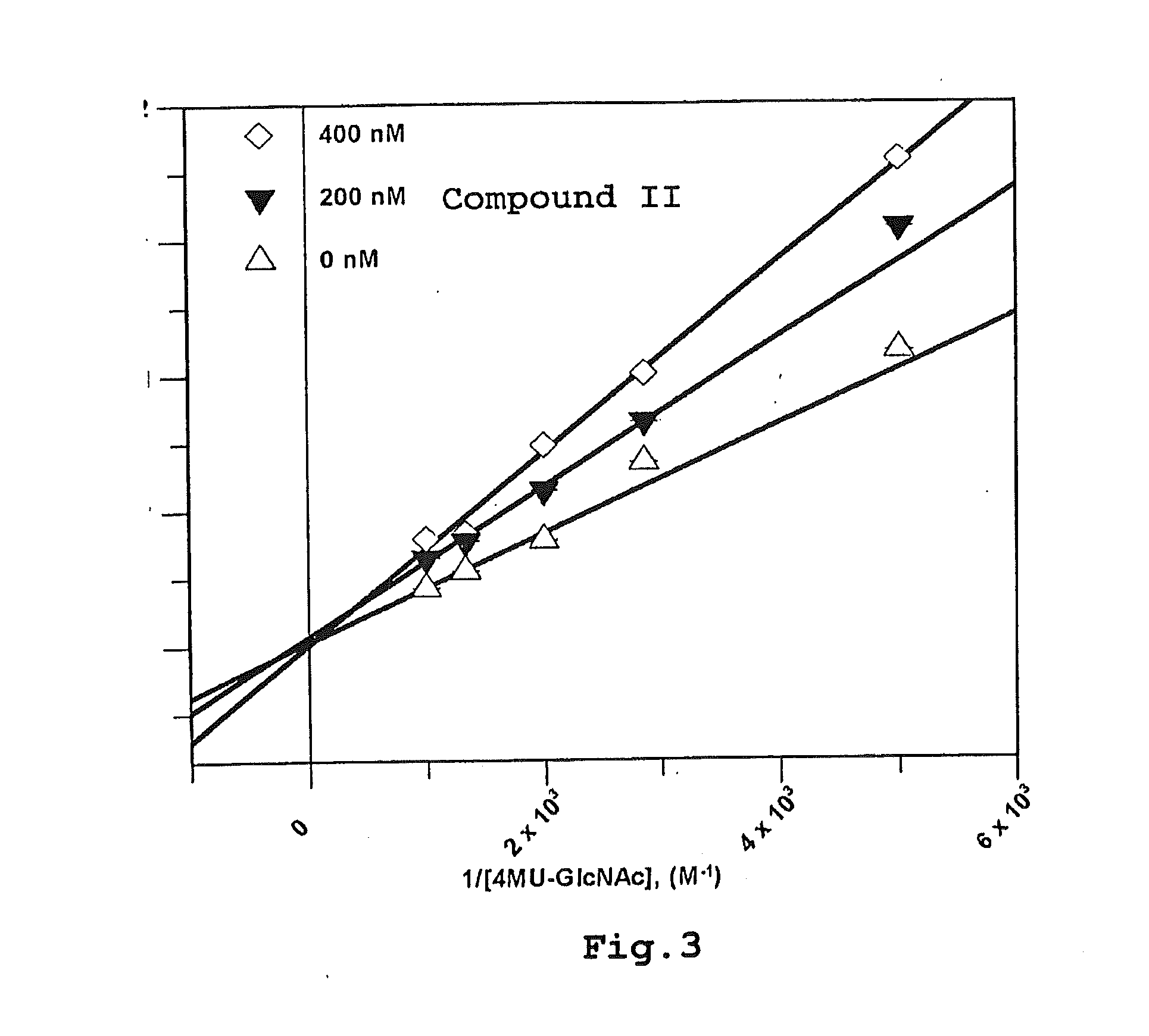
Patents
Literature
35 results about "Hexosaminidase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hexosaminidase (EC 3.2.1.52, beta-acetylaminodeoxyhexosidase, N-acetyl-beta-D-hexosaminidase, N-acetyl-beta-hexosaminidase, N-acetyl hexosaminidase, beta-hexosaminidase, beta-acetylhexosaminidinase, beta-D-N-acetylhexosaminidase, beta-N-acetyl-D-hexosaminidase, beta-N-acetylglucosaminidase, hexosaminidase A, N-acetylhexosaminidase, beta-D-hexosaminidase) is an enzyme involved in the hydrolysis of terminal N-acetyl-D-hexosamine residues in N-acetyl-β-D-hexosaminides.
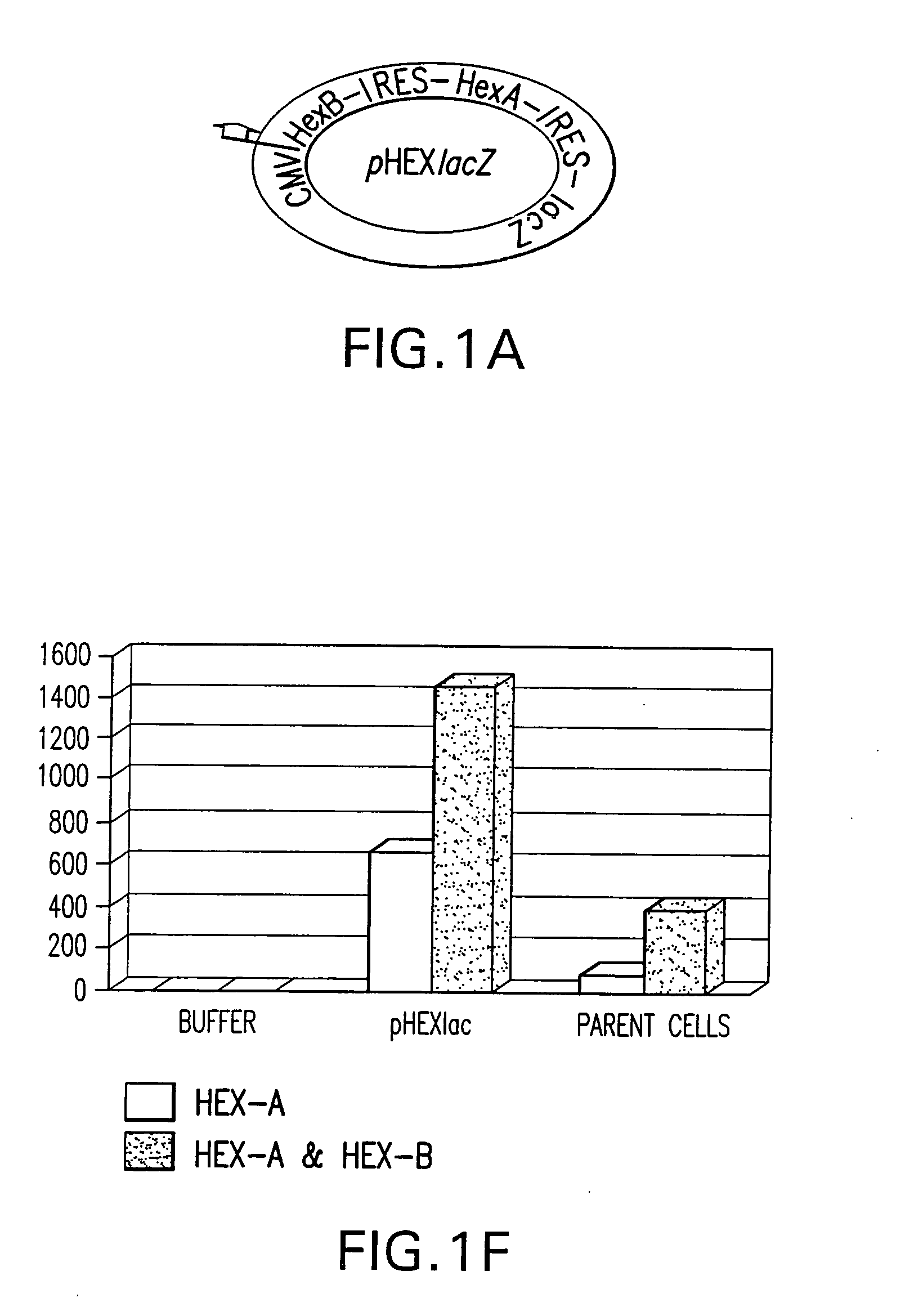
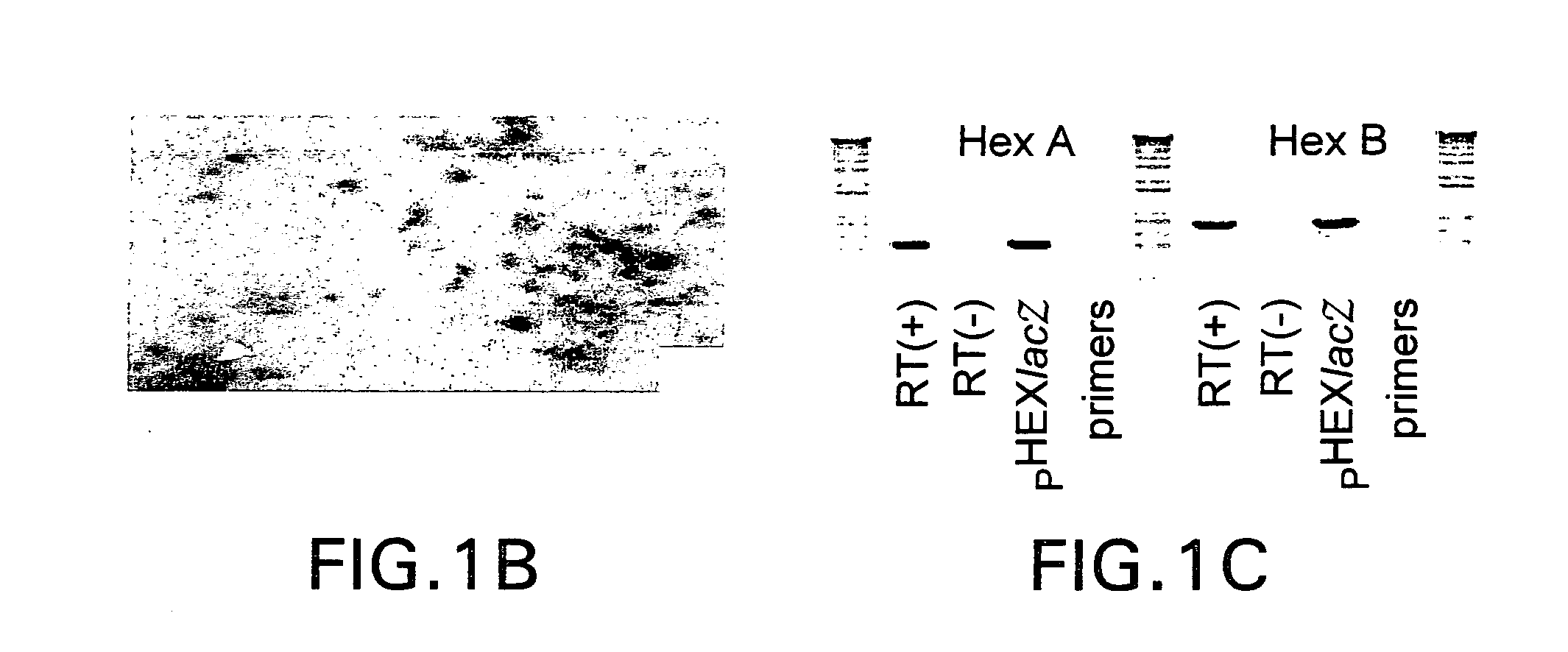
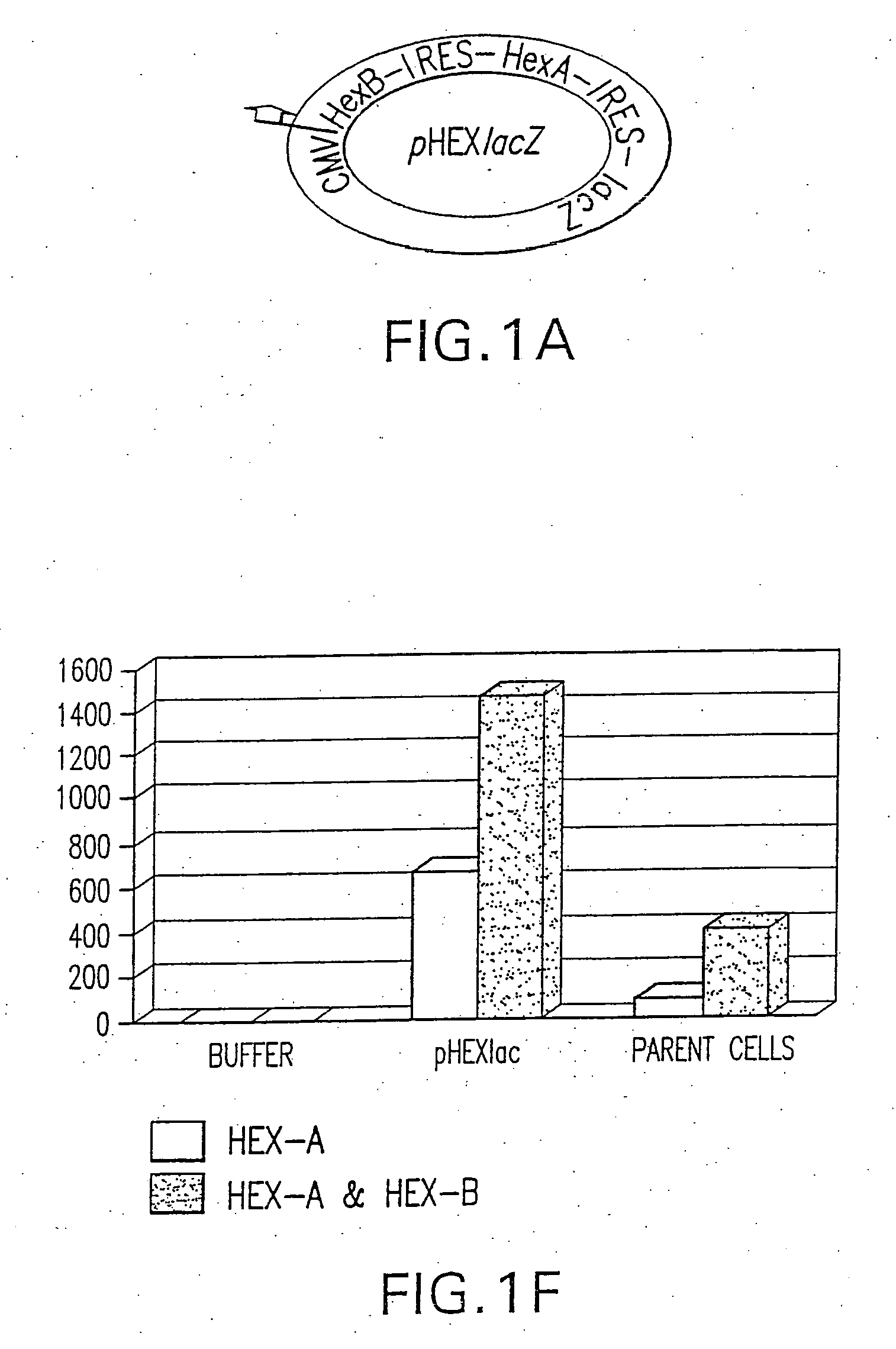
Vectors having both isoforms of beta-hexosaminidase and uses of the same
Disclosed are compositions and methods related to nucleic acid constructs containing a HexB encoding element and a HexA encoding element. These constructs can be used in the treatment of Tay-Sachs and Sandoff disease.
Owner:UNIVERSITY OF ROCHESTER
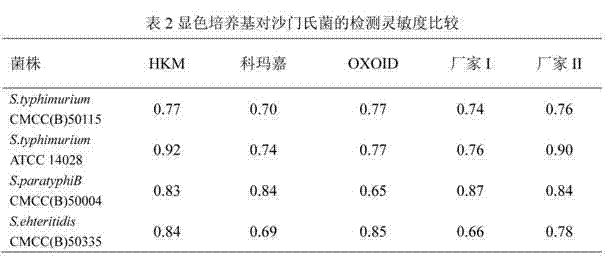
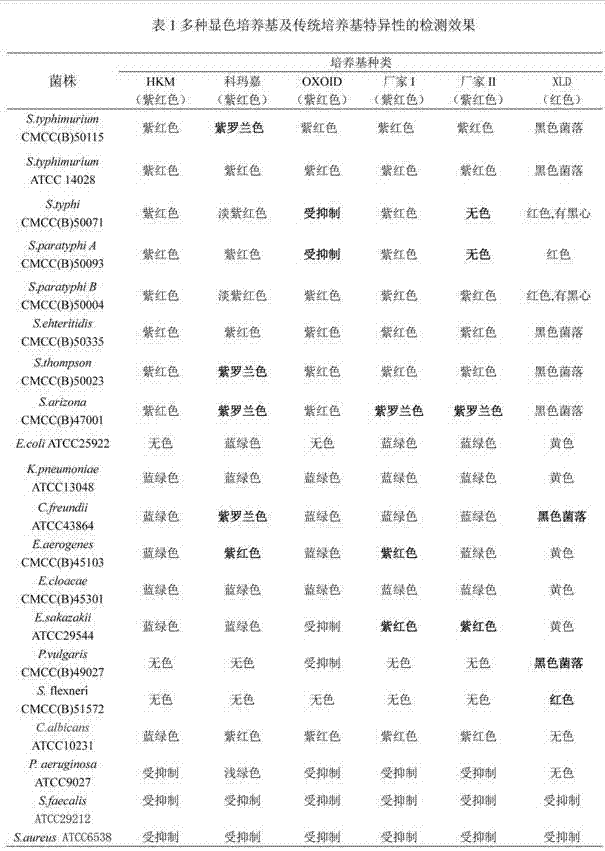
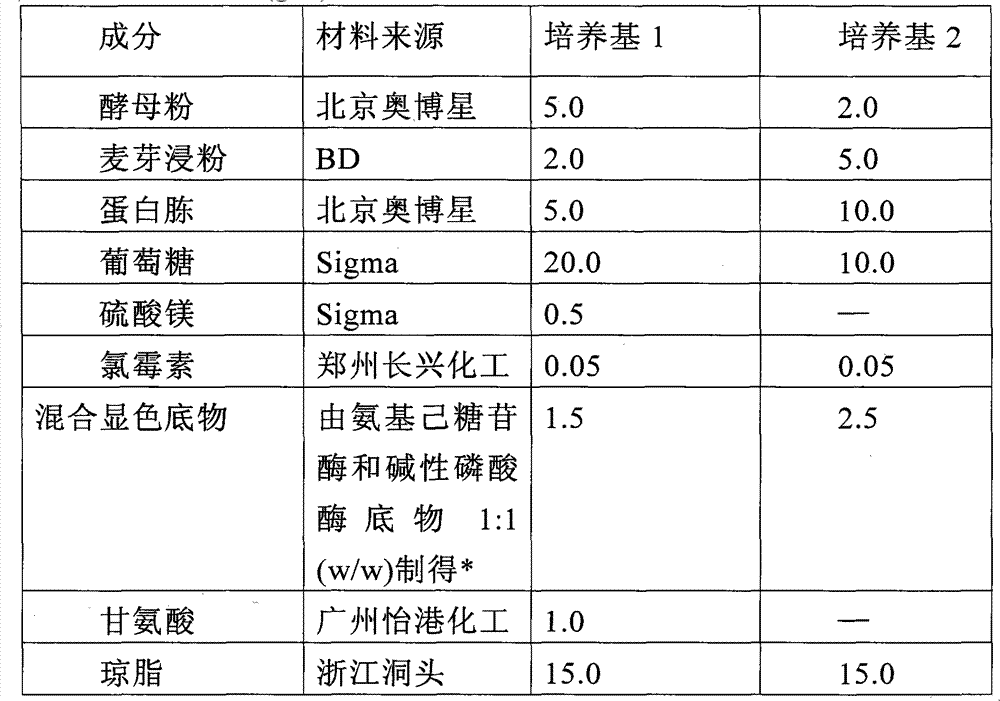
Chromogenic medium for detecting salmonella
InactiveCN102827918AHigh detection sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesBiotechnologyActive agent
The invention discloses a chromogenic medium for detecting salmonella, which belongs to the fields of food safety and clinical microbiological detection. The culture medium contains agar, peptone, beef extract powder, sodium chloride, a surfactant, cholate, an enzyme inducer, an octoate enzyme chromogenic substrate, a beta-galactosidase chromogenic substrate, a hexosaminidase chromogenic substrate and robiocina. The chromogenic medium disclosed by the invention is used for detecting salmonella, has high detection sensitivity and high specificity, and can be used for initially identifying strains directly according to the color of a colony; the chromogenic medium has high operability, and suitable for treating large-reflux samples, and can be used for comprehensively, systematically and accurately detecting and initially identifying salmonella in food production, clinical disease diagnosis and the environment; and a new way is provided for rapid detection of microorganisms.
