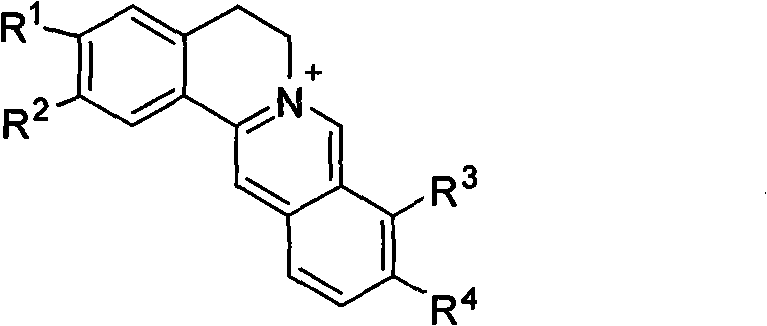
Application of berberine and derivatives thereof in preparation of indole amine 2, 3-dioxygenase inhibitor
A technology of dioxygenase and derivatives, applied in the field of medicine, can solve the problems such as the use of berberine and its derivatives that are not seen, and achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
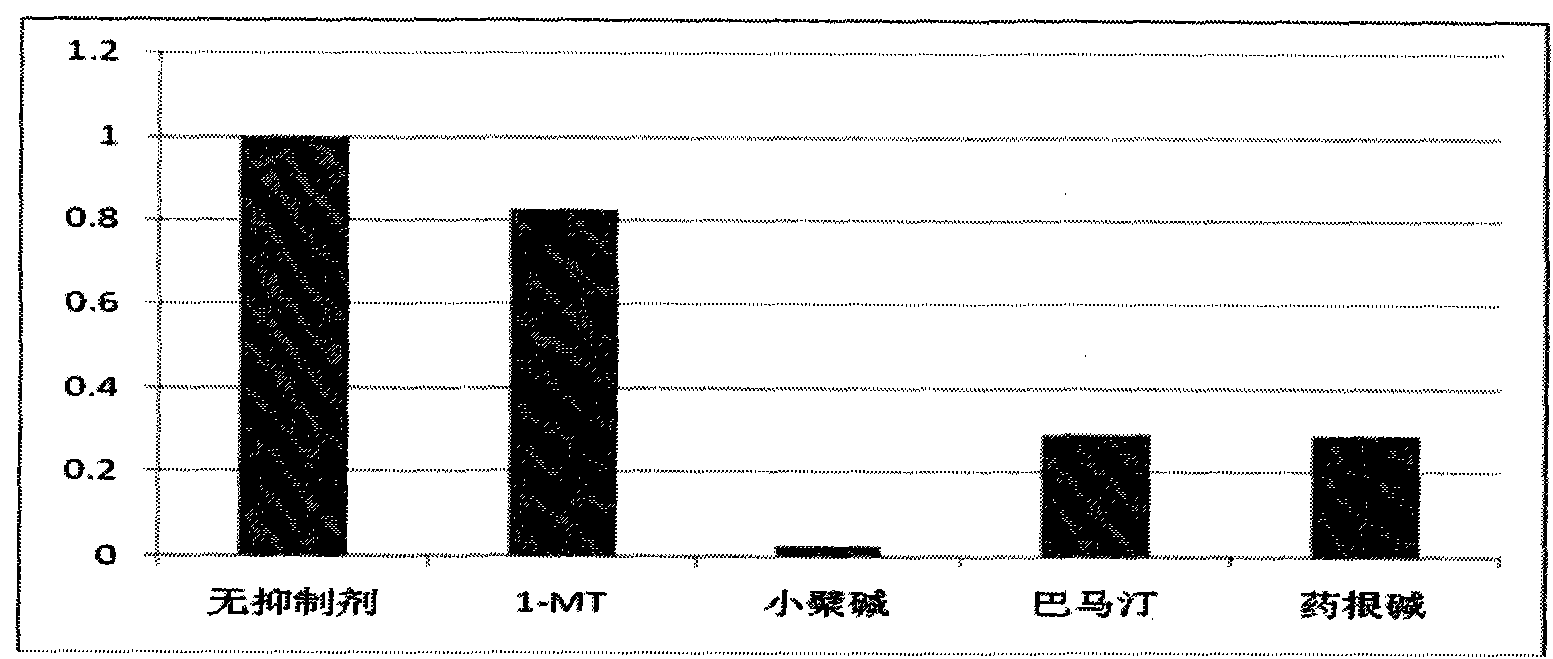
[0039] 1) Detection of IDO inhibitory activity:
[0040] Reaction conditions: 50mM potassium phosphate buffer (pH 6.5), 40mM vitamin C, 400ug / ml catalase, 20uM methylene blue, substrate L-tryptophan and the sample to be tested were mixed, and the mixture was incubated at 37°C for 5 minutes. Then add IDO enzyme to the above mixture, react