 6mmol) was added to the DMF solution of NaH (206mg, 6mmol) under the protection of nitrogen. After stirring for 30min, 1,6-dibromohexane (4.6 mL, 10mmol). After stirring for 12 h, it was quenched with water. The resulting mixture was treated with CH 2 Cl 2 Extracted 3 times, the organic phase was washed with brine, and washed with MgSO 4 dry. After removing the solvent, the product was eluted in a silica gel column with petroleum ether and ethyl acetate at a volume ratio of 40:1 to obtain 3-(2-(6-bromohexyloxy)ethyl)thi...
Embodiment 2
[0081] A formula (I 2 ) / (II) poly 3-(4-bromotrimethylaminobutyl)thiophene / 1,2-bis(5-p-carboxyphenyl-2-methylthiophen-3-yl)perfluorocyclic ring Pentenyl sodium fluorescent switch system.
[0082] Its preparation method comprises the following steps:
[0083] (1) poly 3-(2-(6-bromotrimethylaminobutyl))thiophene (structural formula such as formula (Ⅰ) 2 ) Synthesis shown in ).
[0084] m=100
[0085] It differs from Example 1 in that:
[0086] Thiophene (208.0mg, 2.5mmol), Pd(OAc) 2 (28.0mg, 0.125mmol), 1,4-dibromobutane (10.0mmol), potassium carbonate (7.5mmol, 3eq), pivalic acid (5.1mg, 0.05mmol) were added to 3.0mL of anhydrous tert-amyl alcohol, After being filled with nitrogen, it was heated to 100°C and stirred for 24h. Afterwards, the solvent was removed by rotary evaporation, and 3-(4-bromobutyl)thiophene (248.0 mg, 45%) was purified through a silica gel column with ethyl acetate / hexane=10:90 (v:v) as the eluent. 1 HNMR (400 MHz, DMSO-d6, ppm): 7.59 (s, 1H), 6.7...
Embodiment 3
[0091] A formula (I 3 ) / (II) poly 3-(2-(6-bromotrimethylaminoethoxy)ethyl)thiophene / 1,2-bis(5-p-carboxyphenyl-2-methylthiophene- 3-yl) sodium perfluorocyclopentene fluorescent switch system.
[0092] Its preparation method comprises the following steps:
[0093] (1) Preparation of poly 3-(2-(6-bromotrimethylaminoethoxy)ethyl)thiophene.
[0094] m is 150
[0095] It differs from Example 1 in that:
[0096] 3-Hydroxyethanethiophene (663μL, 6mmol) was added to the DMF solution of NaH (206mg, 6mmol) under the protection of nitrogen, and after stirring for 30min, 1,2-dibromoethane (1.5 mL, 10mmol). After stirring for 12 h, it was quenched with water. The resulting mixture was treated with CH 2 Cl 2 Extracted 3 times, the organic phase was washed with brine, and washed with MgSO 4 dry. After removing the solvent, the product was eluted in a silica gel column with petroleum ether and ethyl acetate at a volume ratio of 40:1 to obtain 3-(2-(2-bromoethoxy)ethyl)thiophene (53...
PUM
| Property | Measurement | Unit |
|---|---|---|
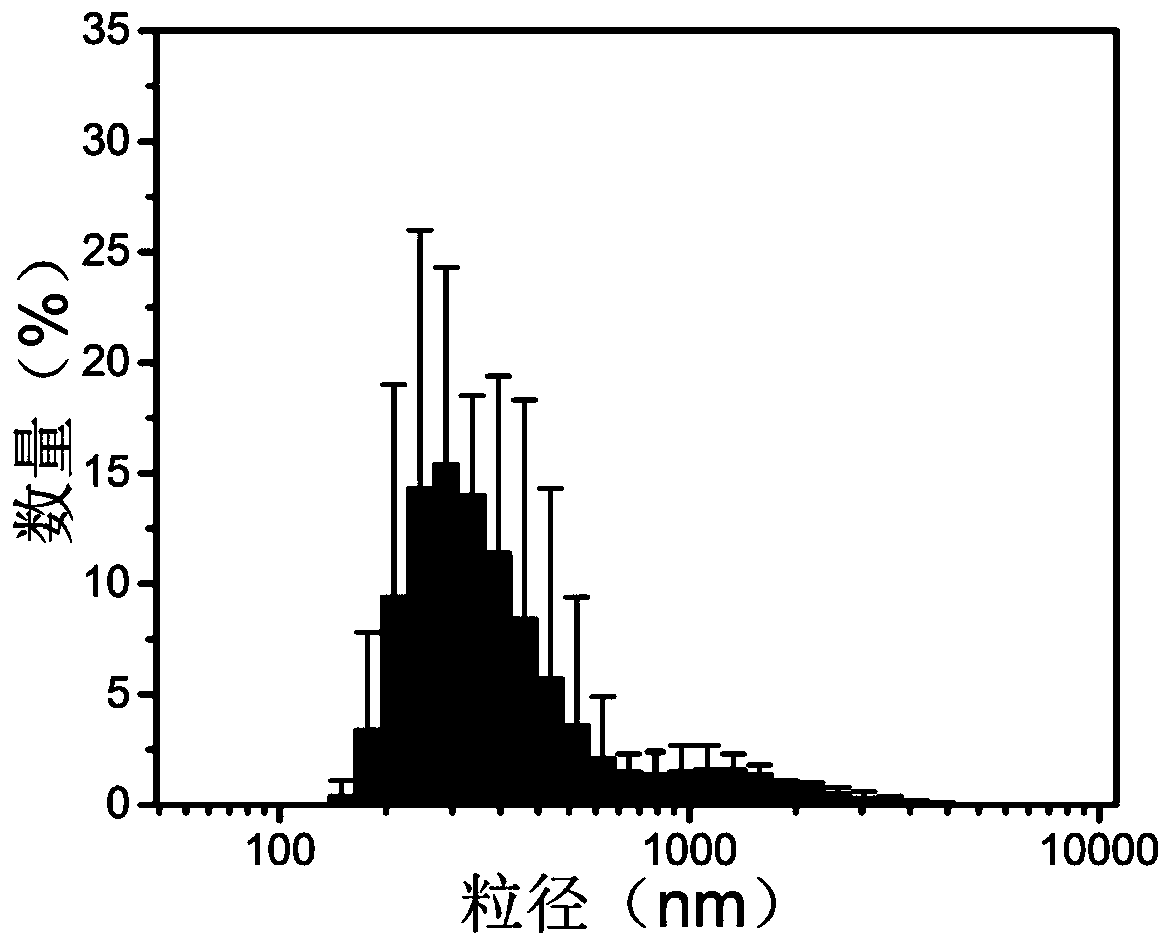
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com