Owner:GUANGDONG HUANKAI MICROBIAL SCI & TECH
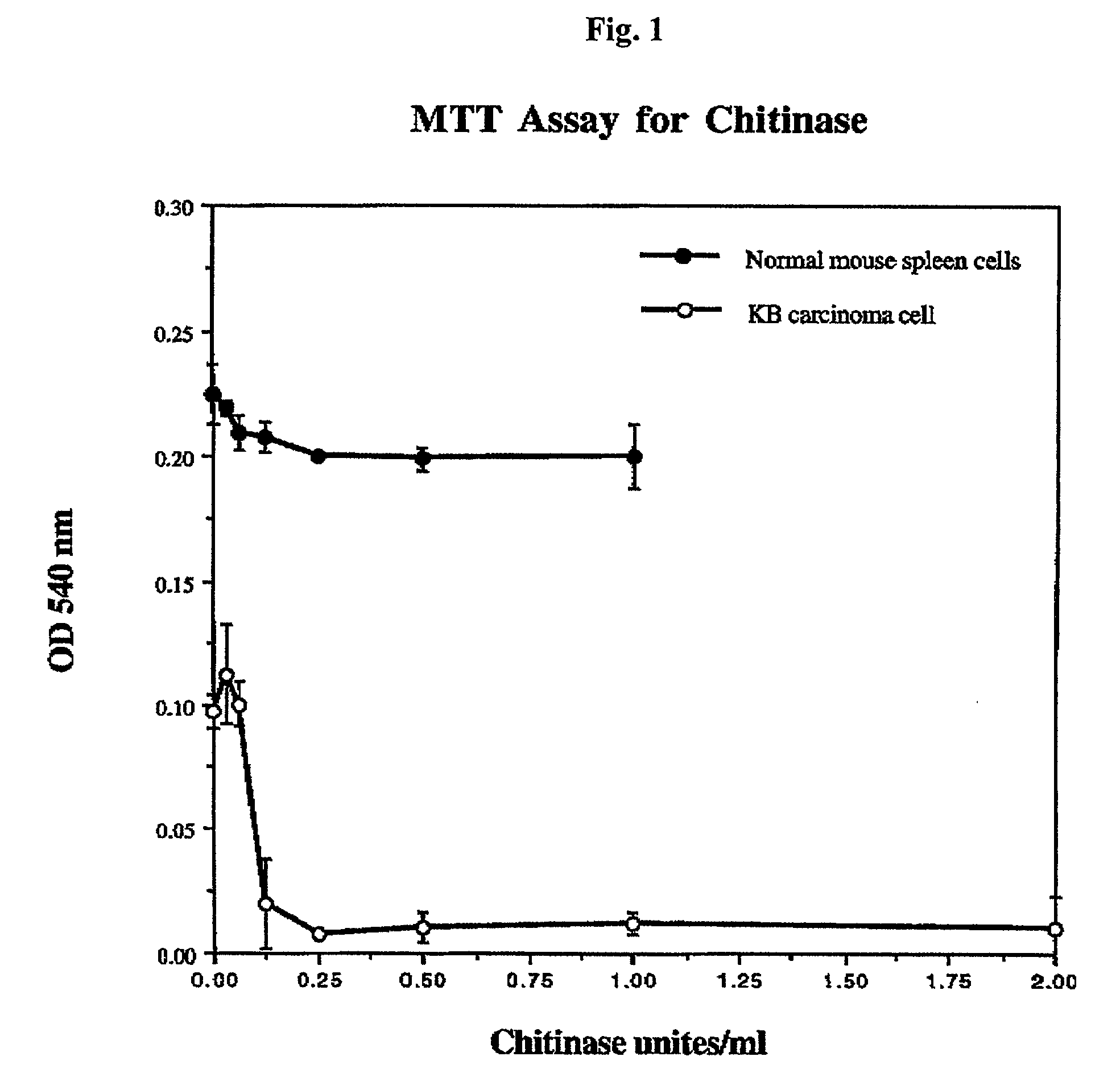
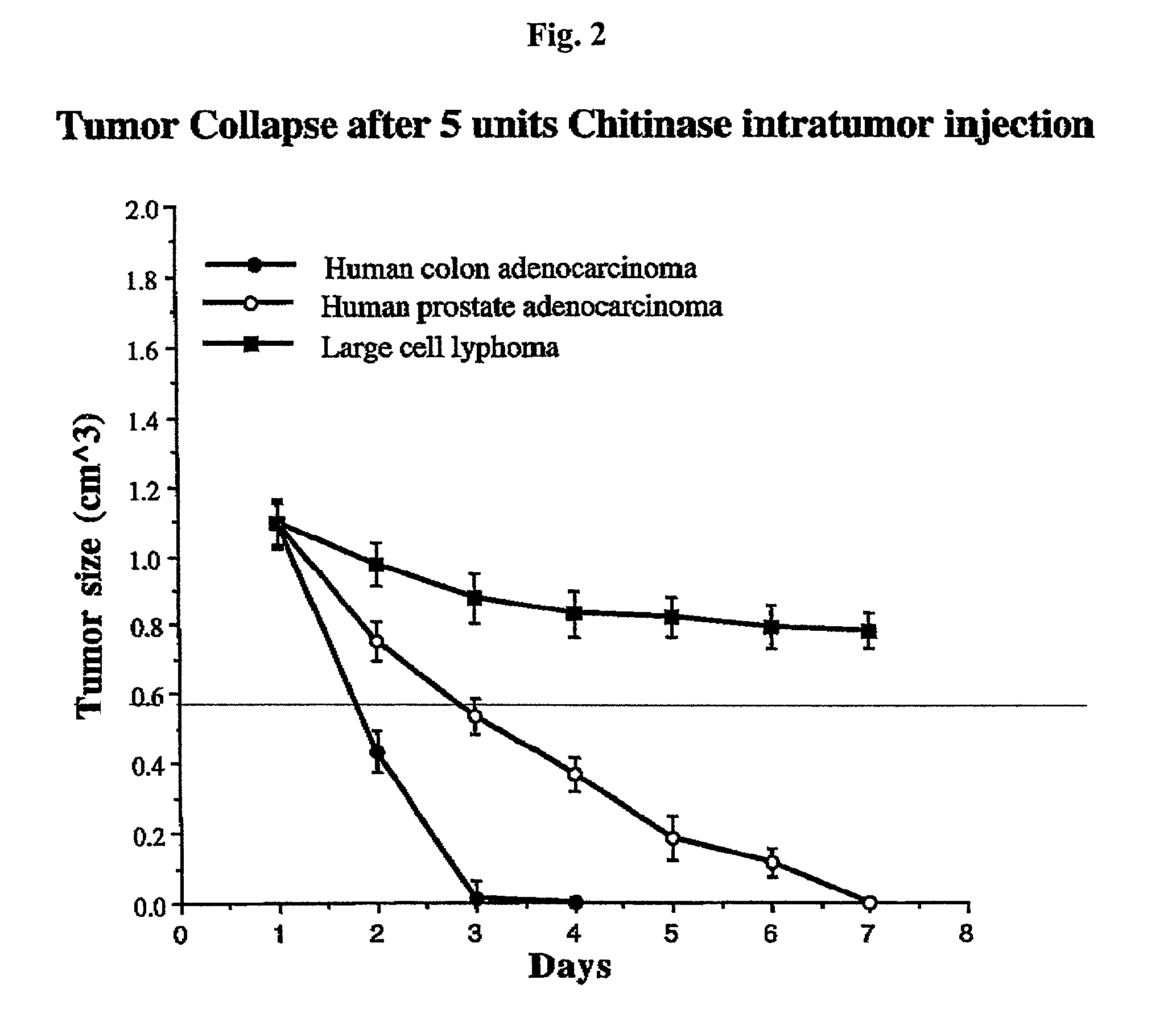
Enzyme-based anti-cancer compositions and methods
InactiveUS20030003114A1Peptide/protein ingredientsGenetic material ingredientsCancer cellAntibody fragments
A composition for treating cancer is provided. The composition is a hexosaminidase covalently attached to a cancer cell targeting ligand and improves selectively of the hexosaminidase for tumor cells. In certain embodiments, the hexosaminidase is alternately chitinase (N-acetyl-glucosaminohydrolase), chitosanase, or N-acetyl-hexosaminidase and the targeting ligand is either a monoclonal antibody, an antibody fragment immunospecific to a tumor cell or cancer cell antigen, epidermal growth factor (EGF), fibroblast growth factor (FGF), transferrin, or folic acid. Also provided is a method for treating cancerous tumors comprising administering the composition to a patient that has a cancerous tumor.
Owner:CITY OF HOPE NAT MEDICAL CENT & BECKMAN RES INST +1
Selective glycosidase inhibitors
The present invention provides glucoimidazole derivatives and methods of making them. The compounds can be used to inhibit the activity of O-GlcNAcase enzymes, including both bacterial OGA (bOGA) and human OGA (hOGA) and can be selective, showing low inhibition of hexosaminidases. The compounds can be used to study the role of the O-GlcNAcase modification in human or animal cells. Furthermore the compounds can have therapeutic uses in the treatment of diseases mediated by the activity of O-GlcNAcase enzymes including type II diabetes, Alzheimers disease, and cancer.
Owner:UNIV COURT OF THE UNIV OF DUNDEE
Antipyrotic
InactiveCN101198341AInhibition releaseInhibit aggregationOrganic active ingredientsCosmetic preparationsOrganic solventMedicine
An anti-inflammatory agent which comprises an oil-soluble licorice extract obtained by extraction treatment of either a leguminous plant of the genus Glycyrrhiza or a water extract of a leguminous plant of the genus Glycyrrhiza with an organic solvent, and has at least one effect selected from an inhibitory effect on hyaluronidase activity, an inhibitory effect on hexosaminidase release (i.e., an inhibitory effect on histamine release), an inhibitory effect on platelet aggregation and an inhibitory effect on phospholipase A2 activity.
Owner:MARUZEN PHARMA
Vectors having both isoforms of beta-hexosaminidase
Disclosed are compositions and methods related to nucleic acid constructs containing a HexB encoding element and a HexA encoding element. These constructs can be used in the treatment of Tay-Sachs and Sandoff disease.
Owner:UNIVERSITY OF ROCHESTER
Development culture medium for separating and identifying pathogens in urogenital tract
ActiveCN102206697AFacilitate early diagnosisGood treatment effectMicrobiological testing/measurementEscherichia coliBacteroides
The invention discloses a development culture medium for separating and identifying pathogens in a urogenital tract, which at least comprises three development substrates, namely a hexosaminidase substrate, a beta-D-galactosidase substrate, a beta-D-glucuroide substrate. The development culture medium added with the three development substrates is prepared into a microbial culture medium agar plate, a sample or a bacterial colony subjected to separate culture is inoculated into the development plate and is incubated, and a result can be directly observed. In the development culture medium, various pathogens including bacteria and fungi in a genital tract can be simultaneously cultured and identified on the same development plate, namely bacterial pathogens such as Escherichia coli, Klebsiella pneumonia, enterococcus, pseudomonas aeruginosa, proteus mirabilis, staphylococcus aureus and the like and fungal pathogens such as candida albicans, candida tropicalis and the like can be simultaneously identified, and judgment can be performed through visual inspection; and the development culture medium is quick and convenient to use, and easy to operate.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Vectors having both isoforms of beta-hexosaminidase and uses of the same
Disclosed are compositions and methods related to delivering a nucleic acid to the central nervous system.
Owner:KYRKANIDES STEPHANOS
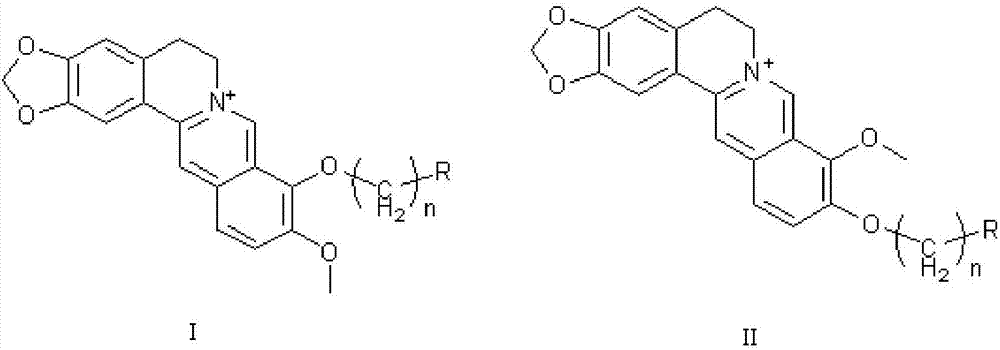
Application of berberine and derivative of berberine as hexosaminidase inhibitor
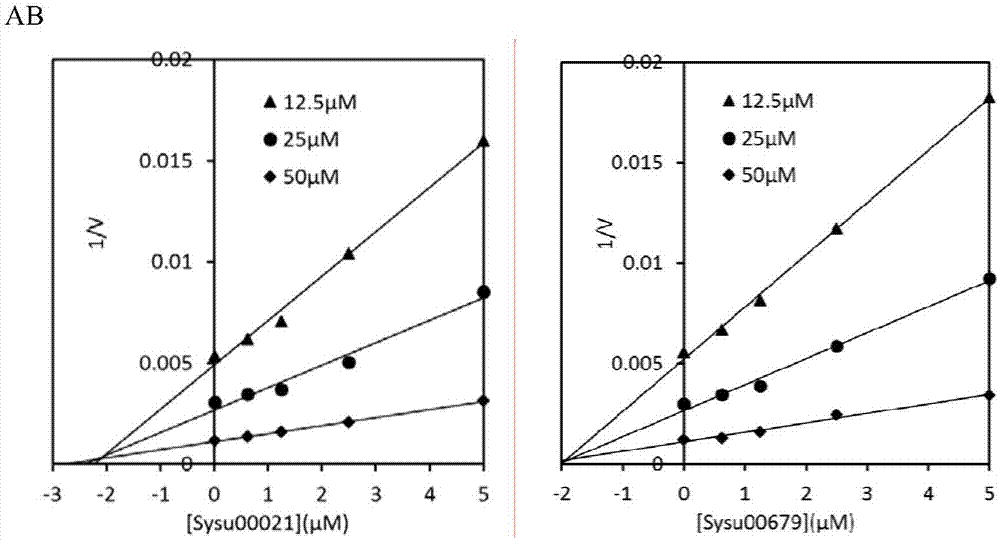
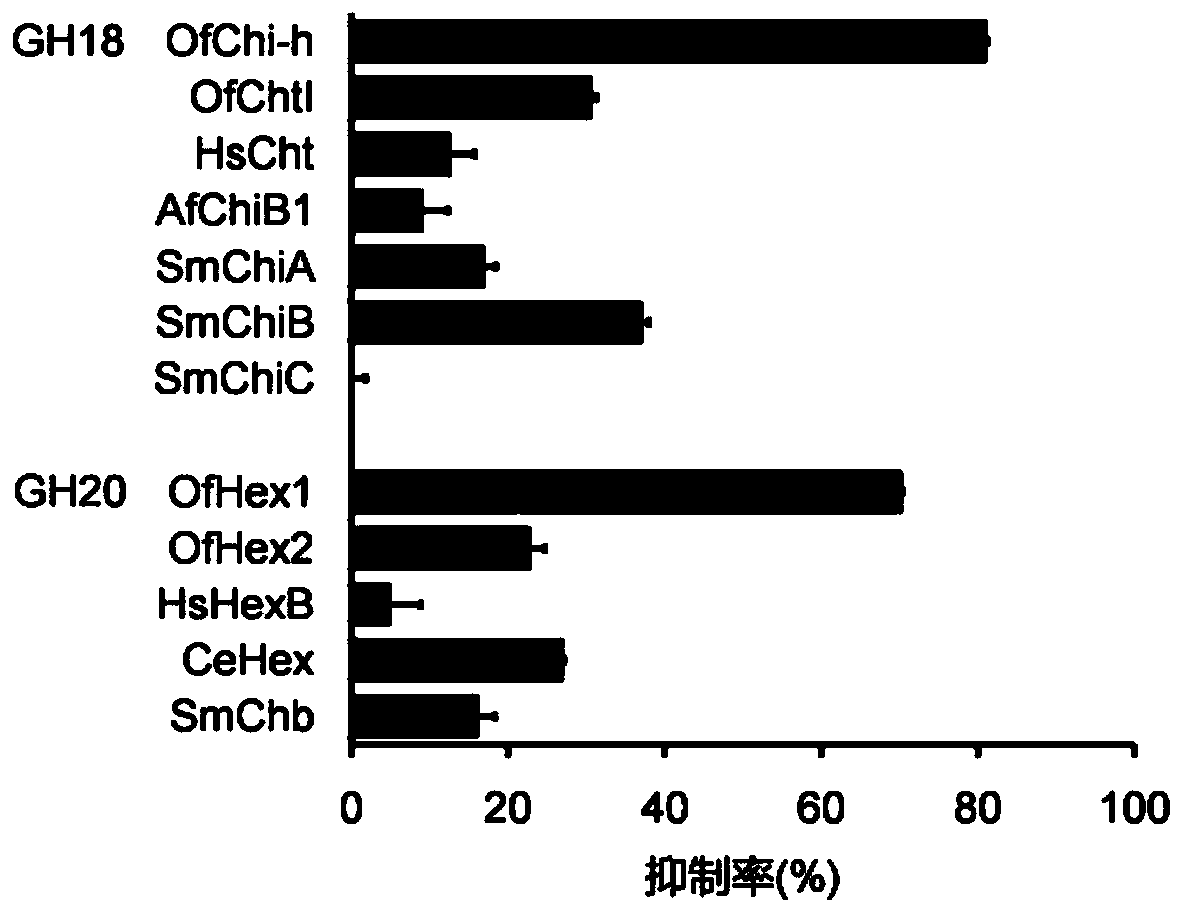
The invention discloses application of berberine and a derivative of the berberine as a hexosaminidase inhibitor. The general structure formula of the hexosaminidase inhibitor is shown as I or II; in all screened 35 compounds, the berberine and the derivative of the berberine show certain inhibition activity on hexosaminidase OfHex1; particularly, the suppression rate of Sysu-00021 and Sysu-00679 on the OfHex1 enzyme is respectively 76 percent and 79 percent, and the inhibition constant Ki is respectively 2mu M and 1.8mu M. Compound applicable object determination shows that under the final concentration of 10mu M, the compound shows higher inhibition activity on the hexosaminidase OfHex1; no inhibition activity is shown on the hexosaminidase HsHexB derived from the human. In a word, the berberine and the derivative of the berberine have wide application prospects in the fields of biology, chemicobiology and the like.
Owner:DALIAN UNIV OF TECH
Novel high functional enzyme having modified substrate specificity of human ß-hexosaminidase b and exhibiting protease resistance
Provided is a modified β-subunit of human β-hexosaminidase which has the activity derived from the α-subunit of wild-type human β-hexosaminidase and has the resistance to protease. A protein comprising an amino acid sequence having substitutions of the 312th to the 318th amino acids with glycine, serine, glutamic acid, proline, serine, glycine and threonine in order, respectively, in an amino acid sequence of a β-subunit of wild-type human β-hexosaminidase.