at 37°C for 30 minutes, add 200ul of 30% (w / v) trichloroacetic acid to terminate the reaction, and heat the reaction system at 65°C for 15 minutes to complete the reaction from formyl The conversion of kynurenine to kynurenine, and then 12000rpm rotation for 10 minutes, the supernatant was mixed with an equal volume of 2% (w / v) p-dimethylaminobenzaldehyde in acetic acid solution, and kynurenine reacted with it The resulting yellow color can be observed at 490nm using a microplate reader.
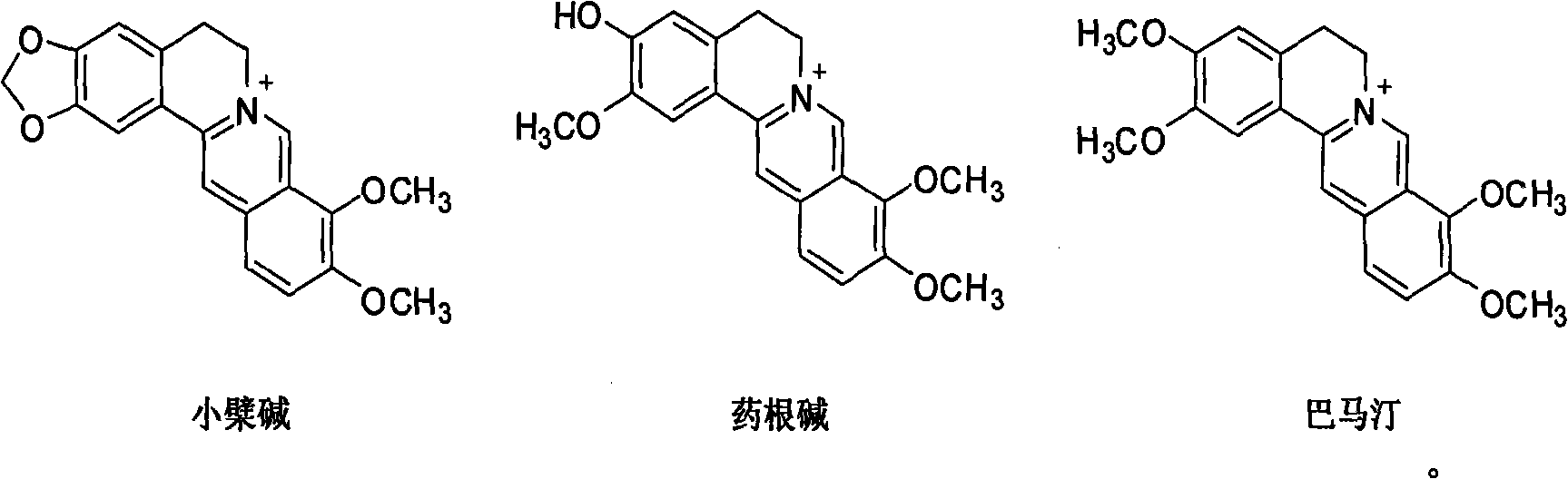
[0041] The test results show that the inhibitory effect of berberine, jatrorrhizine hydrochloride and palmatine hydrochloride on IDO is better than that of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com