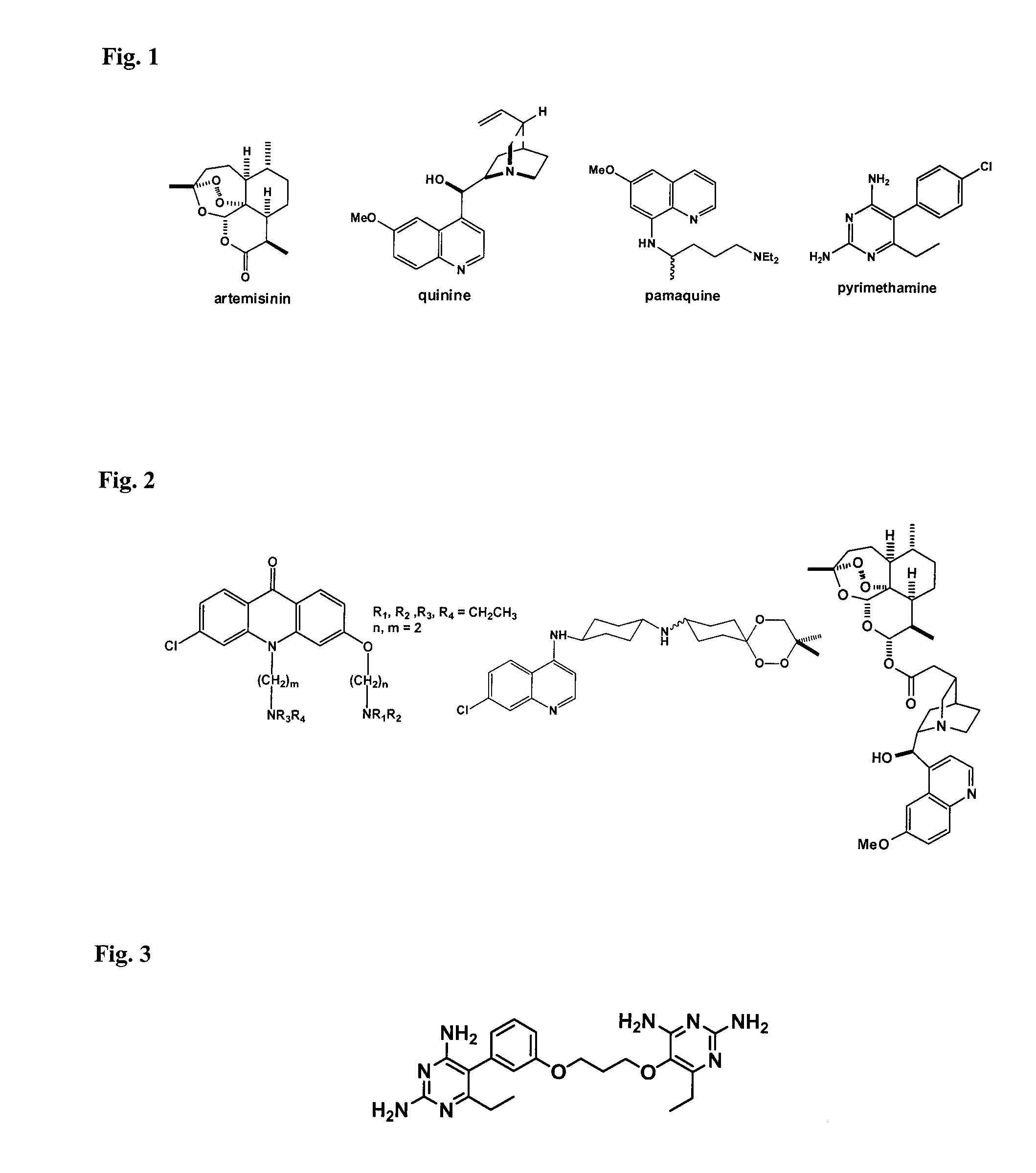
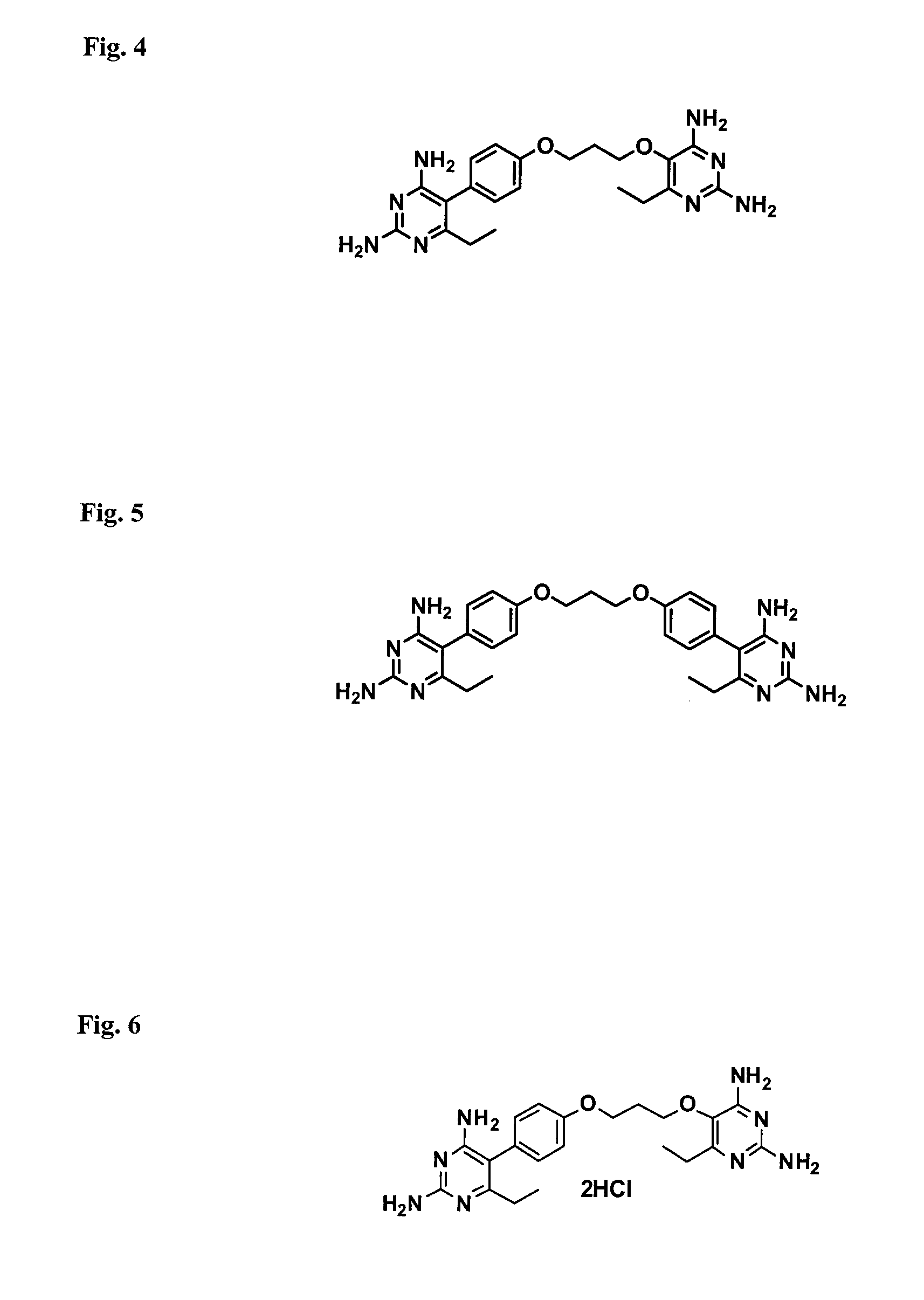
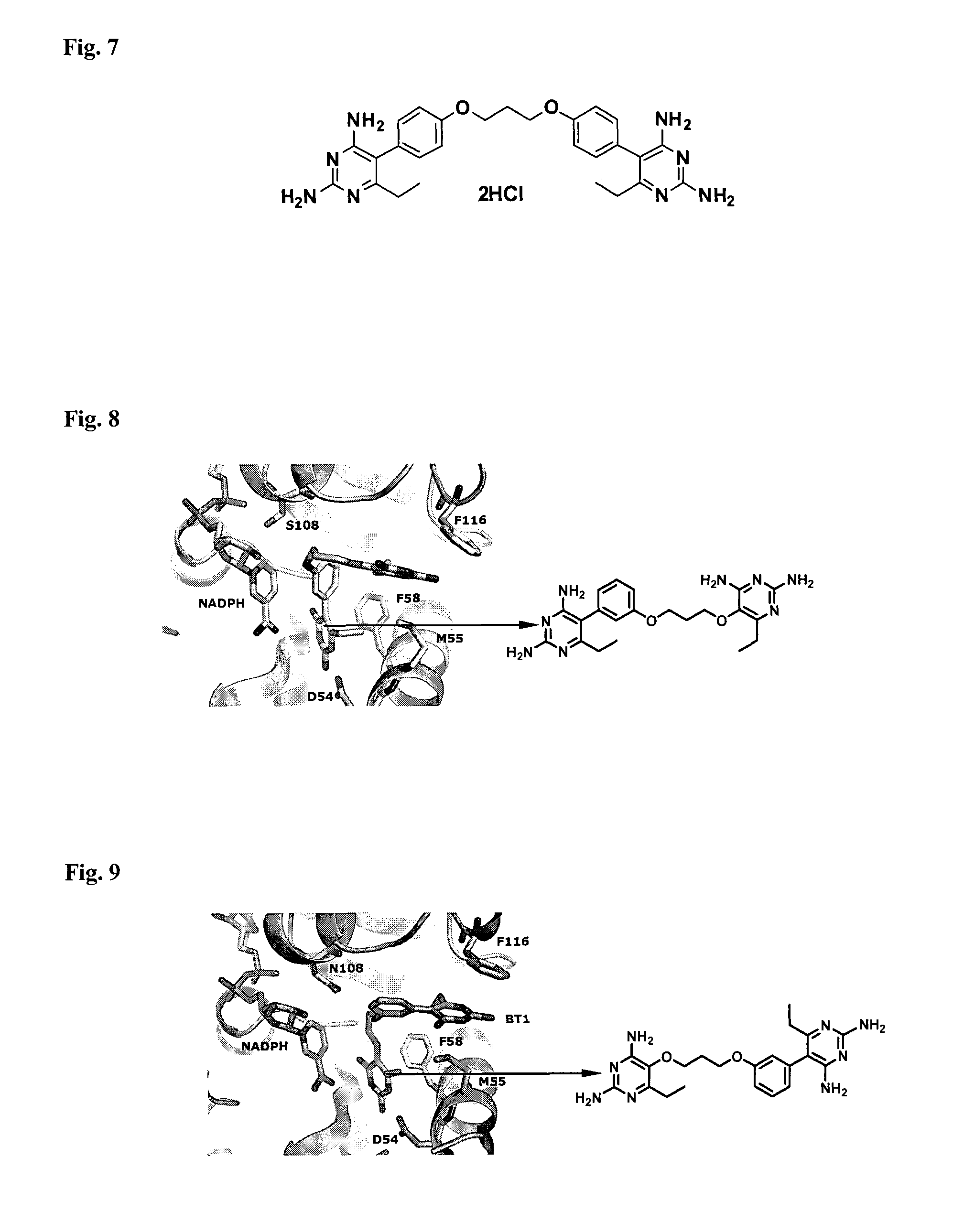
Patents
Literature
31 results about "Inhibition constant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Application of berberine and derivatives thereof in preparation of indole amine 2, 3-dioxygenase inhibitor
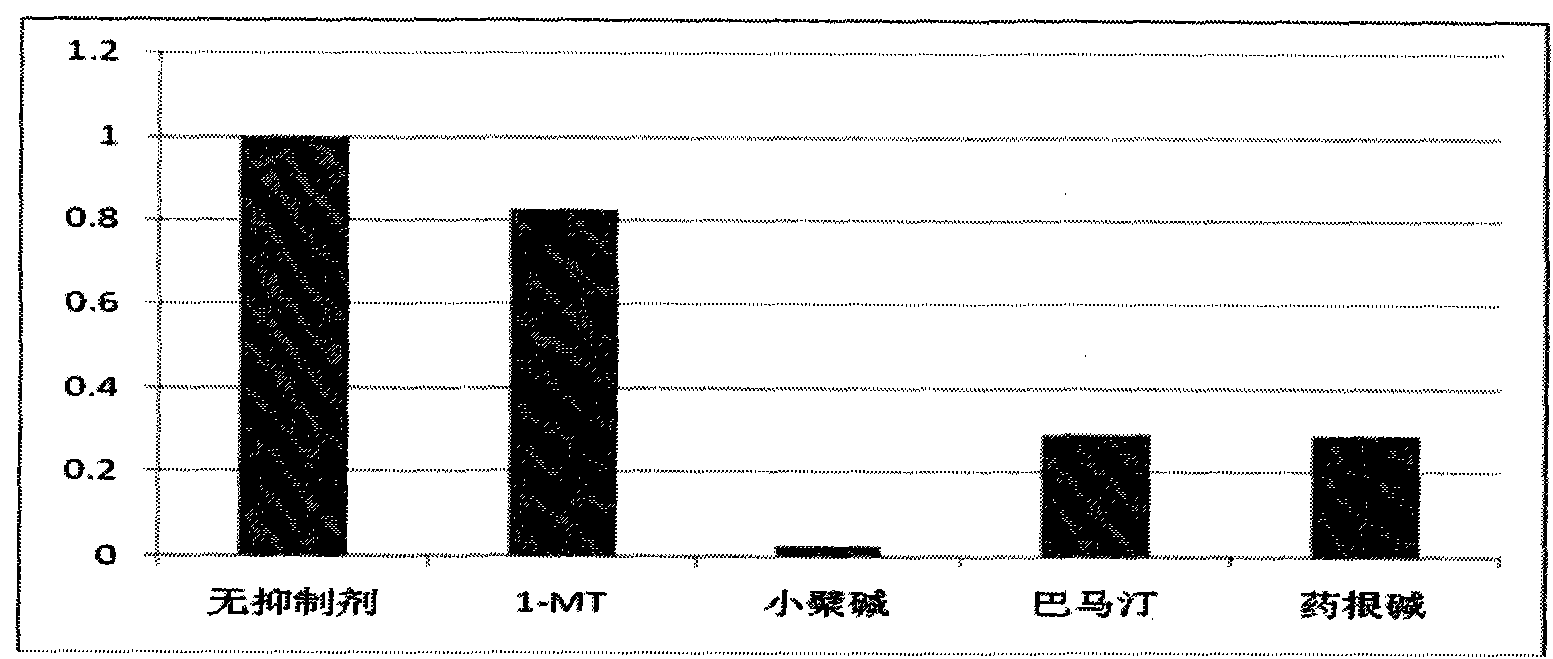
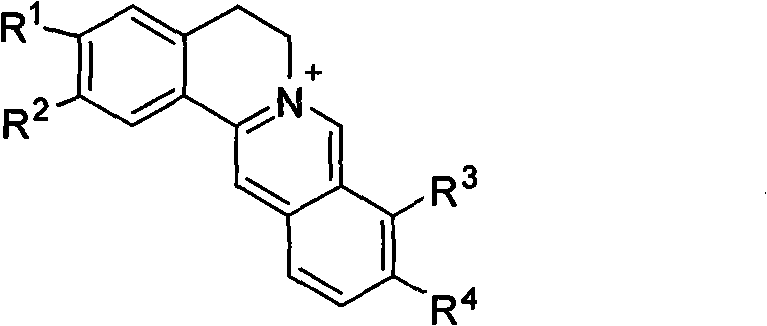
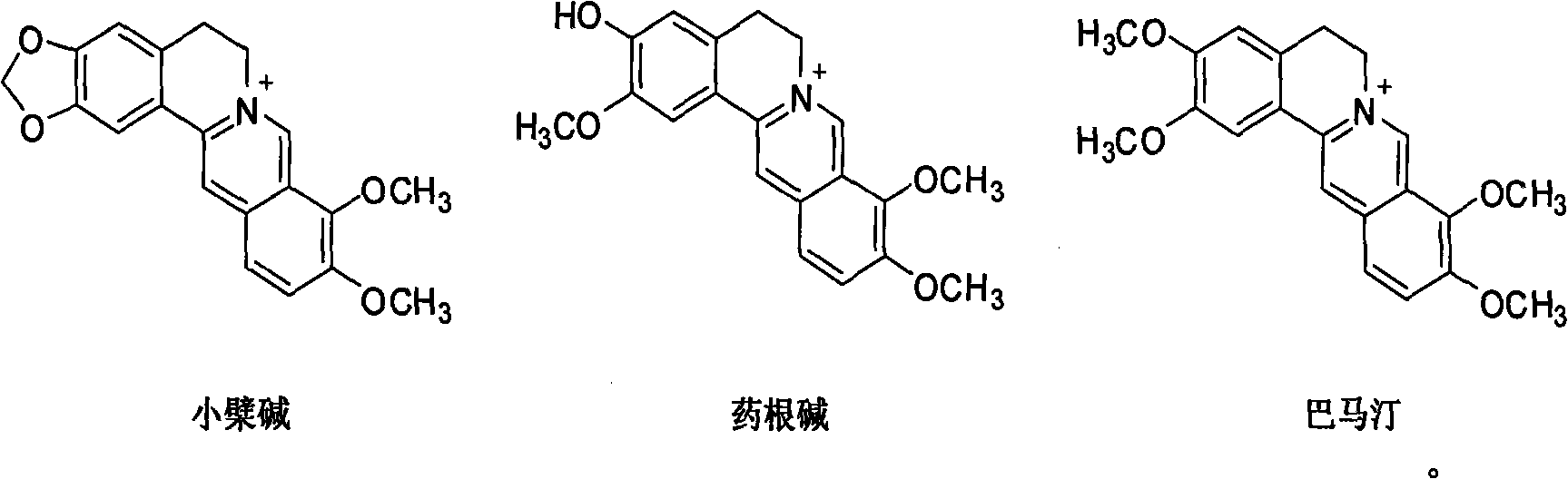
The invention relates to novel application of berberine and derivatives thereof in preparation of medicines, in particular to application of berberine and derivatives thereof in preparation of indole amine 2, 3-dioxygenase IDO inhibitor, belonging to the medicine field. According to IDO inhibition activity detection, reversible inhibition judgment, inhibitor type judgment, Ki value determination and median effective inhibition concentration IC50 determination, the results show that berberine is reversible inhibitor and inhibition constant Ki is 8muM; and jatrorrhizine hydrochloride and palmatine hydrochloride are irreversible inhibitors, and the median effective inhibition concentrations IC50 thereof are respectively 123muM and 126muM. When the berberine and the derivatives thereof disclosed in the invention are used as IDO inhibitors, the application prospect is wide and the IDO inhibitors can be used for treating serious diseases such as cancers, AIDS, Alzheimer diseases, tristimania, cataract and the like with pathological feature of IDO-mediated tryptophan metabolism.