Owner:UNIVERSITY OF TOKUSHIMA +1
Method for assaying the activity of lysosomal enzymes
InactiveUS20090325206A1Inexpensive and simple for determinationSimple and expedite sample collectionMicrobiological testing/measurementLysosomeGlucuronidase
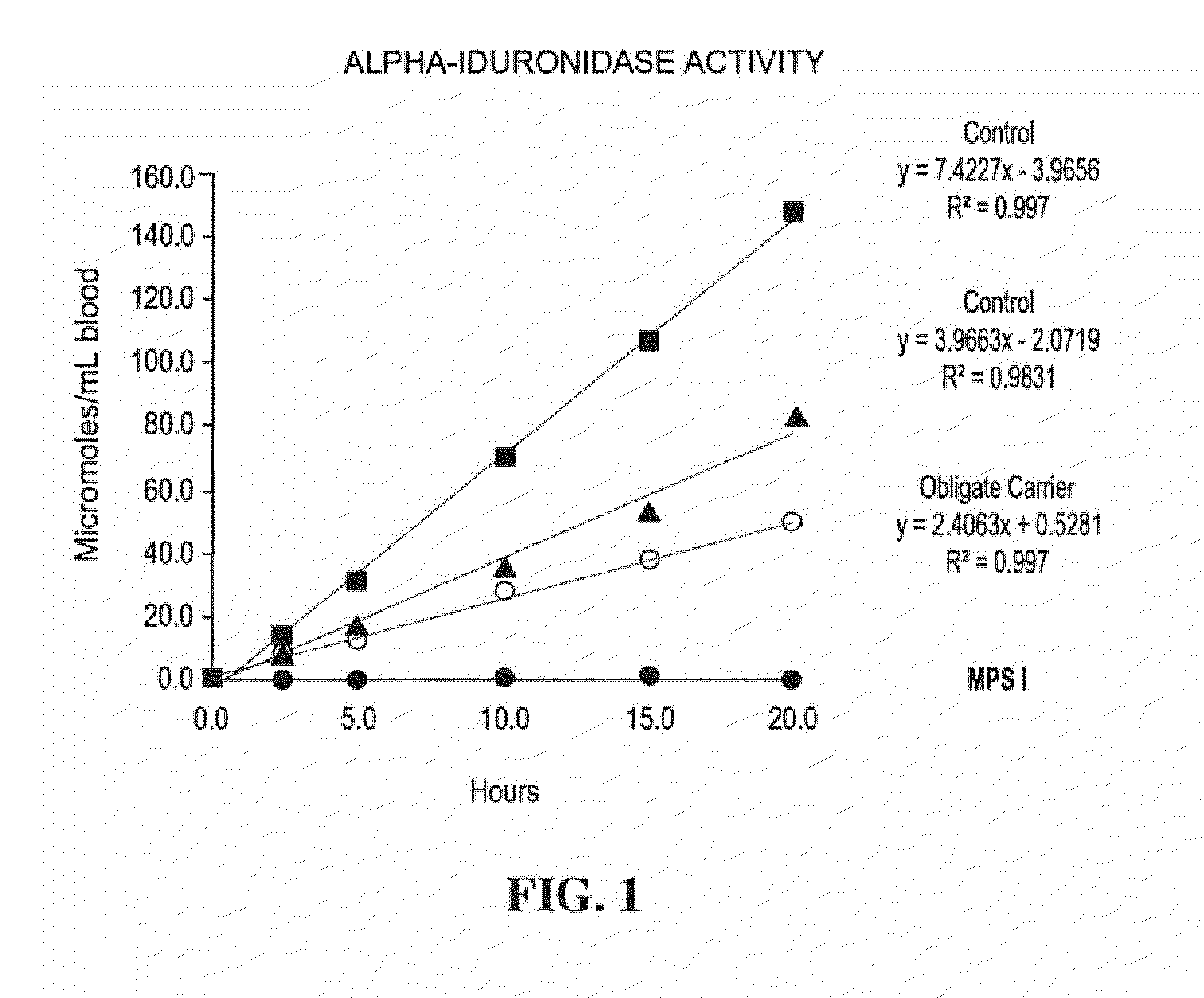
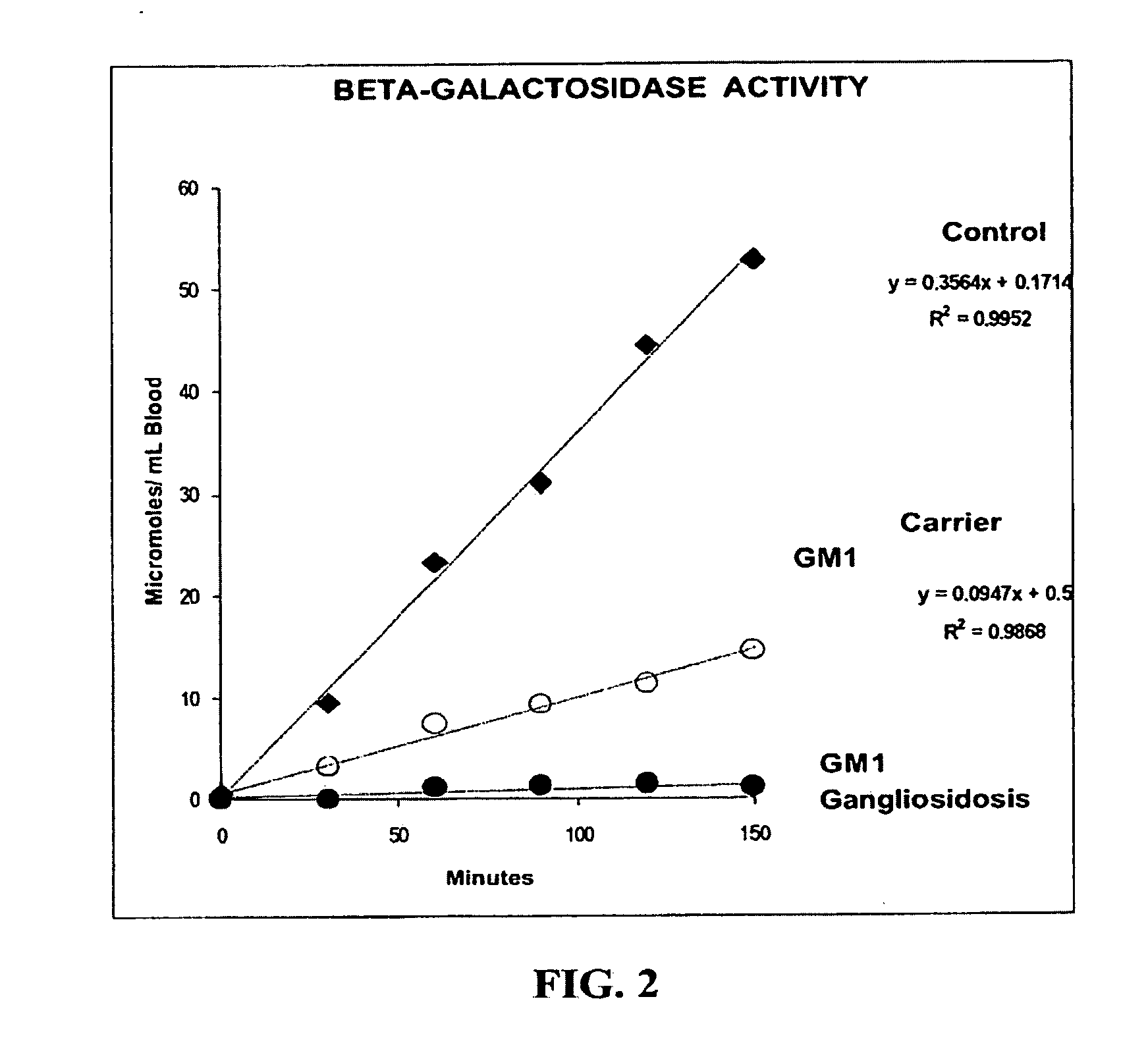
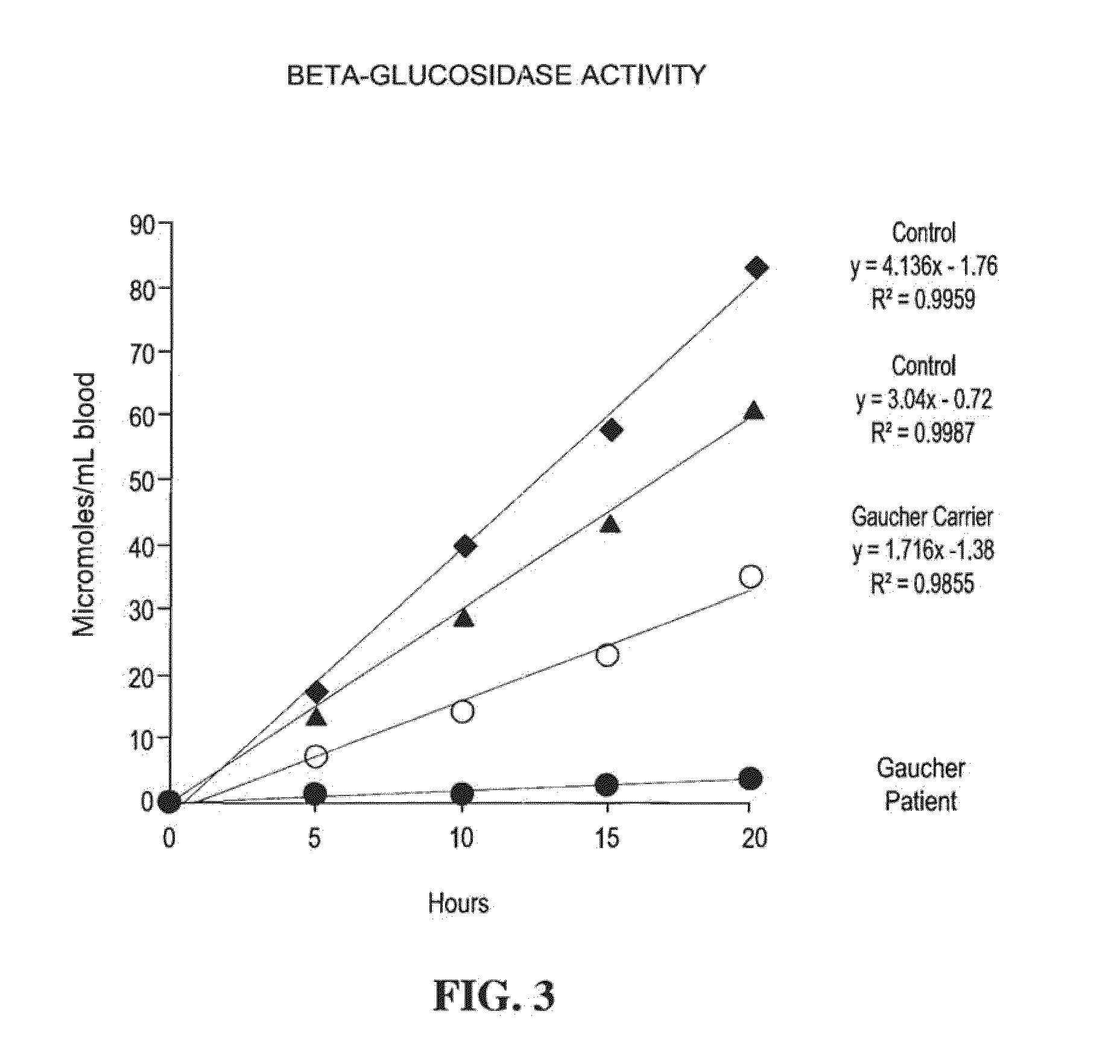
A method, and associated kit, for assaying the activity of lysosomal enzymes present in dried bodily fluids and cell tissue samples, such as α-L-iduronidase, β-D-galactosidase, β-D-glucosidase, chitotriosidase, total α-D-galactosidase and α-D-galactosidase A, hexosaminidase A and B, α-D-mannosidase, β-D-mannosidase, α-L-fucosidase, N-acetyl-α-galactosaminidase, arylsulfatases, sphingomyelinase, β-galactocerebrosidase, iduronate-2-sulfatase and β-D-glucuronidase. The method includes: (a) combining with a dried bodily fluid or cell tissue sample containing at least one type of lysosomal enzyme: (1) an eluent, (2) an incubation buffer and (3) a substrate or substrates capable of reacting with the assayed lysosomal enzymes and producing their corresponding enzyme product or products, (b) allowing the dried bodily fluid or cell tissue sample to react with the eluent, incubation buffer and substrate or substrates for an adequate time and temperature, and (c) applying measuring means to the enzyme product to determine the activities of the lysosomal enzymes present.
Owner:GENZYME CORP
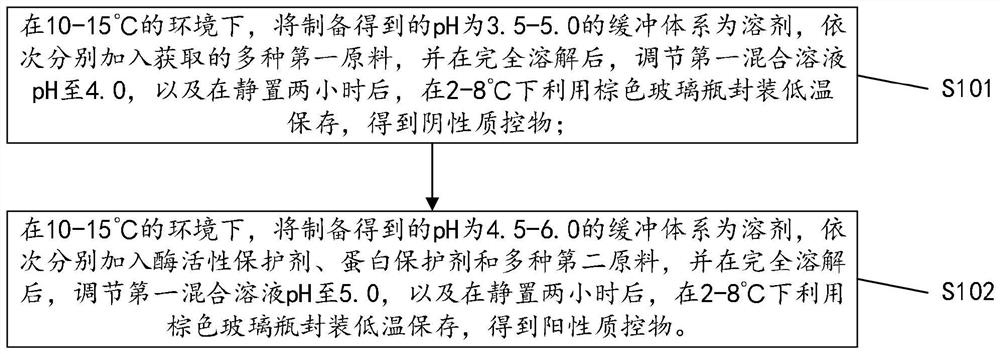
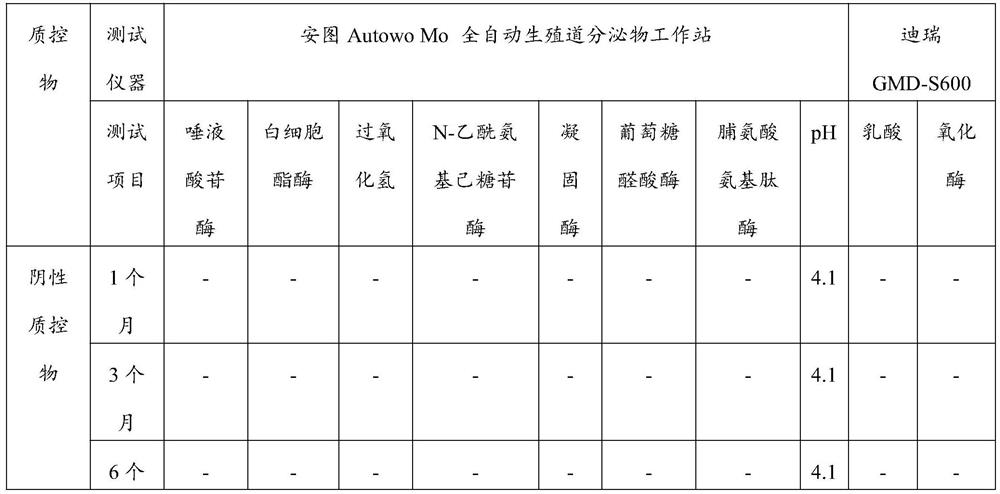
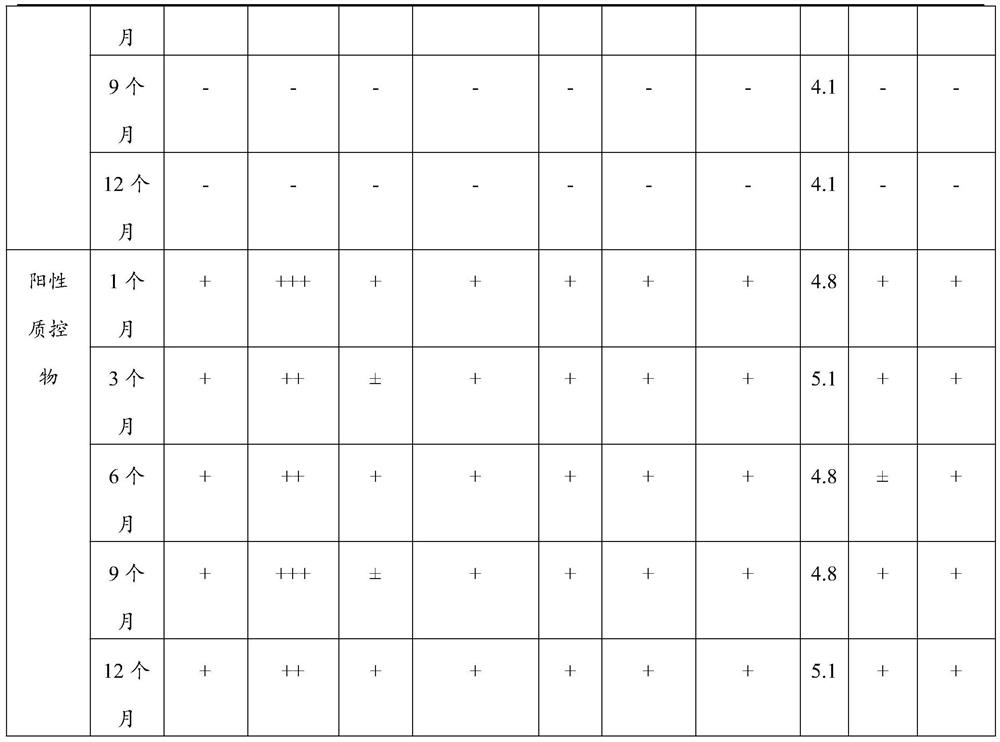
Quality control material for detecting gynecological secreta, and preparation method of quality control material
ActiveCN106872679ASolve few quality control itemsFix stability issuesBiological testingLeukocyte esteraseHydrogen
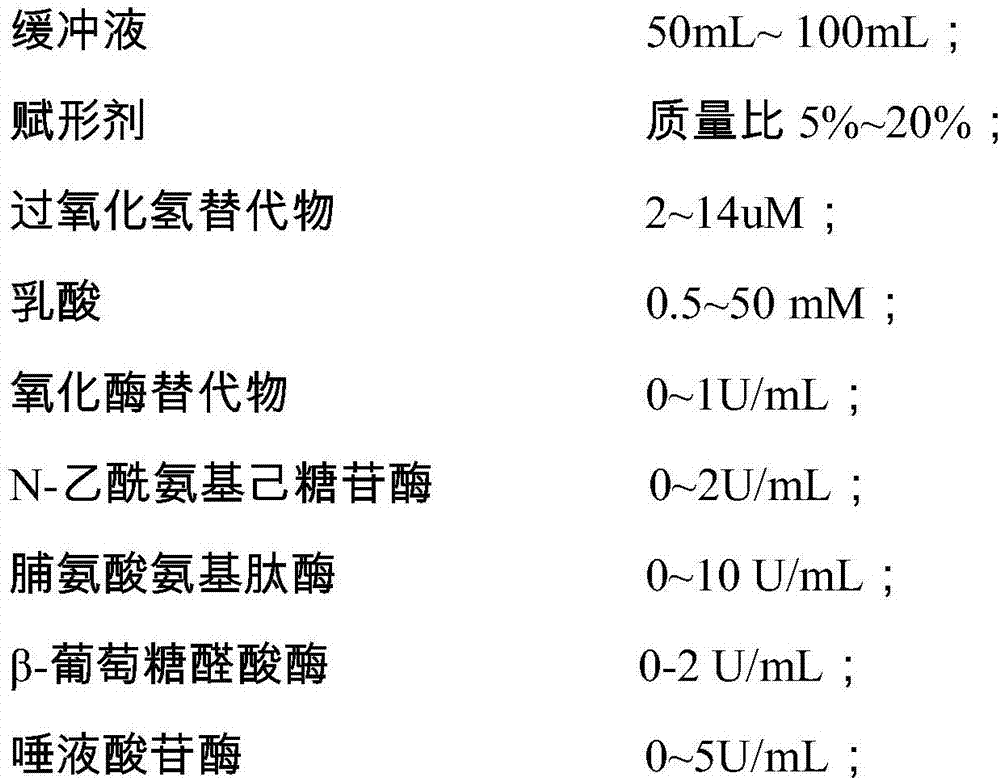
The invention discloses a quality control material for detecting gynecological secreta, and a preparation method of the quality control material. The quality control material comprises a negative quality control material and a positive quality control material. The quality control material can be used for carrying out quality control on nine items including hydrogen peroxide, lactic acid, oxidase, glucuronidase, N-acetyl hexosaminidase, neuraminidase, leukocyte esterase, proline aminopeptidase and potential of hydrogen, thus solving the problems of the existing quality control material that quality control items are fewer and stability is poor. The quality control material provided by the invention has the advantages of more quality control items, stableness and effectiveness, and can be applied to automatic detection equipment such as a gynecological secreta detector. After being subjected to freeze drying, the negative quality control material and the positive quality control material are dry powder-shaped; the quality control material can be made into a kit form. Furthermore, the preparation method provided by the invention is simple and easy to operate, and production cost is low.
