Arginine decarboxylase mutant and application thereof in production of agmatine
A technology of arginine decarboxylase and agmatine, which is applied in the biological field and can solve problems such as the limitation of agmatine production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
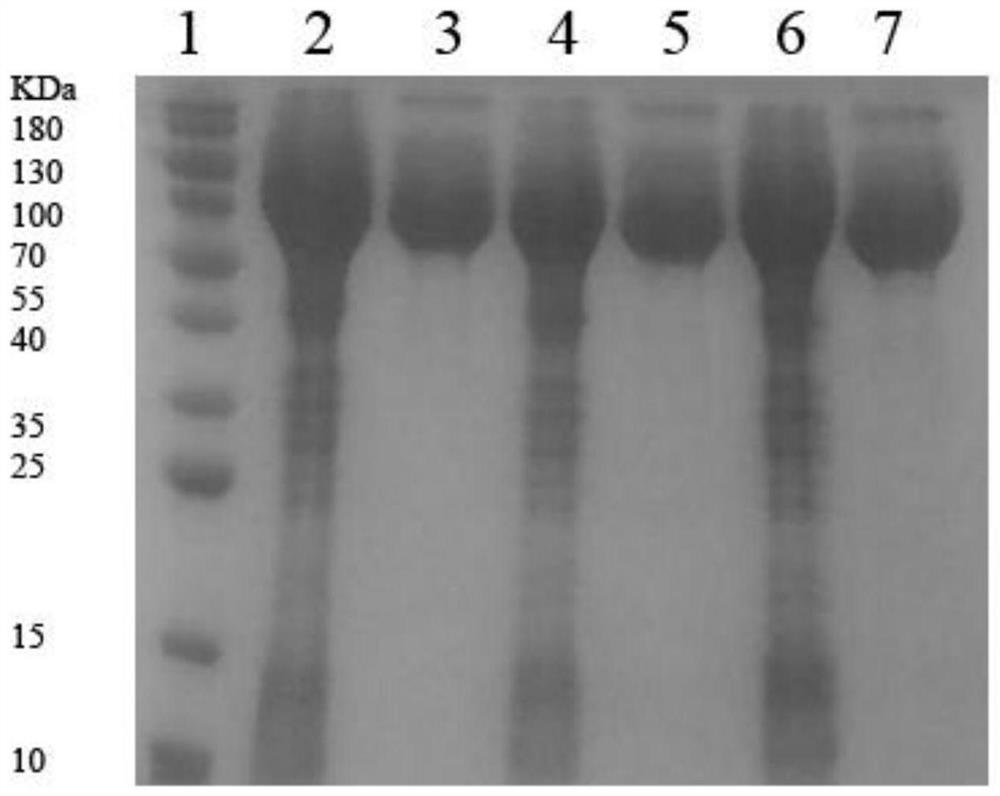
[0045] Example 1: Preparation of Arginine Decarboxylase Mutants
[0046] Specific steps are as follows:
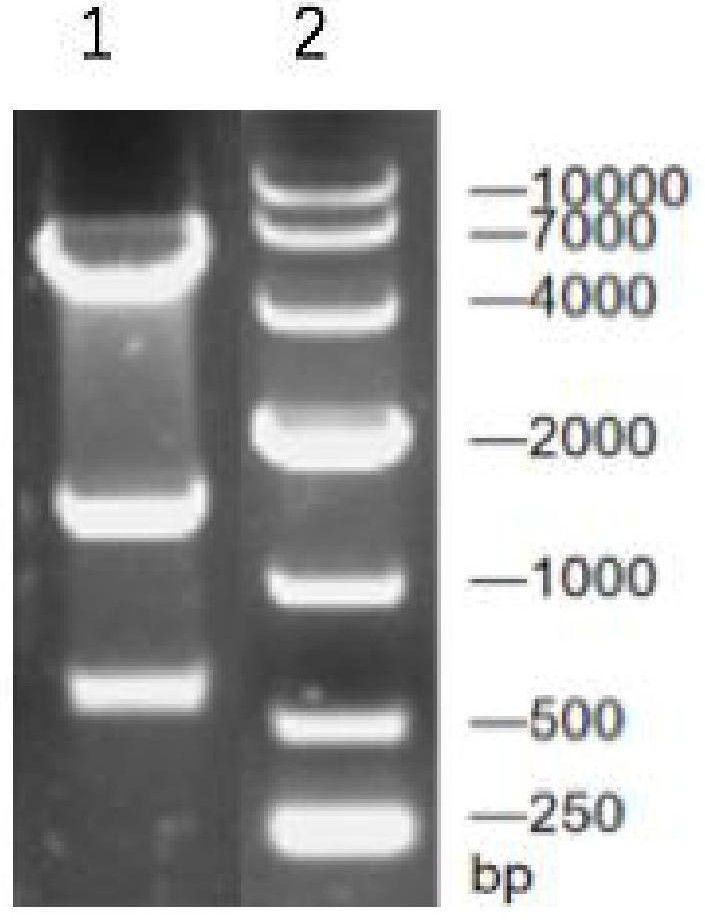
[0047] Synthetic nucleotide sequence is as the gene speA of coding arginine decarboxylase shown in SEQ ID NO.2; The gene speA of the coding arginine decarboxylase that will obtain and pET-28a (+) plasmid pass through EcoR I / BamH I After double enzyme digestion and ligation, the ligation product was obtained; the ligation product was transformed into Escherichia coli BL21 to obtain the transformation product; the transformation product was spread on LB solid medium (containing 50 μg·mL -1 Kanamycin), cultured upside down in a constant temperature incubator at 37°C for 8-12 hours to obtain transformants; pick transformants and inoculate them into LB liquid medium, and shake the flask for 8-12 hours at 37°C and 180rpm Afterwards, the plasmid was extracted for enzyme digestion verification (for the verification results, see figure 1 ) and sequencing verification, if the veri...
