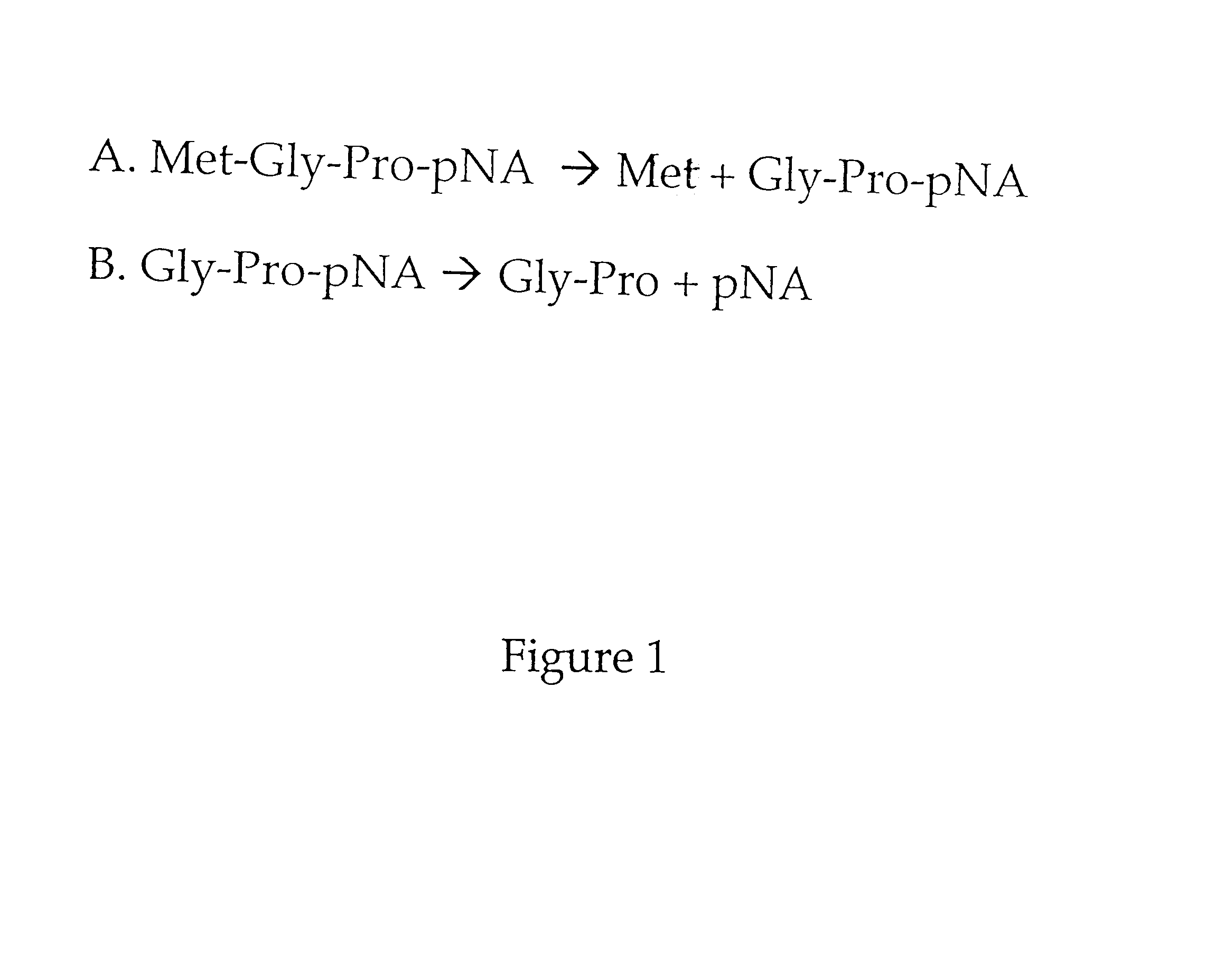Patents
Literature
202 results about "Aminopeptidase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Aminopeptidases are enzymes that catalyze the cleavage of amino acids from the amino terminus (N-terminus) of proteins or peptides(exopeptidases).They are widely distributed throughout the animal and plant kingdoms and are found in many subcellular organelles, in cytosol, and as membrane components. Aminopeptidases are used in essential cellular functions. Many, but not all, of these peptidases are zinc metalloenzymes.
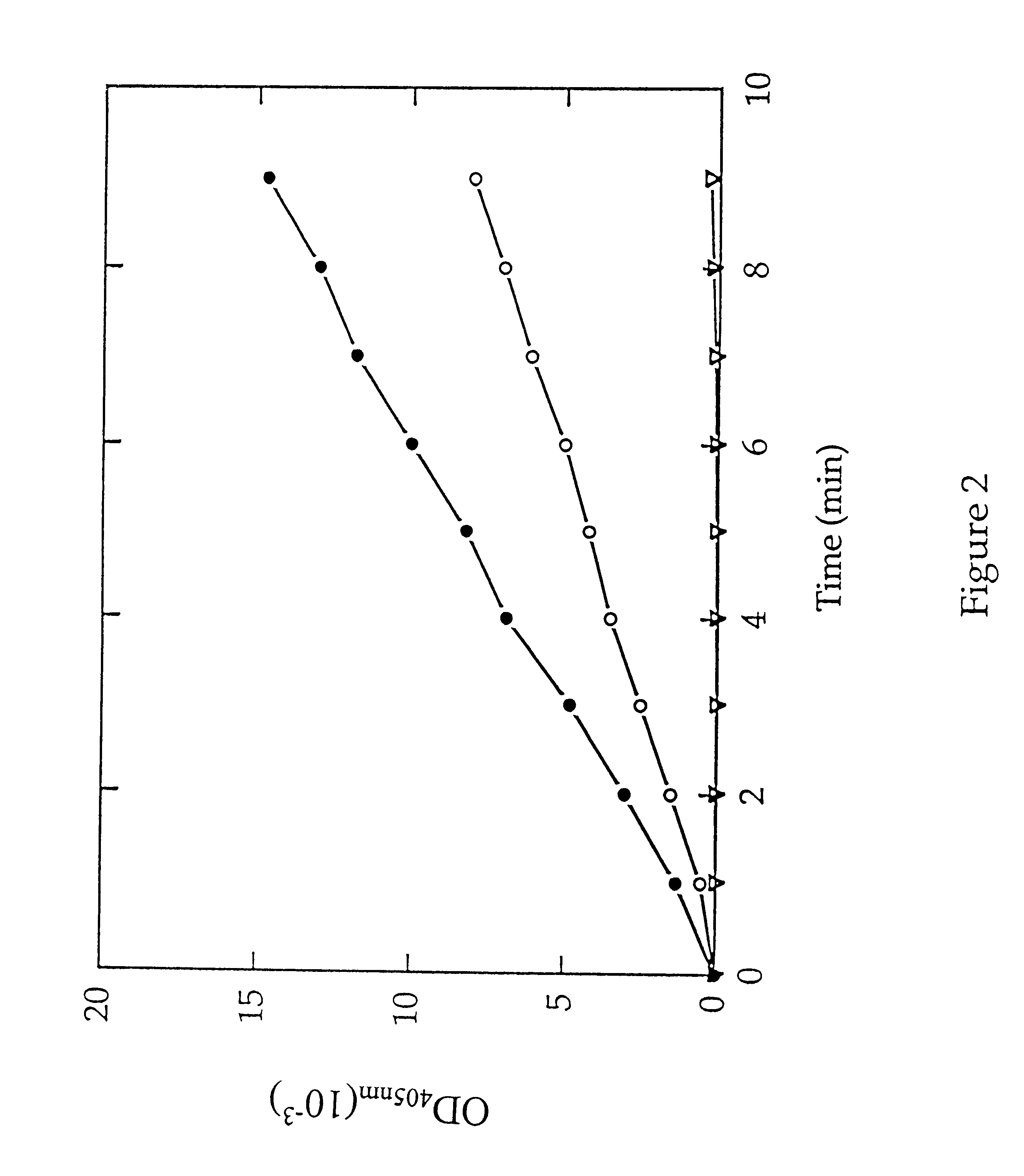
Methods for identifying inhibitors of methionine aminopeptidases
InactiveUS6261794B1Microbiological testing/measurementPeptidesMethionine aminopeptidaseMethionine biosynthesis
Methods are provided for detecting methionine aminopeptidase (MAP) activity and for detecting inhibitors of MAP. The methods utilize a peptide comprising an N-terminal methionine which can be cleaved from the peptide by MAP, and a C-terminal detection moiety which is released by a second peptidase only if the N-terminal methionine has been cleaved from the peptide. When the peptide is combined with MAP and the second peptidase, the detection moiety is released, while the addition of a MAP inhibitor will inhibit the release of the detection moiety. Reaction mixes, peptides, and kits which are useful for practicing the methods are also provided.