Owner:DIRUI MEDICAL TECH CO LTD
Novel branched sialo-sugar molecules and antiviral agents using the same
The present invention provides a novel branched-chain sialose molecule represented by the following formula (I), which is capable of preventing type A from all humans and animals as a host-wide variation or antigenic variation against influenza virus Substances used for adsorbents such as pharmaceuticals infected with influenza virus and influenza B virus, and filters for virus removal. (In the formula, NeuAc represents N-acetylneuraminic acid in which the hydroxyl group, carboxyl group and amide group of the NeuAc may be chemically modified by halogen, alkyl group or acyl group in the same or different ways, Hex represents hexose, HexNAc represents acetylhexosamine , R is a matrix selected from hydrogen atoms, hydrocarbon chains, sugar chains, lipids, proteins, and synthetic polymers, and R may also have substituents. In addition, the combination of N-acetylneuraminic acid and hexose may be naturally occurring The ortho-glycosidic bond may be a chemically transformed bond such as S-glycosidic or Se-glycosidic bond.)
Owner:JAPAN SCI & TECH CORP
Raav vectors for the treatment of gm1 and gm2 gangliosidosis
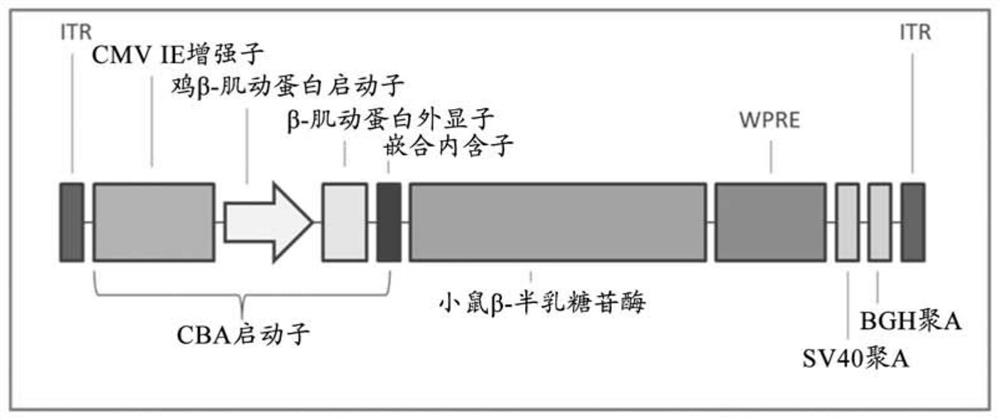
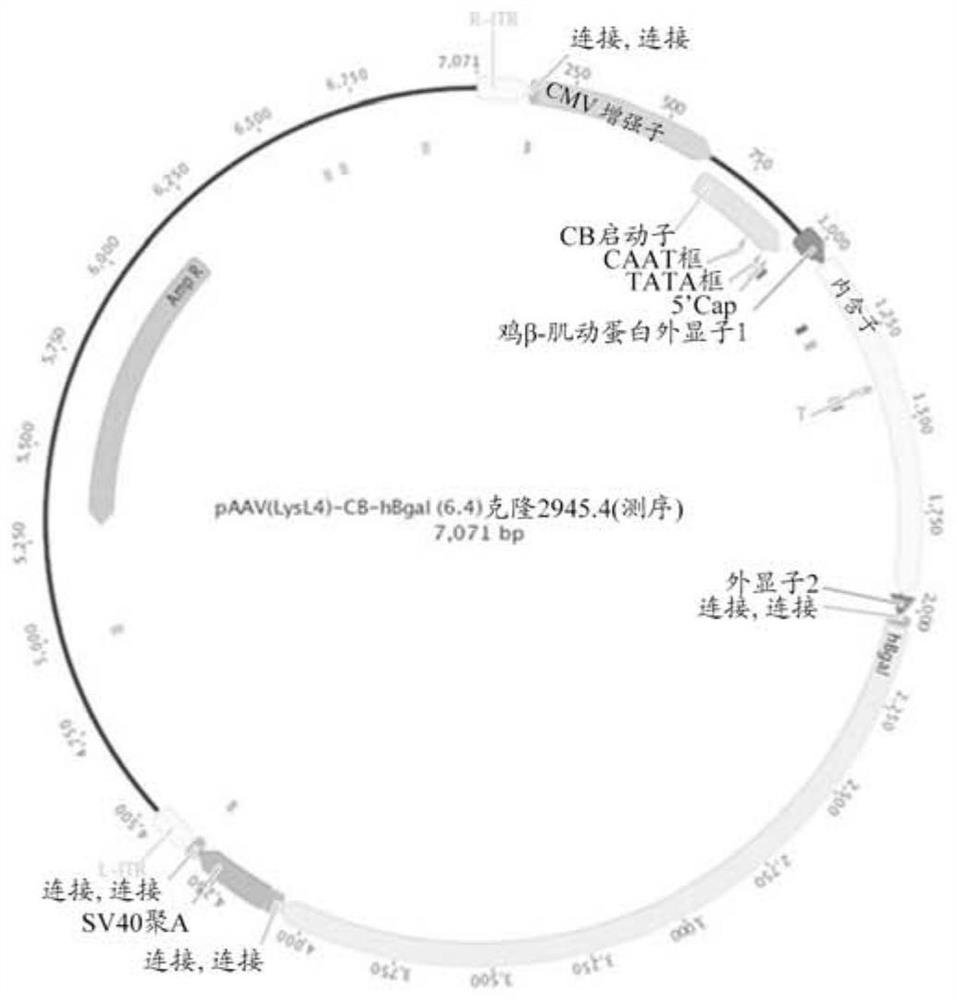
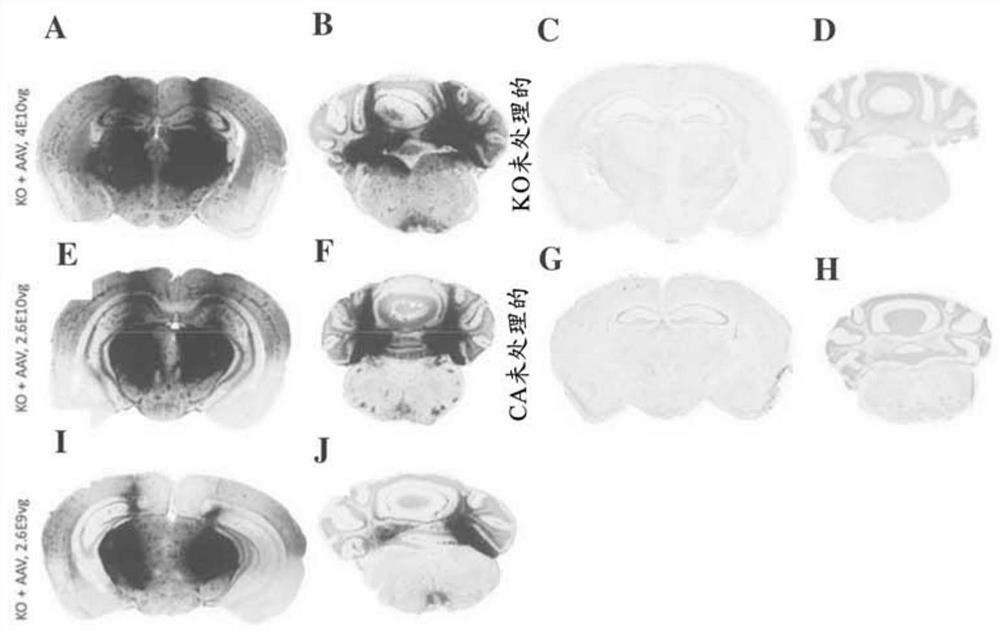
Aspects of the disclosure relate to compositions and methods for the treatment of lysosomal storage disorders, such as GM1 gangliosidosis, Tay Sachs disease, and Sandhoff disease. In some embodiments, the compositions comprise viral vectors encoding beta- galactosidase. In some embodiments, the compositions comprise viral vectors encoding beta- hexosaminidase subunits (e.g. HEXA, HEXB, or combinations thereof).
Owner:UNIV OF MASSACHUSETTS
Hexosaminidase and coding gene related to strawberry softening, preparation and application thereof
ActiveCN109971736AStrong targetingAvoid missingFruit and vegetables preservationGenetic engineeringBiotechnologyHeterologous
The present invention discloses two hexosaminidase gene sequences derived from strawberries (Fragaria x ananassa qing xia) and a preparation method and an application thereof, and a technical method using genetic engineering. The hexosaminidase gene is cloned into a pichia pastoris expression vector to obtain a pichia pastoris recombinant strain heterologously expressing hexosaminidase, abd the hexosaminidase prepared by the heterologous expression of the strain can uses p-nitrophenyl-N-acetyl-beta-D-amino glucosamine or p-nitrophenyl-N-acetyl-beta-D-aminogalactosamine as a substrate for reaction. The present invention also provides the application of the hexosaminidase in controlling postharvest fruit softening and preservative activity screening, and belongs to the field of fruit preservation.
Owner:辽宁精细化工产业技术发展有限公司
Selective glycosidase inhibitors
The present invention provides glucoimidazole derivatives and methods of making them. The compounds can be used to inhibit the activity of O-GlcNAcase enzymes, including both bacterial OGA (bOGA) and human OGA (hOGA) and can be selective, showing low inhibition of hexosaminidases. The compounds can be used to study the role of the O-GlcNAcase modification in human or animal cells. Furthermore the compounds can have therapeutic uses in the treatment of diseases mediated by the activity of O-GlcNAcase enzymes including type II diabetes, Alzheimers Disease, and cancer.
Owner:UNIV COURT OF THE UNIV OF DUNDEE
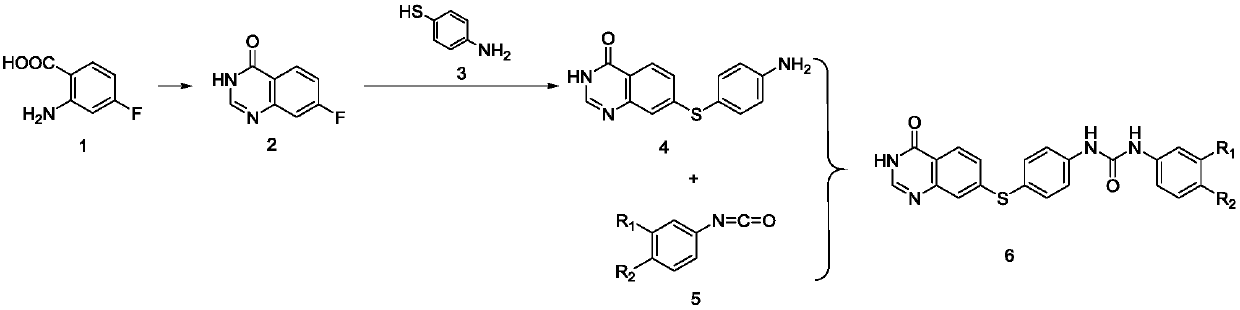
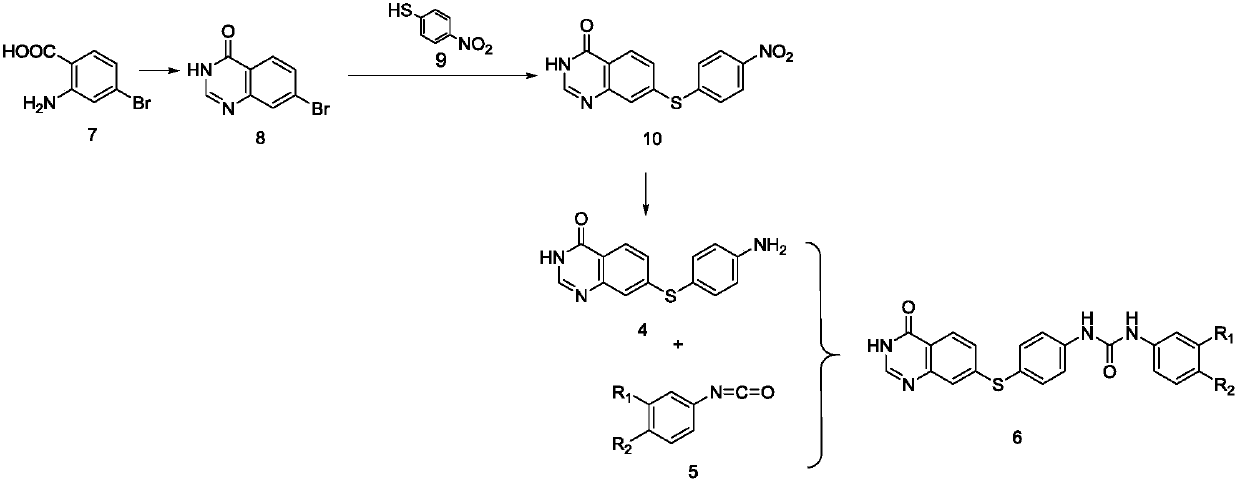
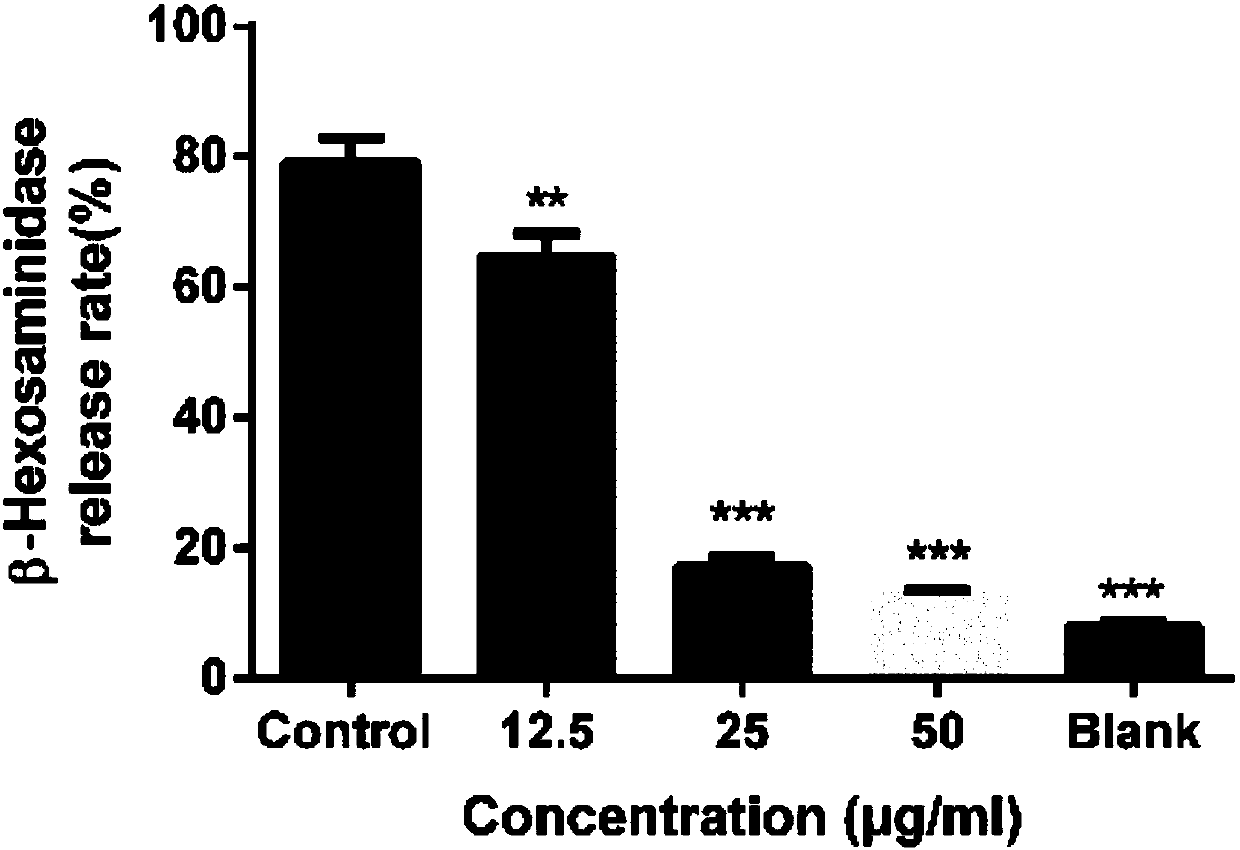
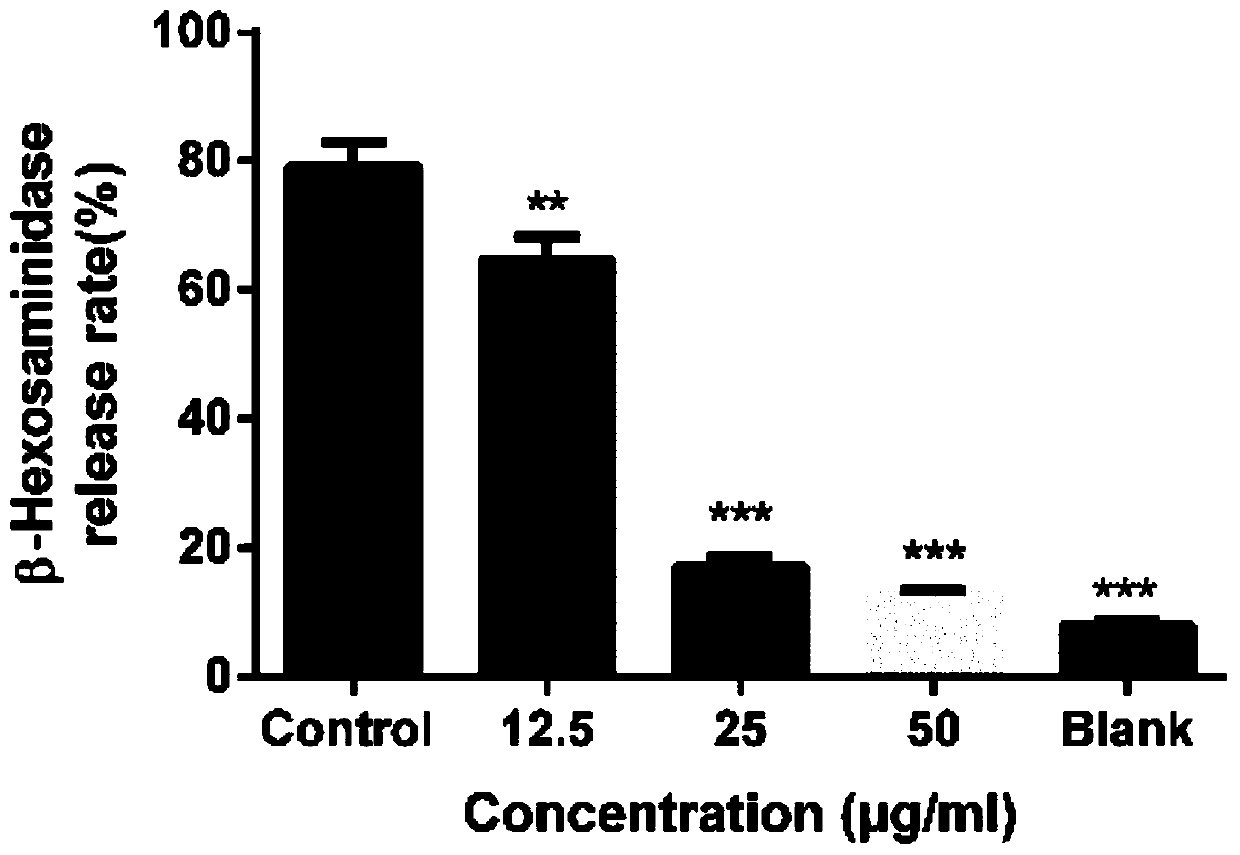
Quinazolinone-containing diaryl urea compound and preparation method and application thereof
ActiveCN107814773AReduce concerns about anaphylaxisRelieve painOrganic active ingredientsOrganic chemistryReagentRaw material
The invention provides a quinazolinone-containing diaryl urea compound and a preparation method and application thereof. The quinazolinone-containing diaryl urea compound can obviously inhibit LAD2 cells from releasing beta-hexosaminidase, shows obvious dosage association, can be used for preparing an antiallergic medicament, and particularly can be used for preparing a medicament for antagonizingLAD2 cells from releasing beta-hexosaminidase so as to reduce pain and a load of a patient. The preparation method provided by the invention is easy in obtaining of raw material sources, mild in reaction condition, simple in operation in the reaction process and cheap in used reagent.