Owner:FUDAN UNIV
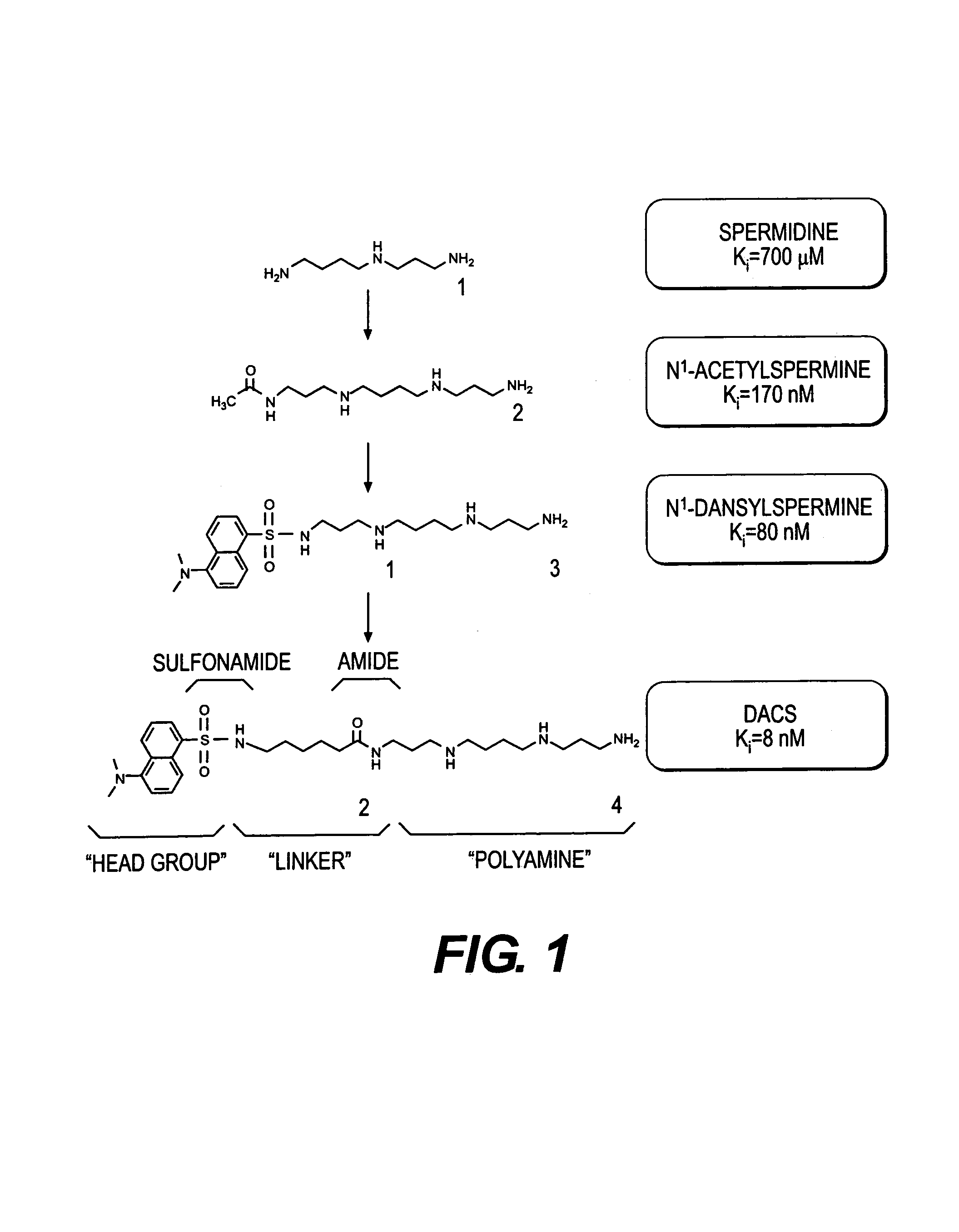
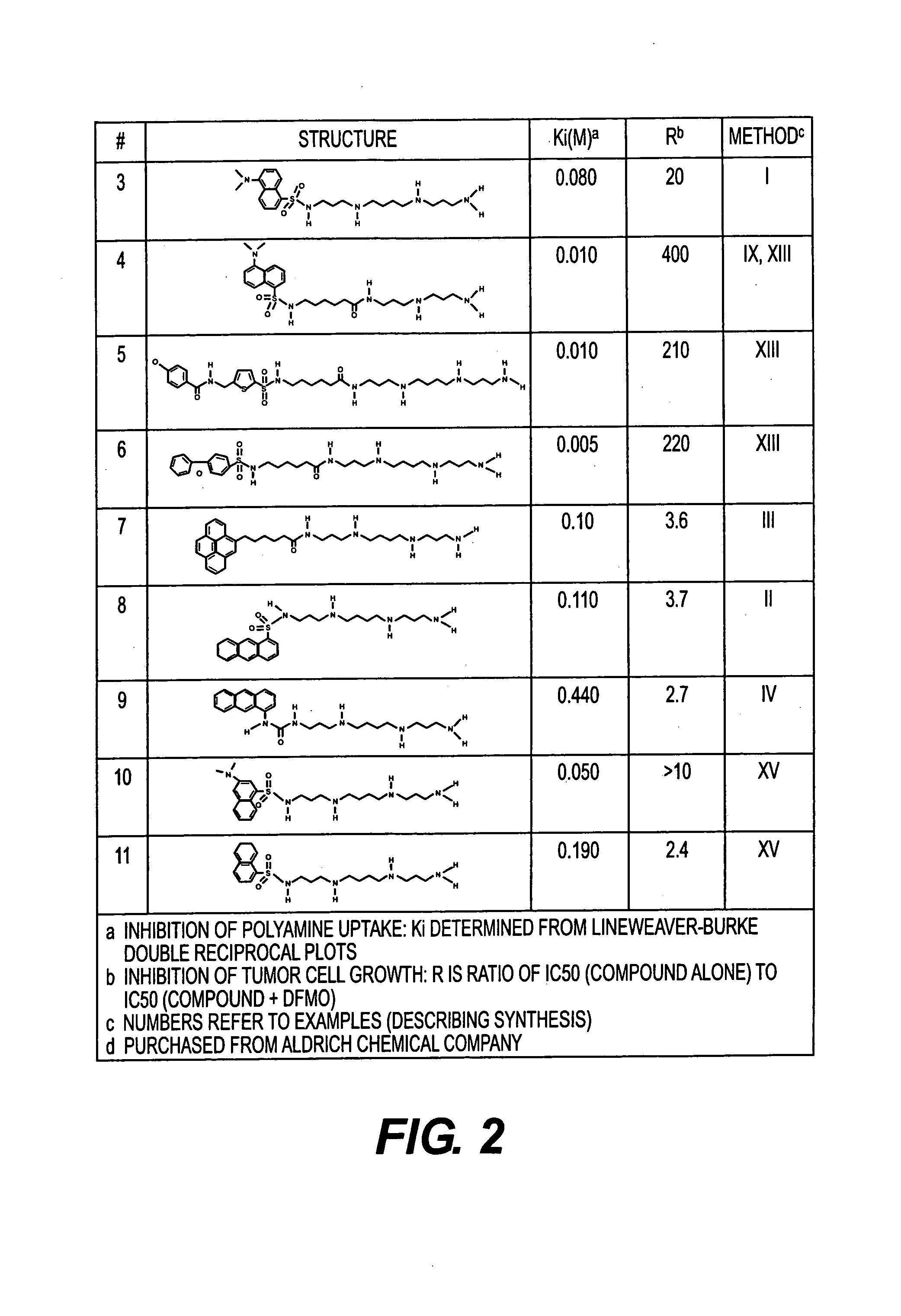
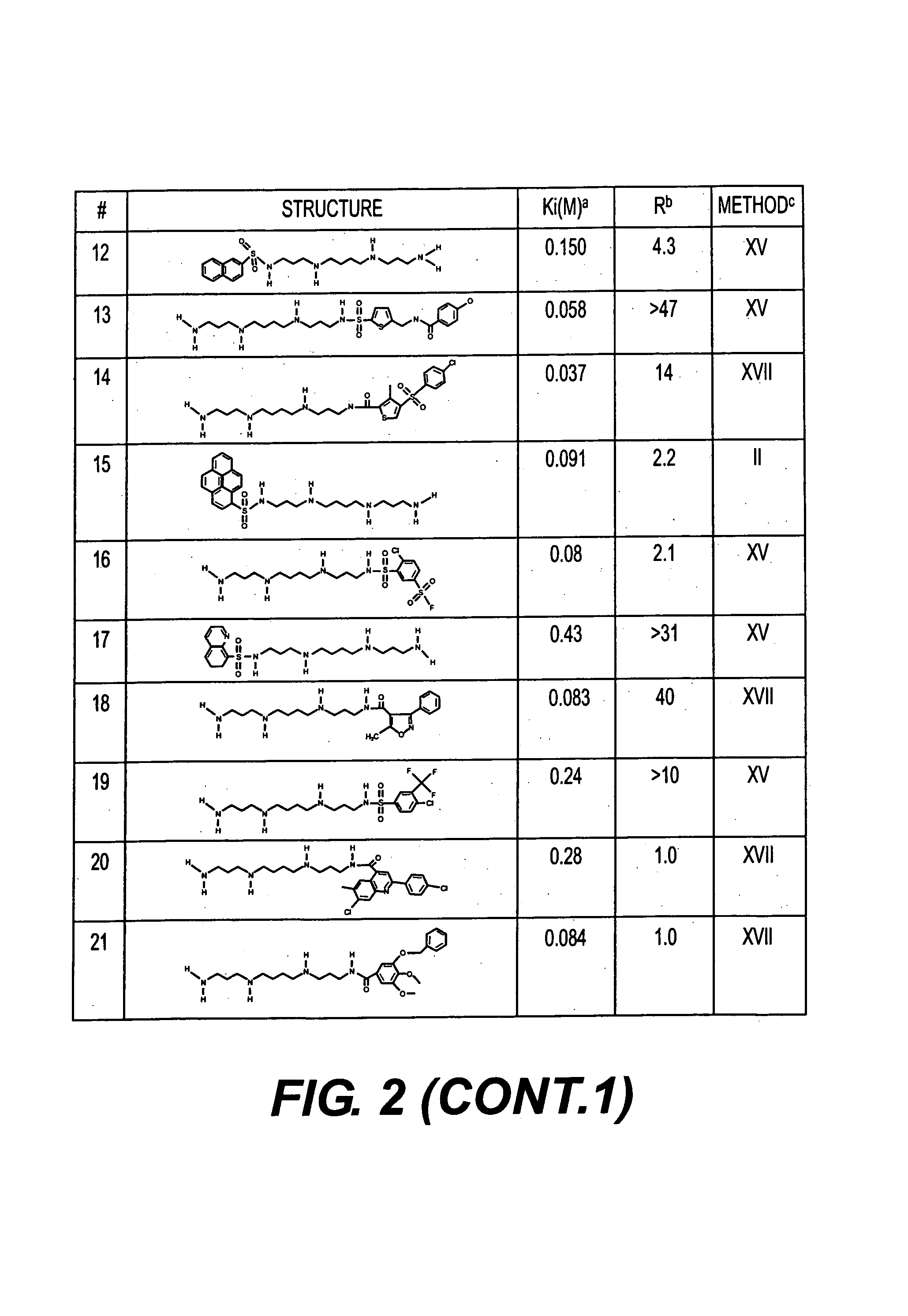
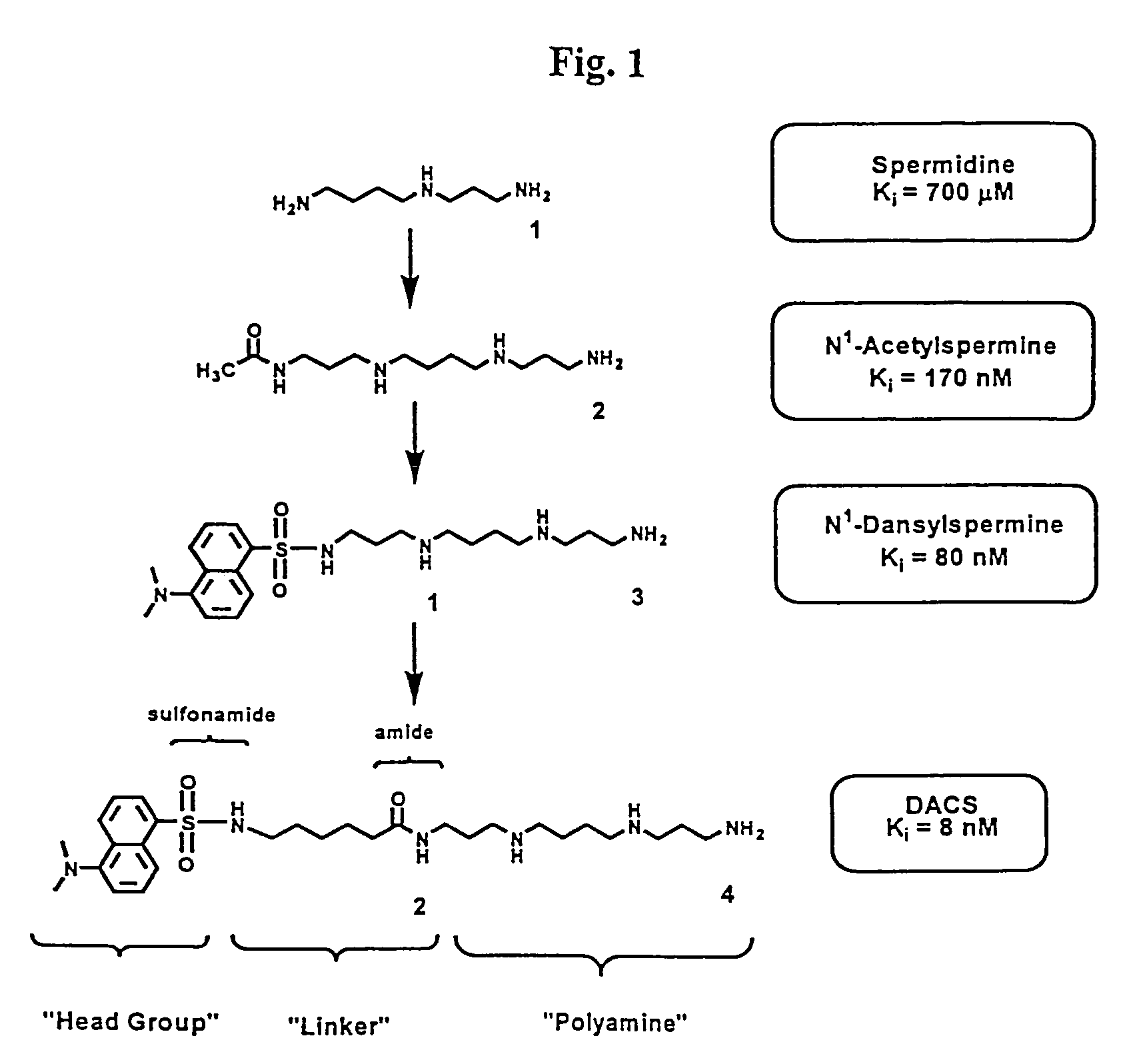
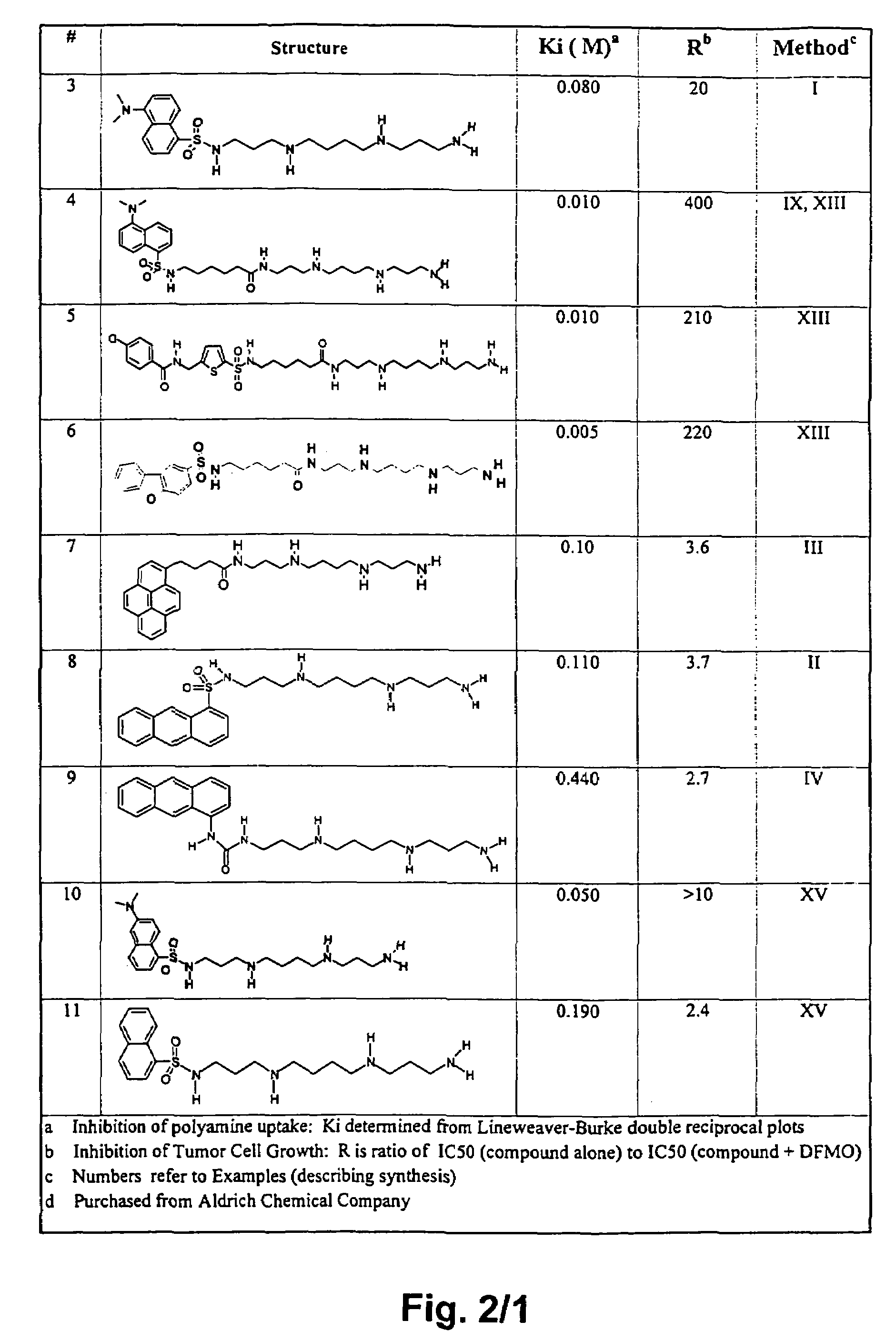
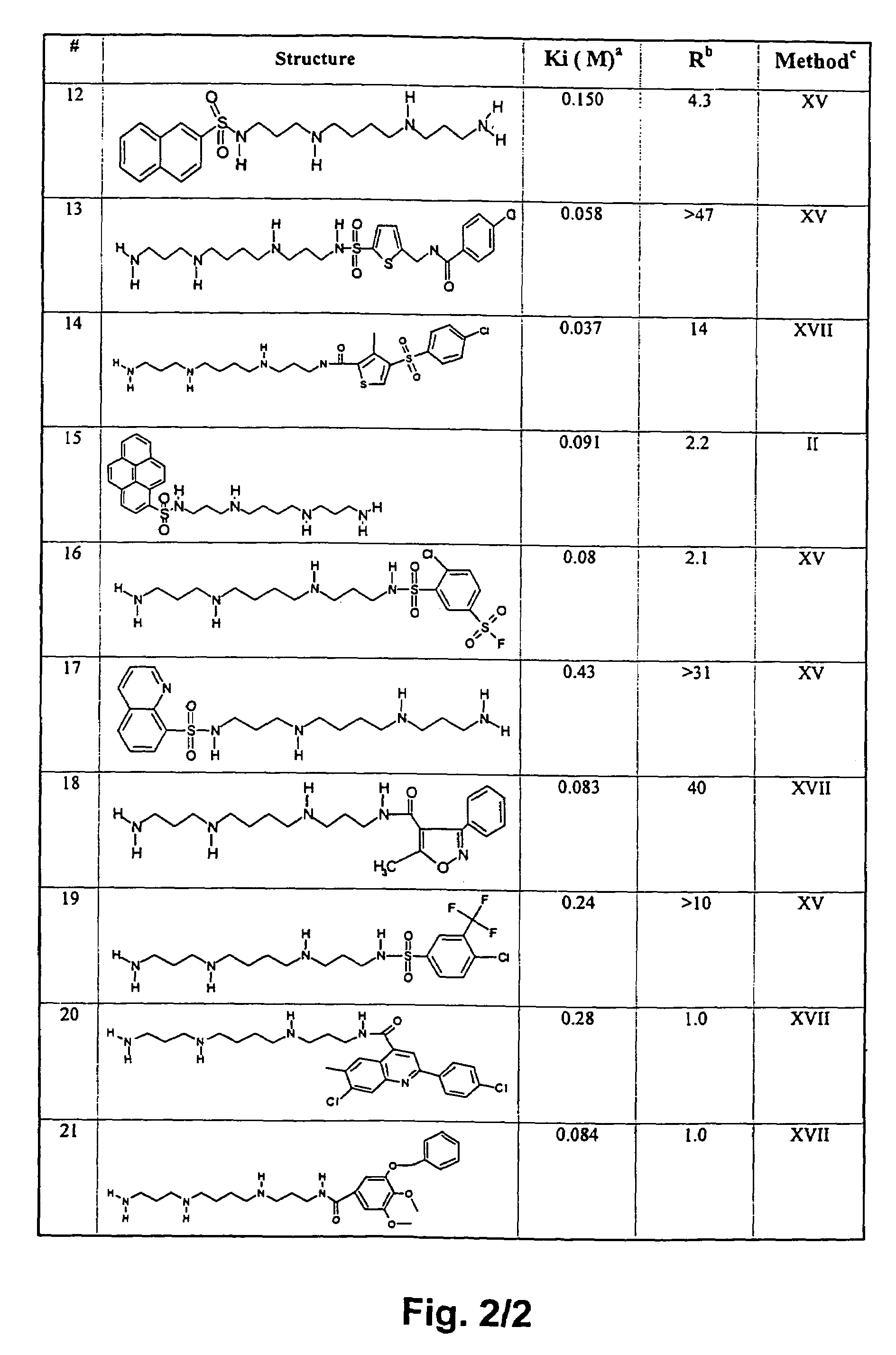
Polyamine analogues as therapeutic and diagnostic agents
InactiveUS7208528B1Rapid and efficient testingIncrease profitUrea derivatives preparationBiocideChemical synthesisDisease
Novel inhibitors of polyamine transport having inhibition constants two orders of magnitude lower than those of known compounds are disclosed. These polyamine analogues are useful pharmaceutical agents for treating diseases where it is desired to inhibit polyamine transport or other polyamine binding proteins, for example cancer and post-angioplasty injury. Novel chemical synthetic methods to obtain polyamine analogues are disclosed, including the production of a combinational polyamine library. These approaches yield analogues with desirable activities both for diagnostic and research assays and therapy. The assays of the invention are useful for high throughput screening of targets in the discovery of drugs that interact with the polyamine system.
