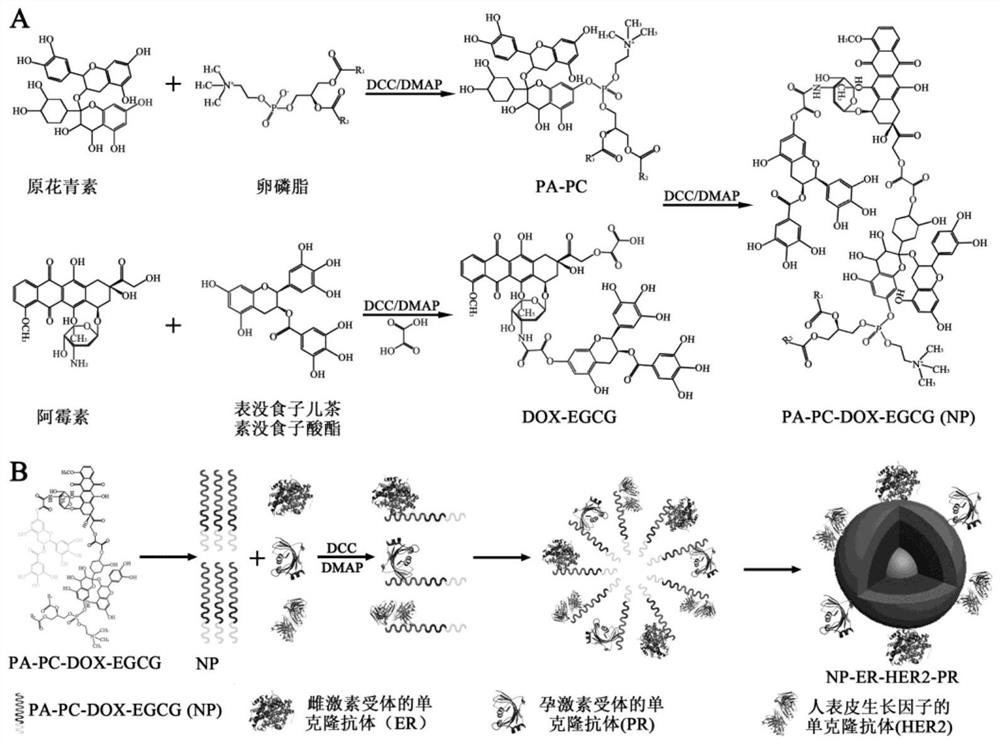
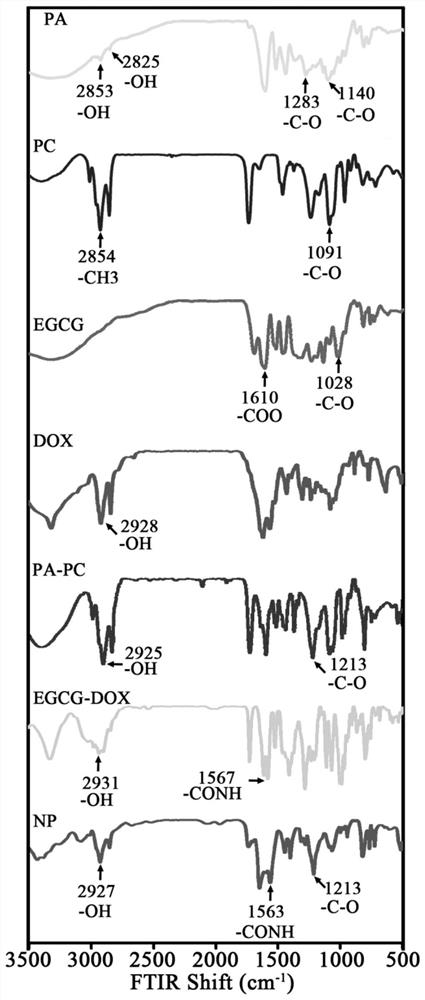
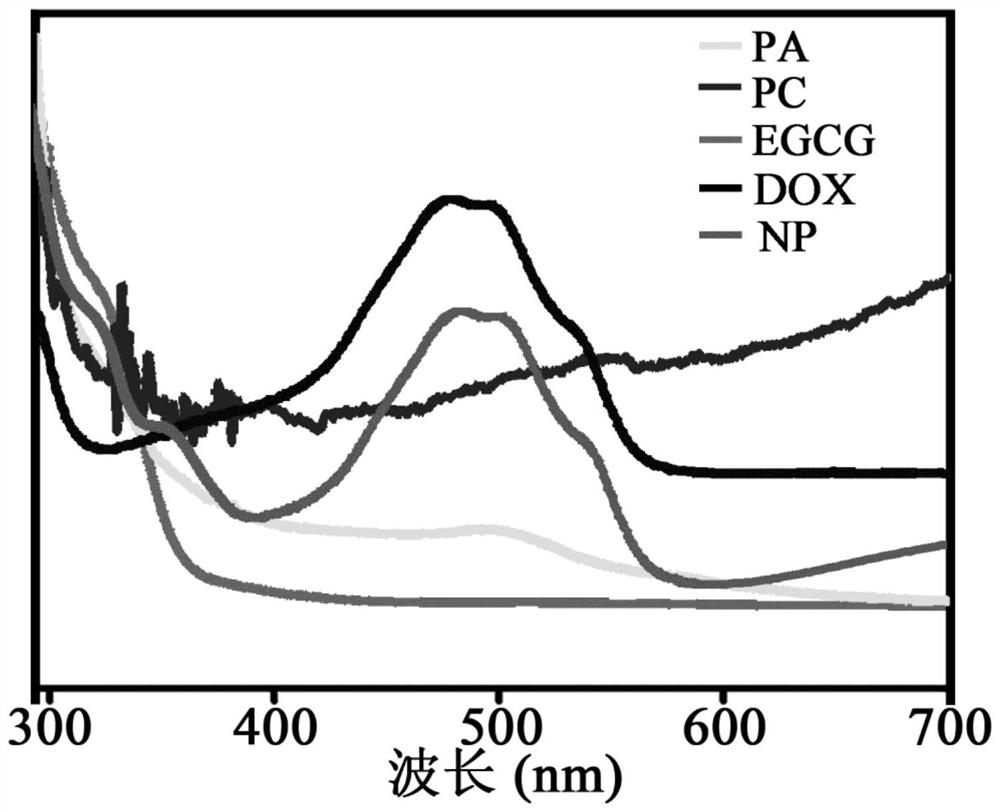
A targeted nanoparticle for inhibiting breast cancer and its preparation and application
A nanoparticle, breast cancer technology, applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, drug combinations, etc., can solve the drug resistance of tumor cell heterogeneity chemotherapy drugs and affect the treatment effect of patients Prognosis and other issues, to achieve the effect of reversing the drug resistance mechanism and enhancing the therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1 Preparation of a targeted nanoparticle for inhibiting breast cancer
[0068] 1. Preparation method
[0069] (1) Carboxylation of doxorubicin:
[0070] Measure 4 ml of dimethylformamide (DMF), add to a beaker, weigh 10 mg of dicyclohexylcarbodiimide (DCC), 1 mg of 4-dimethylaminopyridine (DMAP), 5 mg of Oxalic acid, mixed and stirred for 30 min, added 500 μl 1mg / ml doxorubicin (DOX), stirred at room temperature with a magnetic stirrer for 3 h, collected the reaction solution into a centrifuge tube, centrifuged at 5000 rpm for 10 min, collected the supernatant, added 5 ~7 times the volume of water, shake well, add 2 volumes of ethyl acetate, extract 3 times, collect the ethyl acetate layer, use a dialysis bag for 12 h dialysis, and freeze-dry to obtain orange crystals, which are carboxylated albino Mycin.
[0071] (2) Esterification reaction of proanthocyanidins and lecithin
[0072] Measure 4 ml of DMF, add it into a beaker, weigh 10 mg of DCC, 1 mg of DMAP...
Embodiment 2
[0081] Example 2 Preparation of a targeted nanoparticle for inhibiting breast cancer
[0082] 1. Preparation method
[0083] (1) Carboxylation of doxorubicin:
[0084] Measure 3 ml of dimethylformamide (DMF), add to a beaker, weigh 9 mg of dicyclohexylcarbodiimide (DCC), 0.9 mg of 4-dimethylaminopyridine (DMAP), 4.5 mg of Oxalic acid, mixed and stirred for 30 min, added 450 μl 1mg / ml doxorubicin (DOX), stirred at room temperature with a magnetic stirrer for 3 h, collected the reaction liquid into a centrifuge tube, centrifuged at 5000 rpm for 10 min, collected the supernatant, added 5- 7 times the volume of water, shake well, add 2 volumes of ethyl acetate, extract 3 times, collect the ethyl acetate layer, use a dialysis bag for 12 hours of dialysis, and freeze-dry to obtain orange crystals, which are carboxylated doxorubicin white.
[0085] (2) Esterification reaction of proanthocyanidins and lecithin
[0086] Measure 3 ml of DMF, add it into a beaker, weigh 9 mg of DCC, ...
Embodiment 3
[0095] Example 3 Preparation of a targeted nanoparticle for inhibiting breast cancer
[0096] 1. Preparation method
[0097] (1) Carboxylation of doxorubicin:
[0098] Measure 5 ml of dimethylformamide (DMF), add to a beaker, weigh 11 mg of dicyclohexylcarbodiimide (DCC), 1.1 mg of 4-dimethylaminopyridine (DMAP), 5.5 mg of Oxalic acid, mixed and stirred for 30 min, added 550 μl 1mg / ml doxorubicin (DOX), stirred at room temperature with a magnetic stirrer for 3 h, collected the reaction liquid into a centrifuge tube, centrifuged at 5000 rpm for 10 min, collected the supernatant, added 5- 7 times the volume of water, shake well, add 2 volumes of ethyl acetate, extract 3 times, collect the ethyl acetate layer, use a dialysis bag for 12 hours of dialysis, and freeze-dry to obtain orange crystals, which are carboxylated doxorubicin white.
[0099] (2) Esterification reaction of proanthocyanidins and lecithin
[0100] Measure 5 ml of DMF, add it into a beaker, weigh 11 mg of DCC...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com