Owner:XI AN JIAOTONG UNIV
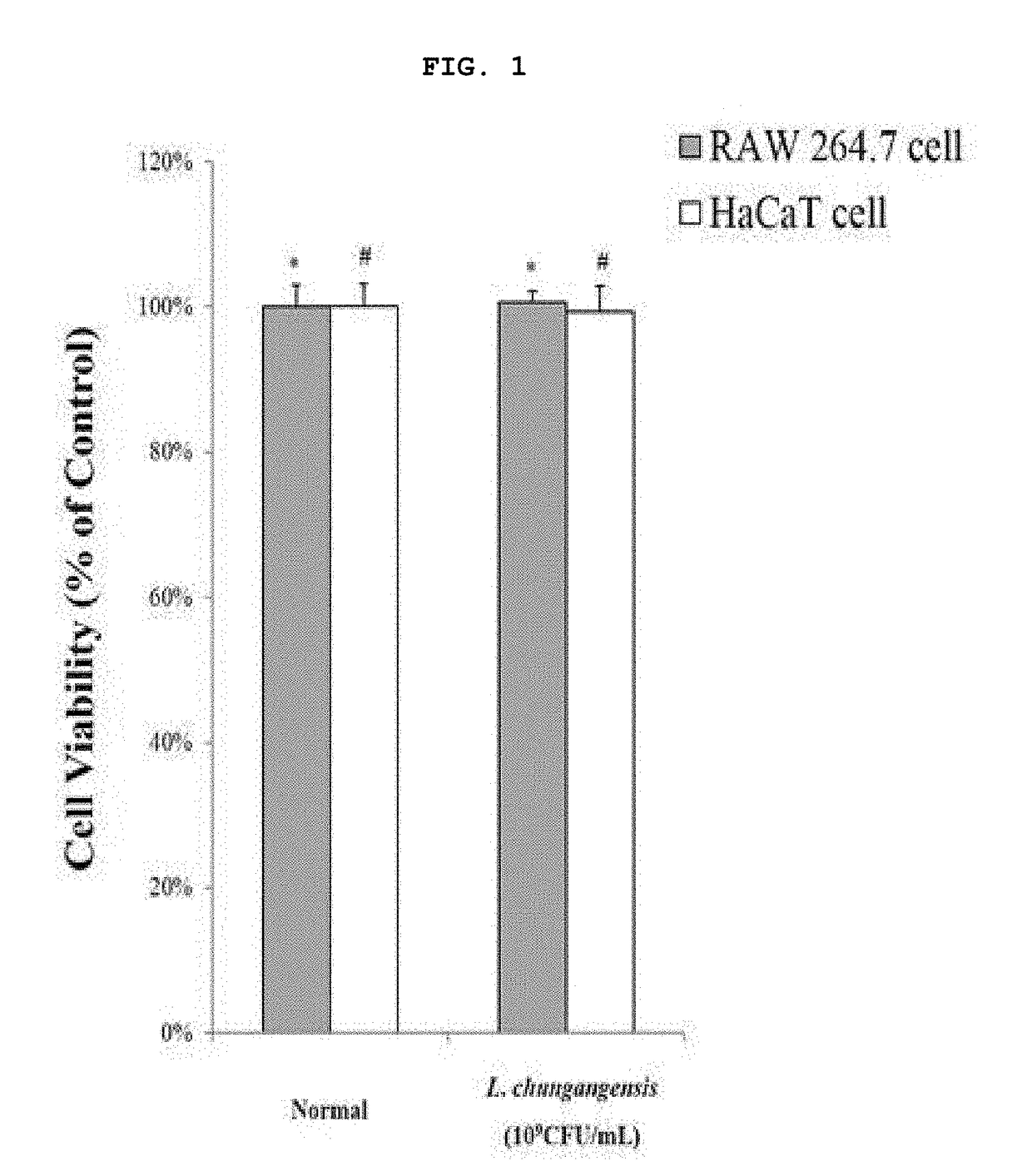
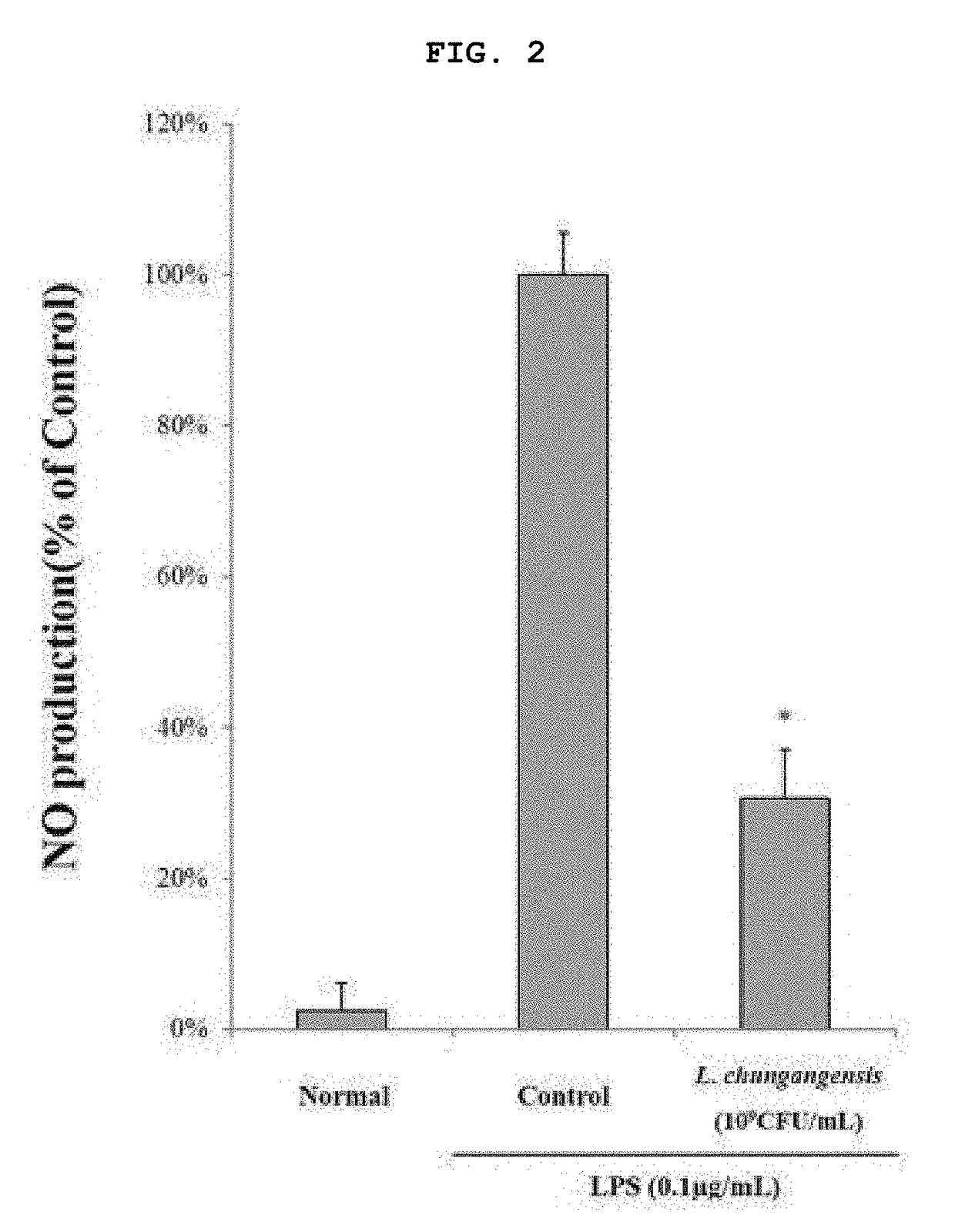
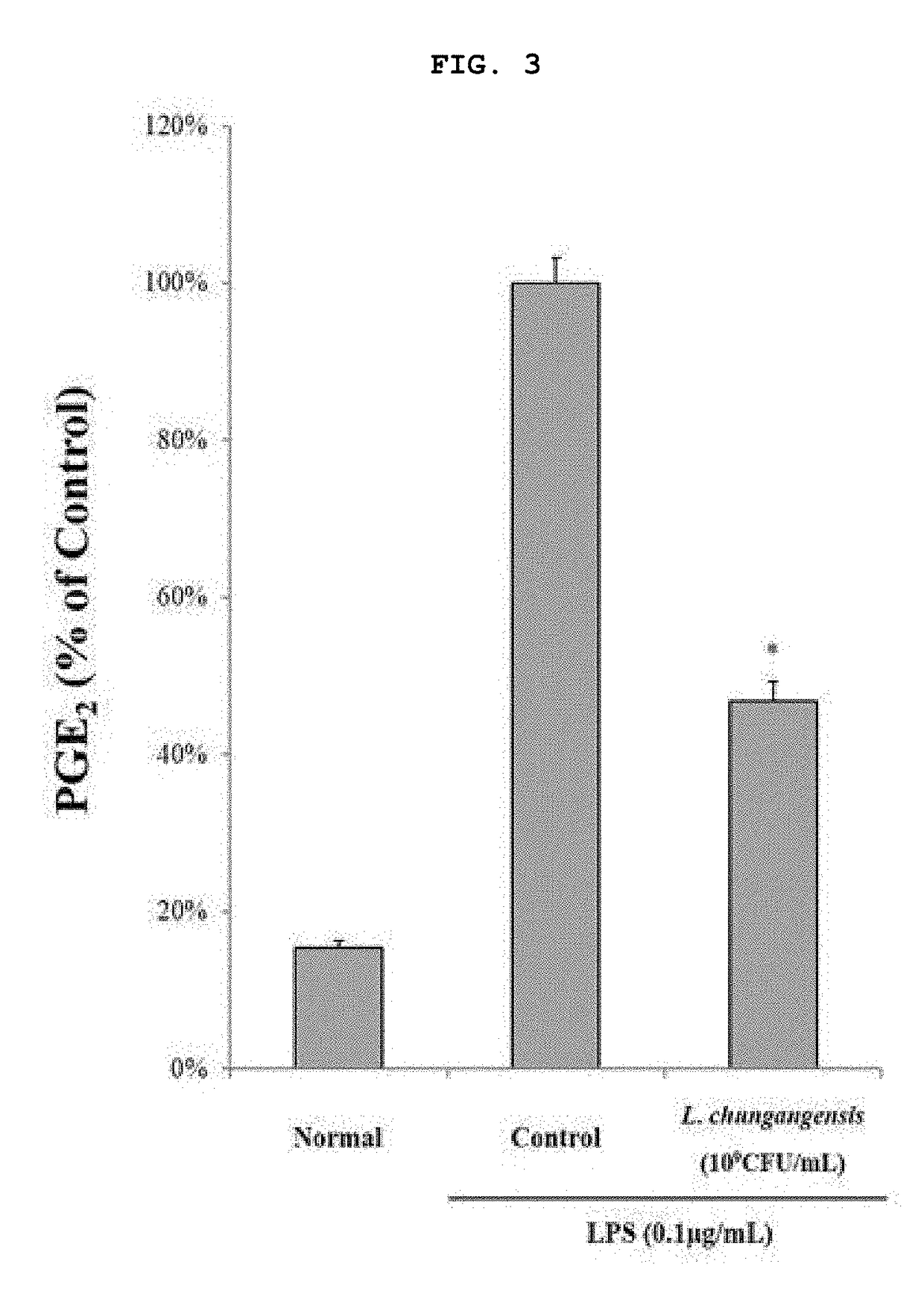
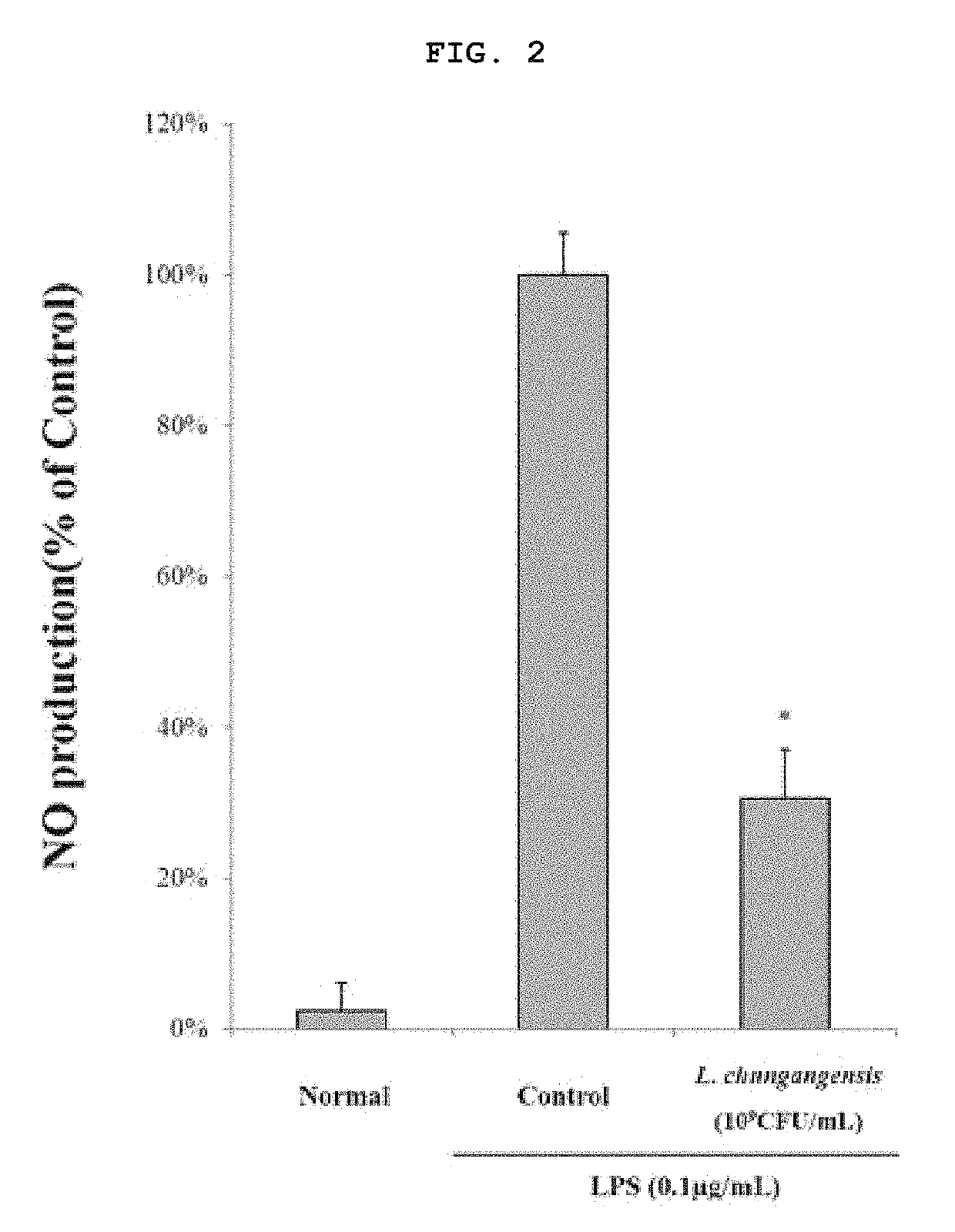
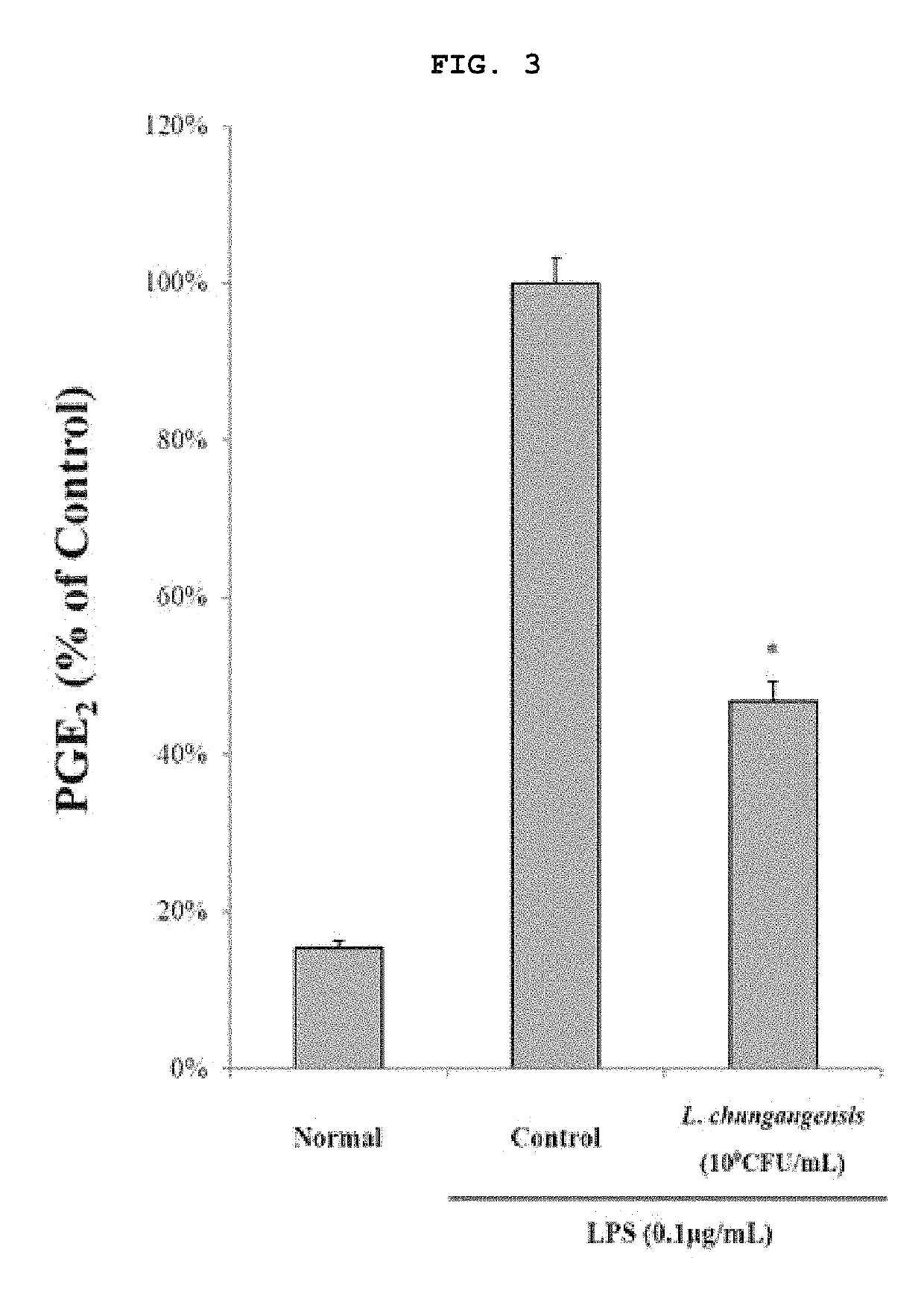
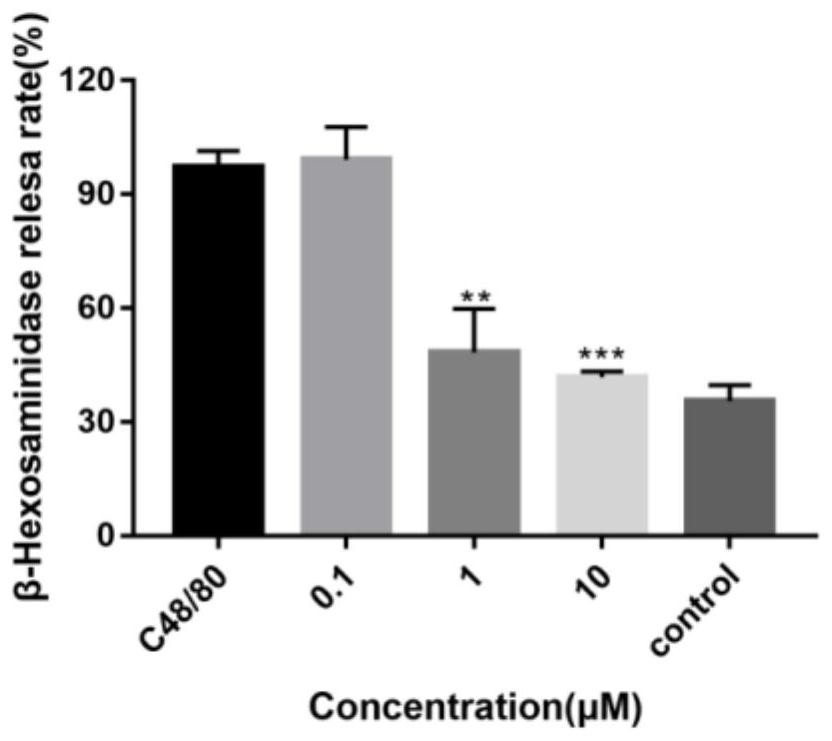
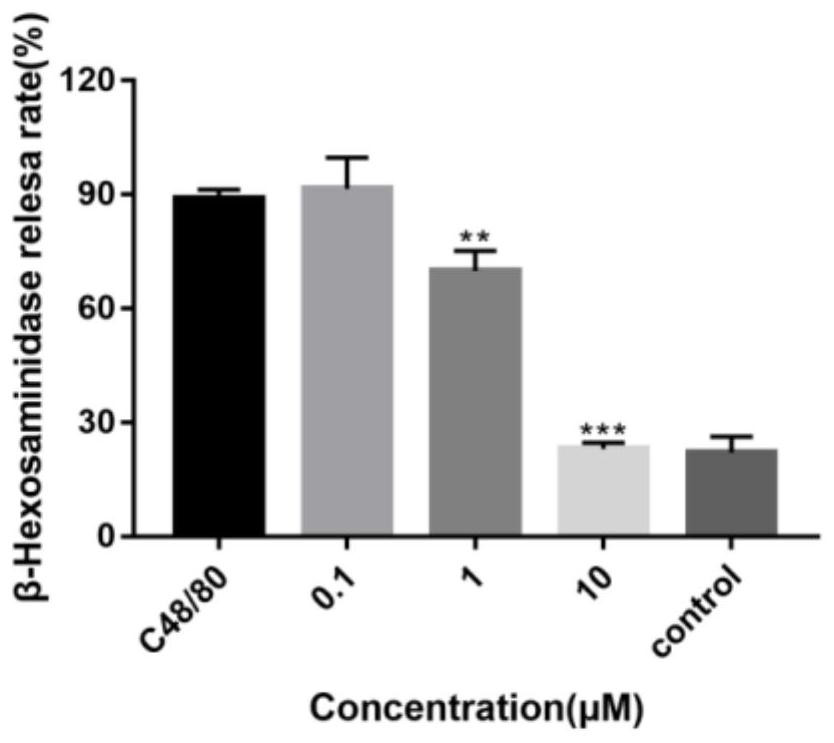
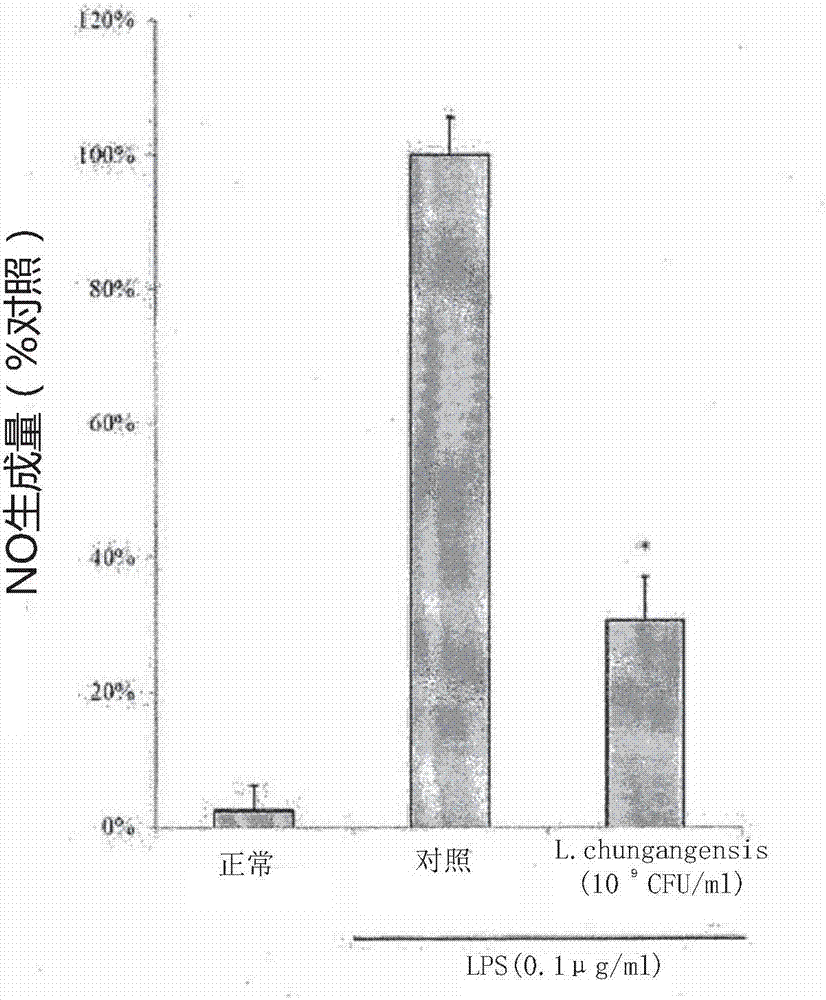
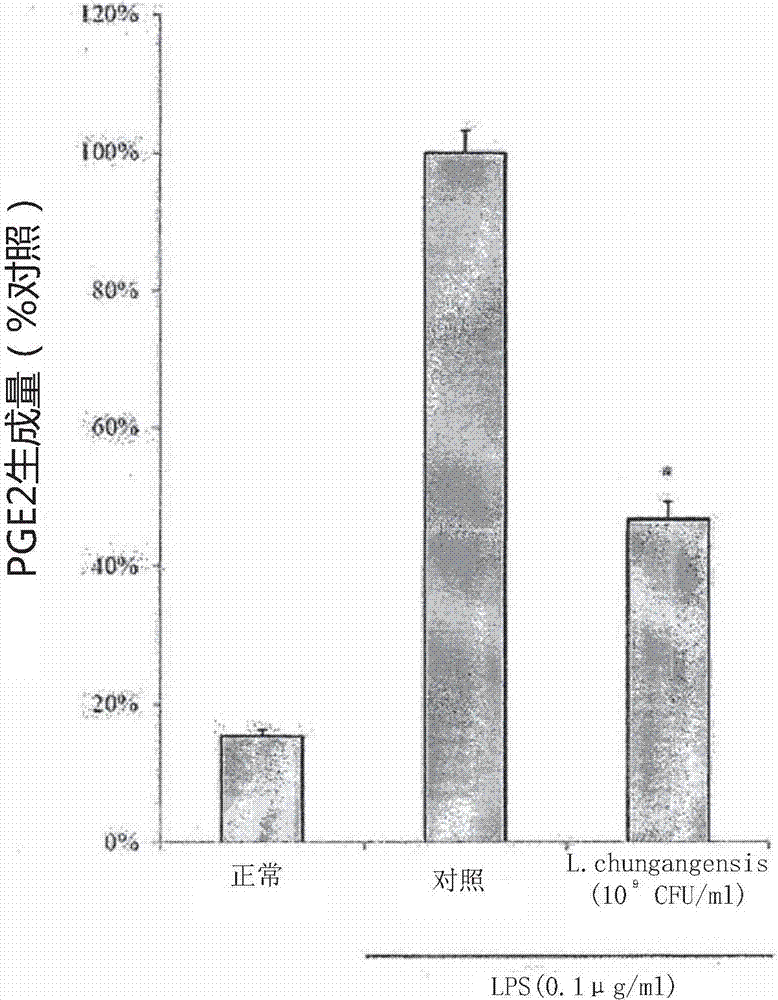
Pharmaceutical composition for preventing or treating inflammatory diseases, containing lactococcus chungangensis as active ingredient
ActiveUS20180085409A1Good effectInhibition releaseAntibacterial agentsCosmetic preparationsAdditive ingredientStaphylococcus aureus
The composition according to the present invention, which comprises Lactococcus chungangensis as an active ingredient, has excellent effects of preventing or treating inflammatory diseases, inhibiting the secretion of nitric oxide and prostaglandin E2, which are major inflammatory factors, and inhibiting the secretion of β-hexosaminidase and histamine, which are major factors related to allergies, and also significantly suppressing the production of skin disease-related cytokines and chemokines. Such effects are at the same level as those of conventional skin disease therapeutic agents (tacrolimus), and thus the composition can be used as a preventive or therapeutic agent against inflammatory diseases. In addition, the composition according to the present invention exhibits an antibacterial activity against Staphylococcus aureus, which is a microorganism inducing a secondary infection of atopic dermatitis and the like, and thus can be used in preventing or treating a bacterial infection.
Owner:CHUNG ANG UNIV IND ACADEMIC COOP FOUND
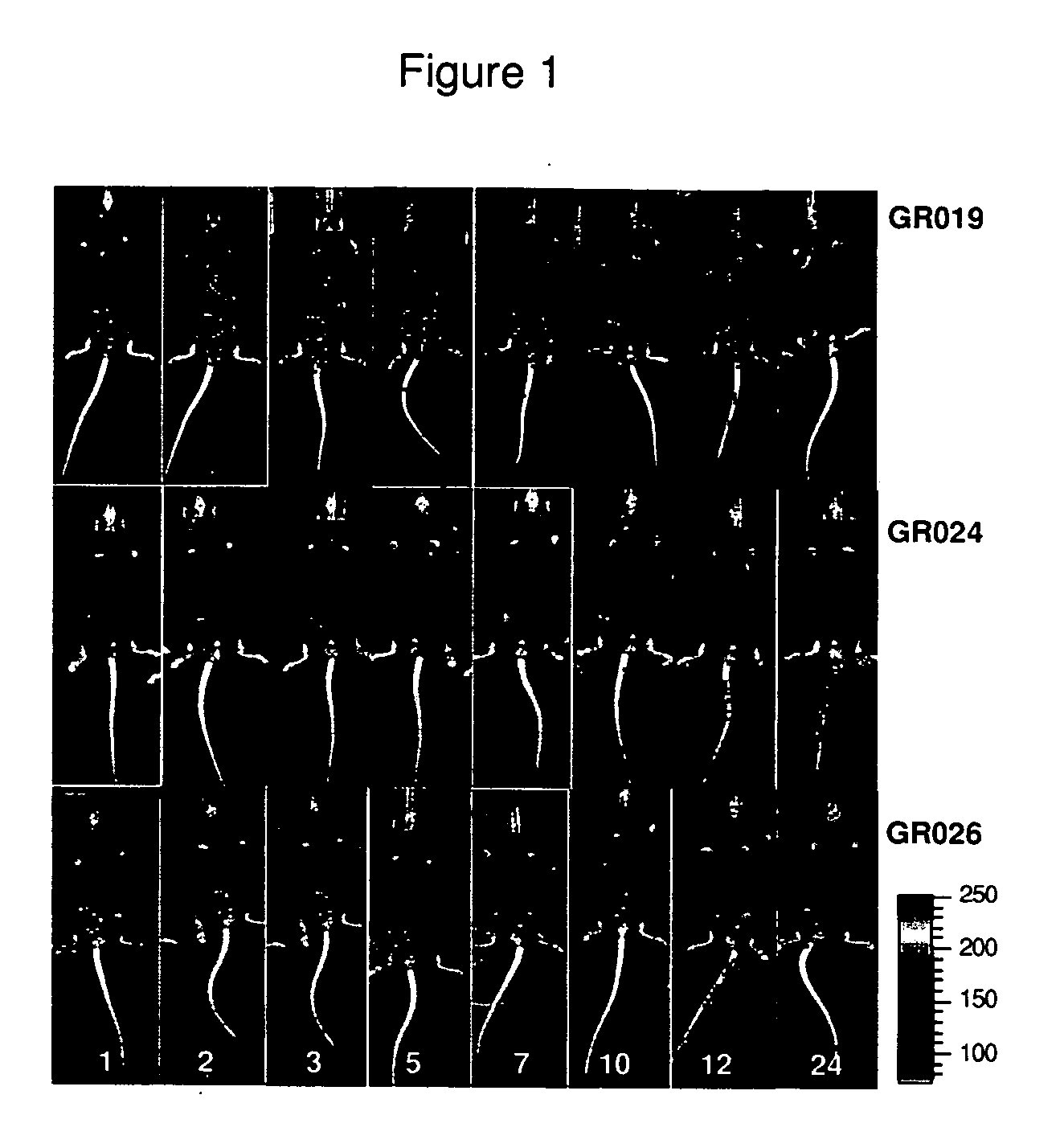
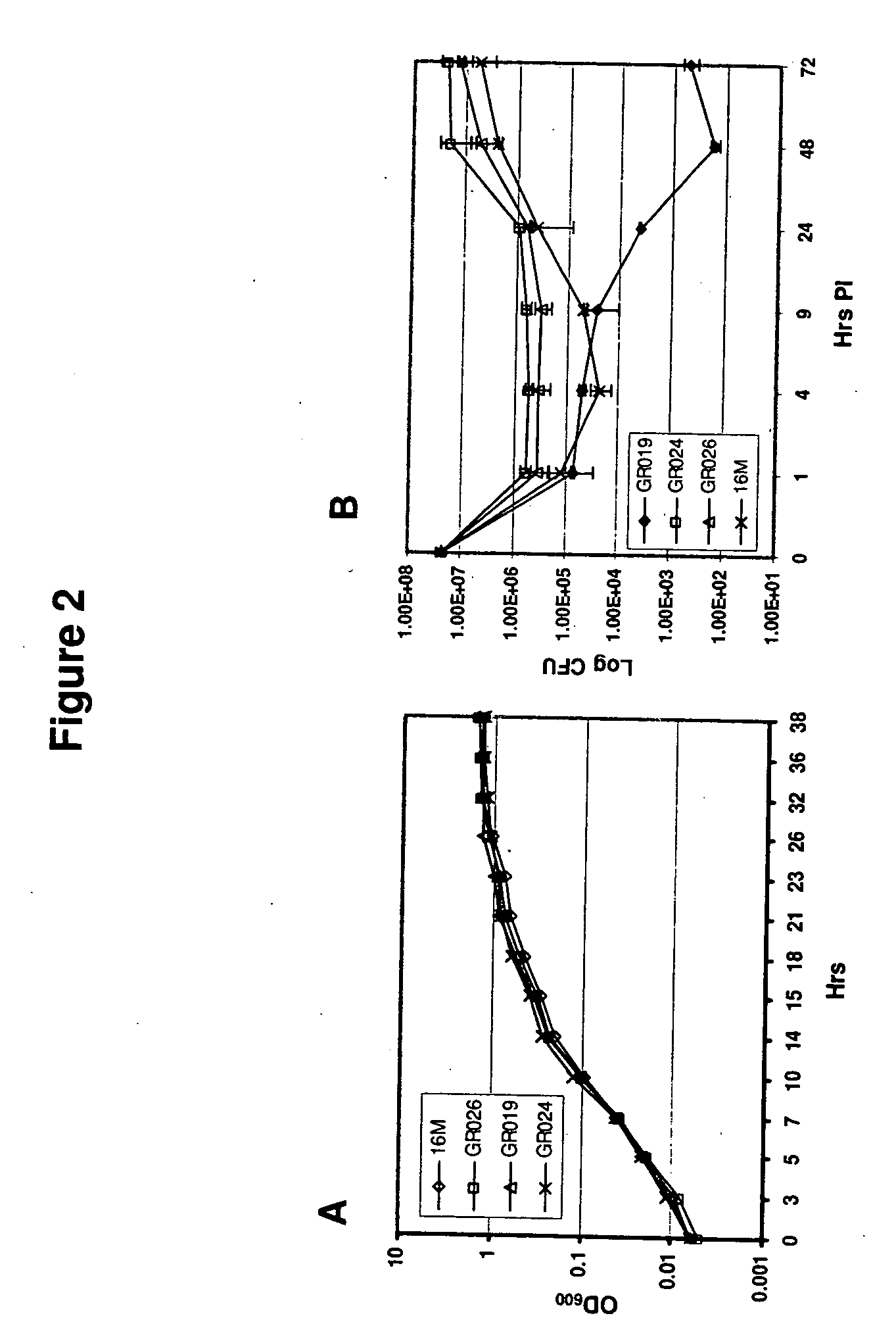
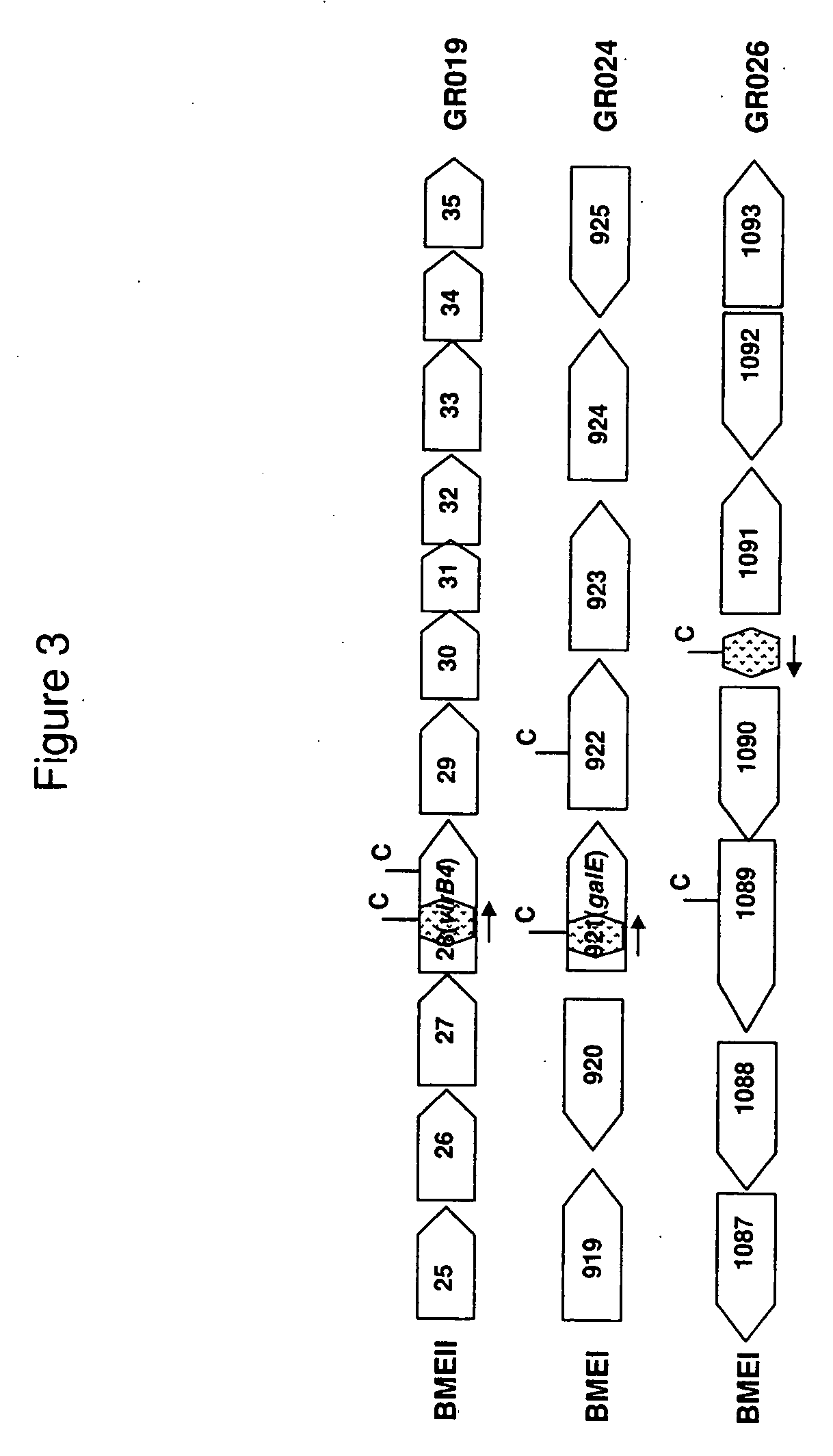
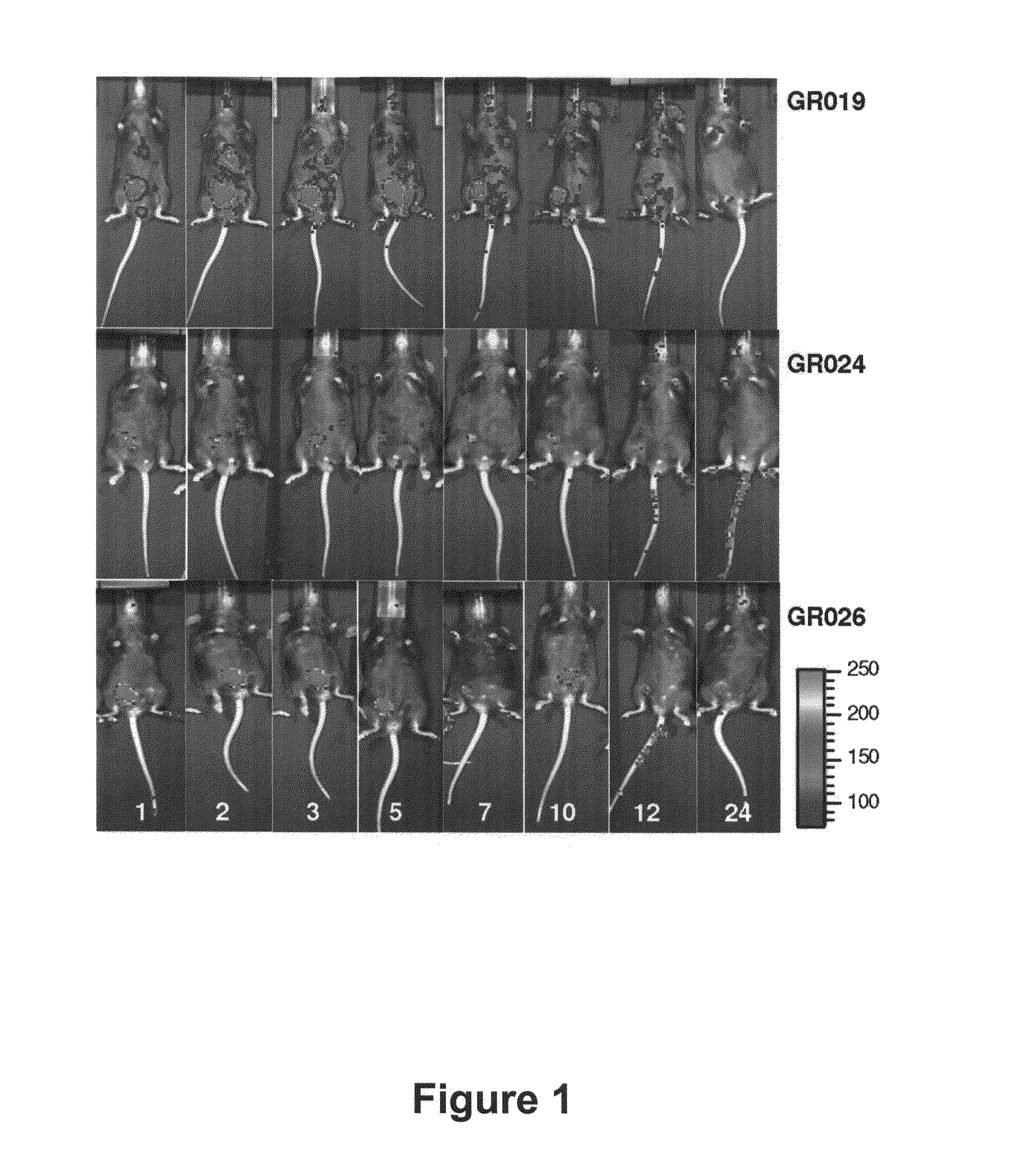
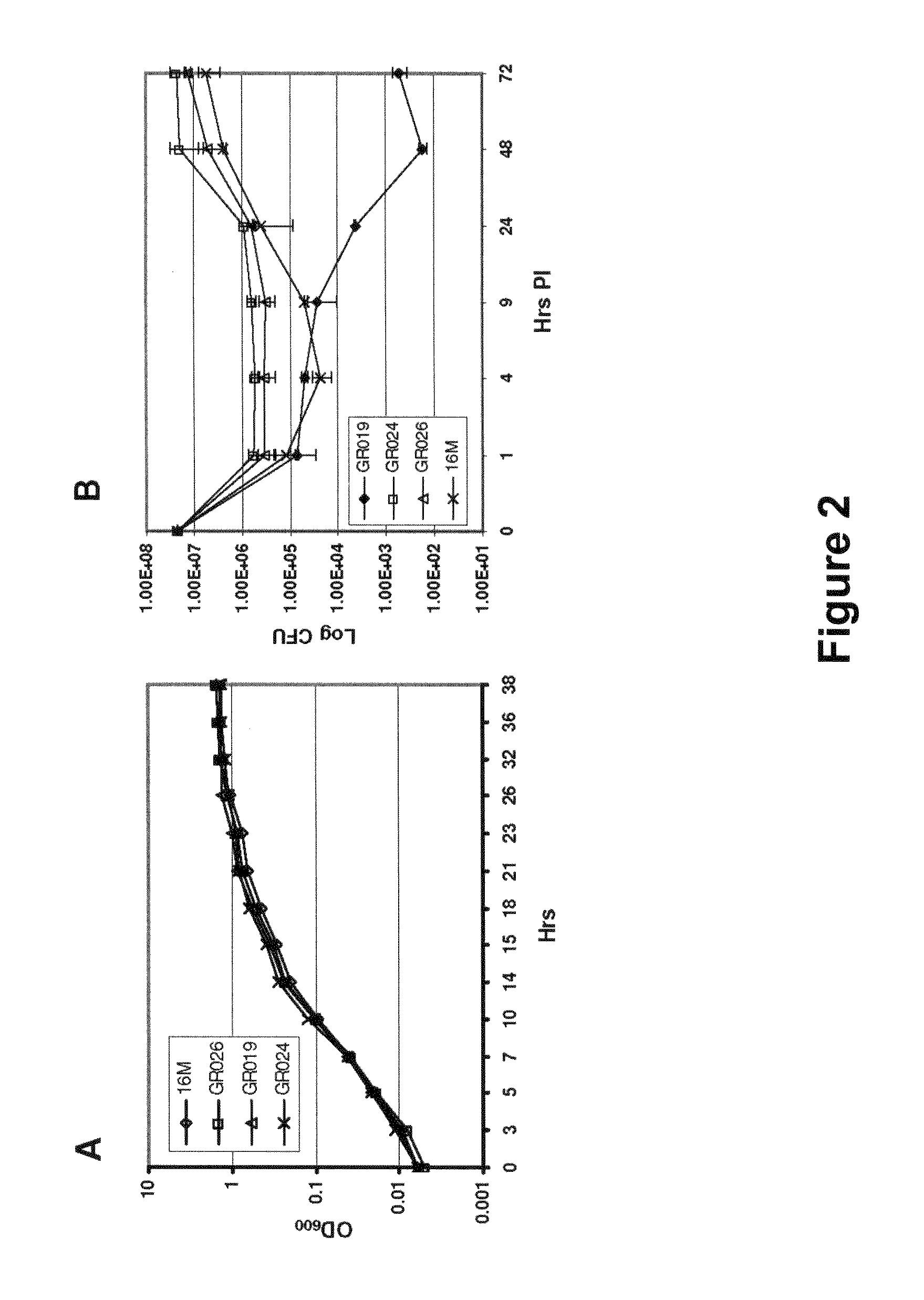
Brucella melitensis mutants and methods
InactiveUS20080107682A1Effective immune responseEfficient responseAntibacterial agentsBacterial antigen ingredientsVaccinationORFS
Certain attenuated mutants of Brucella, especially B. melitensis, B. abortus, B. suis and B. ovis, when administered to a human or animal trigger a protective immune response such that subsequent challenge with virulent Brucella of the same species does not result in disease or results in much less severe symptoms. Functional inactivation of galE, a virB gene or the operon (ORFs 1087-1090) comprising the gene encoding β-hexosaminidase (BMEI1087) and a lytic murein transglycosylase gene (BMEI1088). A specific example of the attenuated galE mutant which produces a protective immune response is B. melitensis GR024. The specific example of an inactivated ORF1087-1090 operon is B. melitensis GR026; it has an insertion mutation in the promoter region upstream of ORF 1090. Vaccination with live cells of either or both of these mutants results in a T cell response which protects the human or animal against challenge with virulent B. melitensis. Similar strategies for protective immunity using live attenuated mutants are useful for B. abortus, B. suis and B. ovis as well.