Owner:AMINEX THERAPEUTICS
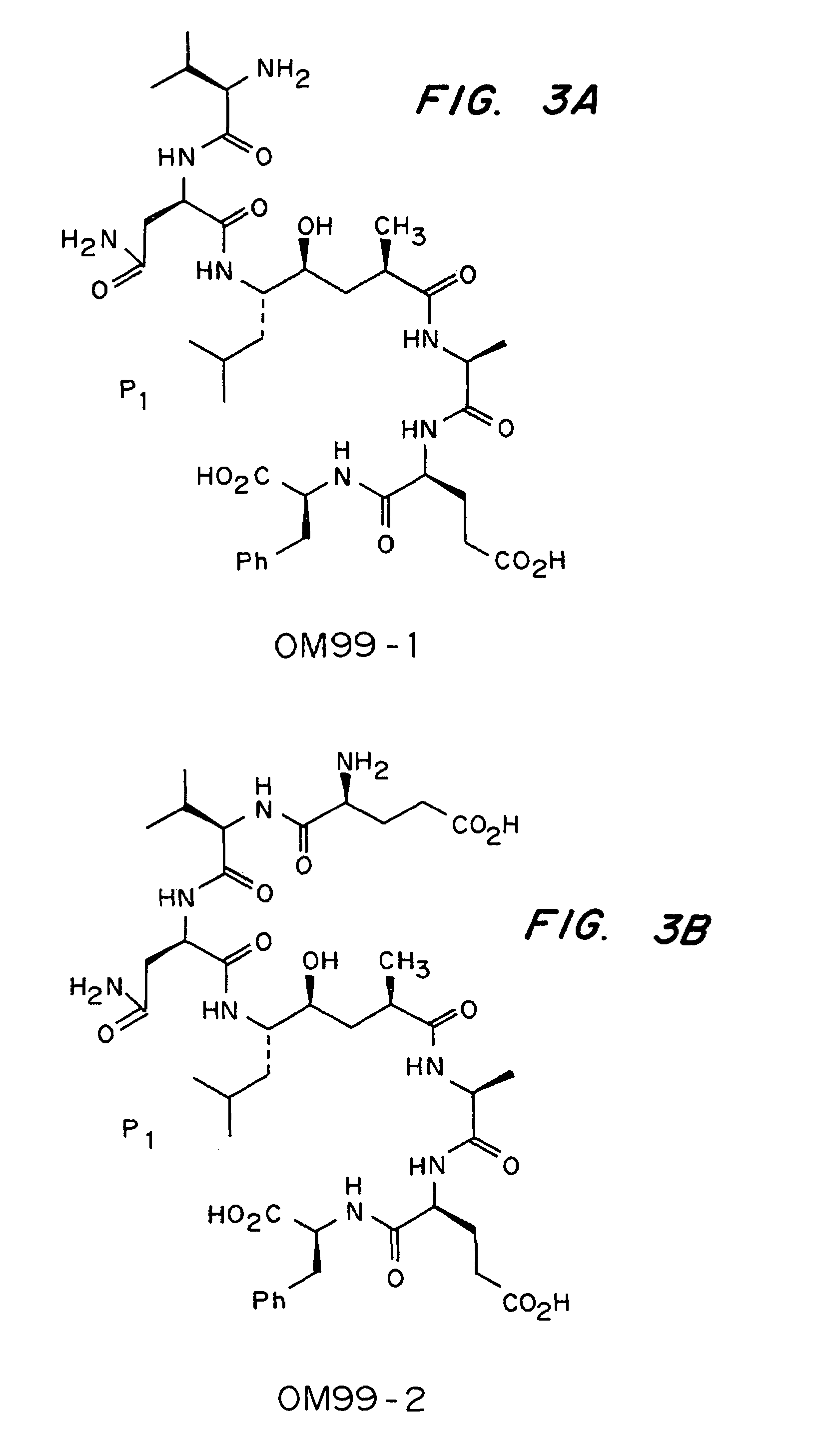
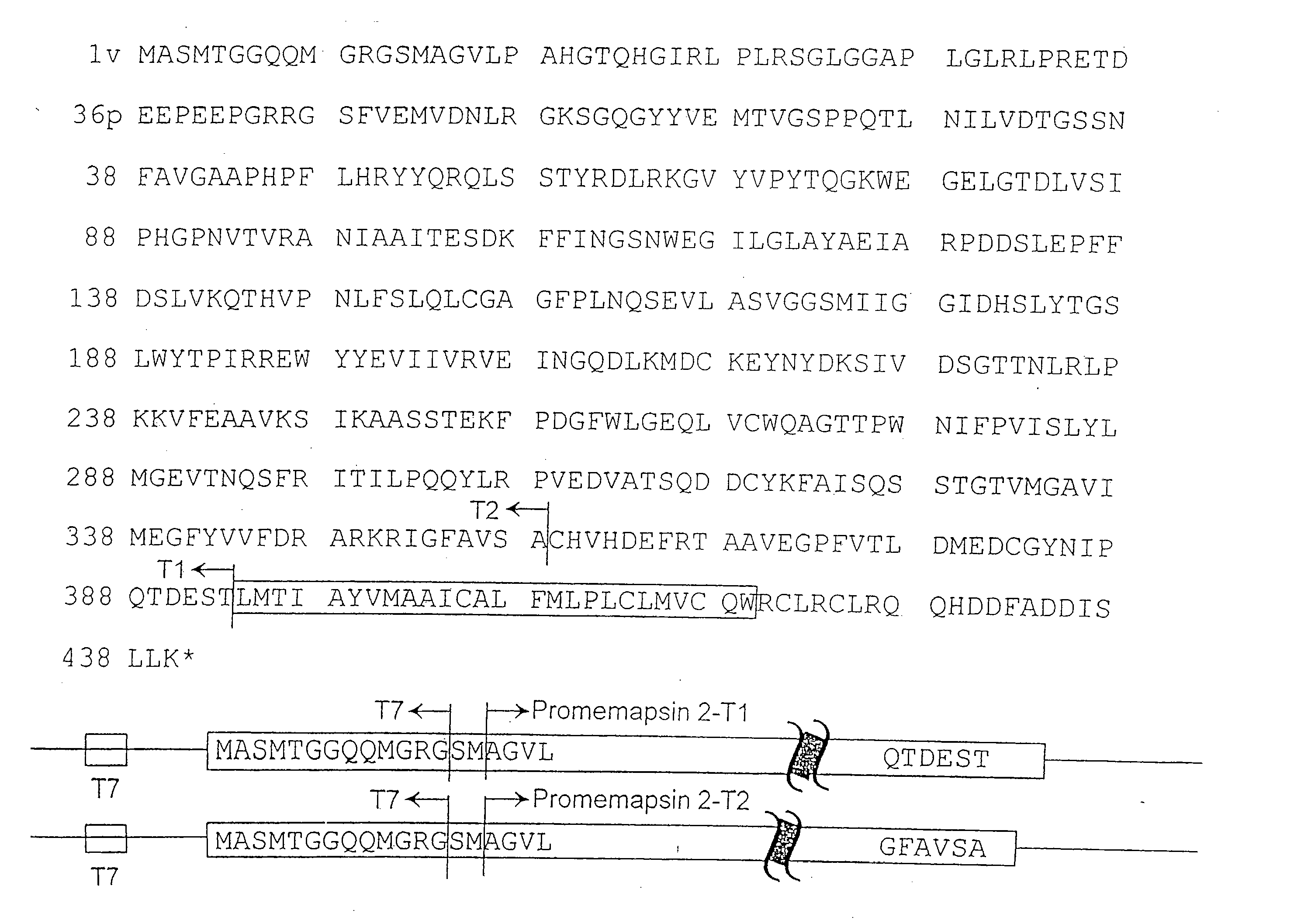
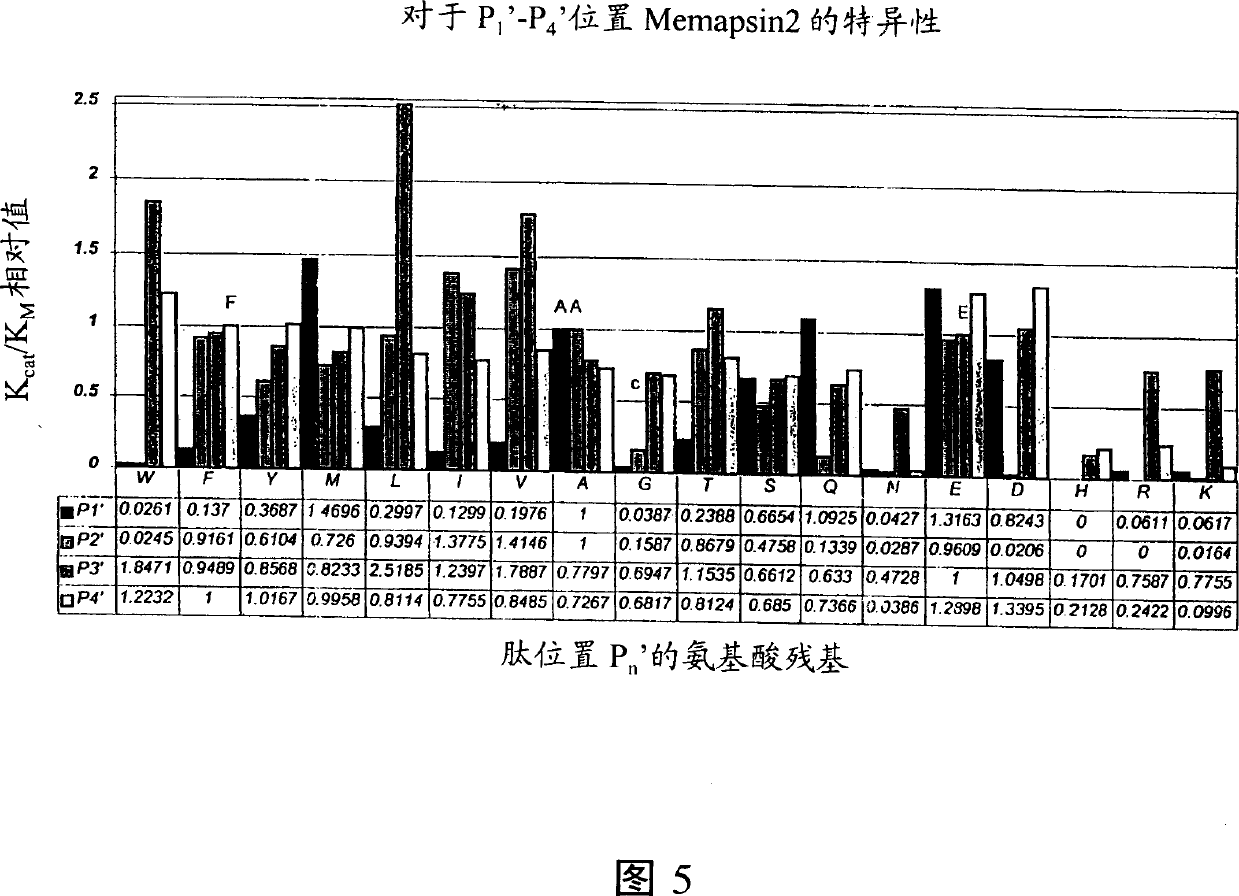
Inhibitors of memapsin 2 and use thereof
InactiveUS7244708B2Reduce molecular weightGrowth inhibitionNervous disorderHydrolasesDiseaseSubstrate analog
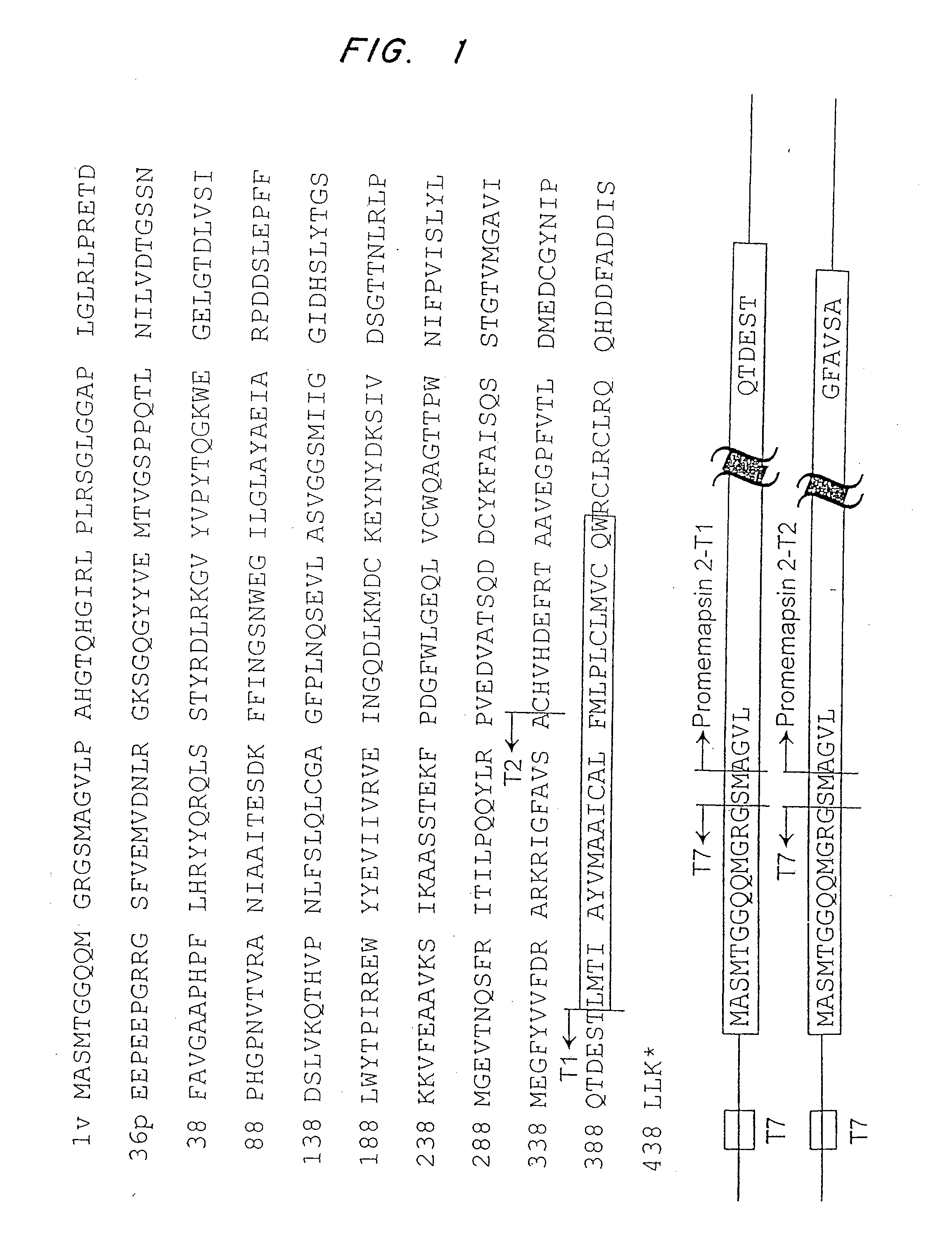
Methods for the production of purified, catalytically active, recombinant memapsin 2 have been developed. The substrate and subsite specificity of the catalytically active enzyme have been determined and were used to design substrate analogs of the natural -2 substrate that can inhibit the function of memapsin 2. Processes for the synthesis of two substrate analogues including isosteres at the sites of the critical amino acid residues were developed and the substrate analogues, OMR99-1 and OM99-2, were synthesized. The inhibition constant of OM99-2 is 1.6×10−9 M against recombinant pro-memapsin 2. Crystallography of memapsin 2 bound to this inhibitor was used to determine the three dimensional structure of the protein, and the importance of the various residues in binding. This information is useful for designing new inhibitors to memapsin 2, for diagnosing and treating and / or preventing Alzheimer's disease.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS +1
Use of tryptanthrin compound as indoleamine 2,3-dioxygenase (IDO) inhibitor
InactiveCN103054870AExcellent inhibitory effectOrganic active ingredientsNervous disorderDiseaseInhibition constant
The invention belongs to the medicinal field, and concretely relates to a use of a 8-nitrotryptanthrin compound as an IDO inhibitor. The 8-nitrotryptanthrin compound is a reversible IDO inhibitor, has an inhibition constant Ki of 0.054muM, has in-vitro and cell-based median effective inhibition concentrations IC50 of 0.103muM and 1.80*10<-5>muM respectively, and has an inhibition effectiveness obviously better than a present inhibitor 1-methyltryptophan (Ki of 34muM and IC50 of 340muM). 8-nitrotryptanthrin disclosed in the invention can effectively lower the abnormally-increasing IDO activity in a tumor animal model as the IDO inhibitor, and also has tumor treatment effects comprising tumor growth delaying, tumor volume reduction and in-vitro tumor cell killing. 8-nitrotryptanthrin disclosed in the invention has a wide application prospect, and can be used for treating serious diseases having the IDO mediated tryptophan metabolism approach pathology characteristics, such as cancers, the Alzheimer disease, tristimania, cataract and the like as the IDO inhibitor.
Owner:FUDAN UNIV
Polyamine analogues as therapeutic and diagnostic agents
InactiveUS7160923B1High throughput screeningHigh sensitivityUrea derivatives preparationBiocideChemical synthesisDisease
Novel inhibitors of polyamine transport having inhibition constants two orders of magnitude lower than those of known compounds are disclosed. These polyamine analogues are useful pharmaceutical agents for treating diseases where it is desired to inhibit polyamine transport or other polyamine binding proteins, for example cancer and post-angioplasty injury. Novel chemical synthetic methods to obtain polyamine analogues are disclosed, including the production of a combinatiorial polyamine library. These approaches yield analogues with desirable activities both for diagnostic and research assays and therapy. The assays of the invention are useful for high throughput screening of targets in the discovery of drugs that interact with the polyamine system.
Owner:MEDIQUEST THERAPEUTICS INC