Owner:SAINT LOUIS UNIVERSITY
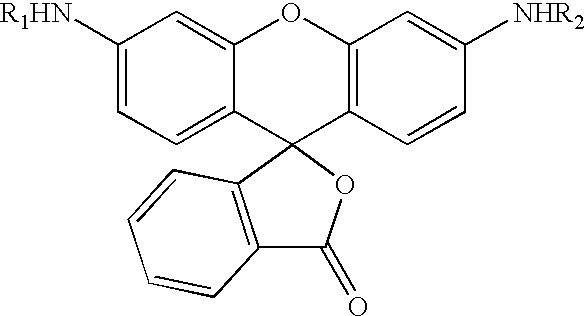
Novel fluorogenic or fluorescent reporter molecules and their applications for whole-cell fluorescence screening assays for caspases and other enzymes and the use thereof
InactiveUS20020150885A1Microbiological testing/measurementChemiluminescene/bioluminescenceScreening proceduresApoptosis
The present invention relates to novel fluorescent dyes, novel fluorogenic and fluorescent reporter molecules and new enzyme assay processes that can be used to detect the activity of caspases and other enzymes involved in apoptosis in whole cells, cell lines and tissue samples derived from any living organism or organ. The reporter molecules and assay processes can be used in drug screening procedures to identify compounds which act as inhibitors or inducers of the caspase cascade in whole cells or tissues. The reagents and assays described herein are also useful for determining the chemosensitivity of human cancer cells to treatment with chemotherapeutic drugs. The present invention also relates to novel fluorogenic and fluorescent reporter molecules and new enzyme assay processes that can be used to detect the activity of type 2 methionine aminopeptidase, dipeptidyl peptidase IV, calpain, aminopeptidase, HIV protease, adenovirus protease, HSV-1 protease, HCMV protease and HCV protease.
Owner:CYTOVIA INC
Amino acid-fluorophore compound and application thereof
ActiveCN102603695AOrganic chemistryMicrobiological testing/measurementNaphthalene diimideAbnormal expression
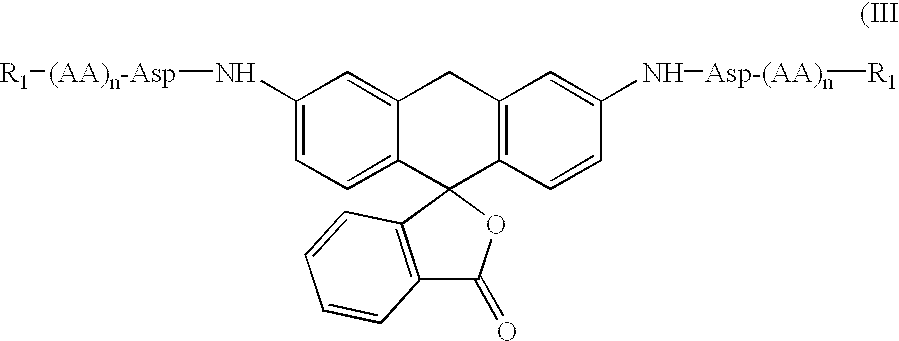
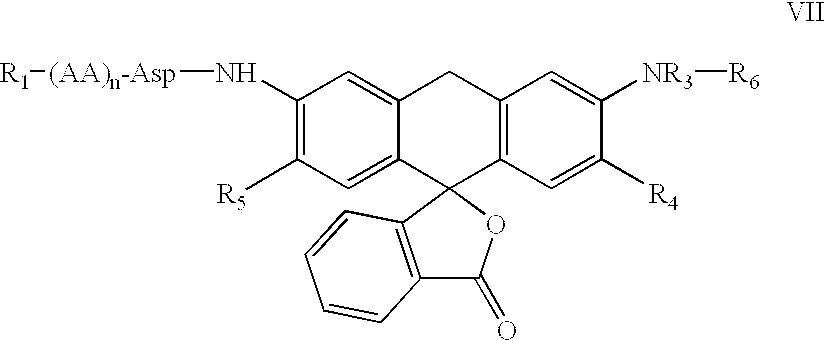
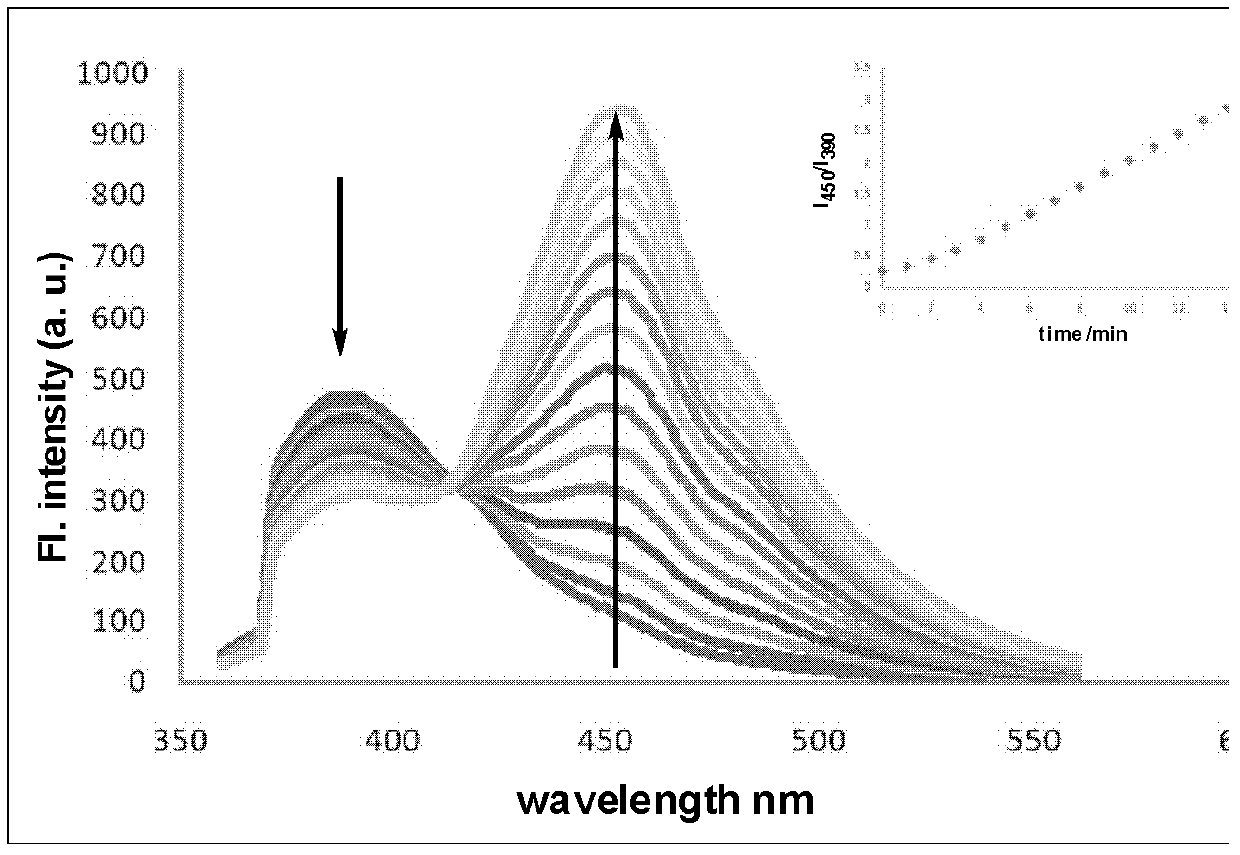
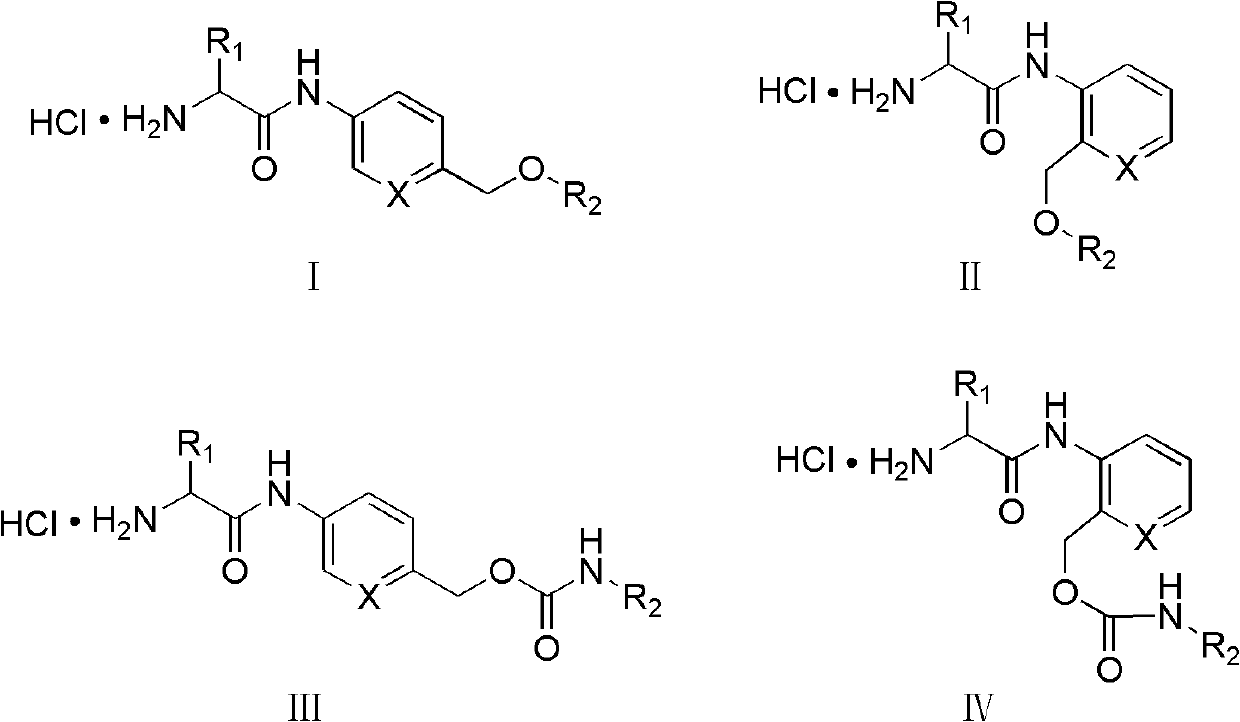
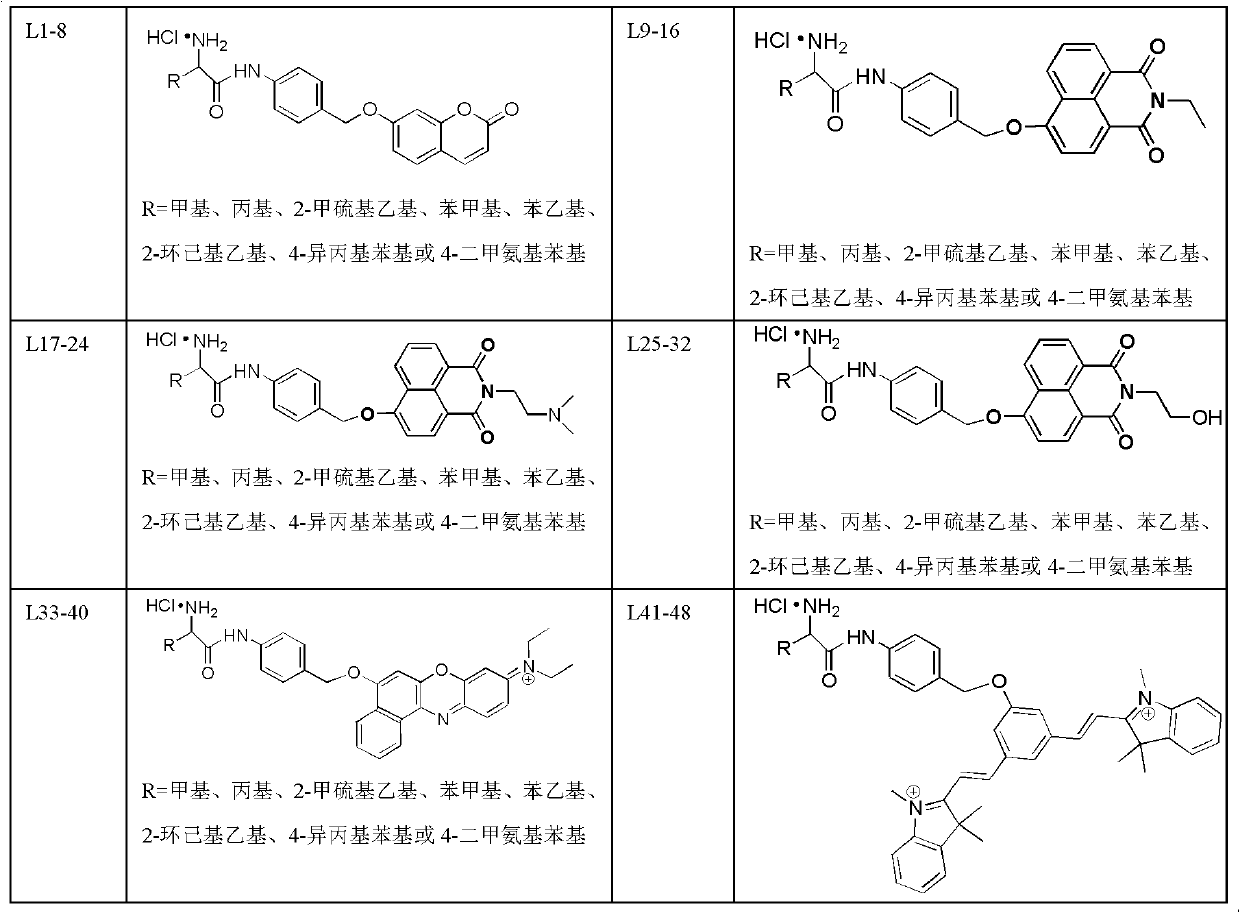
The invention relates to an amino acid-fluorophore compound and application of the compound, wherein the general structural formula of the amino acid-fluorophore compound is (I), (II), (III), and (IV) or a free form thereof, wherein R1 is selected from various amino acid side chains, preferably methyl, propyl, 2-methylthio ethyl, benzyl, phenylethyl, 2-cyclohexyl ethyl, 4-isopropyl phenyl and 4-dimethyl amino phenyl; R2 is selected from various fluorophores, preferably 7-hydroxyl coumarin, naphthalimide fluorophores, Nile red series and Cy fluorophores; and X is C or N. Fluorescent probe molecules can be used for detecting the activity (enzyme level and cell level) of aminopeptidase N, can be used as a probe tool for detecting the tissue distribution of aminopeptidase N and tumor tissue imaging, and can be used as a diagnosis tool for various diseases due to abnormal expression of aminopeptidase N. In addition, the preparation method of the compound has the advantages of mild reaction conditions, easily-accessible and cheap raw materials and simplicity in operation and after-treatment.