Embodiment 2
[0063] Example 2: Product inhibition of arginine decarboxylase mutants to agmatine
[0064] Specific steps are as follows:
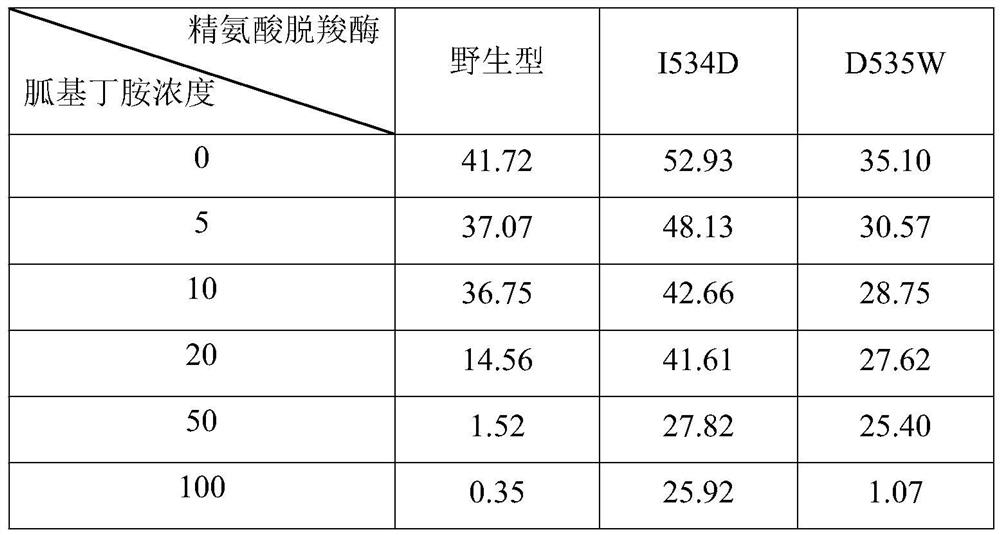
[0065] Add concentrations of 0, 5, 10, 20, 50, 100 g / L (corresponding to 0.0000, 0.0385, 0.0769, 0.1538, 0.3846, 0.7692 mol / L) agmatine, to obtain reaction systems containing different concentrations of agmatine; use the reaction systems containing different concentrations of agmatine to detect the wild-type arginine decarboxylase and arginine decarboxylation obtained in Example 1, respectively. The specific enzyme activity of the pure enzyme of enzyme mutant I534D and arginine decarboxylase mutant D535W (detection result is shown in Table 1); Obtain wild-type arginine decarboxylase, arginine decarboxylase mutation according to the detection result of specific enzyme activity Half-inhibition constant of body I534D and arginine decarboxylase mutant D535W to agmatine; wherein, half-inhibition constant=the corresponding concentration of agmatine when the ...
Embodiment 3
[0069] Embodiment 3: the production of agmatine (whole cell transformation method+recombinant Escherichia coli)
[0070] Specific steps are as follows:
[0071]The transformation system was added with 20g / L L-arginine, 4mmol / L MgSO 4 , the Tris-HCL buffer (pH 8.5, 50mmol / L) of 7mmol / L pyridoxal phosphate (PLP); the recombinant Escherichia coli BL21 / pET-28a-speA, BL21 / pET-28a-speA that embodiment 1 obtains -1 and BL21 / pET-28a-speA-2 cells were added to the transformation system in an amount of 30g / L, and transformed at 37°C and 220r / min for 12h to obtain transformation liquid.
[0072] Detect the content of agmatine and the conversion rate of L-arginine in the conversion liquid, and the detection results are shown in Table 2;
[0073] Wherein, the calculation formula of L-arginine conversion rate is as follows:
[0074] The conversion rate of L-arginine=the amount of agmatine produced (mol) / the initial amount of L-arginine added (mol)×100%.
[0075] It can be seen from Tabl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com