Vaginal secretion dry chemical analysis liquid quality control material and preparation method thereof
A technology of vaginal secretions and dry chemistry, applied in the field of clinical examination of vaginal secretions, can solve the problems of cumbersome operation, many factors affected by quality control substances, and easy confusion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0016] Embodiments of the present invention are described in detail below, examples of which are shown in the drawings, wherein the same or similar reference numerals designate the same or similar elements or elements having the same or similar functions throughout. The embodiments described below by referring to the figures are exemplary and are intended to explain the present invention and should not be construed as limiting the present invention.
[0017] In the description of the present invention, "plurality" means two or more, unless otherwise specifically defined.
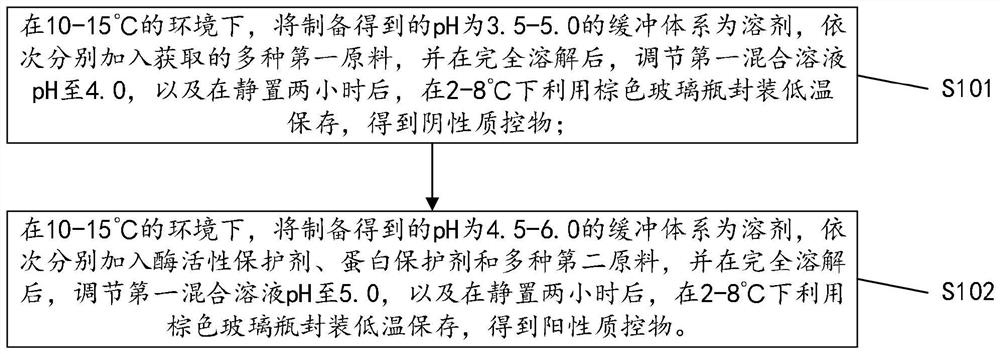
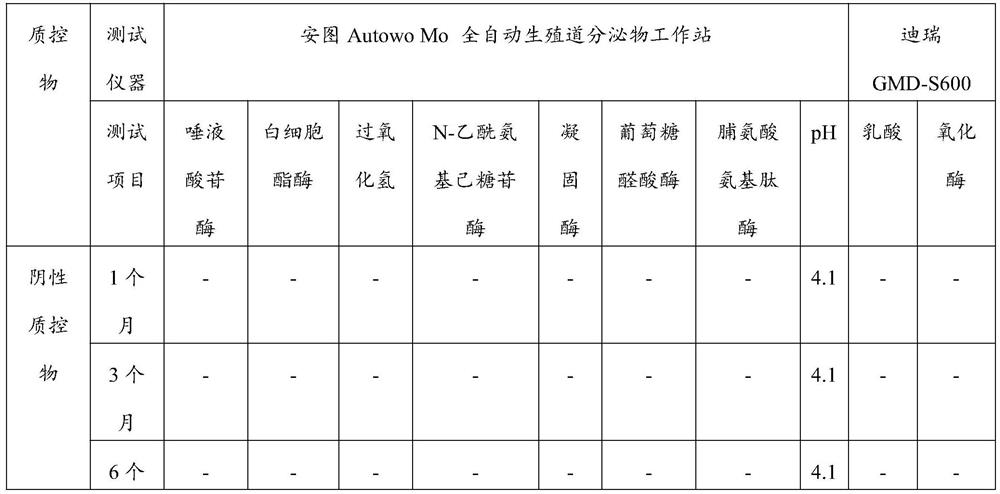
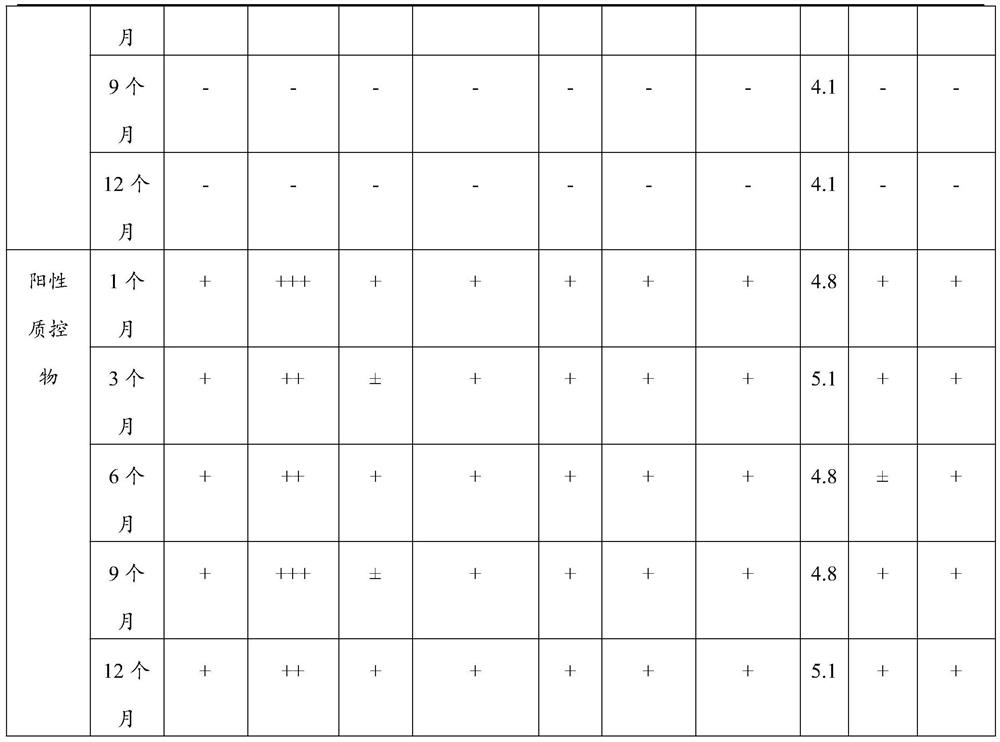
[0018] The invention provides a liquid quality control substance for dry chemical analysis of vaginal secretions. The liquid quality control substance for dry chemical analysis of vaginal secretions includes a negative quality control substance and a positive quality control substance. The negative quality control substance includes a pH value of 3.5- 5.0 buffer system 50~500mmol / L, preservative 0.01~0.5g / L,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com