Owner:SHANDONG UNIV
Protease-deficient cells
A gram-negative bacterial cell is described that is deficient in a chromosomal gene present in a wild-type such cell which gene shares at least 80% sequence identity with the native sequence of the yfcK gene and encodes an aminopeptidase. Alternatively, a gram-negative bacterial cell is deficient in a chromosomal gene present in a wild-type such cell which gene encodes an aminopeptidase that shares at least 80% sequence identity with the native sequence of aminopeptidase b2324. Either of these types of cells, when comprising a nucleic acid encoding a heterologous polypeptide, produces an N-terminal unclipped polypeptide when it is cultured and the polypeptide recovered, with virtually no N-terminal clipped polypeptide produced as an impurity. Conversely, a method is provided for cleaving an N-terminal amino acid from a polypeptide comprising contacting the polypeptide with an aminopeptidase sharing at least 80% sequence identity with the native sequence of aminopeptidase b2324.
Owner:GENENTECH INC
Breast homing peptides and methods of identifying same using aminopeptidase P
InactiveUS20030232762A1Peptide/protein ingredientsRadioactive preparation carriersAmino acidMedicine
The present invention provides a method of directing a moiety to breast vasculature in a subject by administering to the subject a conjugate which contains a moiety linked to a homing molecule that selectively homes to breast vasculature, whereby the moiety is directed to breast vasculature. In one embodiment, the homing molecule is a peptide containing the amino acid sequence PGPEGAG (SEQ ID NO: 1), or a peptidomimetic thereof.
Owner:BURNHAM INST FOR MEDICAL RES
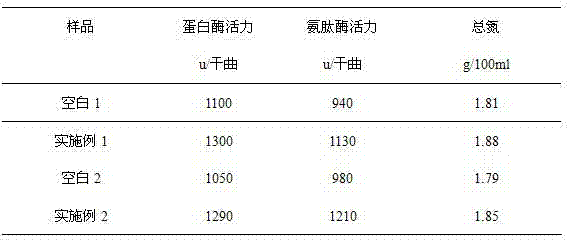
Fermentation preparation and extraction method for bacillus subtilis debitterized aminopeptidase
ActiveCN101492663ANo bitternessReduce or eliminate bitternessMicroorganism based processesEnzymesFood additiveDipeptide