Owner:WISCONSIN ALUMNI RES FOUND
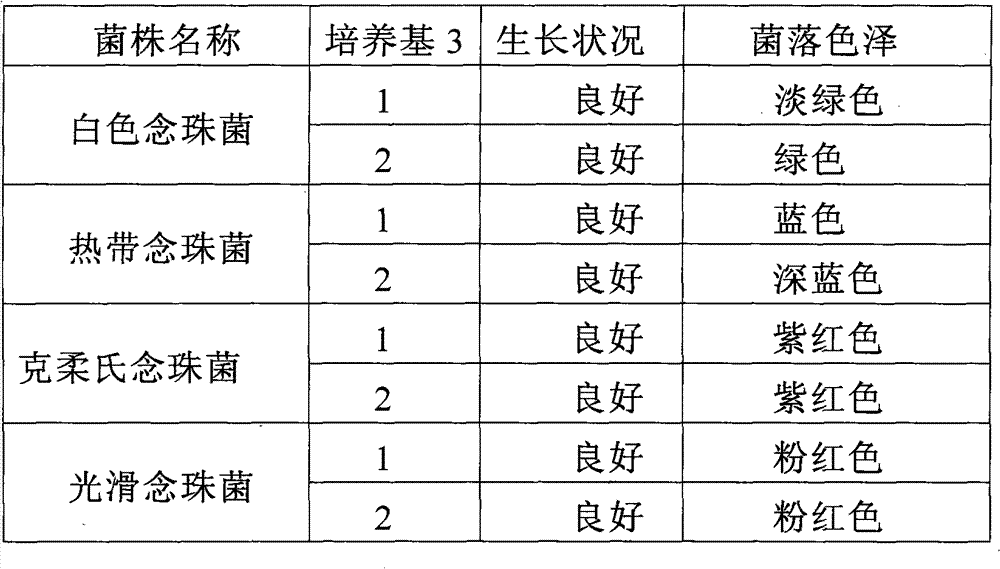
Candida chromogenic medium, detection kit and detection method
ActiveCN101948902BGuaranteed specificityGrowth inhibitionMicrobiological testing/measurementBiotechnologyMedical equipment
The invention relates to candida chromogenic medium, a detection kit and a detection method, belonging to the technical field of microbial diagnosis, in particular to the cultivation and identification of yeast in medical microbiology specimens, drugs and medical equipment sanitary inspection samples, public health surveillance samples and food (including cosmetics) sanitary inspection samples. The chromogenic medium of the invention is composed of basic medium, mixed chromogenic substrate and bacteriostat, wherein the mixed chromogenic substrate consists of aminocaproic glucosidase and alkaline phosphatase substrate, and candida specific enzyme is added into the mixed chromogenic substrate. The detection kit of the invention consists of the chromogenic medium, identification paper A containing enzyme substrate 5-bromo-4-chloro-3-indolyl-N-acetyl-beta-D-aminogalactose and identification paper B containing enzyme substrate 5-bromo-4-chloro-3-indolyl-beta-D- glycopyranoside. In the invention, the candida chromogenic medium and the detection kit have the advantages of low cost and simple configuration, and the method can be applied to the separation and identification of candida rapidly, simply and accurately.
Owner:BEIJING JUNLIKANG BIOTECHNOLOGY CO LTD
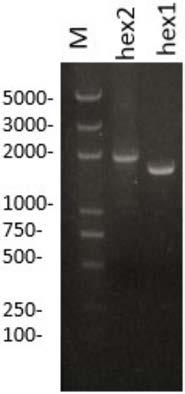
Method, substrate and reagents for beta-hexosaminidase A activity detection
InactiveCN103484526AMicrobiological testing/measurementFluorescence/phosphorescenceReagentLaboratory facility
The present invention provides a method for beta-hexosaminidase A (EC3.2.1.52) activity detection, and further provides a substrate and reagents for beta-hexosaminidase A activity determination. The method, the substrate and the reagents can be used for analyzing and determining beta-hexosaminidase A activity in a sample (including human body fluid or tissue or cell samples) requiring determination, and are mainly used in the field of clinical laboratory. The determination reagent prepared by using the method has characteristics of convenience, rapidness and high sensitivity, and is easily promoted and applied.
Owner:北京中科非凡生物技术有限公司
Vaginal secretion dry chemical analysis liquid quality control material and preparation method thereof
PendingCN112903986AEliminate the tedious steps of reconstitutionGood effectMicrobiological testing/measurementBiological testingLactate oxidaseWhite blood cell