Inhibitors of memapsin 2 and use thereof
InactiveUS20080021196A1Reduce molecular weightGrowth inhibitionNervous disorderHydrolasesDiseaseActive enzyme
Methods for the production of purified, catalytically active, recombinant memapsin 2 have been developed. The substrate and subsite specificity of the catalytically active enzyme have been determined. The substrate and subsite specificity information was used to design substrate analogs of the natural memapsin 2 substrate that can inhibit the function of memapsin 2. The substrate analogs are based on peptide sequences, shown to be related to the natural peptide substrates for memapsin 2. The substrate analogs contain at least one analog of an amide bond which is not capable of being cleaved by memapsin 2. Processes for the synthesis of two substrate analogues including isosteres at the sites of the critical amino acid residues were developed and the substrate analogues, OMR99-1 and OM99-2, were synthesized. OM99-2 is based on an octapeptide Glu-Val-Asn-Leu-Ala-Ala-Glu-Phe (SEQ ID NO:28) with the Leu-Ala peptide bond substituted by a transition-state isostere hydroxyethylene group (FIG. 1). The inhibition constant of OM99-2 is 1.6×10−9 M against recombinant pro-memapsin 2. Crystallography of memapsin 2 bound to this inhibitor was used to determine the three dimensional structure of the protein, as well as the importance of the various residues in binding. This information can be used by those skilled in the art to design new inhibitors, using commercially available software programs and techniques familiar to those in organic chemistry and enzymology, to design new inhibitors to memapsin 2, useful in diagnostics and for the treatment and / or prevention of Alzheimer's disease.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS +1
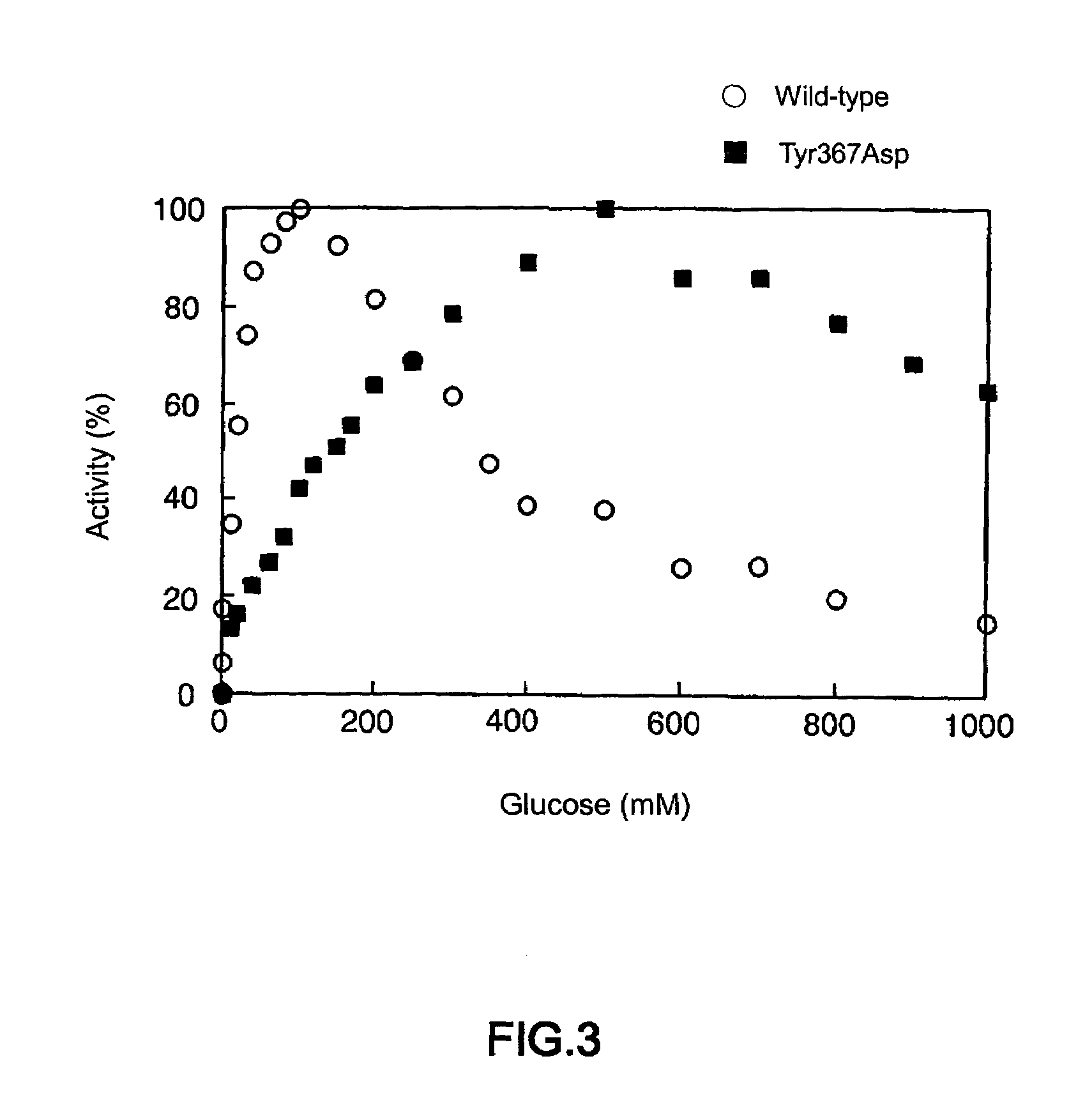
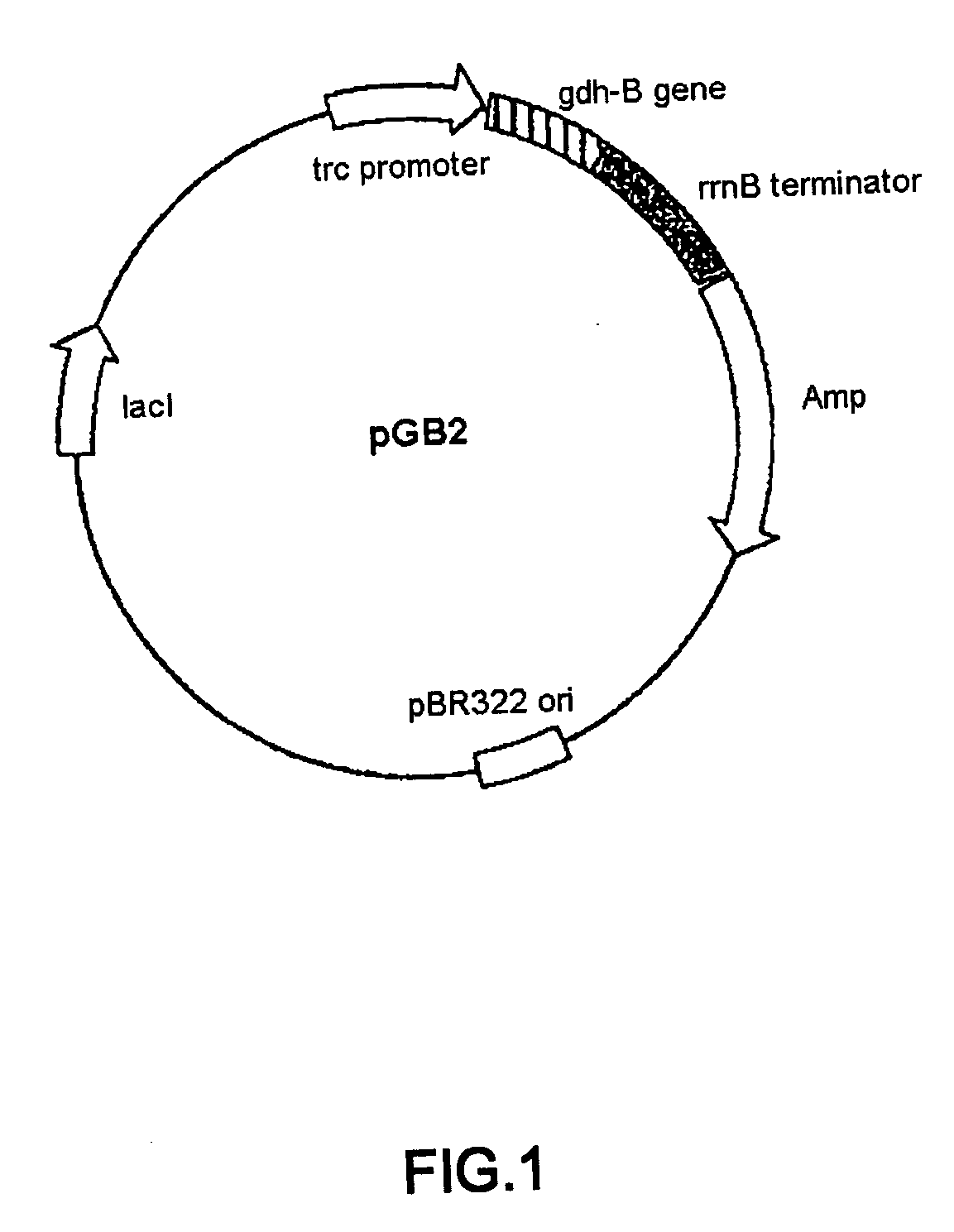
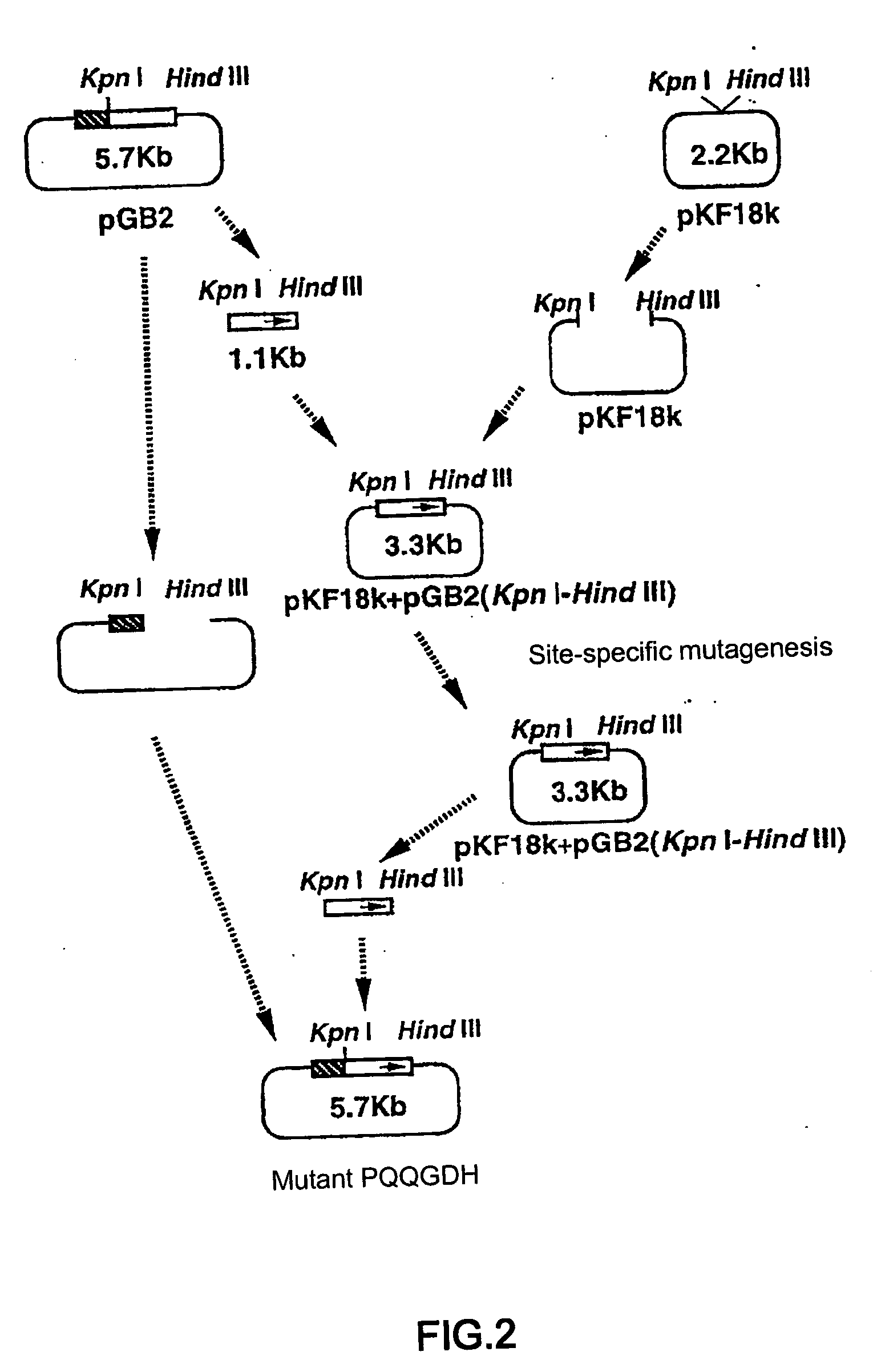
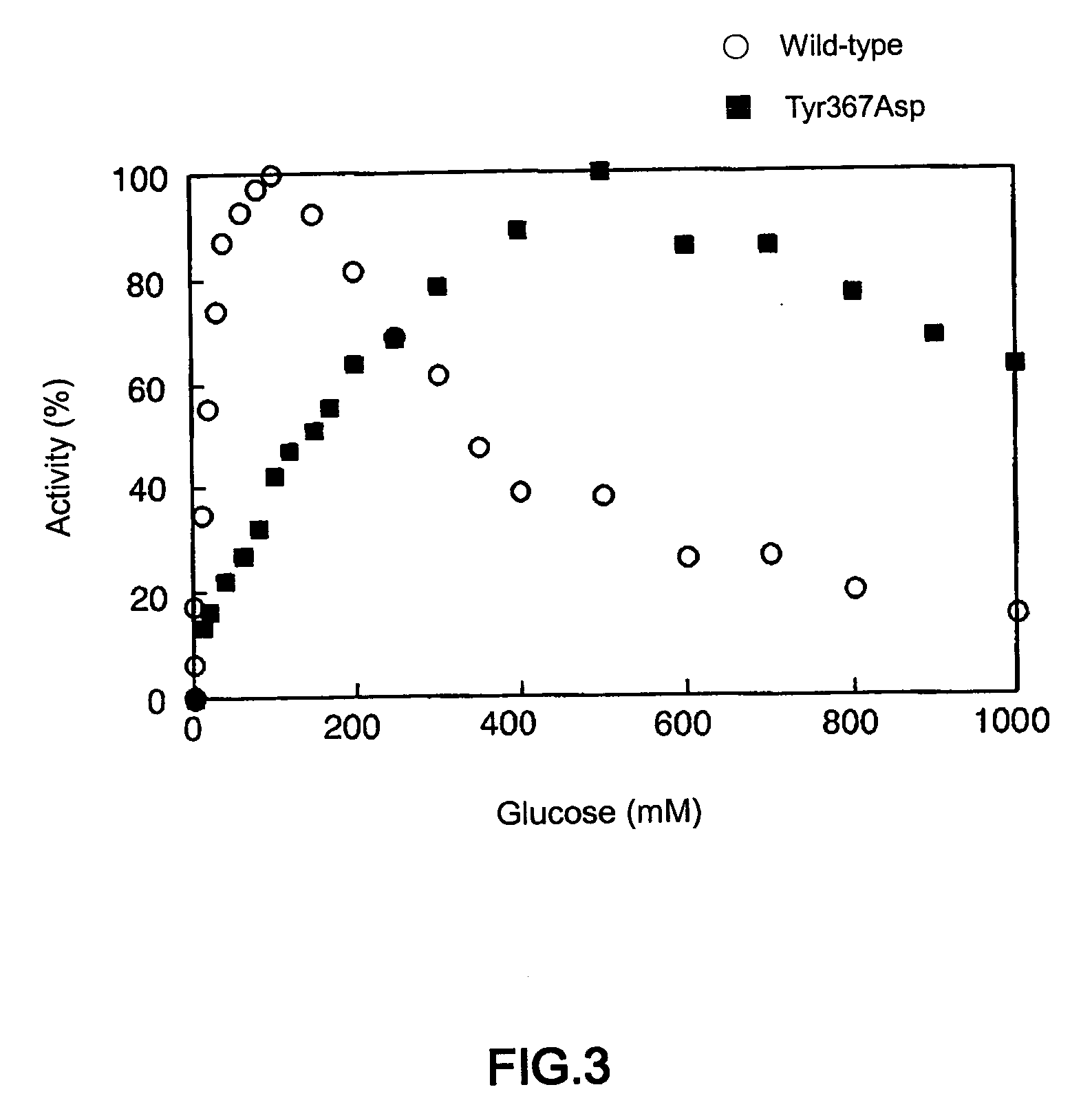
Glucose dehydrogenase
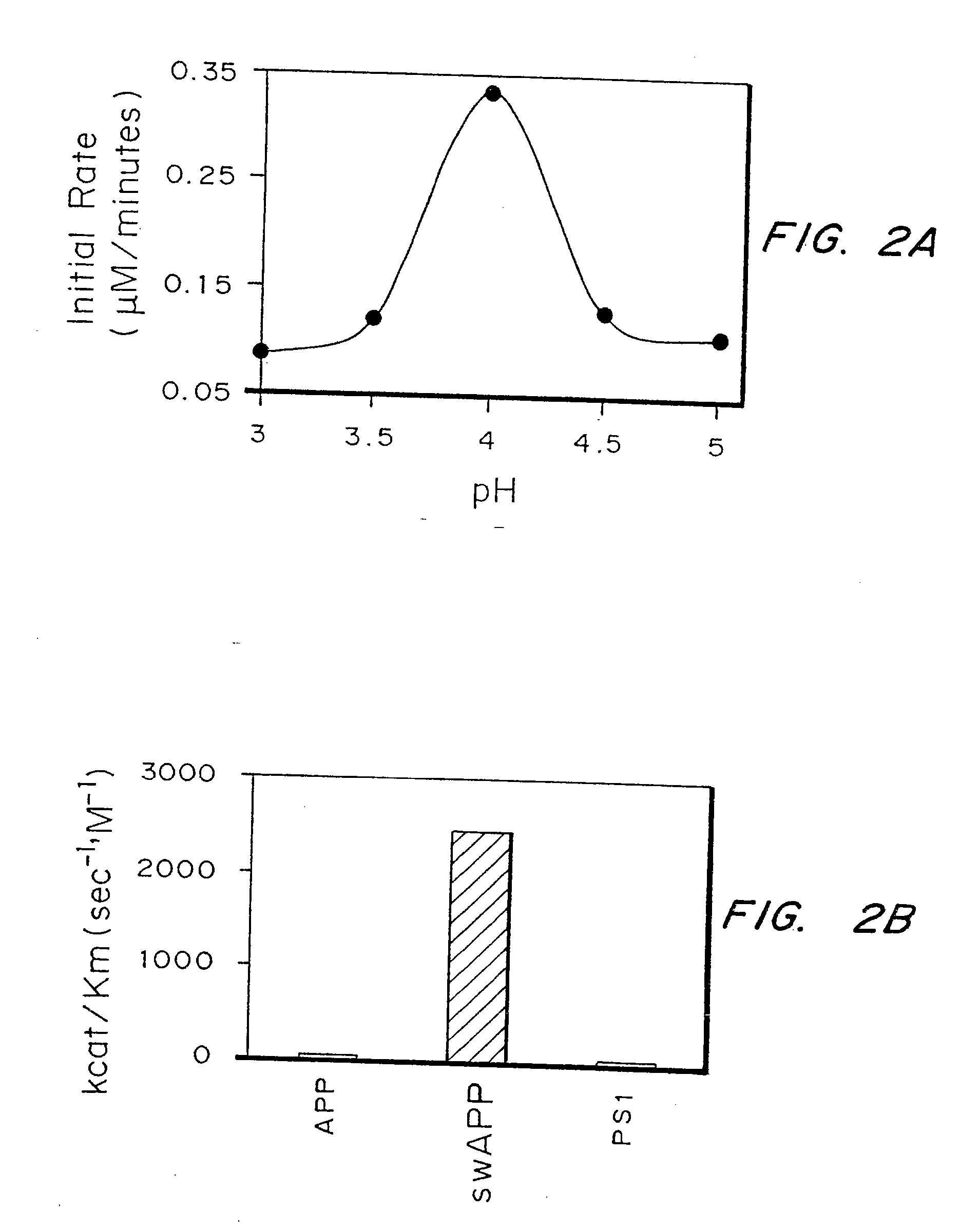
Disclosed is a modified glucose dehydrogenase having pyrroloquinoline quinone as a coenzyme, wherein one or more amino acid residues in a region of amino acid 349-377 of water-soluble PQQGDH derived from Acinetobacter calcoaceticus is replaced with other amino acid residues and has an inhibition constant (Ksi) of 200 mM or more. The modified water-soluble PQQGDH of the invention can be utilized for measuring glucose levels in the presence of high concentrations of glucose because of the low substrate inhibition by glucose.
Owner:SODE
Inhibitor and application thereof in inhibiting chitinase and hexosaminidase activity
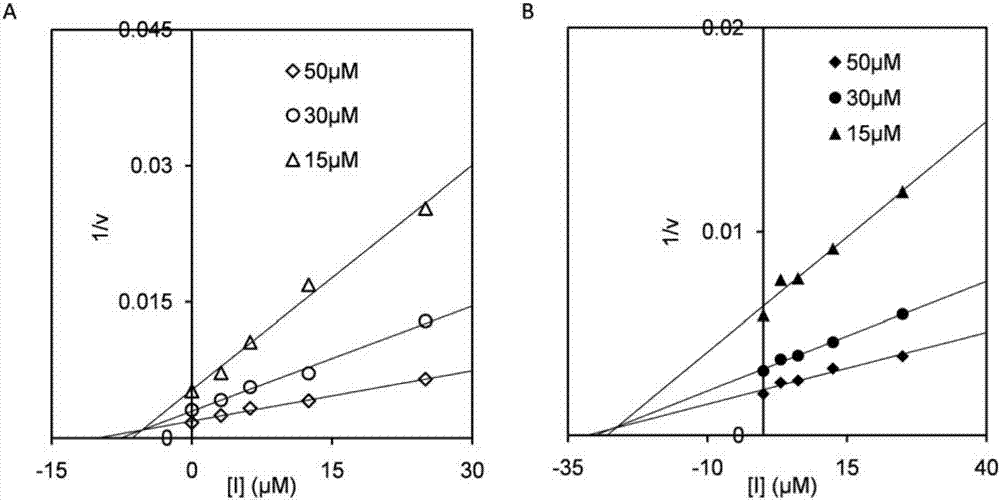
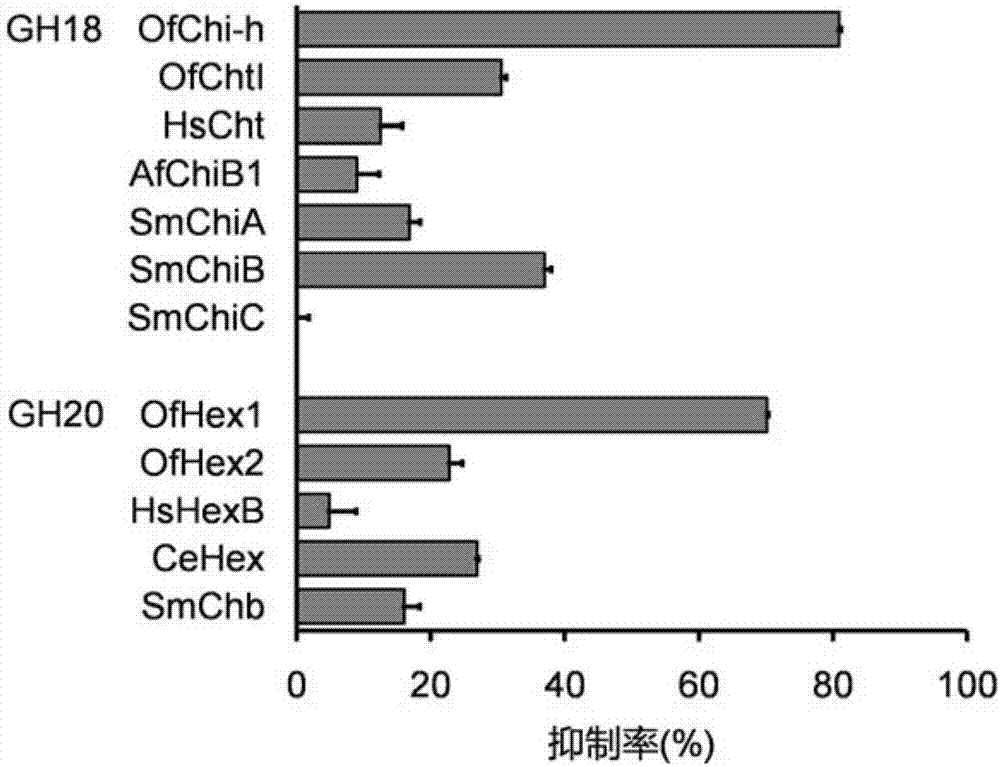
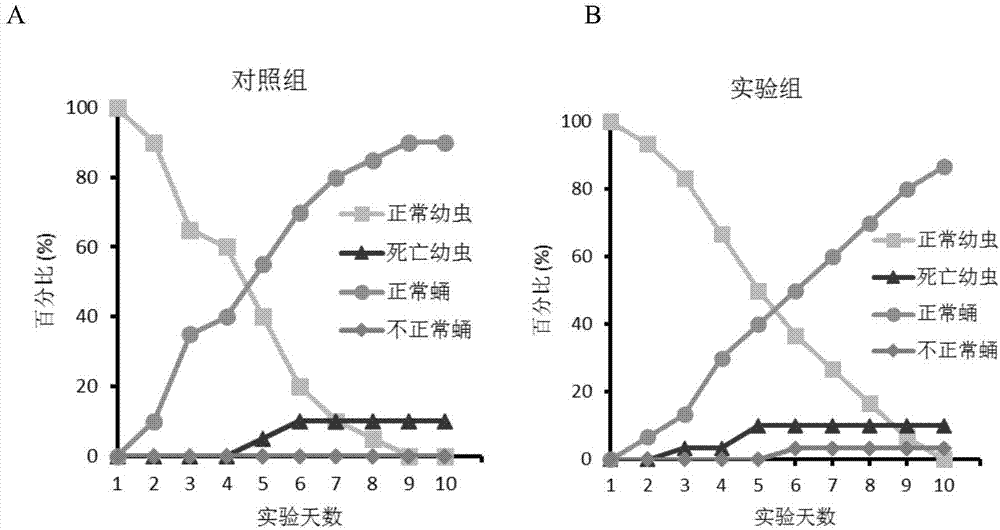
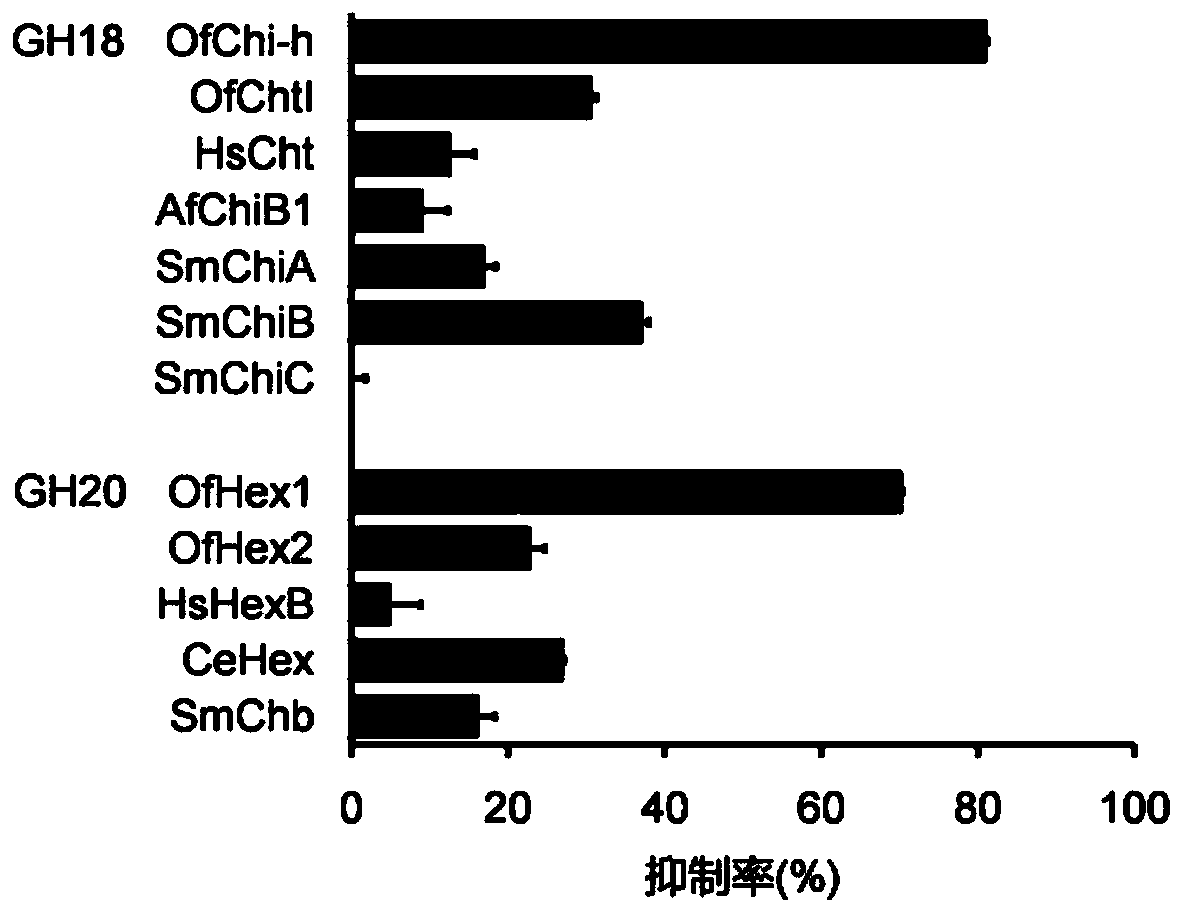
ActiveCN106854144AImprove solubilityConvenient researchBiocideOrganic chemistryChitinaseInhibition constant
The invention discloses application of a compound for efficient inhibition of chitinase OfChi-h and hexosaminidase OfHex1 activity, and relates to application of a compound phlegmacin B1 and its derivatives in inhibiting chitinase OfChi-h and hexosaminidase OfHex1 activity. The used final concentration is not less than 13ppm, under the concentration, the measured inhibition rates are 90.4% and 71.4% respectively, and inhibition constants Ki are 5.5 microM and 26 microM respectively. The invention proves that the compound phlegmacin B1 has good application prospect in the prevention and control of agricultural pests, especially in prevention and control of lepidoptera insects.