The invention discloses a method for preparing and extracting Bacillus subtilis debittering aminopeptidase by fermentation, which belongs to the technical field of enzyme preparation and food additive. The invention uses the fermentation cylinder production condition of Bacillus subtilis Zj016 to collect fermentation liquor and obtain the product of debittering aminopeptidase by carrying out clarifying, ultrafiltration concentration and salting out on the fermentation liquor. The aminopeptidase produced by the selected Bacillus subtilis Zj016 is exopeptidase which can dissociate amino acid from a polypeptide chain amino terminal. Researched by the laboratory, the aminopeptidase cooperates with alkali protease to hydrolyze soybean protein isolate; the prepared soybean peptide does not have bitterness, and the contents of oligopeptide like dipeptide and tripeptide are higher. Besides, researches on the enzyme lines produced by the Bacillus subtilis show that the Bacillus subtilis only produces circumscribed prolease and does not have the activity of endo protease; moreover, the circumscribed prolease has a stronger hydrolysis capacity to the terminal amino acid with strong hydrophobicity. The research further explains that the invention can reduce or eliminate the bitterness generated during hydrolysis of the soybean protein.
Owner:江苏博立生物制品有限公司
Aminopeptidase for catalytic synthesis of carnosine, and preparation method and application thereof
InactiveCN107217048ALow cost of industrializationHigh reaction rateHydrolasesGenetic engineeringEnzyme GeneUltrafiltration
The invention provides aminopeptidase for catalytic synthesis of carnosine, and a preparation method and application thereof. The aminopeptidase has the amino acid sequence shown by SEQ ID NO:2. For the preparation of biological enzymes, firstly, a gene engineering strain of the biological enzymes is built; the biological enzyme gene fragment obtained through full gene synthesis is subjected to linearized plasmid recombination by using restriction enzymes SalI. The built gene-engineered strain is suitable for high-density culture; the exoenzyme is biologically synthesized; solid-liquid separation is performed; ultrafiltration concentration is performed to obtain enzyme liquid; histidine and beta-alanine can be catalyzed in normal-temperature and normal-pressure water solution to efficiently synthesize carnosine; the reaction environment is friendly; the production cost is low; the conversion time is short; the process operation is simple; the reaction system impurity content is low; the post treatment is easy; the wide prospects of large-scale industrial application are realized.