The invention discloses a vaginal secretion dry chemical analysis liquid quality control material and a preparation method thereof. A corresponding buffer system is prepared first, and then, with the buffer system as a solvent, a plurality of raw materials of a negative quality control material and a positive quality control material are added and dissolved; when different components are added, the next component can be added after the previous component is completely dissolved; in order to ensure the enzyme activity, a liquid negative quality control material and a liquid positive quality control material are prepared in an environment of 4-15 DEG C, and the effect is better; after the preparation is completed, subpackaging and storage are carried out within 24 hours, and quality control can be carried out on 10 items such as coagulase, hydrogen peroxide, lactic acid, oxidase, glucuronidase, N-acetyl hexosaminidase, sialidase, leukocyte esterase, proline aminopeptidase and pH value at the same time, so that the stability is good, the period is long, the use is convenient and fast, complex step of redissolving before the freeze-dried product is used is omitted, and errors caused by redissolving are reduced; and the liquid quality control material has a good practical application value.
Owner:URIT MEDICAL ELECTRONICS CO LTD
Pharmaceutical composition for preventing or treating inflammatory diseases, containing Lactococcus chungangensis as active ingredient
ActiveUS10413575B2Good effectInhibition releaseAntibacterial agentsCosmetic preparationsInflammatory factorsStaphylococcus cohnii
The composition according to the present invention, which comprises Lactococcus chungangensis as an active ingredient, has excellent effects of preventing or treating inflammatory diseases, inhibiting the secretion of nitric oxide and prostaglandin E2, which are major inflammatory factors, and inhibiting the secretion of β-hexosaminidase and histamine, which are major factors related to allergies, and also significantly suppressing the production of skin disease-related cytokines and chemokines. Such effects are at the same level as those of conventional skin disease therapeutic agents (tacrolimus), and thus the composition can be used as a preventive or therapeutic agent against inflammatory diseases. In addition, the composition according to the present invention exhibits an antibacterial activity against Staphylococcus aureus, which is a microorganism inducing a secondary infection of atopic dermatitis and the like, and thus can be used in preventing or treating a bacterial infection.
Owner:CHUNG ANG UNIV IND ACADEMIC COOP FOUND
Halogenated diarylurea compounds and application thereof in preparation of antiallergic drugs
InactiveCN112076185AAllergic reactions suppressedRich types of anti-allergic drugsOrganic chemistryAmide active ingredientsAnaphylactoid reactionsBasophilic Granulocyte
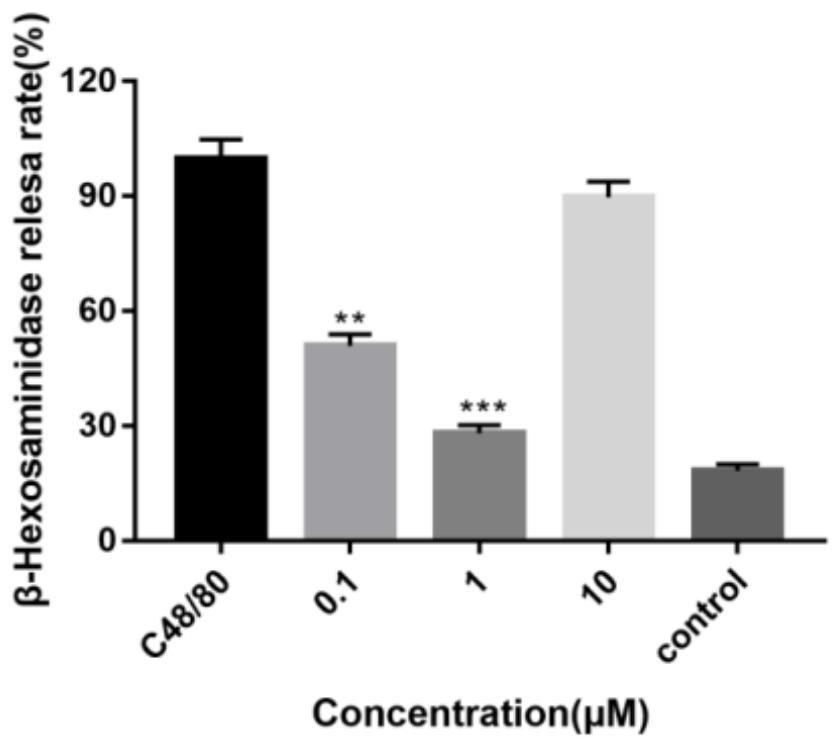
The invention discloses halogenated diarylurea compounds and application thereof in preparation of an antiallergic drug, and discloses for the first time that the halogenated diarylurea compounds caneffectively antagonize beta-aminohexylglycosidase release and histamine release of human basophilic granulocyte KU812 caused by C48 / 80, so as to inhibit anaphylactoid reaction. Therefore, when the halogenated diarylurea compounds are used as an anti-allergic preparation, the types of anti-allergic medicines are enriched, and more possible treatment schemes are provided for clinical anti-allergic treatment.