Owner:DALIAN UNIV OF TECH
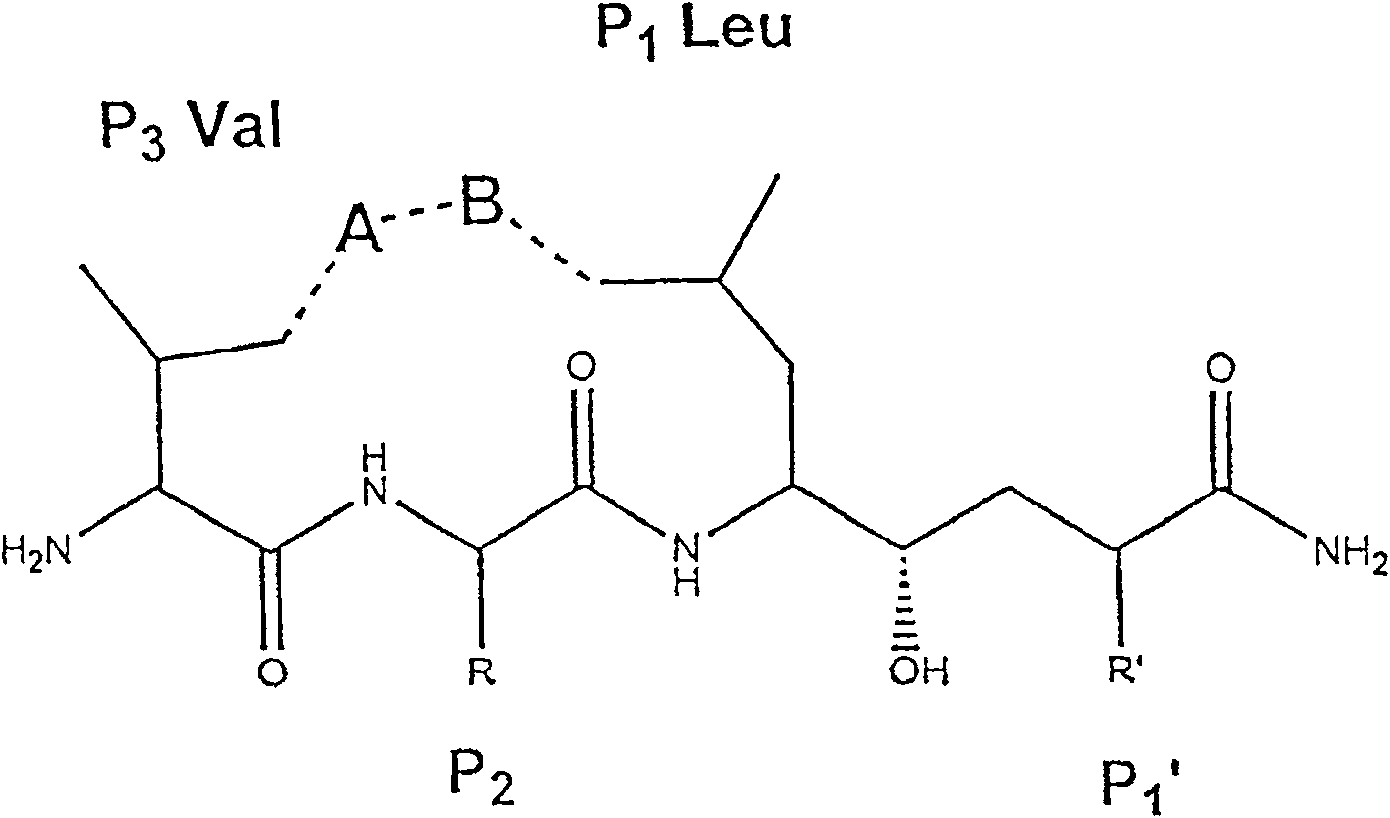
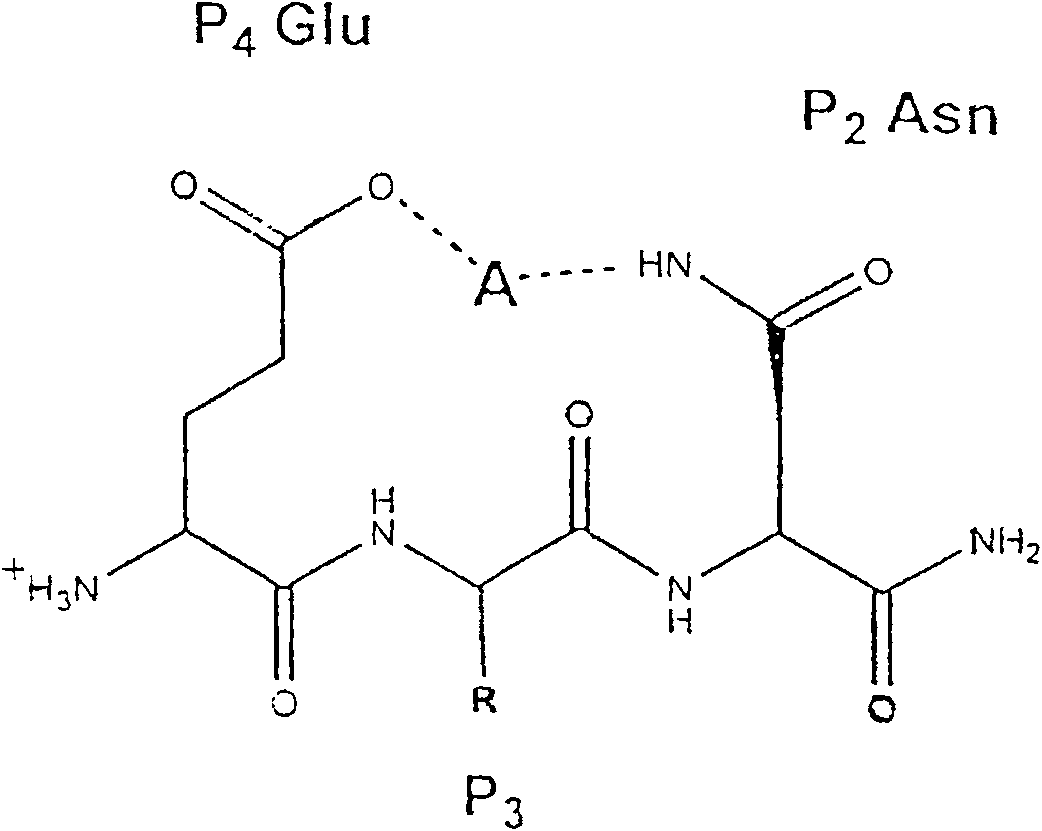
Hydrazine nitrile cathepsin inhibitors with different P<3> structures, and application thereof
InactiveCN102731344AEasy to synthesizeSimple structureOrganic chemistrySkeletal disorderArylHydrazine compound
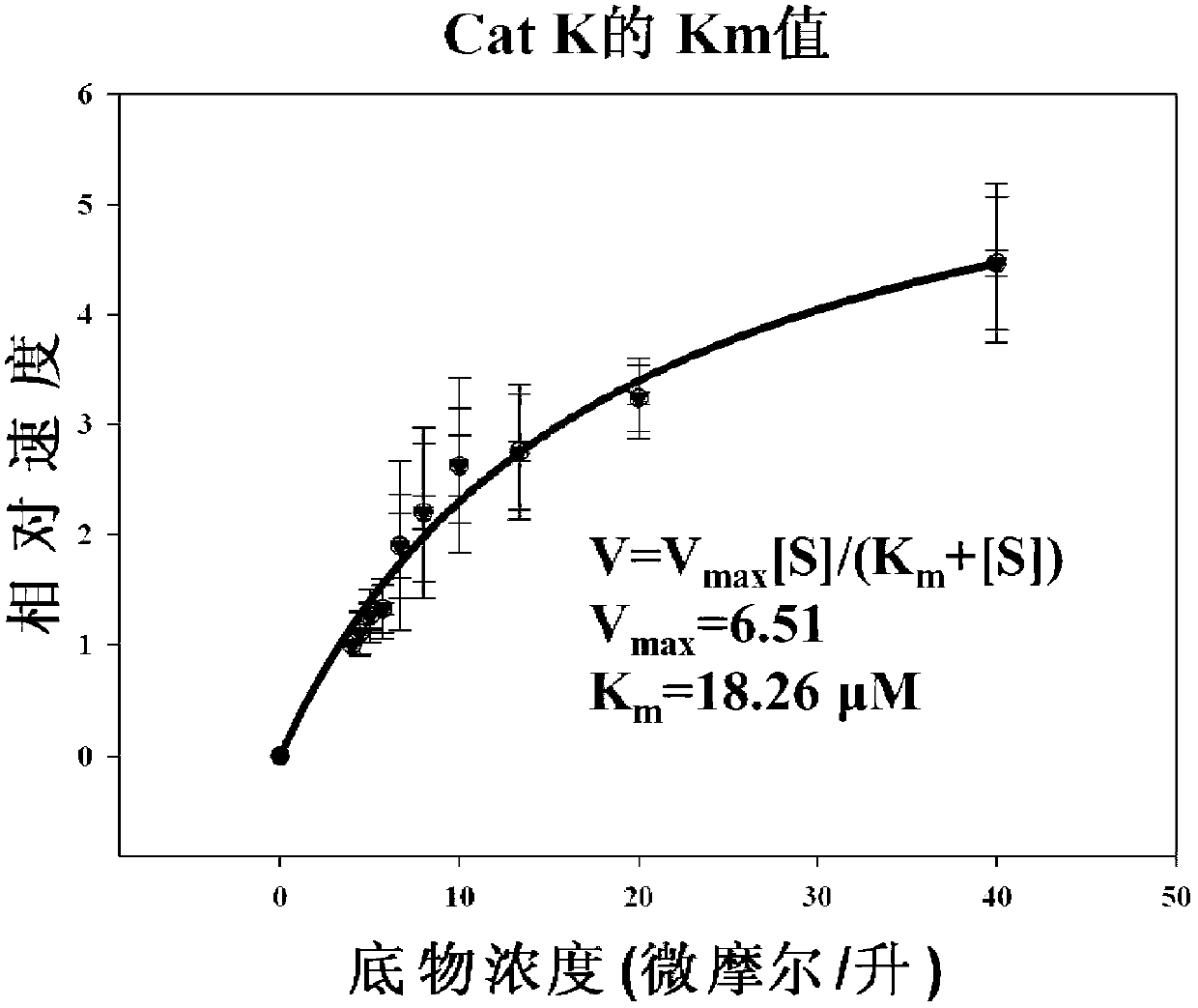
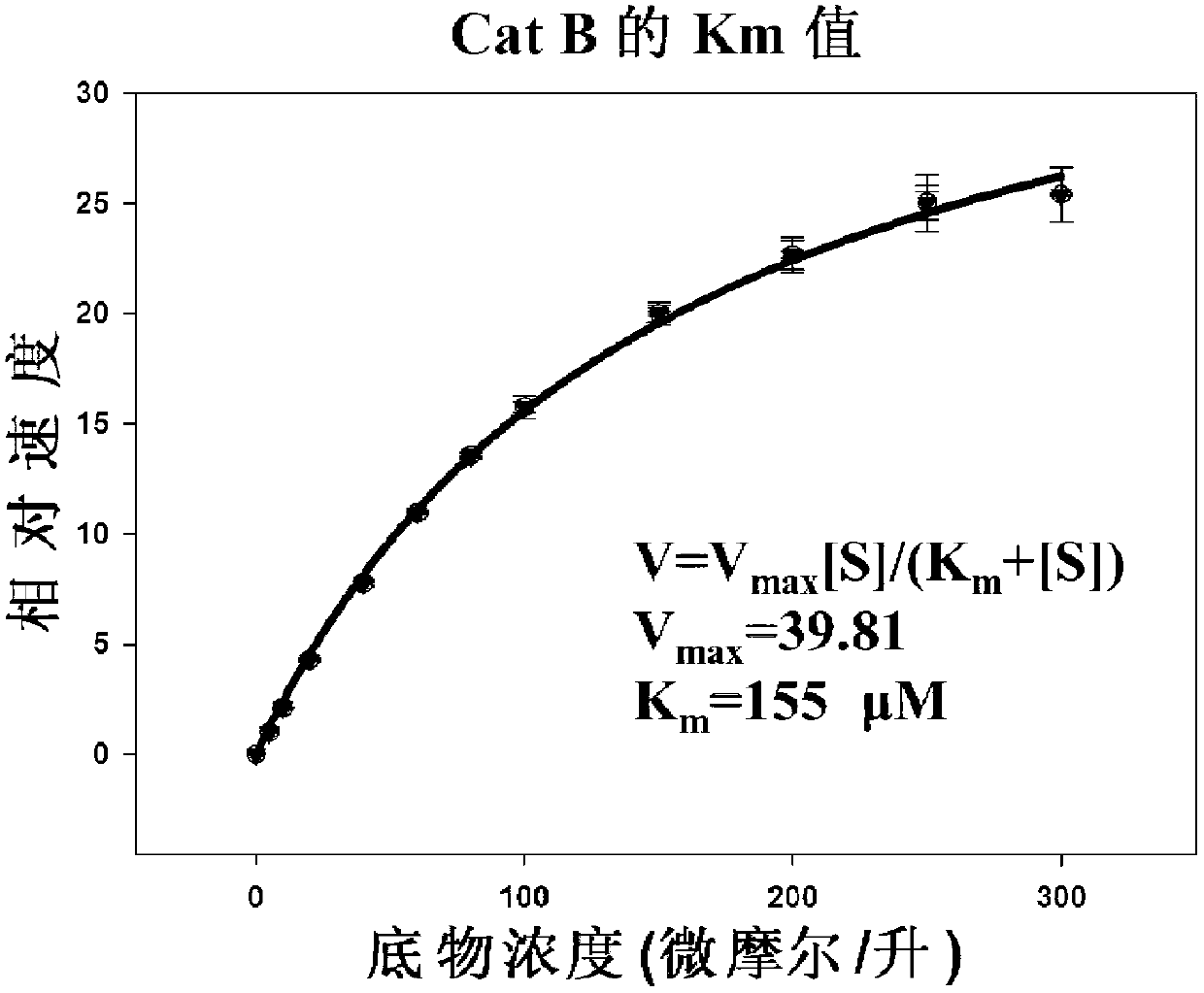
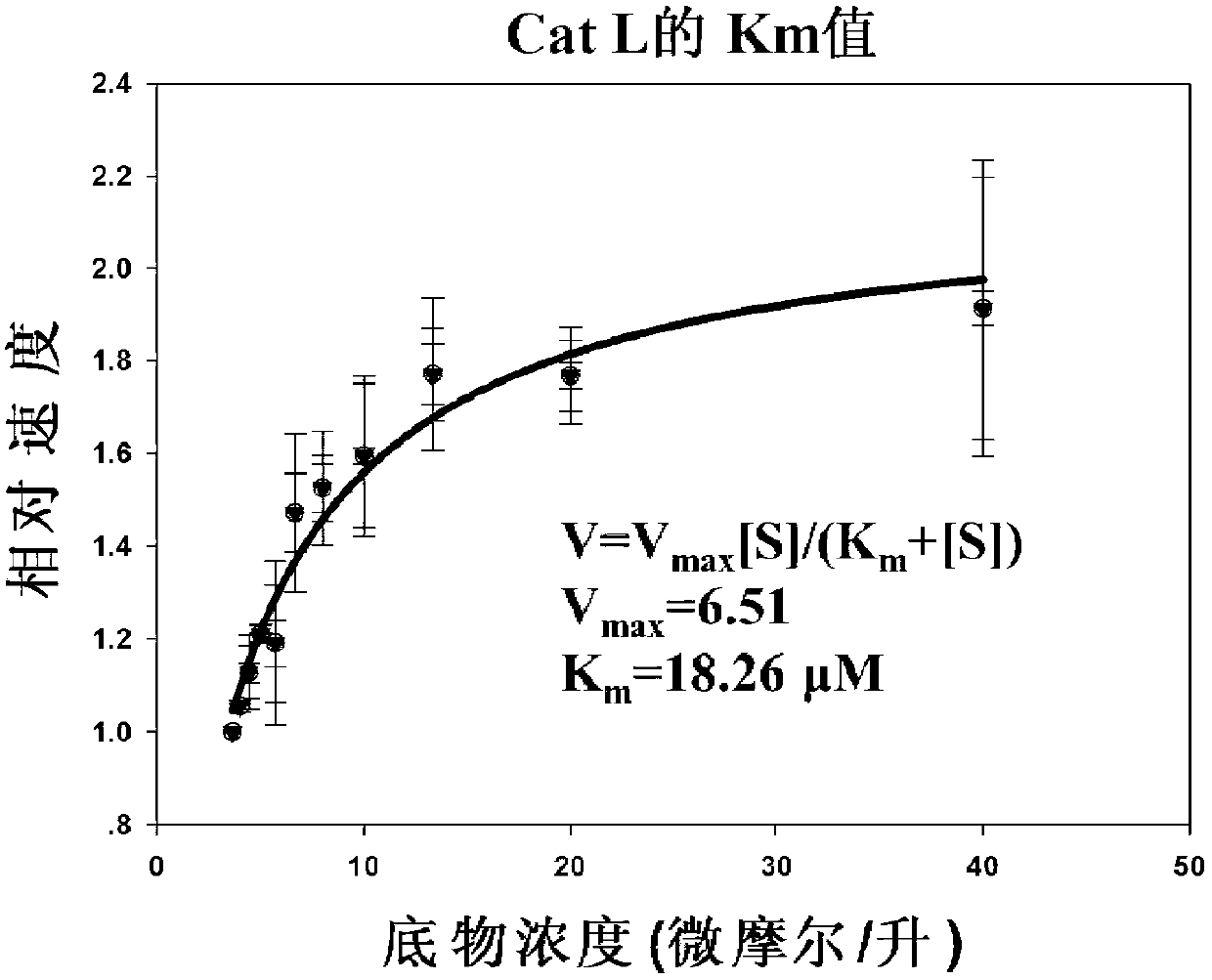
The invention which belongs to the field of the design and the synthesis of cathepsin (Cat for short) inhibitors concretely relates to novel and efficient hydrazine nitrile inhibitors with different P<3> structures directed to four Cats (K, B, L and S). The main structure of the hydrazine nitrile inhibitors in the invention is represented by a formula in the specification; and in the formula, R1 is isobutyl, and R2 is alkyl, aryl, or cycloalkyl. The peptidomimetic hydrazine nitrile inhibitors designed and synthesized in the invention have the advantages of relatively simple structure, and easy synthesis; and the design and the synthesis of the maternal structure provide great conveniences for the synthesis of subsequent end products. Synthesized compounds, which can effectively inhibit actives of the Cat K, the Cat B, the Cat S and the Cat L, have an inhibition constant to the Cat K and the Cat L being in a picomole concentration (10<-12>M) level; and the synthesized compounds, which have weak inhibition effects on the Cat B and the Cat S have an inhibition constant being in a nanomole concentration (10<-9>M) level.
Owner:JILIN UNIV
Application of berberine and derivative of berberine as hexosaminidase inhibitor
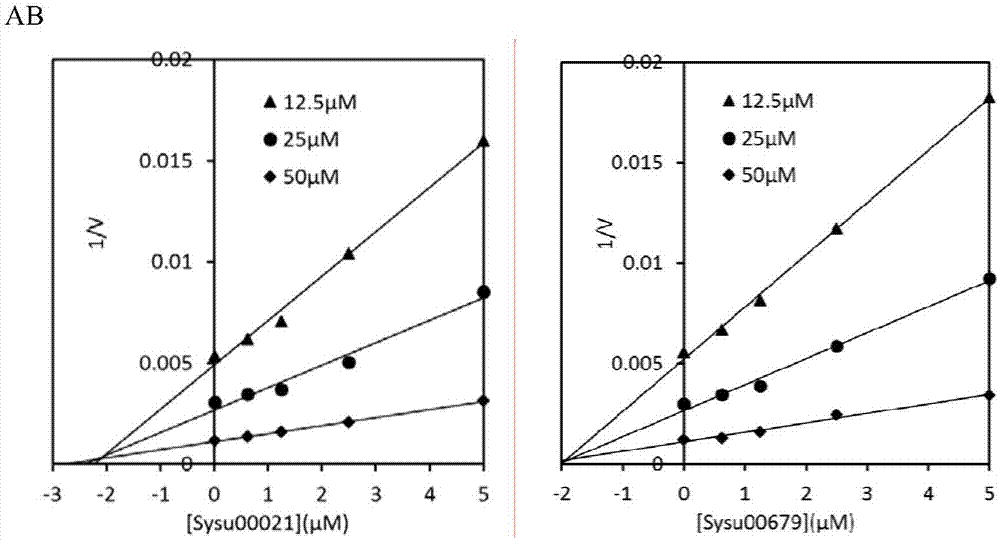
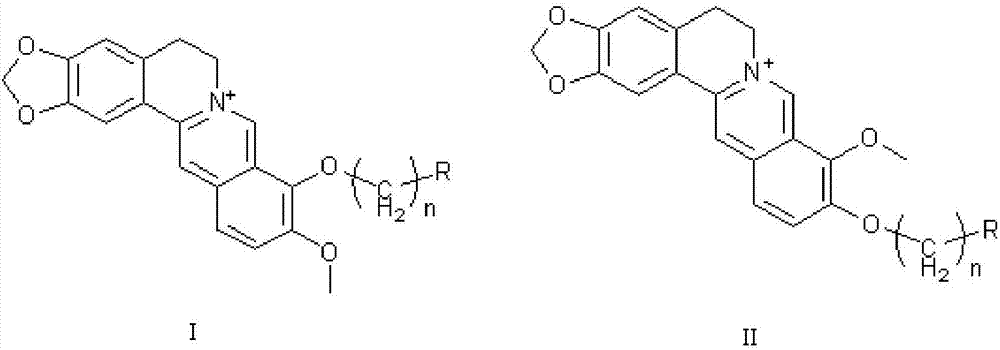
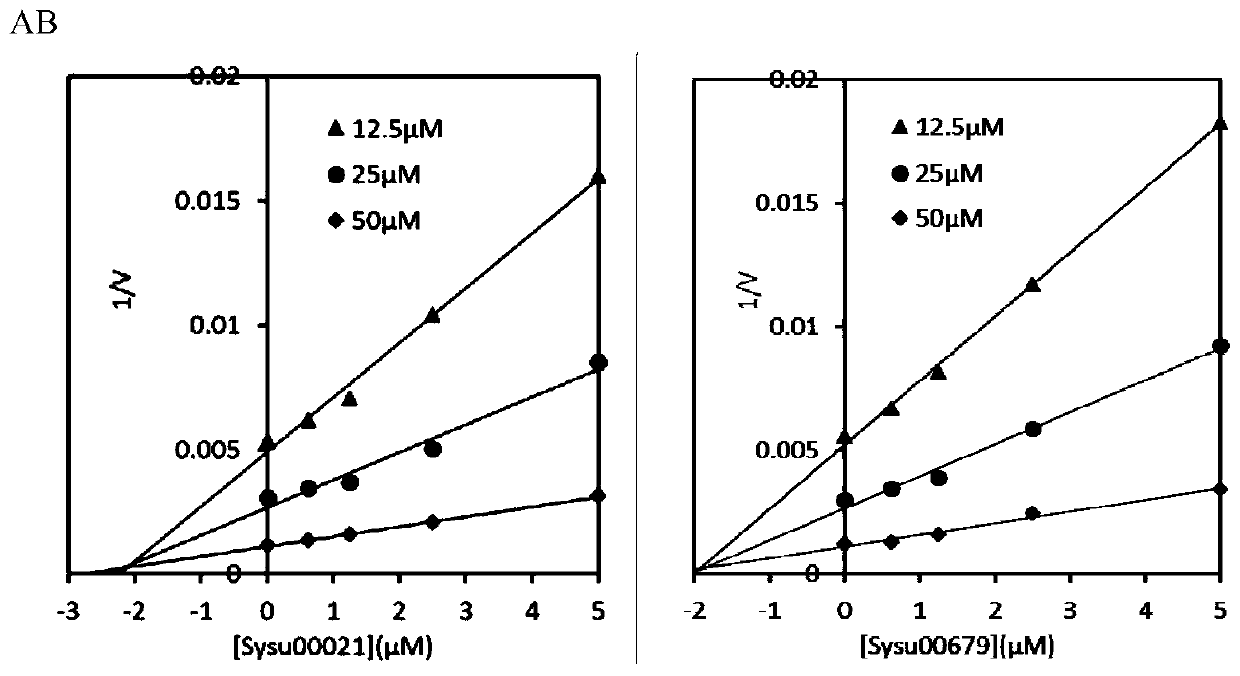
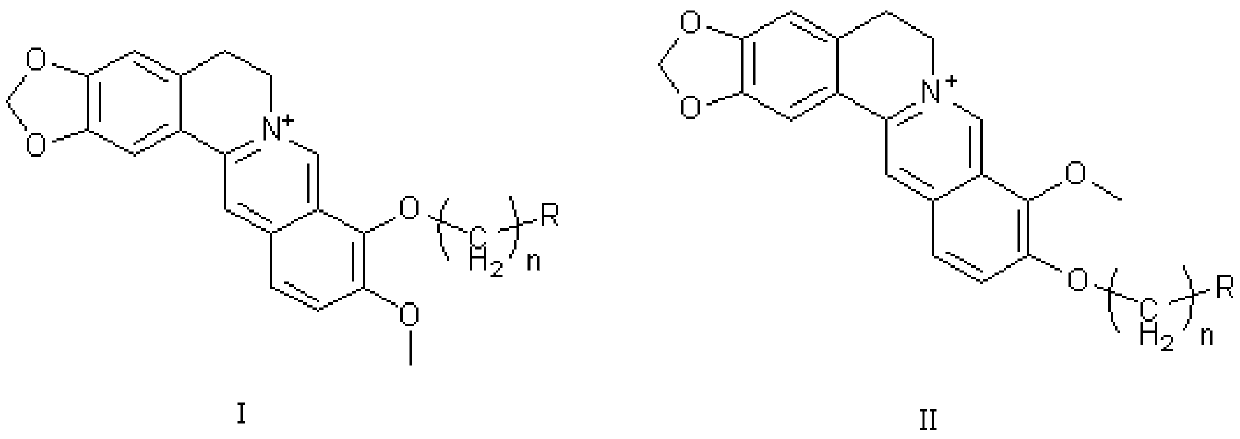
The invention discloses application of berberine and a derivative of the berberine as a hexosaminidase inhibitor. The general structure formula of the hexosaminidase inhibitor is shown as I or II; in all screened 35 compounds, the berberine and the derivative of the berberine show certain inhibition activity on hexosaminidase OfHex1; particularly, the suppression rate of Sysu-00021 and Sysu-00679 on the OfHex1 enzyme is respectively 76 percent and 79 percent, and the inhibition constant Ki is respectively 2mu M and 1.8mu M. Compound applicable object determination shows that under the final concentration of 10mu M, the compound shows higher inhibition activity on the hexosaminidase OfHex1; no inhibition activity is shown on the hexosaminidase HsHexB derived from the human. In a word, the berberine and the derivative of the berberine have wide application prospects in the fields of biology, chemicobiology and the like.