Owner:JIANGSU CHENGXIN PHARMA
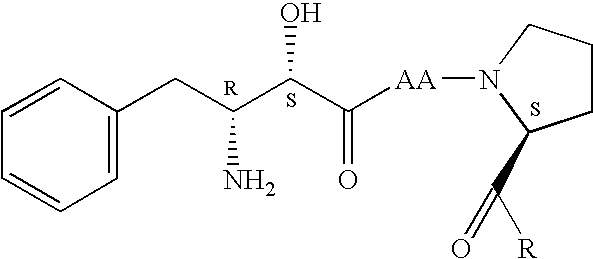
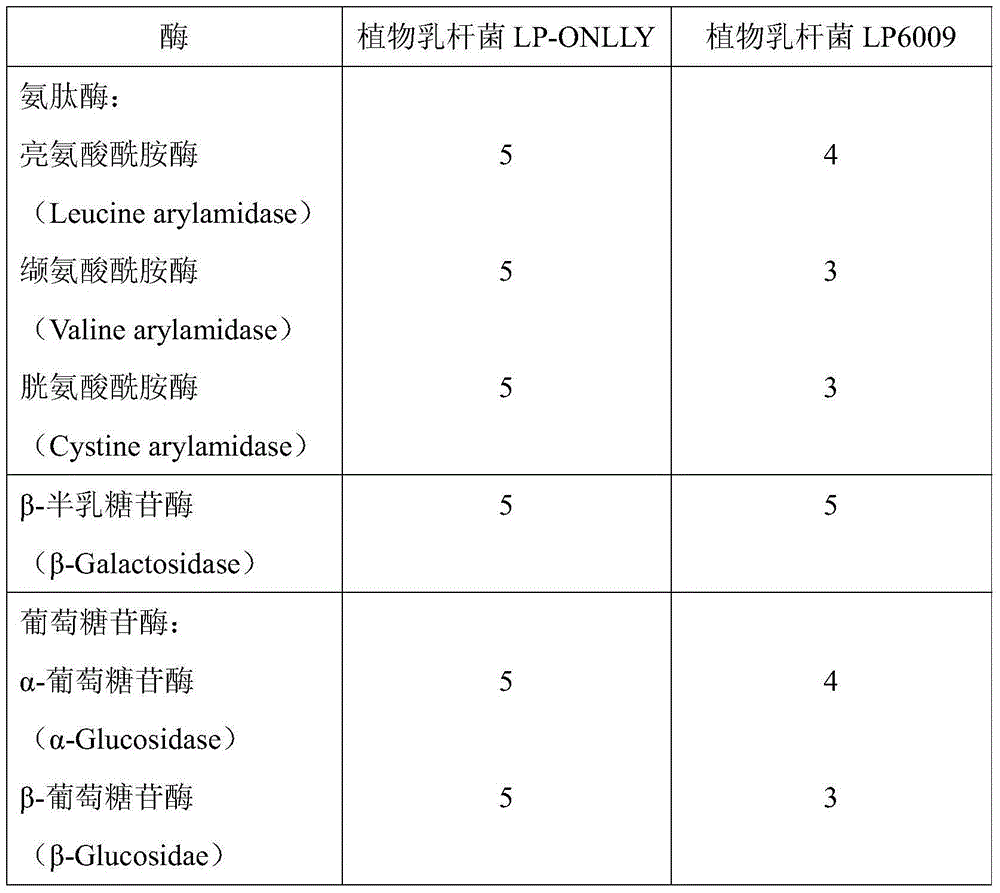
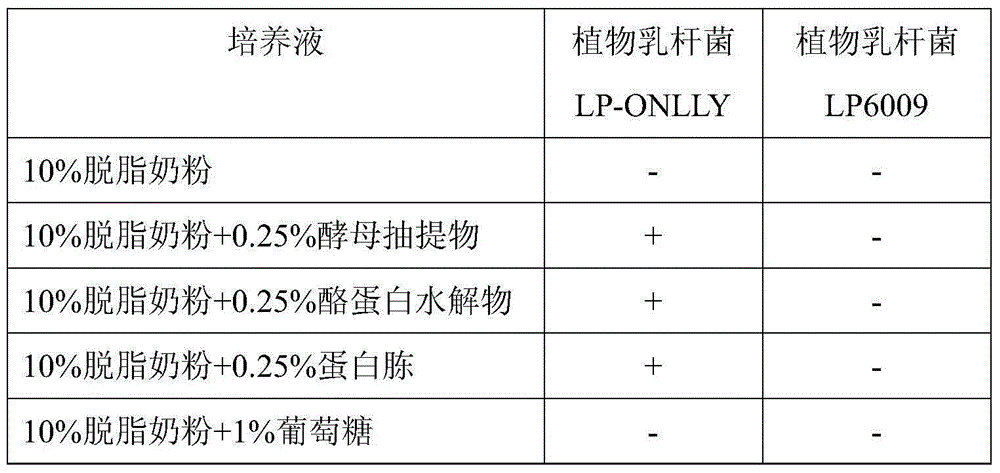
Lactobacillus plantarum (LP-ONLLY) and application thereof in active fermented milk
ActiveCN104531587AFunction healthyHigh aminopeptidase activityMilk preparationBacteriaMicroorganismAlglucerase
The invention discloses a lactobacillus plantarum (LP-ONLLY) and the application thereof in active fermented milk. The LP-ONLLY is preserved in China General Microbiological Culture Collection Center on December 6, 2004, and the preservation register number is CGMCC NO.1258. The LP-ONLLY has the high aminopeptidase activity, high beta-galactosidase activity and high glucosidase activity, and frozen and dried living bacterium powder is used as an independent leavening agent which can be applied for preparation of the active fermented milk. The active fermented milk contains the high probiotics live bacteria, the live bacteria have the high stability and can supplement probiotics, and the health function of the LP-ONLLY is achieved.
Owner:SHANGHAI JIAODA ONLLY CO LTD +1
Preparation method of low-biogenic amine soy sauce
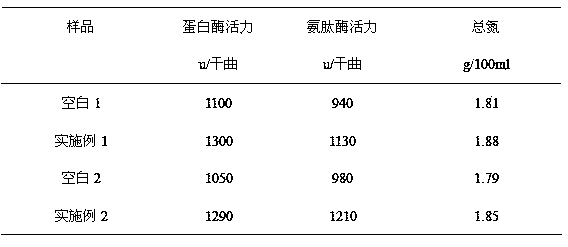
The invention provides a preparation method of low-biogenic amine soy sauce. The preparation method comprises the following steps: adding glycine and citric acid in starter propagation process, fermenting for 60-90 days by adopting a high-salt diluted-state fermented soy sauce brewing method, then squeezing and filtering, thereby being capable of obviously reducing the number of microorganisms in the yeast of the soy sauce. With the adoption of the method disclosed by the invention, the total content of biogenic amine in the crude soy sauce can be reduced by more than 60%, the activity of neutral protease and acid protease in the yeast of the soy sauce can be increased by more than 10%, the aminopeptidase activity can be increased by more than 20%, and the recovery rate of the protein can be increased by more than 3%.