Owner:XI AN JIAOTONG UNIV
A kind of inhibitor and its application in inhibiting chitinase and hexosaminidase activity
ActiveCN106854144BImprove solubilityConvenient researchBiocideOrganic chemistryBiotechnologyOrder Lepidoptera
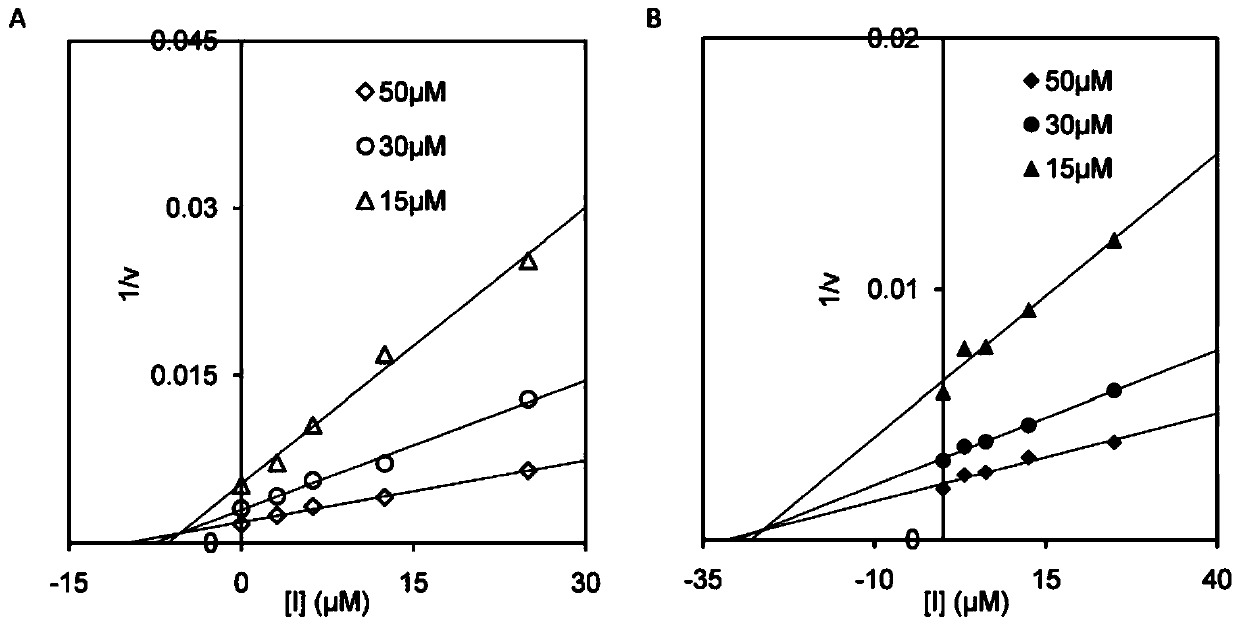
The invention discloses application of a compound for efficient inhibition of chitinase OfChi-h and hexosaminidase OfHex1 activity, and relates to application of a compound phlegmacin B1 and its derivatives in inhibiting chitinase OfChi-h and hexosaminidase OfHex1 activity. The used final concentration is not less than 13ppm, under the concentration, the measured inhibition rates are 90.4% and 71.4% respectively, and inhibition constants Ki are 5.5 microM and 26 microM respectively. The invention proves that the compound phlegmacin B1 has good application prospect in the prevention and control of agricultural pests, especially in prevention and control of lepidoptera insects.
Owner:DALIAN UNIV OF TECH
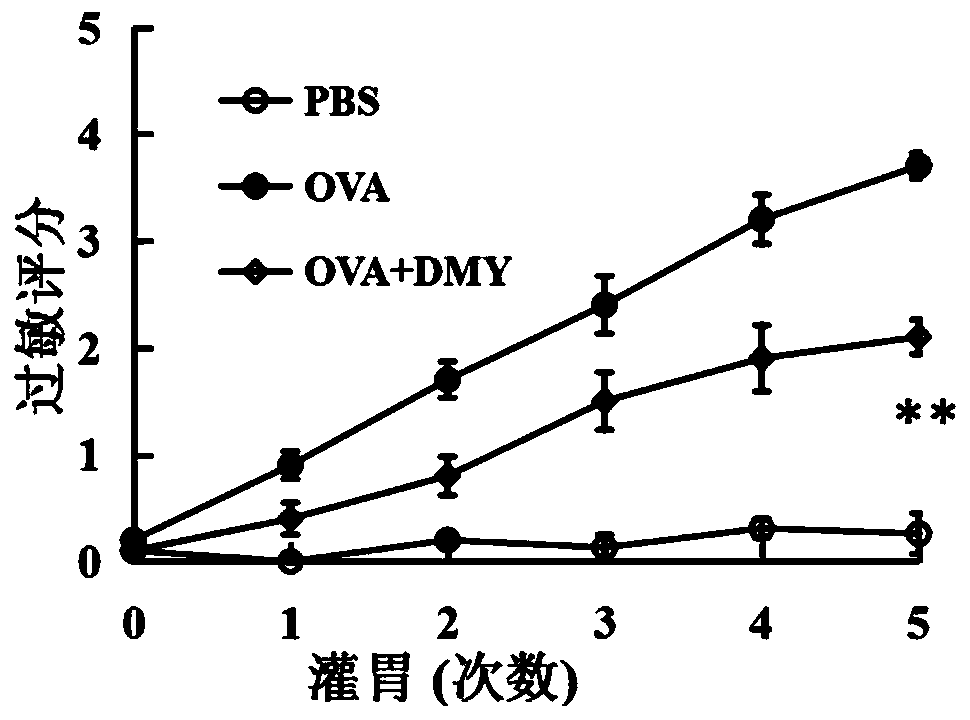
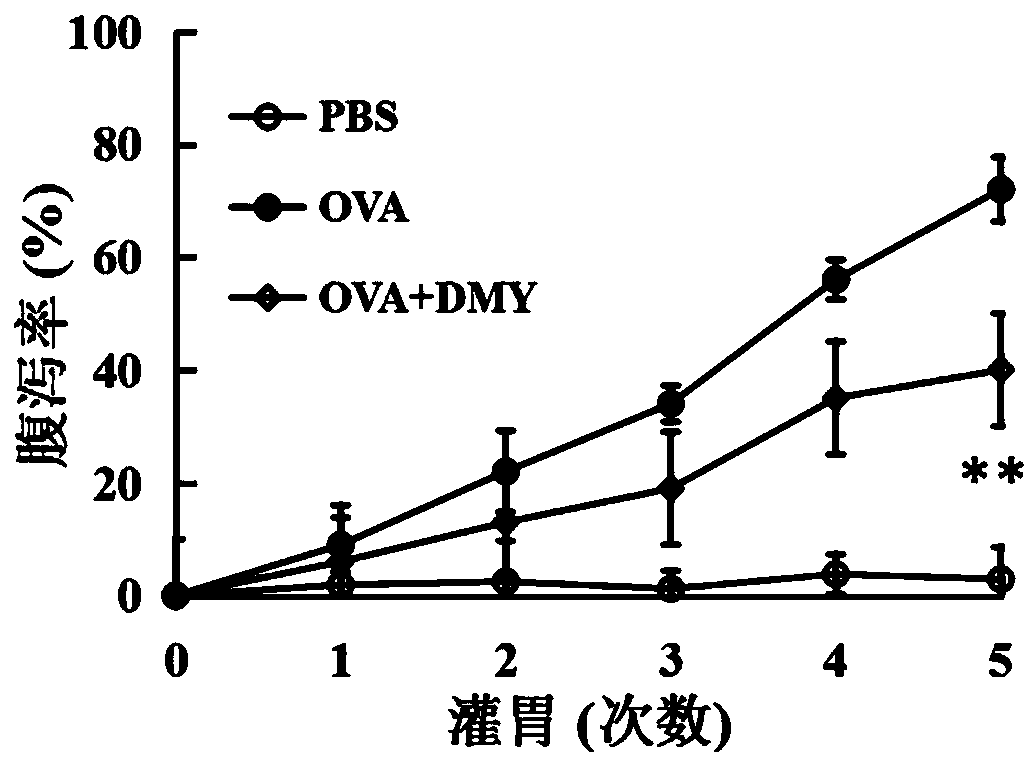
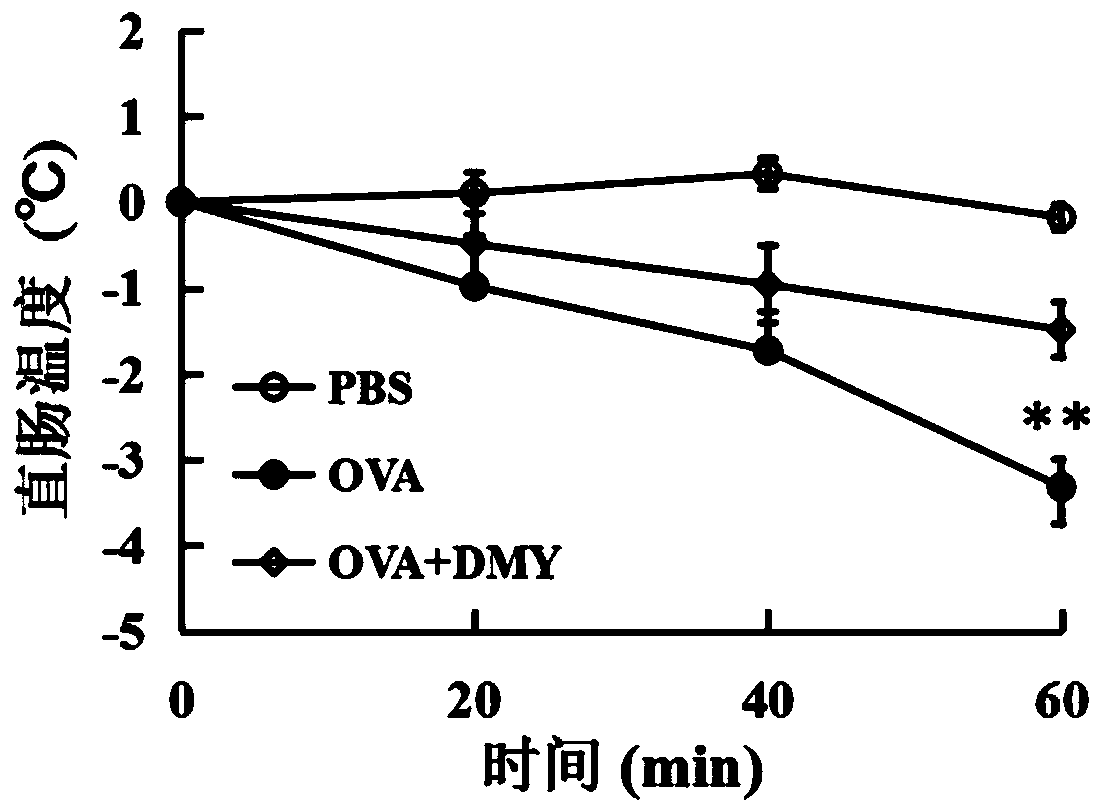
Application of dihydromyricetin to preparation of medicines for resisting food allergy
InactiveCN110693871AImprove stabilityGood anti-food allergy activityOrganic active ingredientsImmunological disordersBasophilic GranulocyteFood sensitization
The invention provides an application of dihydromyricetin to preparation of medicines for resisting food allergy. The dihydromyricetin as one of active components or an only active component is used for preparing the medicines for resisting food allergy, and the dihydromyricetin is subjected to alkaline modification treatment. Through a food sensitinogen induced mouse allergy model and an in vitrorat basophilic granulocyte model, the situation that the dihydromyricetin can relieve allergy symptoms of mice, can reduce the level of specific IgE, histamine and mastocyte protease in serum and canrestrain rat basophilic granulocyte from releasing beta-hexosaminidase is determined, and the dihydromyricetin has favorable activity of resisting food allergy, so that a new effective active component for treating the food allergy is provided; and besides, after being subjected to alkaline modification treatment, the dihydromyricetin has the characteristics of being stable under neutral condition and capable of maintaining favorable activity for resisting the food allergy.
Owner:JIMEI UNIV
Brucella Melitensis Mutants and Methods
Certain attenuated mutants of Brucella, especially B. melitensis, B. abortus, B. suis and B. ovis, when administered to a human or animal trigger a protective immune response such that subsequent challenge with virulent Brucella of the same species does not result in disease or results in much less severe symptoms. Functional inactivation of galE, a virB gene or the operon (ORFs 1087-1090) comprising the gene encoding β-hexosaminidase (BMEI1087) and a lytic murein transglycosylase gene (BMEI1088). A specific example of the attenuated galE mutant which produces a protective immune response is B. melitensis GR024. The specific example of an inactivated ORF1087-1090 operon is B. melitensis GR026; it has an insertion mutation in the promoter region upstream of ORF 1090. Vaccination with live cells of either or both of these mutants results in a T cell response which protects the human or animal against challenge with virulent B. melitensis. Similar strategies for protective immunity using live attenuated mutants are useful for B. abortus, B. suis and B. ovis as well.
Owner:WISCONSIN ALUMNI RES FOUND
Diaryl urea compounds containing quinazolinone and preparation method and application thereof
ActiveCN107814773BReduce concerns about anaphylaxisRelieve painOrganic active ingredientsOrganic chemistryHexosaminidasePharmaceutical Substances
The invention provides a quinazolinone-containing diaryl urea compound and a preparation method and application thereof. The quinazolinone-containing diaryl urea compound can obviously inhibit LAD2 cells from releasing beta-hexosaminidase, shows obvious dosage association, can be used for preparing an antiallergic medicament, and particularly can be used for preparing a medicament for antagonizingLAD2 cells from releasing beta-hexosaminidase so as to reduce pain and a load of a patient. The preparation method provided by the invention is easy in obtaining of raw material sources, mild in reaction condition, simple in operation in the reaction process and cheap in used reagent.
Owner:XI AN JIAOTONG UNIV
Lignan compound derived from radix paeoniae rubra and preparation method and application thereof
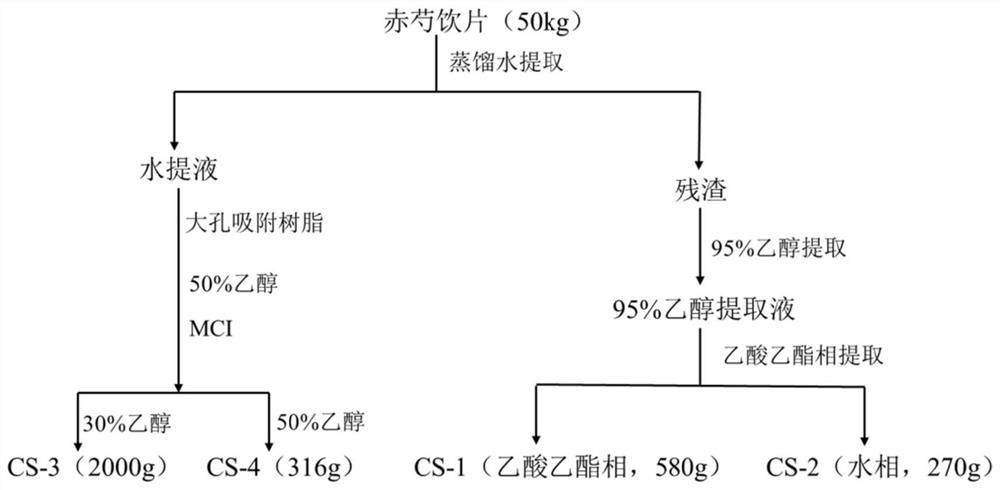
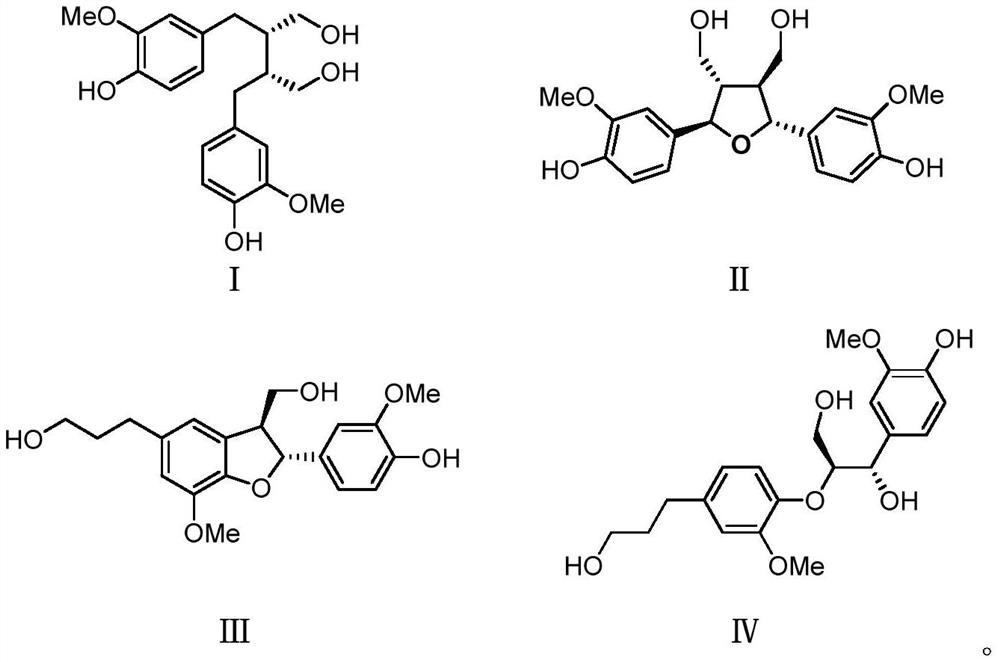
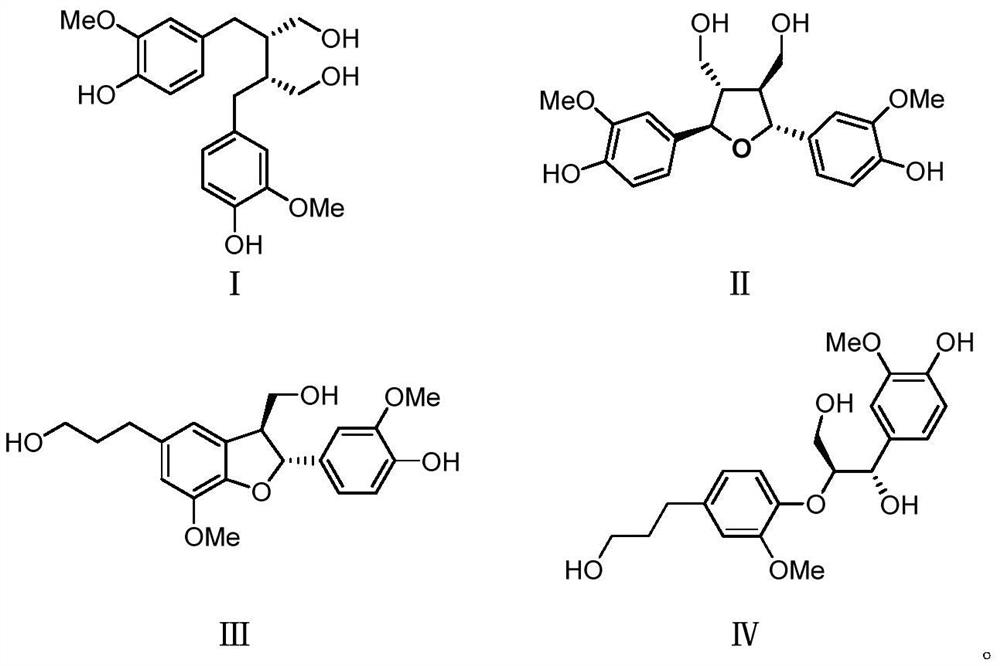
ActiveCN112972438AVisibly anti-allergicObvious anti-allergic effectAntipyreticAnalgesicsDiseaseLignan
The invention belongs to the field of medicines, and relates to a lignan compound derived from radix paeoniae rubra, a preparation method and application thereof in prevention and treatment of allergic diseases. Pharmacological experiments prove that the compound can effectively inhibit release of beta-hexosaminidase (beta-HEX) and histamine (His) in sensitized RBL-2H3 cells, can significantly reduce the content of inflammatory mediators in RBL-2H3 cell supernatant, has good anti-allergic, anti-anaphylactoid and anti-inflammatory effects, and has an effective dose of 5 [mu]mol / L. The compound can be used for preparing the medicine for preventing and treating allergic diseases.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
Pharmaceutical composition for preventing or treating inflammatory diseases, containing lactococcus chungangensis as active ingredient
ActiveCN107405370AEasy to solveGood treatment effectAntibacterial agentsCosmetic preparationsStaphylococcus aureusCytokine
The composition, of the present invention, containing Lactococcus chungangensis as an active ingredient, has excellent effects of preventing or treating inflammatory diseases, inhibiting the secretion of nitric oxide and prostaglandin E2, which are major inflammatory factors, and inhibiting the secretion of beta-hexosaminidase and histamine, which are major factors related to allergies, and also significantly inhibits the production of skin disease-related cytokines and chemokines. The effects are on the same level as those of conventional therapeutic agents, even compared therewith, for skin diseases (tacrolimus), and thus the composition can be used as a preventive or therapeutic agent for inflammatory diseases. In addition, the composition according to the present invention exhibits an antibacterial activity against Staphylococcus aureus, which is a microorganism inducing secondary infection and the like of atopic dermatitis, and thus can be used in preventing or treating bacterial infection.
Owner:CHUNG ANG UNIV IND ACADEMIC COOP FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com