Owner:DALIAN UNIV OF TECH
Glucose dehydrogenase
Disclosed is a modified glucose dehydrogenase having pyrroloquinoline quinone as a coenzyme, wherein one or more amino acid residues in a region of amino acid 349-377 of water-soluble PQQGDH derived from Acinetobacter calcoaceticus is replaced with other amino acid residues and has an inhibition constant (Ksi) of 200 mM or more. The modified water-soluble PQQGDH of the invention can be utilized for measuring glucose levels in the presence of high concentrations of glucose because of the low substrate inhibition by glucose.
Owner:SODE
D-3-phosphoglycerate-dehydrogenase as well as coding gene and construction method thereof
The invention relates to D-3-phosphoglycerate-dehydrogenase and a coding gene thereof. The amino acid sequence of the D-3-phosphoglycerate-dehydrogenase is shown as a sequence 1; the inhibition constant Ki of L-serine of the D-3-phosphoglycerate-dehydrogenase is greater than 250mM; and the sequence of the coding gene is shown as a sequence 2. The difference between the amino acid sequence of the D-3-phosphoglycerate-dehydrogenase (PGDH) provided by the invention and the amino acid sequence (sequence 3) of the escherichia coli wild type PGDH lies in that amino acid at 344th site is not histidine but lactamine and amino acid at 346th site is not asparagines but lactamine and the PGDH generated by mutation is not inhibited by the L-serine. In addition, the enzyme activity of a PGDH variant disclosed by the invention has no change compared with the wild type PGDH variant.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
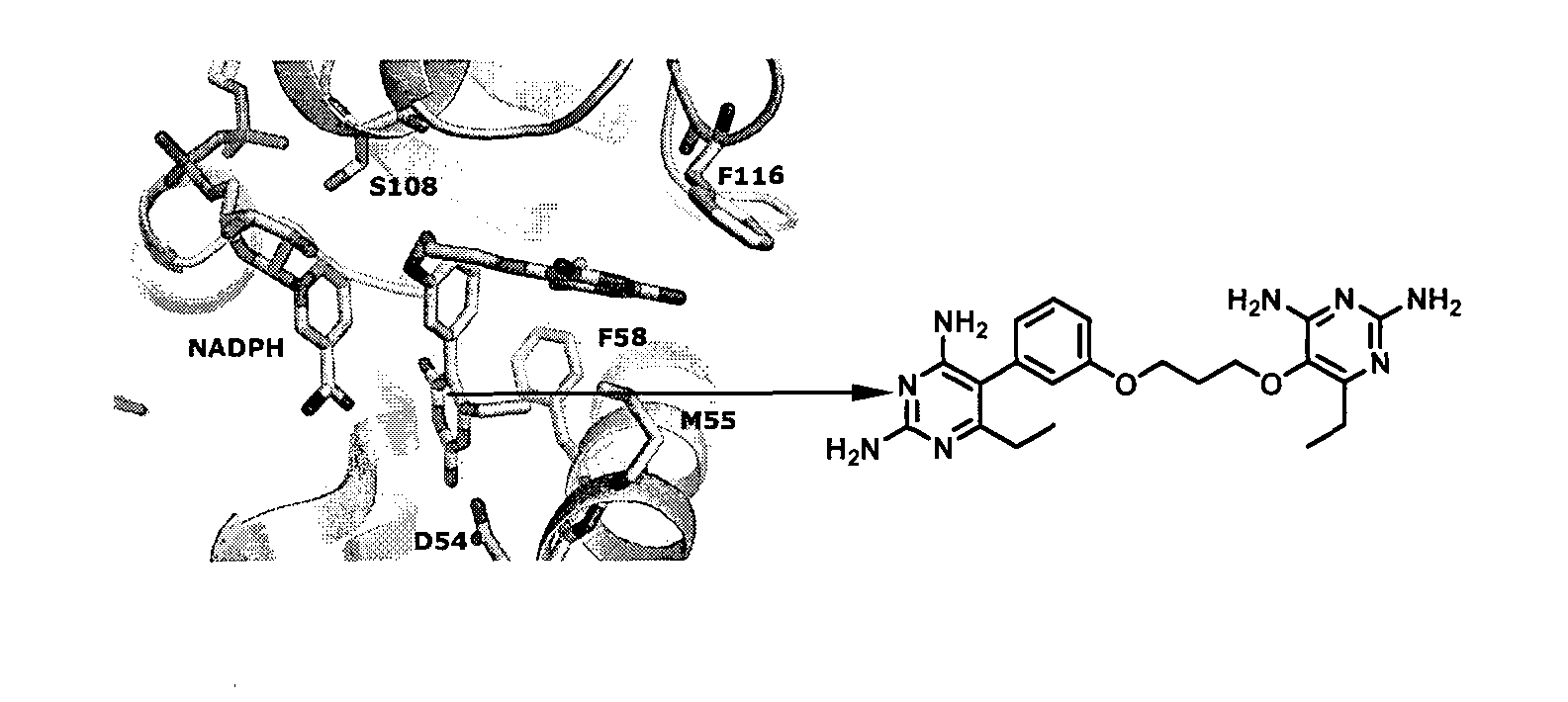
Anti-folate antimalarials with dual-binding modes and their preparation
InactiveUS20130324727A1Avoid developmentOrganic chemistryAgainst vector-borne diseasesWild typeDouble bond
The present invention is anti-folate antimalarials with dual-binding modes of the general formula (I) [refer to structure in the abstract] wherein R1 and R2 which may be the same or different are independently selected from methyl or ethyl or alkyl-phenyl, R3 is independently hydrogen, halide, lower alkyl substituted with ester, carboxylic, amide, and ether. Linker is X(CH2)nY wherein X and Y which may be the same or different are independently selected from oxygen, carbon, nitrogen, substituted phenyl where n is an integer from 1 to 2-6, or pharmaceutically acceptable salts therefore. The anti-folate antimalarials with dual-binding modes act as novel inhibitors with good inhibition constants against wild-type, double (C59R+SIOSN), triple (N51+C59R+SIOSN, C59R+S 1 OSN+I164L), and quadruple (N51+C59R+S108N+I164L) mutant enzymes. The compounds are also effective against wild type (Tm4 / S.2) and mutants (K1CB1, W2, Cs1-2 and V1 / S) malaria parasites.
Owner:NAT SCI & TECH DEV AGENCY
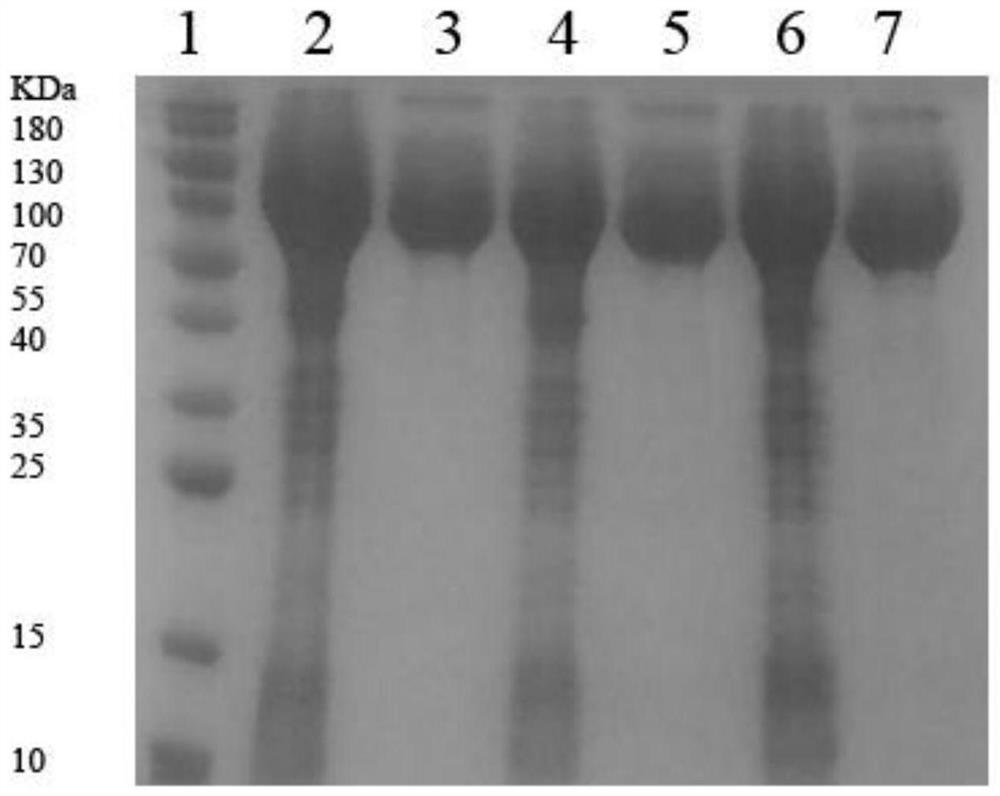
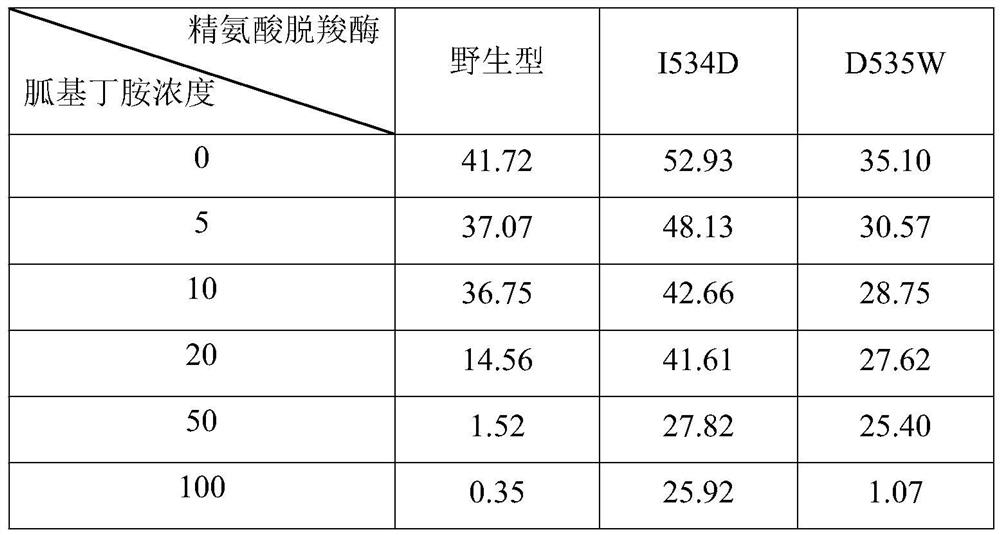
Arginine decarboxylase mutant and application thereof in production of agmatine
ActiveCN111748548AIncreased half-inhibition constantIncrease contentBacteriaMicroorganism based processesArginineArginine decarboxylase
The invention discloses an arginine decarboxylase mutant and an application of the arginine decarboxylase mutant in production of agmatine, which belong to the technical field of biology. The invention provides arginine decarboxylase mutants I534D and D535W which are less influenced by product inhibition. The arginine decarboxylase mutant I534D is obtained by mutating isoleucine at the 534th siteof wild-type arginine decarboxylase of which the amino acid sequence is shown as SEQ ID NO.1 into aspartic acid. The half inhibition constant of the arginine decarboxylase can reach 0.66 mol / L and isincreased by 5.5 times compared with that of wild arginine decarboxylase; the arginine decarboxylase mutant D535W is obtained by mutating aspartic acid at the 535th site of wild type arginine decarboxylase with an amino acid sequence shown as SEQ ID NO.1 into tryptophan, the half inhibition constant of the arginine decarboxylase can reach 0.50 mol / L, and is improved by 4.2 times compared with thatof wild arginine decarboxylase.
Owner:JIANGNAN UNIV
Inhibitors of MEMAPSIN2 and use thereof
The substrate and subsite specificity of the catalytically active enzyme was determined by measuring the initial hydrolysis rate of the substrate using MALDI-TOF / MS. In addition, memapsin subsite specificity was determined by probing the inhibitor library with memapsin2 and detecting bound memapsin2 with a memapsin2 antibody and alkaline phosphatase-conjugated secondary antibody. Substrate and subsite specificity information can be used to design analogs of natural memapsin2 substrates capable of inhibiting memapsin2 function. Substrate analogs are based on peptide sequences related to the native peptide substrate of memapsin2. Substrate analogs contain at least one analog whose amide bond cannot be broken by memapsin2. A synthetic method for analogues containing isosteric substrates at key amino acid residue positions has been developed, and more than 70 substrate analogues have been synthesized, including MM1-005, MM1-012, MM1-017, MM1- 018, MM1-025, MM1-026, MM1-037, MM1-039, MM1-040, MM1-066, MM1-070 and MM1-071 correspond to recombinant pro-memapsin2 with inhibition constants in the range of 1.4-61.4×109M . These inhibitors are useful in the diagnosis, treatment and / or prevention of Alzheimer's disease.
Owner:OKLAHOMA MEDICAL RES FOUND +1
Virtual screening method for micromolecular reversible inhibitor of alkaline metalloproteinase from flavobacterium YS-80-122
InactiveCN103646191AReduce in quantityReduce wasteSpecial data processing applicationsMacromolecular dockingVirtual screening