Owner:SOUTH CHINA UNIV OF TECH
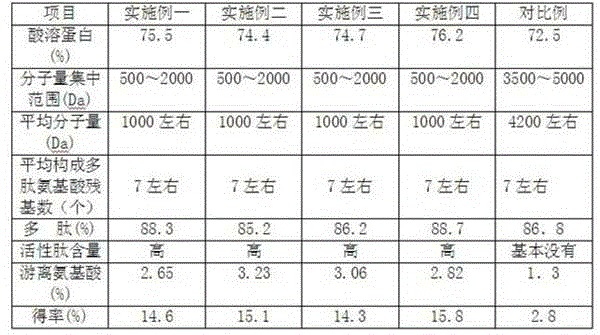
Method for extracting low molecular weight active collagen peptide from pigskin
InactiveCN102911991AHigh activityHigh yieldPeptide preparation methodsFermentationCollagenanOrganic chemistry
The invention relates to the technical field of deep processing of pigskin, particularly relates to a method for extracting pigskin collagen peptide. The method uses fresh pigskin to serve as a raw material, and a pure biology method is used for obtaining the pigskin collagen peptide. The method for extracting low molecular weight active collagen peptide from the pigskin comprises the following steps of: taking the fresh pigskin to serve as the raw material, firstly conducting outer cutting on collagen in the pigskin by adopting aminopeptidase and carboxypeptidase; separating the collagen from other tissue elements in the pigskin, then the pigskin is in a scattered state, and using endo protease to conduct enzymolysis on protein to enable the protein to form micromolecular protein peptides. Different protein peptides are scientifically selected in different stages, processing steps are optimized, production efficiency is improved, yield of the collagen peptide is promoted, and activity of the collagen peptide is improved.
Owner:HUZHOU JIAMEI BIOCHEM PRODS
Method for preparing leucine aminopeptidase through fermentation of bacillus subtilis engineering bacteria
InactiveCN102703407AIncrease enzyme activityEnhancing Fermentation EnzymesHydrolasesMicroorganism based processesFood additiveUltrafiltration
The invention relates to a method for preparing leucine aminopeptidase through the fermentation of bacillus subtilis engineering bacteria, belonging to the technical field of enzymic preparations and food additives. The method is characterized in that the enzyme powder of the leucine aminopeptidase is obtained by using the fermentation production of the bacillus subtilis engineering bacteria and carrying out extraction operations such as flocculation, filtration sterilization, ultrafiltration and concentration, and freeze drying on fermentation liquor, and the basic enzymology characteristics of the prepared leucine aminopeptidase are studied. According to the method, the bacillus subtilis engineering bacteria PMA5-SAP for the high yield of the leucine aminopeptidase, which is structured on the basis of the wild bacillus subtilis engineering bacteria zj016, is further optimized in the fermentation process, the enzyme activity of the leucine aminopeptidase produced in a 7L-fermentation tank is substantially increased by adopting strategies such as the pretreatment of a culture medium, the adjustment of culture medium components, the control of dissolved oxygen level and the like, and the level of enzymes produced through fermentation is increased from 7000U / L (unit for enzyme activity) to about 200000U / L.
Owner:JIANGNAN UNIV
Cyclic NGR polypeptide, radionuclide labeled molecular probe and application thereof
ActiveCN112079900ARealize the purpose of videoImprove targetingRadioactive preparation carriersPeptide preparation methodsIn vivoStructural formula
