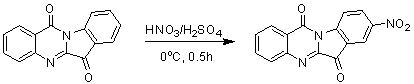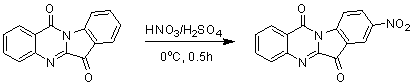Use of tryptanthrin compound as indoleamine 2,3-dioxygenase (IDO) inhibitor
A dioxygenase and compound technology, applied in the field of medicine, can solve the problems of difficulty in meeting research and clinical medication, long separation process, low extraction rate, etc., and achieve the effect of good potential for research and development of new drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
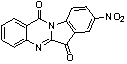
[0021] Embodiment 1: the preparation of 8-nitrotryptanthrin
[0022]
[0023]First add 1ml of concentrated sulfuric acid to the reaction flask, under ice cooling, add tryptanthrin (248mg, 1mmol) in batches, then add dropwise (0.15ml) concentrated nitric acid, and react for 1h. After the reaction, it was poured into ice water and drained to obtain a yellow-green 8-nitrotryptanthrin solid. Yield 90%. The characterization data are as follows: 1 H NMR (400 MHz, CDCl 3 ): δ= 8.86(d, 1H), 8.75(d, 1H), 8.69(m, 1H), 8.49(m, 1H), 8.09(d, 1H), 7.95(m, 1H), 7.76(d, 1H).
Embodiment 2
[0024] Example 2: Preliminary detection of IDO inhibitory activity
[0025] The construction, expression, extraction and purification of the plasmid containing human IDO gene were carried out according to the method reported by Littlejohn et al. (Protein Exp Purif, 2000. 19(1): 22-9). The inhibitory activity of compounds against IDO was tested according to the method introduced. Mix 50 mM potassium phosphate buffer (pH 6.5), 40 mM vitamin C, 400 μg / ml catalase, 20 μM methylene blue, substrate L-tryptophan and the sample to be tested, and incubate the mixture at 37 °C for 5 minutes , then add IDO enzyme to the above mixture, react at 37 °C for 30 minutes, add 200 μl of 30% (w / v) trichloroacetic acid to terminate the reaction, and heat the reaction system at 65 °C for 15 minutes to complete from The conversion of formylkynurenine to kynurenine was followed by centrifugation at 12,000 rpm for 10 min, and the supernatant was mixed with an equal volume of 2% (w / v) p-dimethylaminob...
Embodiment 3
[0027] Example 3: Determination of whether it is reversible inhibition
[0028] In the case of a fixed inhibitor concentration, a series of different concentrations of the enzyme are reacted with the inhibitor and the reaction rate is determined. Plot the reaction speed against the enzyme concentration (ν~[E]), and judge whether it is a reversible inhibitor or not according to the characteristics of the curve.
[0029] Reaction conditions: In a 500 μl reaction system, first add 50 mM potassium phosphate buffer (pH 6.5), 40 mM vitamin C, 400 μg / ml catalase, 20 μM methylene blue, 300 mM substrate L-tryptamine Acid or 100 mM inhibitor (8-nitrotryptanthrin) was added at the same time, and the mixture was incubated at 37 °C for 5 minutes, and then different volumes of IDO enzyme were added to the above mixture (for 8-nitrotryptanthrin, the amount of enzyme added The volumes were 0.5, 1, 2, 3, 4, 5, 6, 7 μl), the reaction was carried out at 37 °C for 30 minutes, and 200 μl of 30% (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com