The invention relates to a virtual screening method for a micromolecular reversible inhibitor of an alkaline metalloproteinase from flavobacterium YS-80-122 and belongs to the field of marine biotechnologies. The virtual screening method comprises the following steps: determining the type of the micromolecular reversible inhibitor according to the known structural data of the alkaline metalloproteinase from the flavobacterium YS-80-122; measuring the inhibition constant of the micromolecular reversible inhibitor of the type to the alkaline metalloproteinase from the flavobacterium YS-80-122, and determining compounds composing a training set; conducting molecular docking on the compounds of the training set and the alkaline metalloproteinase from the flavobacterium YS-80-122; acquiring the theoretical binding free energy and the theoretical reversible inhibition constant of the compounds of the training set and the alkaline metalloproteinase from the flavobacterium YS-80-122; in combination with the measured data, making a screening rule and establishing a screen model; conducting virtual screening. The virtual screening method is high in screening speed, can quickly reduce the quantity of candidate molecules, and reduce waste of time and test materials.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
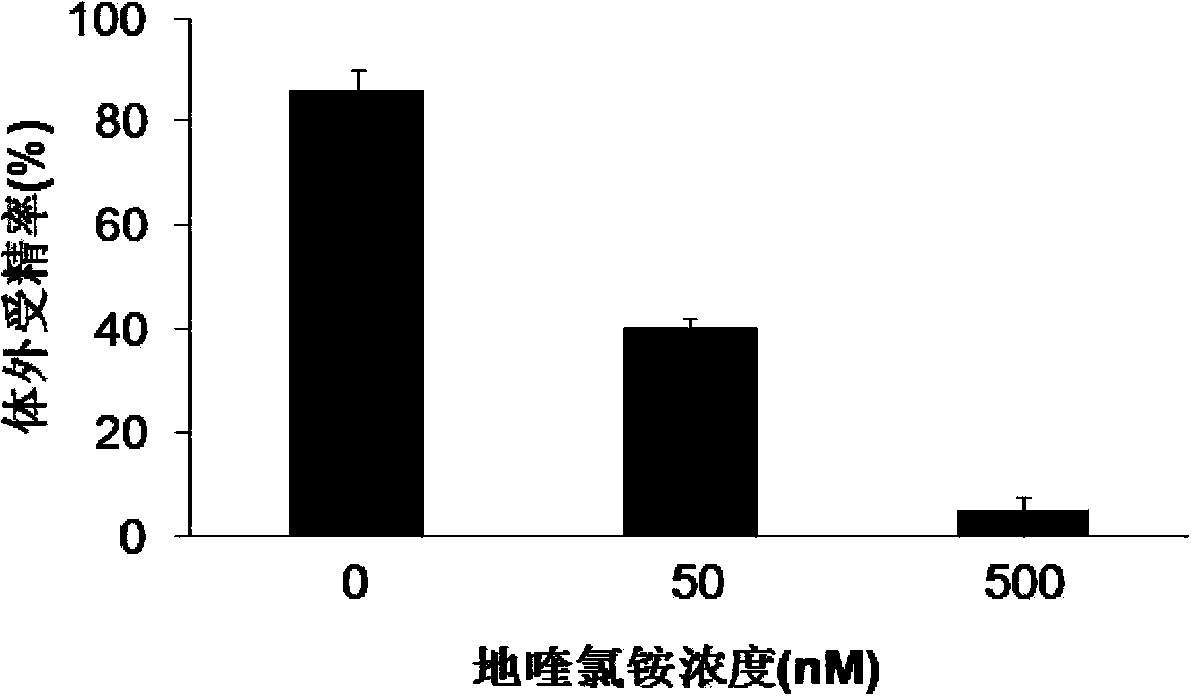
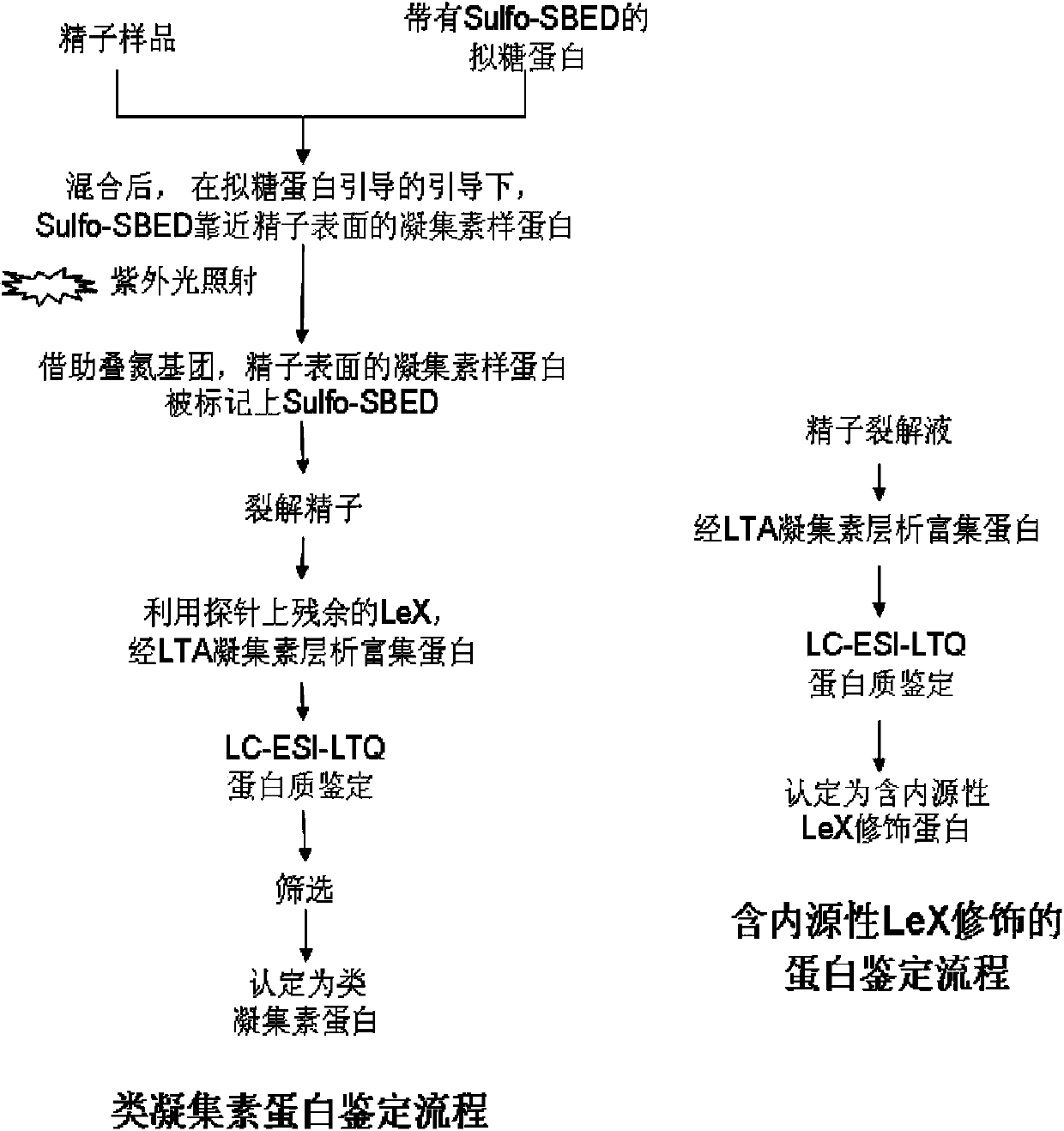
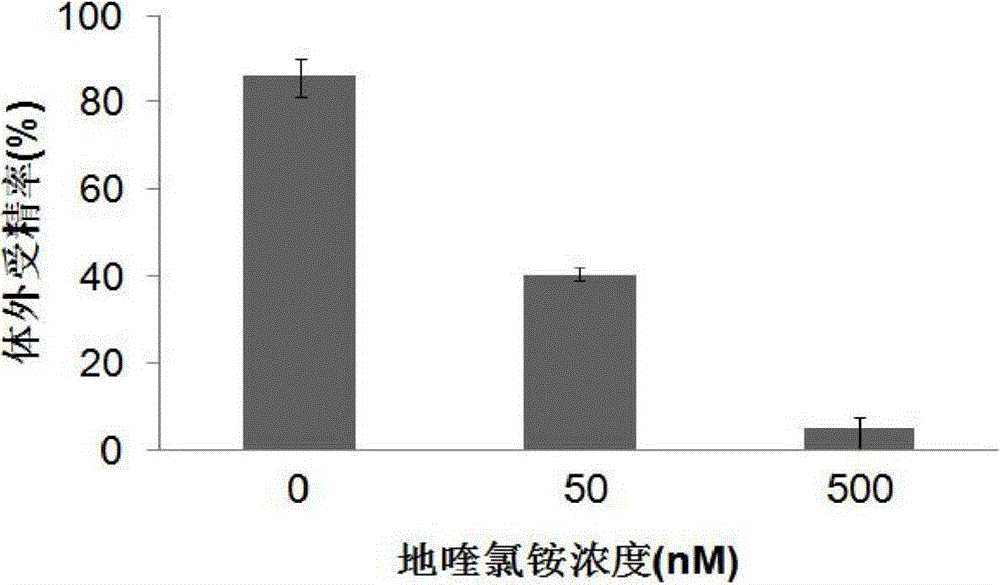
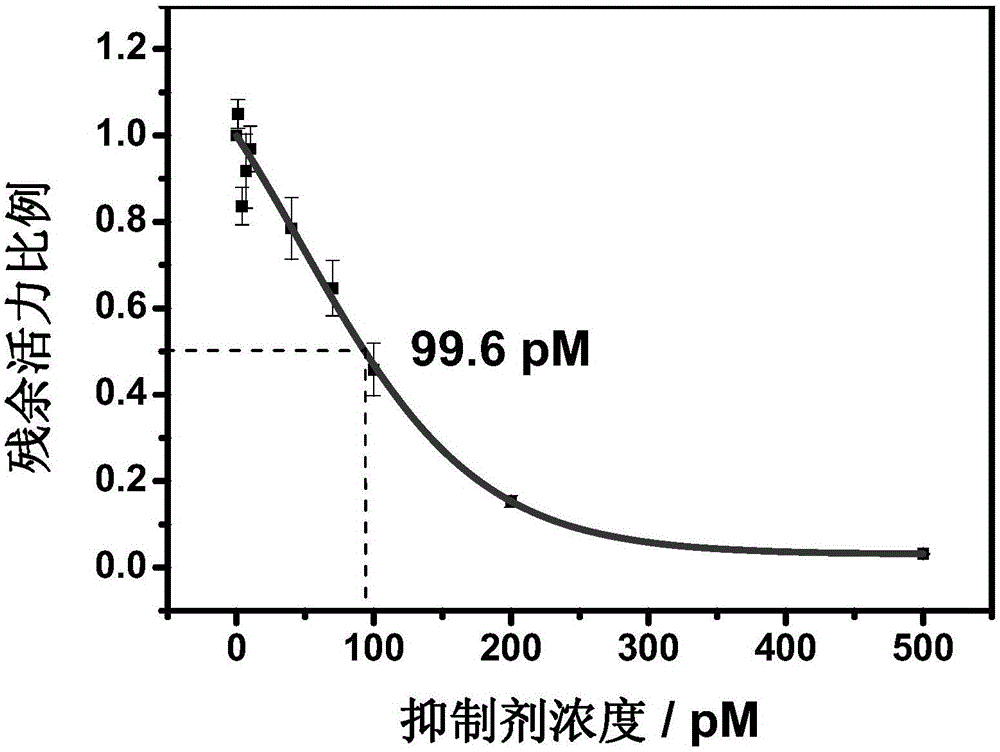
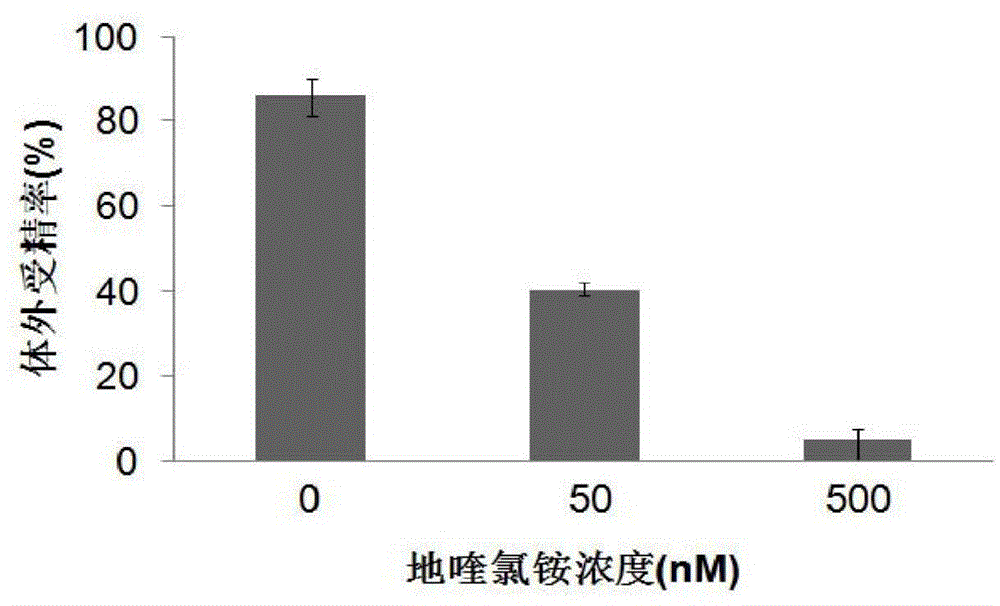
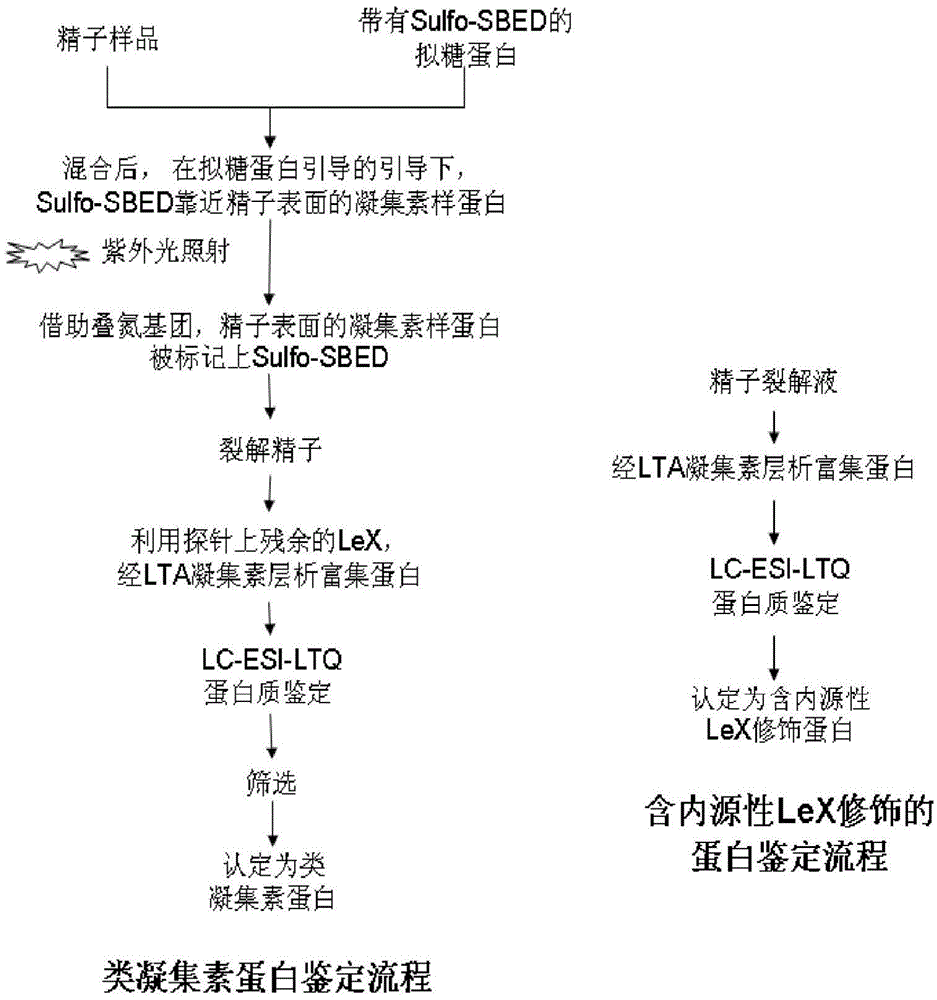
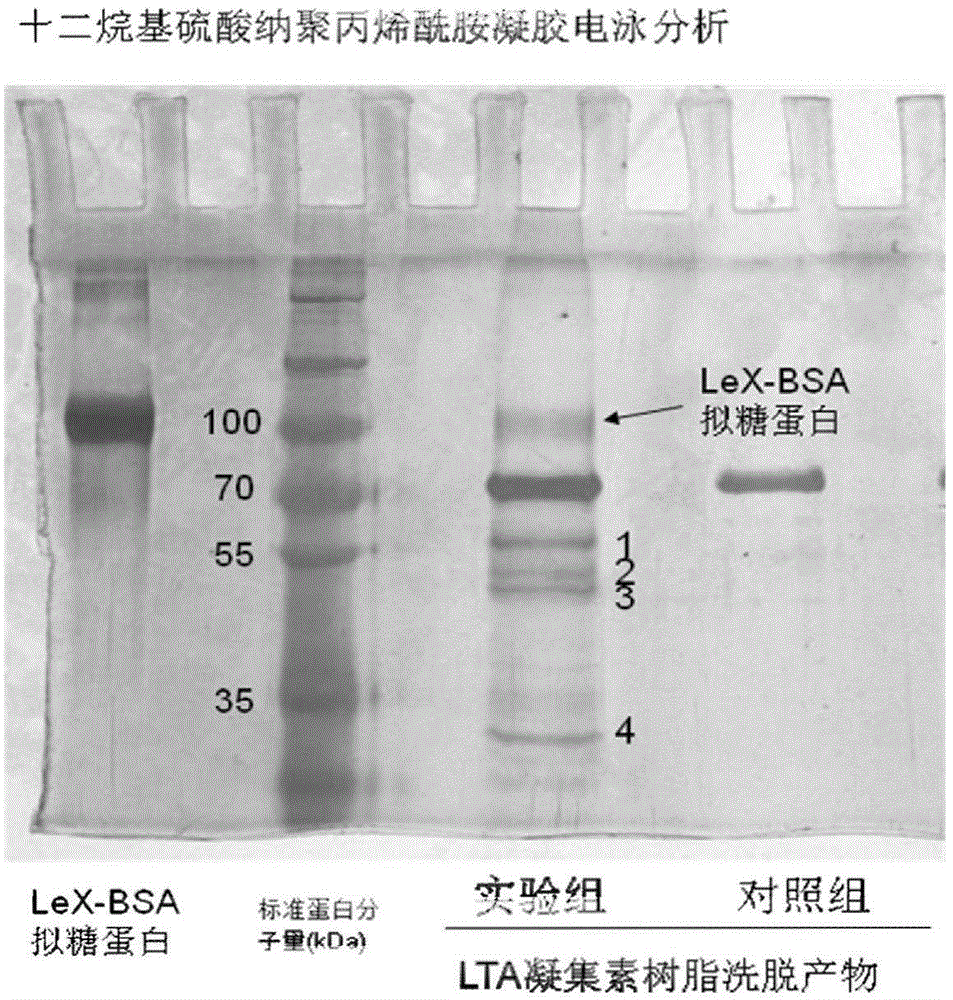
Application of CTBS (Di-N-acetylchitobiase) protein serving as target spot of sperm-egg binding acceptor to screening of sperm-egg binding inhibitor
InactiveCN103743910AControl populationNo harmBiological testingInhibition constantDequalinium chloride
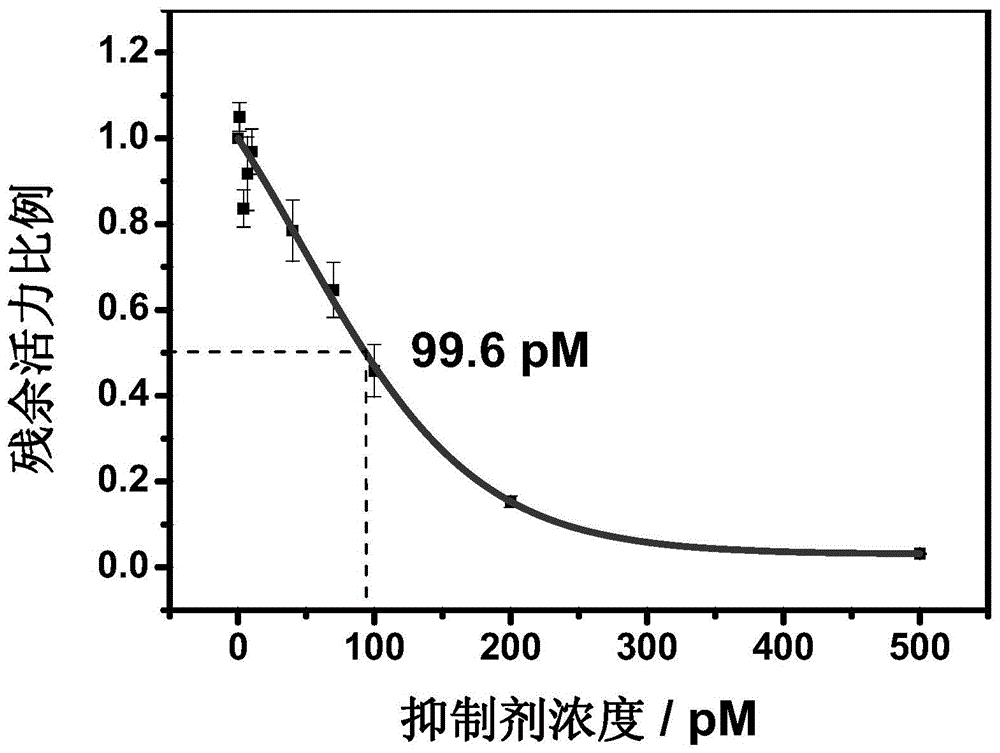
The invention relates to an application of a CTBS (Di-N-acetylchitobiase) protein serving as a target spot of a sperm-egg binding acceptor to screening of a sperm-egg binding inhibitor. According to the application, CTBS is a special sperm-egg binding acceptor on rodent sperm; the CTBS protein only has the specific expression on the sperms of rodents and the protein is not expressed on sperms of human beings; a CTBS antibody can inhibit the sperm-egg binding of mice. The screened sperm-egg binding acceptor is dequalinium chloride and the semi-inhibition constant is less than 100nM. The dequalinium chloride serves as fertility regulation medicine of the mice and the sperm-egg binding of the rodents can be effectively controlled, thus controlling the population quantity of the mice; the aim of controlling mouse damages without pesticides is realized; the damages are not caused when natural enemies of the mice eat the medicine.
Owner:SHANGHAI INST OF PLANNED PARENTHOOD RES +1
Application of king cobra toxin protease inhibitor and its derivatives
InactiveCN101186646AActive specialSimple structurePeptide/protein ingredientsDigestive systemInhibition constantChymotrypsin
The invention relates to a king cobra viper venom inhibitor, and relative derivative. Compared with other natural low-molecular-weight prolease inhibitor, the inventive inhibitor has simple structure, high gene expression yield, and special activity. The preparation method of the king cobra viper venom inhibitor can be prepared by separating and purifying crude venom or prepared by gene engineering. The invention further provides the application of the king cobra viper venom inhibitor and relative derivative, which has double inhibition activity of parenzyme and chymotrypsin, in same magnitude order as the inhibition constant on two proleases, and the inhibition activity with sodium channel.
Owner:KUNMING INST OF ZOOLOGY CHINESE ACAD OF SCI
Kit and method for clinical detection of myeloperoxidase
ActiveCN101914611BGuaranteed accuracyImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsPeroxidaseMedicine
The invention discloses a kit and a method for clinical detection of myeloperoxidase, which belong to the field of biochemical test reagents. The kit provided by the invention consists of three reagents. In the invention, the activity of the myeloperoxidase in a sample is restrained by a myeloperoxidase inhibitor with high specificity, high inhibition and low inhibition constant Ki, the activities of total peroxidase and non-myeloperoxidase are determined respectively, and the difference between the activities of total peroxidase and non-myeloperoxidase is the activity of the myeloperoxidase.The kit provided by the invention has the advantages of low detection cost, small amount of required sample, high determining precision, simple operation and easy popularization.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Hydrazonil Cathepsin K Inhibitor Using Trifluoroethylamine as P2-P3 Linking Group and Its Application
The invention belongs to the technical field of cathepsin K inhibitors, and particularly relates to novel nitrile cathepsin K inhibitors using cathepsin K as target protein and trifluoroethylamino groups as P2-P3 linkers and having different P3 position structures. The P2-P3 linkers are trifluoroethylamino groups, P3 positions are aryl groups comprising a bromophenyl group, a p-biphenyl group, a p-terphenyl group and a p-methyl biphenyl group. The nitrile inhibitors have the advantages that the structures are simple relatively, the synthesis is convenient, and the yield is high; inhibition constants (Ki) of the compounds (the inhibitors) to cathepsin K are in the picomole level, so that the effective inhibiting ability to the cathepsin K is reflected; when the P3 position is the P-terphenyl group, the inhibiting ability and the selectivity of the inhibitor to the Cat K (the cathepsin K) are better than those of corresponding amide inhibitors. In vitro cytotoxicity tests show that the inhibitors are free of cytotoxicity, and have the possibility of being applied to clinical trials and being used as potential anti-osteoporosis drug.