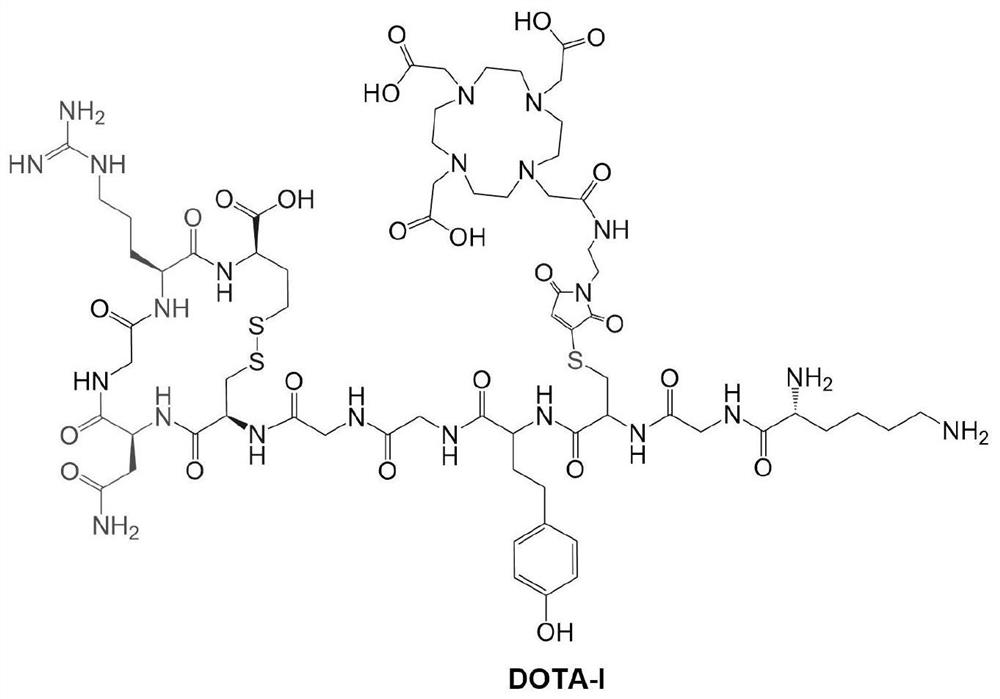
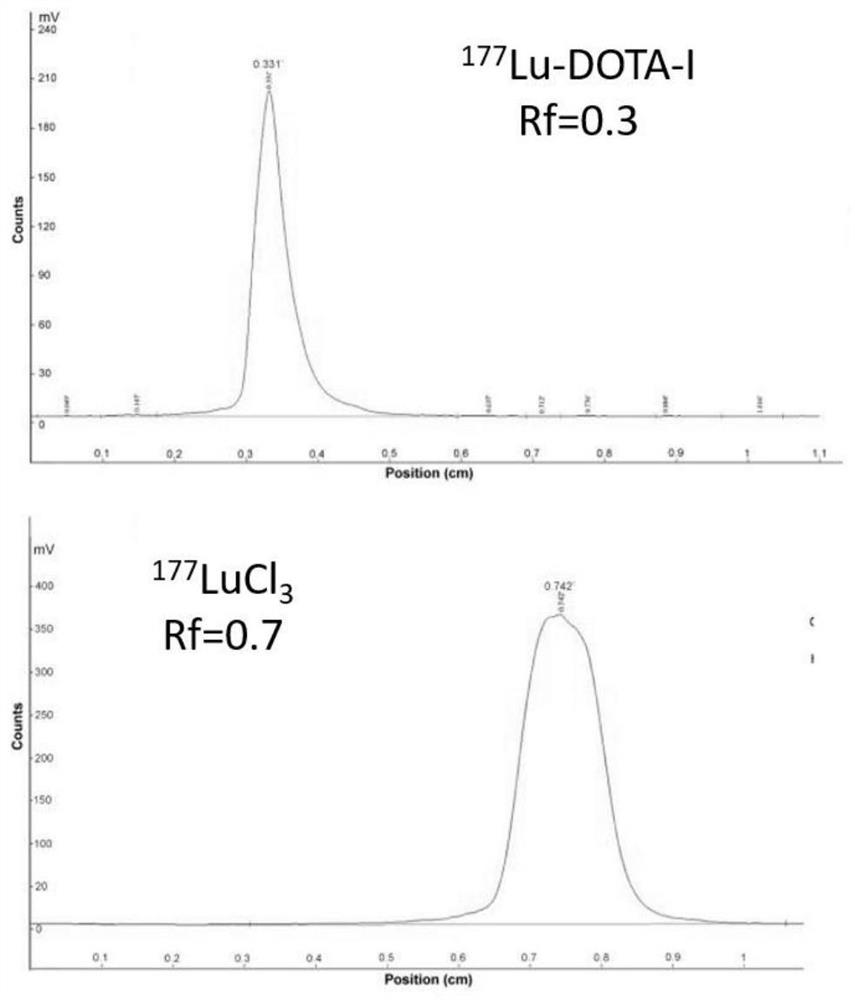
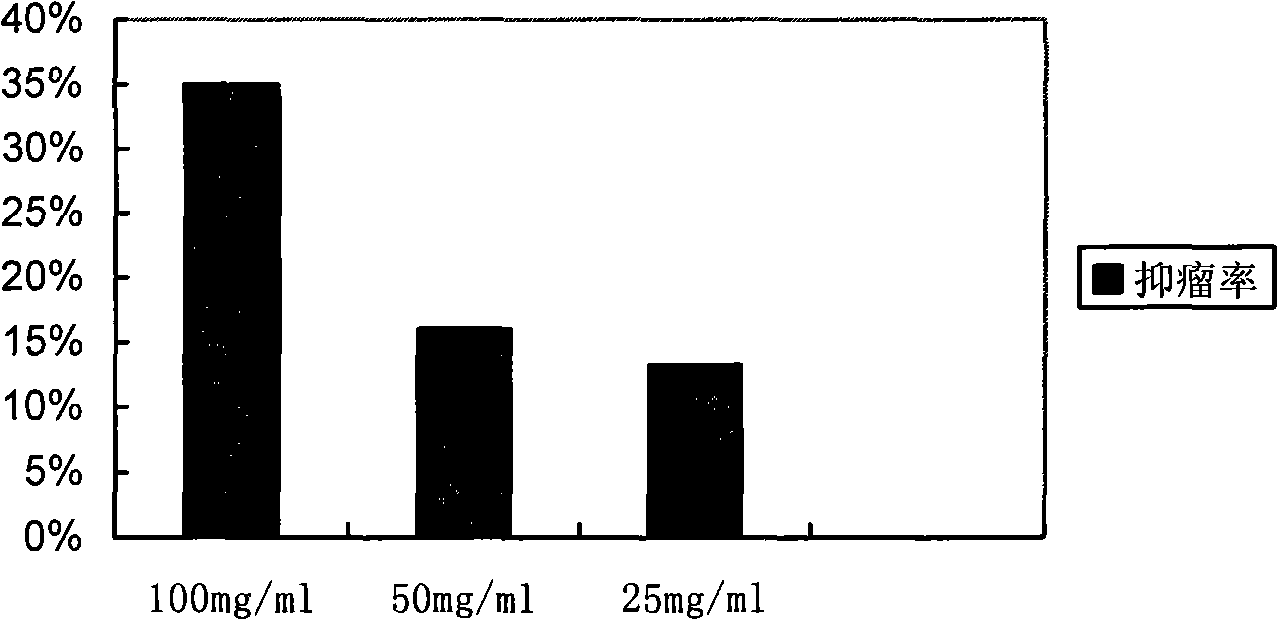
The invention provides a cyclic NGR polypeptide, a radionuclide-labeled aminopeptidase targeting molecular probe and application thereof. The molecular probe is obtained by labeling the cyclic NGR polypeptide with radionuclide, or coupling the cyclic NGR polypeptide with a bifunctional chelating group, and then labeling the coupled product with radionuclide. The radionuclide-labeled aminopeptidasetargeting molecular probe can be used for preparing a developing agent for diagnosing aminopeptidase N (CD13) high-expression tumors or preparing a drug for biological targeting treatment of aminopeptidase N (CD13) high-expression tumors. The integrin receptor can be accurately positioned in vivo, the aim of tumor molecular imaging is achieved through nuclear medicine imaging, and the in-vivo targeting property and the retention time thereof can be remarkably improved. And the aminopeptidase N (CD13) tumor can be accurately positioned as a carrier of radioactive therapy nuclide, so that the aim of treating tumors is fulfilled. The structural formula of the cyclic NGR polypeptide is shown in the specification.
Owner:INST OF NUCLEAR PHYSICS & CHEM CHINA ACADEMY OF
Aminopeptidase N inhibitor bestatin dino ester, synthesis and application thereof
InactiveCN101531613AStrong noveltySignificantly inhibitory activityOrganic active ingredientsOrganic compound preparationSolubilityCurative effect
The invention provides an intensive antitumor medicament, namely bestatin deanol ester, a preparation method and application thereof. The bestatin deanol ester not only can effectively inhibit ectopic expression of aminopeptidase N activity, but also greatly improve the water solubility of the parent drug bestatin of the bestatin deanol ester, has slow release effect, is more excellent than the parent drug bestatin either from healing effect or preparation, and has wide application. Particularly, the invention mainly relates to the following three aspects: (1) design and synthesis of the bestatin deanol ester; (2) N'N deanol which is a micromolecule fragment, can be connected with other medicaments containing carboxyl, improves the pharmacokinetic property of the medicaments, and improves water solubility; and (3) a method for synthesizing an ester substance to which the invention relates with wide application and mild condition.
Owner:SHANDONG UNIV +1
Aminopeptidase derived from Bacillus licheniformis and process for preparation of natural type proteins
The present invention relates to a process for removing methionine (Met) residue at N-terminus of proteins specifically. Particularly, the present invention relates to an aminopeptidase which is purified from Bacillus licheniformis removes a methionine residue from N-terminus of peptide and proteins. And the present invention relates to a process for preparing a natural type protein from proteins produced in microorganisms by recombinant DNA technology. Various kinds of natural type proteins such as human growth hormone (HGH) can be prepared massively and easily by the process of the present invention.