Owner:JILIN UNIV
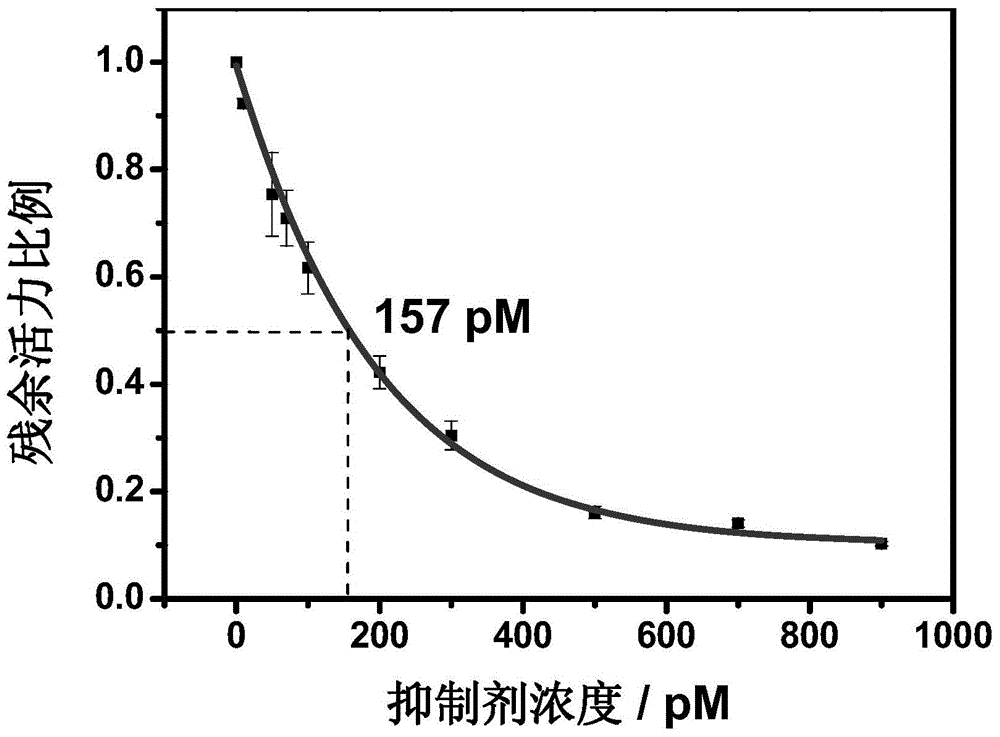
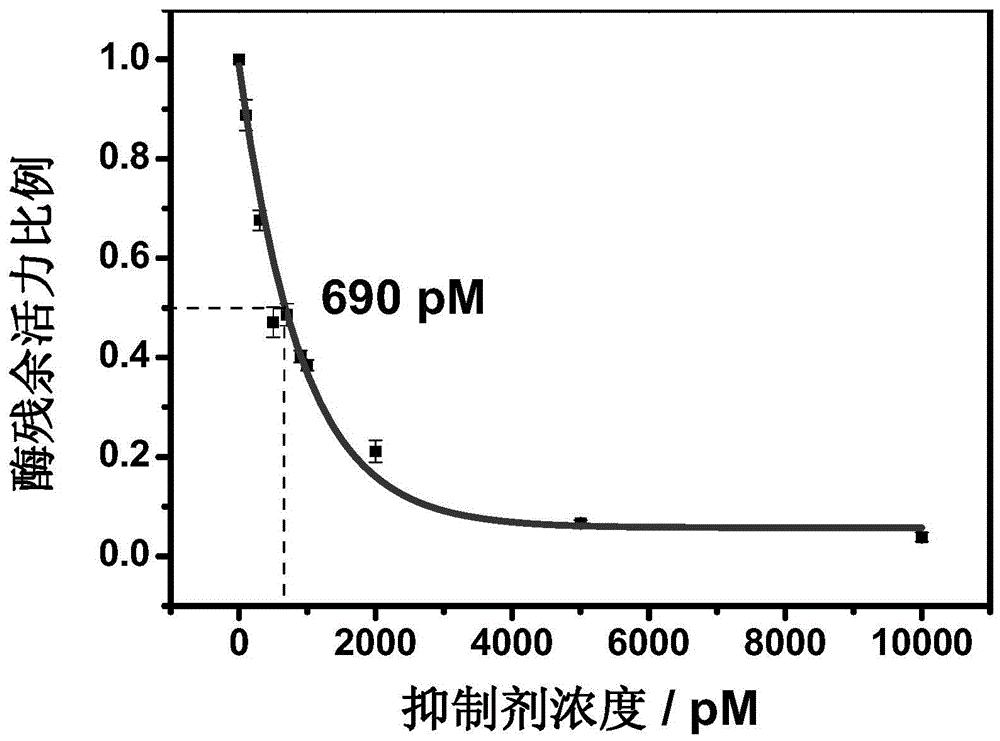
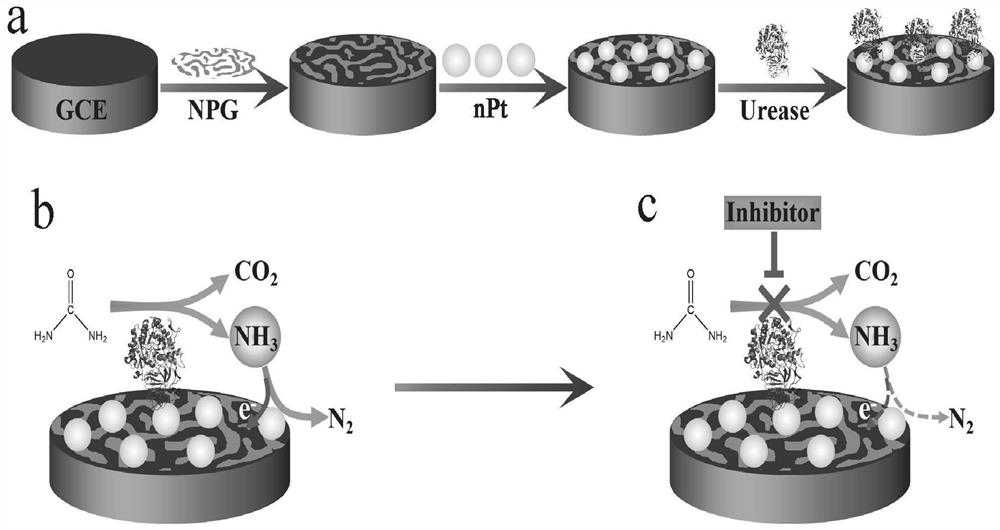
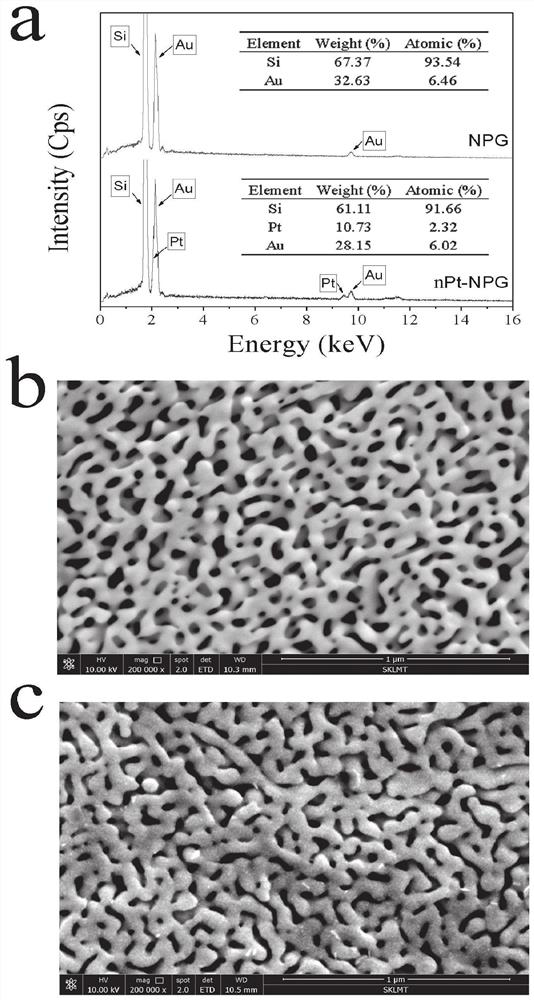
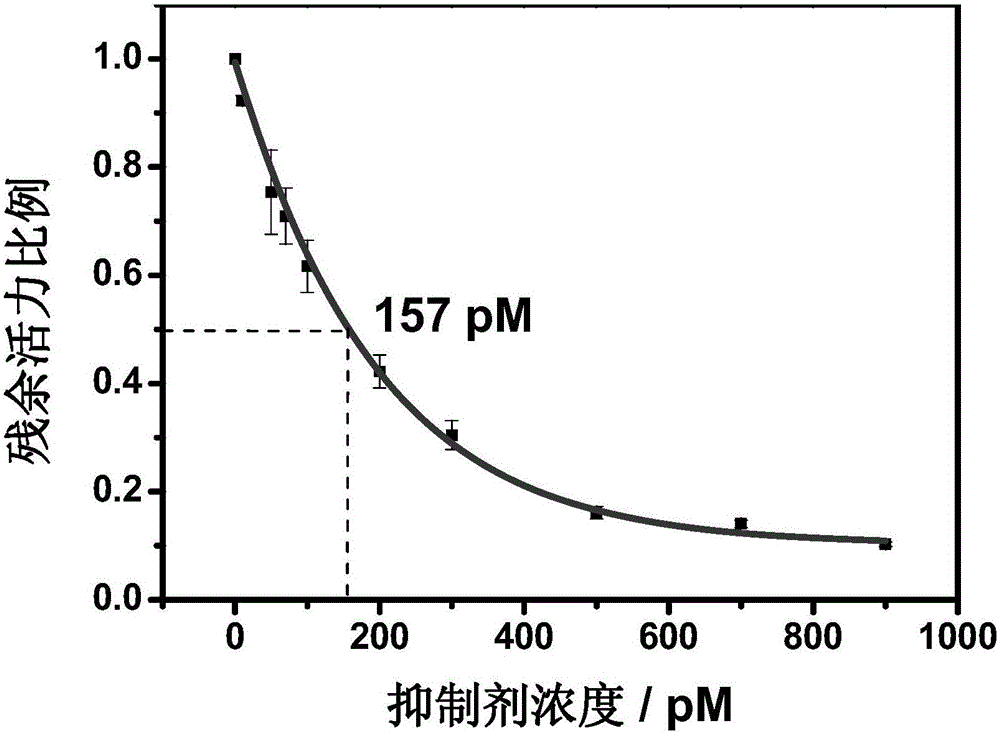
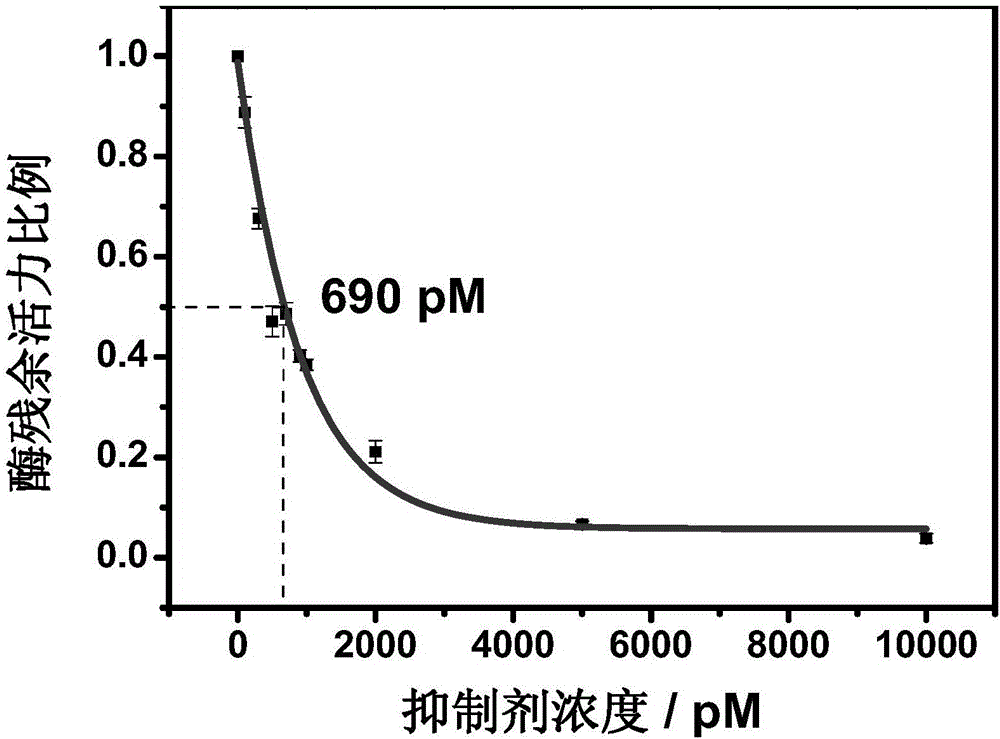
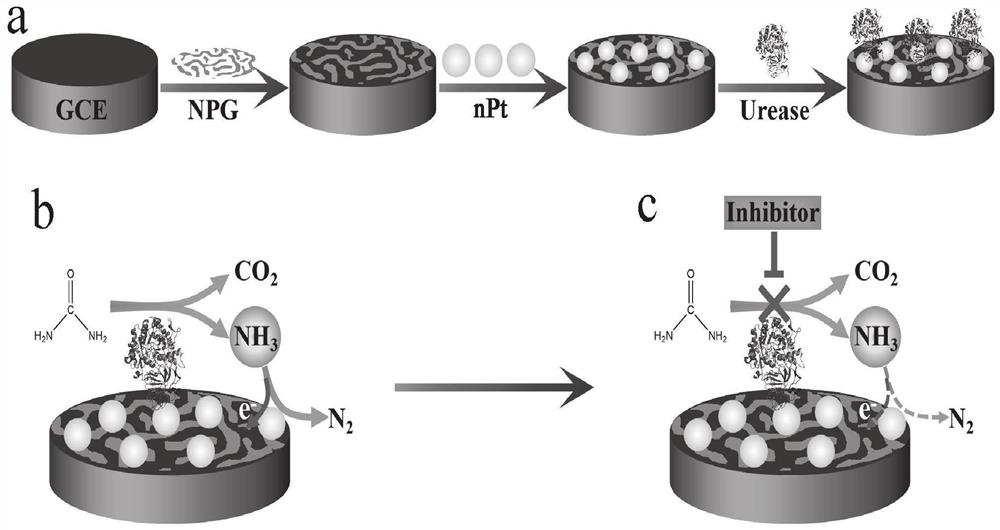
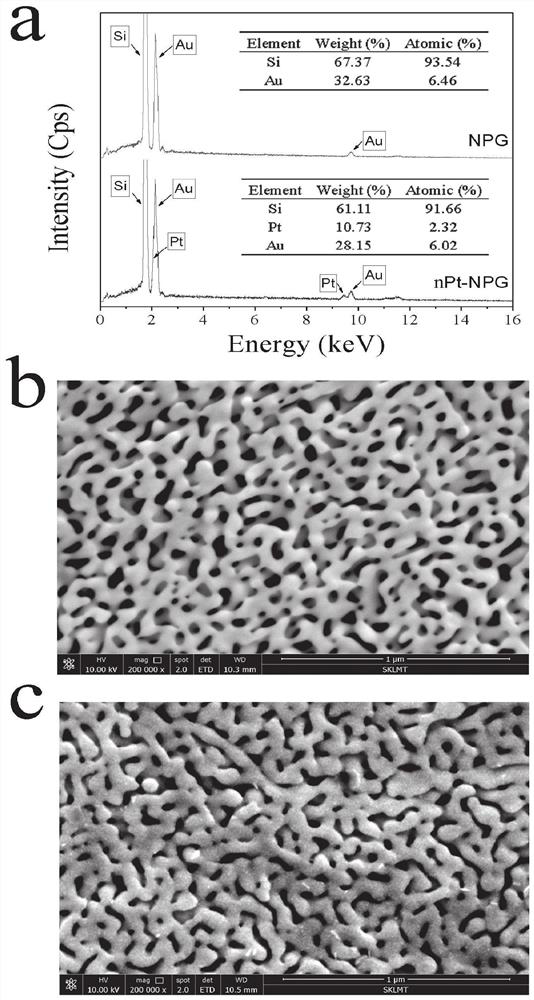
Method for screening helicobacter pylori urease inhibitor
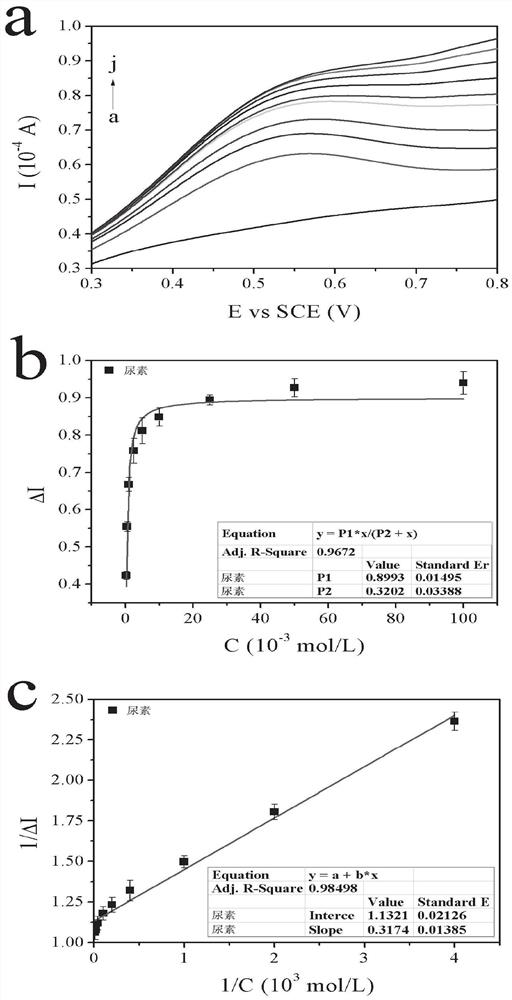
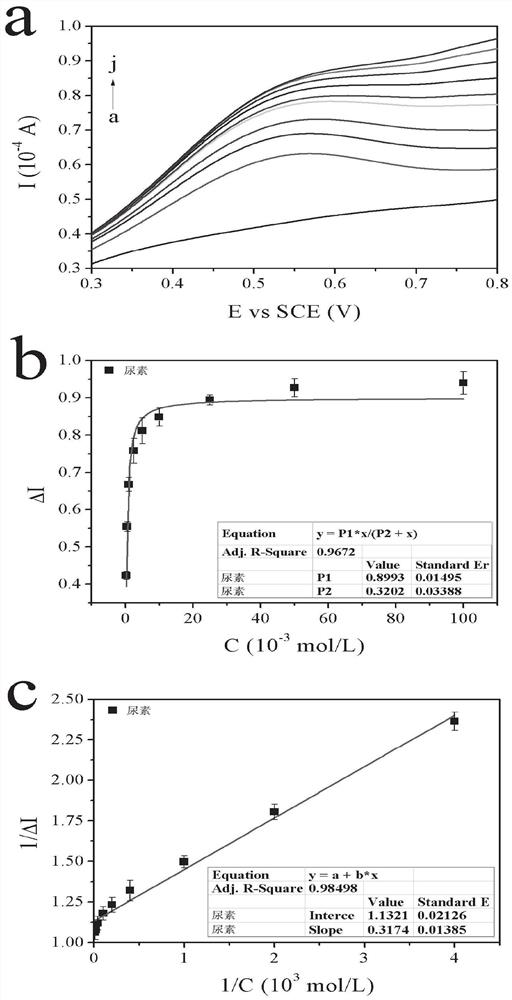
ActiveCN113484388ARealize screeningEfficient screeningMicrobiological testing/measurementBiological material analysisEnzyme Inhibitor AgentHelicobacter pylori
The invention discloses a method for screening a helicobacter pylori urease inhibitor by using an HPUb / nPt / NPG / GCE biosensor. The method comprises the following steps of: (1) preparing and representing a HPUb / nPt / NPG / GCE biosensor; (2) detecting a response current generated by urea with the gradient concentration of 0-100mM by using the HPUb / nPt / NPG / GCE biosensor, and calculating according to a Mie's equation to obtain K<a, urea> and V<max, urea> of the urea detected by the biosensor; and (3) detecting different urease inhibitors, and screening and analyzing inhibition types and inhibition constants of the inhibitors. The HPUb / nPt / NPG / GCE biosensor developed in the method is simple in preparation process, high in detection stability and easy to store. Meanwhile, compared with other detection methods, the screening method provided by the invention does not need a complex sample pretreatment process, is simple to operate and rapid in detection, effectively shortens the potential urease inhibitor screening time, and has a wide application prospect.
Owner:SHANDONG UNIV
A kind of inhibitor and its application in inhibiting chitinase and hexosaminidase activity
ActiveCN106854144BImprove solubilityConvenient researchBiocideOrganic chemistryBiotechnologyOrder Lepidoptera
The invention discloses application of a compound for efficient inhibition of chitinase OfChi-h and hexosaminidase OfHex1 activity, and relates to application of a compound phlegmacin B1 and its derivatives in inhibiting chitinase OfChi-h and hexosaminidase OfHex1 activity. The used final concentration is not less than 13ppm, under the concentration, the measured inhibition rates are 90.4% and 71.4% respectively, and inhibition constants Ki are 5.5 microM and 26 microM respectively. The invention proves that the compound phlegmacin B1 has good application prospect in the prevention and control of agricultural pests, especially in prevention and control of lepidoptera insects.
Owner:DALIAN UNIV OF TECH
Application of ctbs protein as the target of sperm-egg binding receptors in the screening of sperm-egg binding inhibitors
Owner:SHANGHAI INST OF PLANNED PARENTHOOD RES +1
Nitrile cathepsin K inhibitors using trifluoroethylamino groups as P2-P3 linkers, and application of inhibitors
The invention belongs to the technical field of cathepsin K inhibitors, and particularly relates to novel nitrile cathepsin K inhibitors using cathepsin K as target protein and trifluoroethylamino groups as P2-P3 linkers and having different P3 position structures. The P2-P3 linkers are trifluoroethylamino groups, P3 positions are aryl groups comprising a bromophenyl group, a p-biphenyl group, a p-terphenyl group and a p-methyl biphenyl group. The nitrile inhibitors have the advantages that the structures are simple relatively, the synthesis is convenient, and the yield is high; inhibition constants (Ki) of the compounds (the inhibitors) to cathepsin K are in the picomole level, so that the effective inhibiting ability to the cathepsin K is reflected; when the P3 position is the P-terphenyl group, the inhibiting ability and the selectivity of the inhibitor to the Cat K (the cathepsin K) are better than those of corresponding amide inhibitors. In vitro cytotoxicity tests show that the inhibitors are free of cytotoxicity, and have the possibility of being applied to clinical trials and being used as potential anti-osteoporosis drug.
Owner:JILIN UNIV
A kind of method of screening Helicobacter pylori urease inhibitor
ActiveCN113484388BRealize screeningEfficient screeningMicrobiological testing/measurementBiological material analysisUrease enzymeHelicobacter pylori
The invention discloses a method for screening Helicobacter pylori urease inhibitors using a HPUb / nPt / NPG / GCE biosensor. The steps include 1) preparation and characterization of the HPUb / nPt / NPG / GCE biosensor; / NPG / GCE biosensor detects the response current generated by urea with gradient concentration of 0-100mM, and calculates the K of urea detected by the biosensor according to the Mie equation a,尿素 and V max,尿素 3) Detecting different urease inhibitors, screening and analyzing the inhibition type and inhibition constant of each inhibitor. The HPUb / nPt / NPG / GCE biosensor developed in the method of the present invention has a simple preparation process, high detection stability, and is easy to store. At the same time, compared with other detection methods, the screening method provided by the present invention does not require complicated sample pretreatment processes. Moreover, the operation is simple, the detection is rapid, the screening time of potential urease inhibitors is effectively shortened, and the application prospect is broad.
Owner:SHANDONG UNIV
Inhibitors of MEMAPSIN2 and use thereof
Methods for the production of purified, catalytically active, recombinant memapsin 2 have been developed. The substrate and subsite specificity of the catalytically active enzyme have been determinedby a method which determines the initial hydrolysis rate of the substrate by using MALDI-TOF / MS. Alternatively, the subsite specificity of mepapsin can be determined by probing a library of inhibitorswith memapsin 2 and subsequently detecting the bound memapsin 2 with an antibody raised to memapsin 2 and an alkaline phosphatase conjugated secondary antibody. The substrate and subsite specificityinformation was used to design substrate analogs of the natural memapsin 2 substrate that can inhibit the function of memapsin2. The substrate analogs are based on peptide sequences, shown to be related to the natural peptide substrates for memapsin 2. The substrate analogs contain at least one analog of an amide bond which is not capable of being cleaved by memapsin 2. Processes for the synthesisof substrate analogues including isoteres at the sites of the critical amino acid residues were developed and the more than seventy substrate analogues were synthetized, among which MMI-005, MMI-012,MMI-017, MMI-018, MMI-025, MMI-026, MMI-037, MMI-039, MMI-040, MMI-066, MMI-070, and MMI-071 have inhibition constants in the range of 1.4-61.4 x 10<9> M against recombinant pro-memapsin 2. These inhibitors are useful in diagnostics and for the treatment and / or prevention of Alzheimer's disease.
Owner:OKLAHOMA MEDICAL RES FOUND +1
Anti-folate antimalarials with dual-binding modes and their preparation
The present invention is anti-folate antimalarials with dual-binding modes of the general formula (I) [refer to structure in the abstract] wherein R1 and R2 which may be the same or different are independently selected from methyl or ethyl or alkylphenyl, R3 is independently hydrogen, halide, lower alkyl substituted with ester, carboxylic, amide, and ether. Linker is X(CH2)nY wherein X and Y which may be the same or different are independently selected from oxygen, carbon, nitrogen, substituted phenyl where n is an integer from 1 to 2-6, or pharmaceutically acceptable salts therefore. The anti-folate antimalarials with dual-binding modes act as novel inhibitors with good inhibition constants against wild-type, double (C59R+SIOSN), triple (N51+C59R+SIOSN, C59R+S 1 OSN+I164L), and quadruple (N51+C59R+S108N+I164L) mutant enzymes. The compounds are also effective against wild type (Tm4 / S.2) and mutants (K1CB1, W2, Cs1-2 and V1 / S) malaria parasites.
Owner:NAT SCI & TECH DEV AGENCY
Application of dequalinium chloride as mouse birth control medicine
InactiveCN103749457BControl populationControl sperm-egg unionBiocideAnimal repellantsHuman bodyInhibition constant
The invention relates to an application of dequalinium chloride as a mouse birth control medicine. Researches find that CTBS (di-N-acetylchitobiase) is a unique sperm-egg binding receptor on the sperms of rodents, the inhibitor dequalinium chloride can effectively inhibit in vitro fertilization of a mouse, the semi-inhibition constant is less than 100nM, and the toxic effect on a human body is tiny; the researches also find that the CTBS is expressed on the sperms of rodents, the CTBS antibody can inhibit in vitro fertilization of the mouse, and the protein is not expressed on the sperms of the human body. According to the application provided by the invention, dequalinium chloride is used as a mouse birth control medicine and can effectively control the sperm-egg binding of rodents to achieve the aims of controlling the population quantity of mice and realizing mouse damage control without needing pesticides; moreover, after eating the medicine, the natural enemy of the mice does not cause harm.
Owner:SHANGHAI INST OF PLANNED PARENTHOOD RES +1
High activity cellobiase, coding gene and applications thereof
InactiveCN104312997AStrong decompositionHigh activityBacteriaMicroorganism based processesFiberCellobiase activity
The invention discloses high activity cellobiase, a coding gene and applications thereof. The cellobiase is a (a) protein or a (b) protein. The (a) protein is composed of amino acid sequences represented by the SEQ ID No.2 of the sequence table; and the (b) protein is derived from the (a) protein by substituting and / or losing and / or adding one or several amino acid residues to the amino acid sequences represented by the SEQ ID No.2 of the sequence table, and has the high cellobiase activity. The cellobiase has a strong activity on decomposing cellobiose, and the maximal reaction speed can reach 740.39 + / - 10.23 U / mg. In the aspect of product glucose inhibition, the cellobiase has a very good tolerant performance, and the inhibition constant (Ki) is as high as 800 mM. Researches show that the provided cellobiase has the advantages of high catalytic activity, high tolerance, and heat resistance, and has a great potential in production, application, and promotion.
Owner:SOUTH CHINA UNIV OF TECH
Application of berberine and its derivatives as hexosaminidase inhibitors
The invention discloses application of berberine and a derivative of the berberine as a hexosaminidase inhibitor. The general structure formula of the hexosaminidase inhibitor is shown as I or II; in all screened 35 compounds, the berberine and the derivative of the berberine show certain inhibition activity on hexosaminidase OfHex1; particularly, the suppression rate of Sysu-00021 and Sysu-00679 on the OfHex1 enzyme is respectively 76 percent and 79 percent, and the inhibition constant Ki is respectively 2mu M and 1.8mu M. Compound applicable object determination shows that under the final concentration of 10mu M, the compound shows higher inhibition activity on the hexosaminidase OfHex1; no inhibition activity is shown on the hexosaminidase HsHexB derived from the human. In a word, the berberine and the derivative of the berberine have wide application prospects in the fields of biology, chemicobiology and the like.
Owner:DALIAN UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com