Owner:LG LIFE SCI LTD
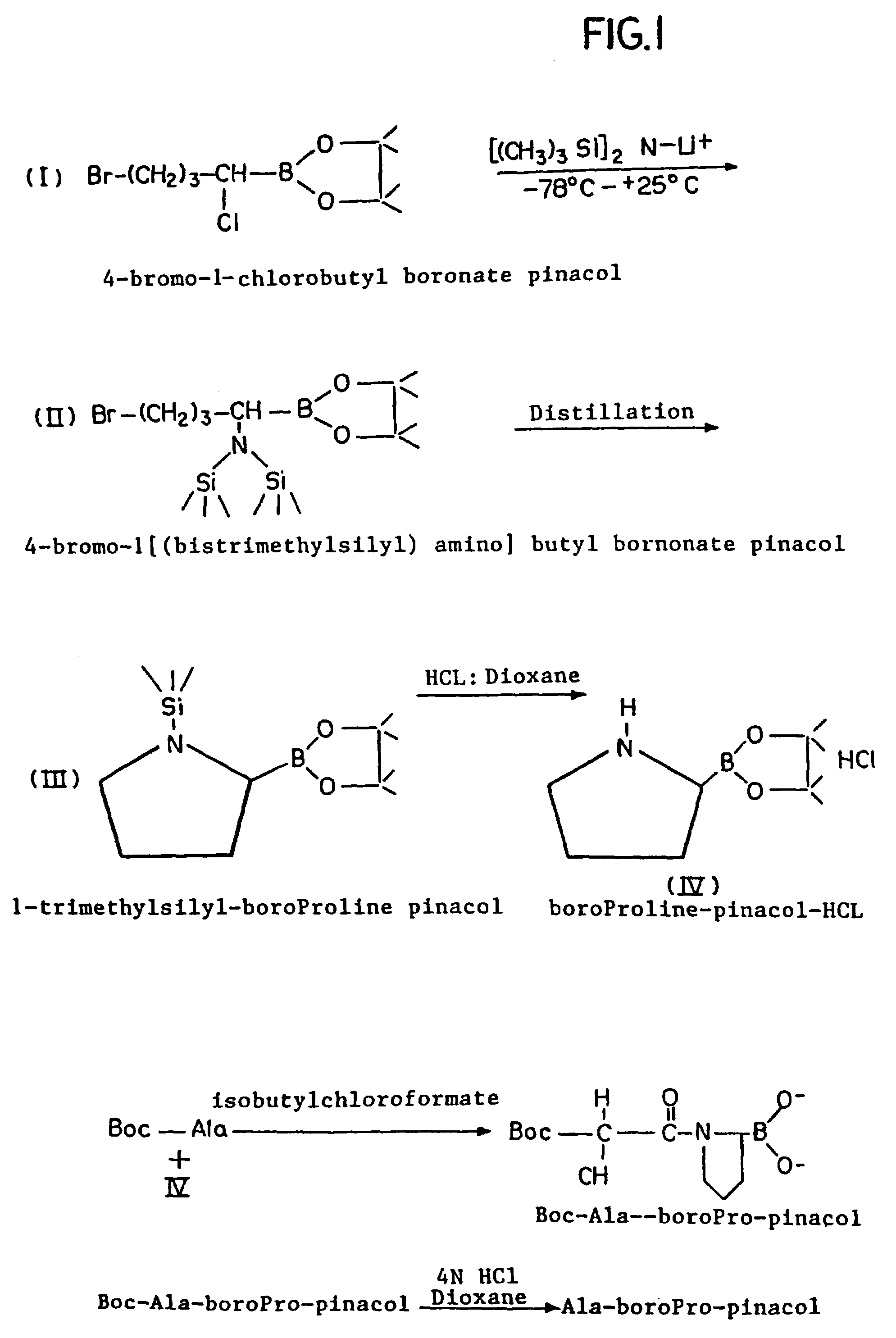
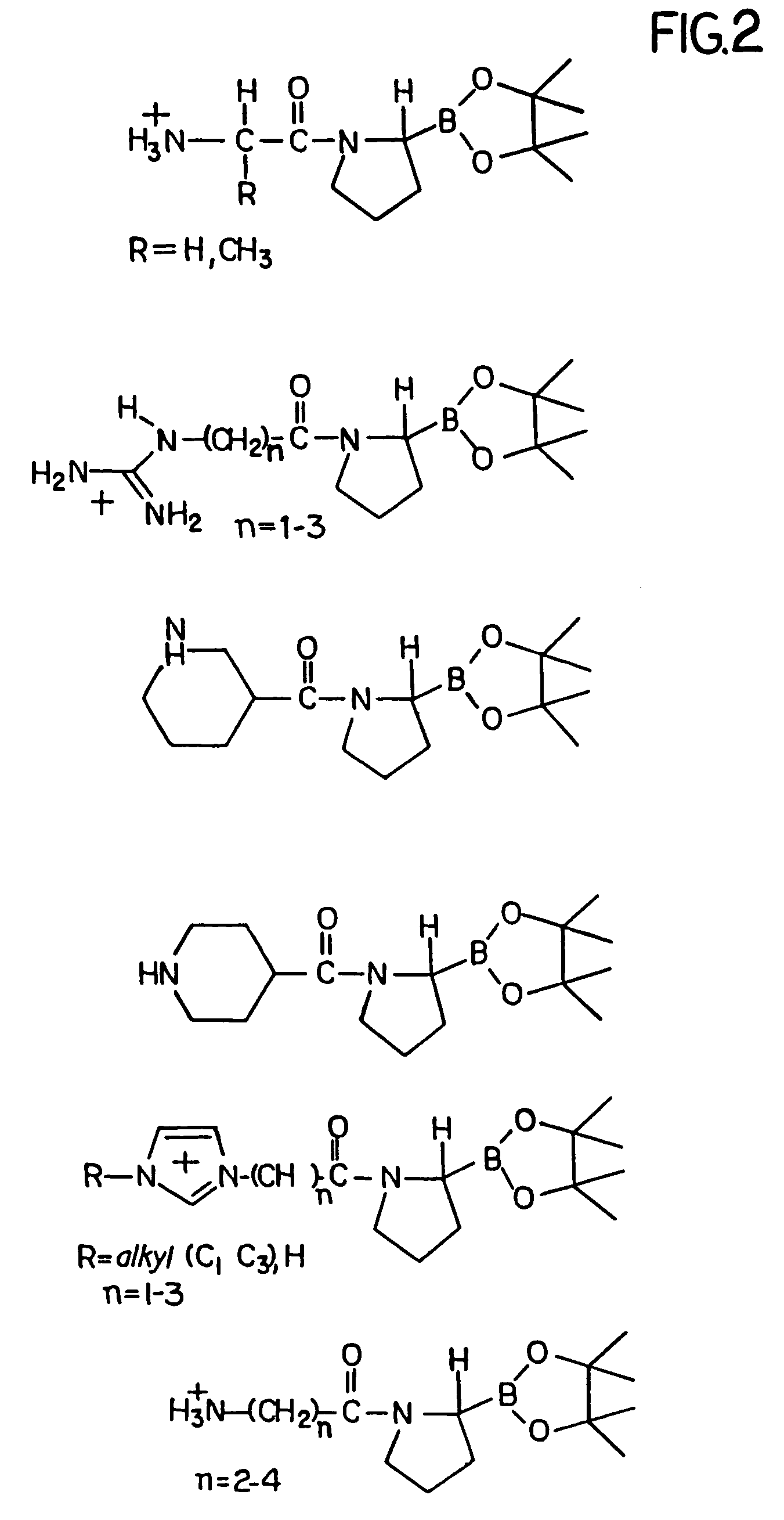
Inhibitors of dipeptidyl-aminopeptidase type IV
Peptide inhibitors of dipeptidyl-aminopeptidase type IV (DP-IV) are provided. The peptide inhibitors have an isomeric purity of about 96–99 percent. The peptide inhibitors include one or more amino acids covalently coupled to boroproline moiety. The compounds are useful as DP-IV inhibitors, in vivo and in vitro.
Owner:TRUSTEES OF TUFTS COLLEGE
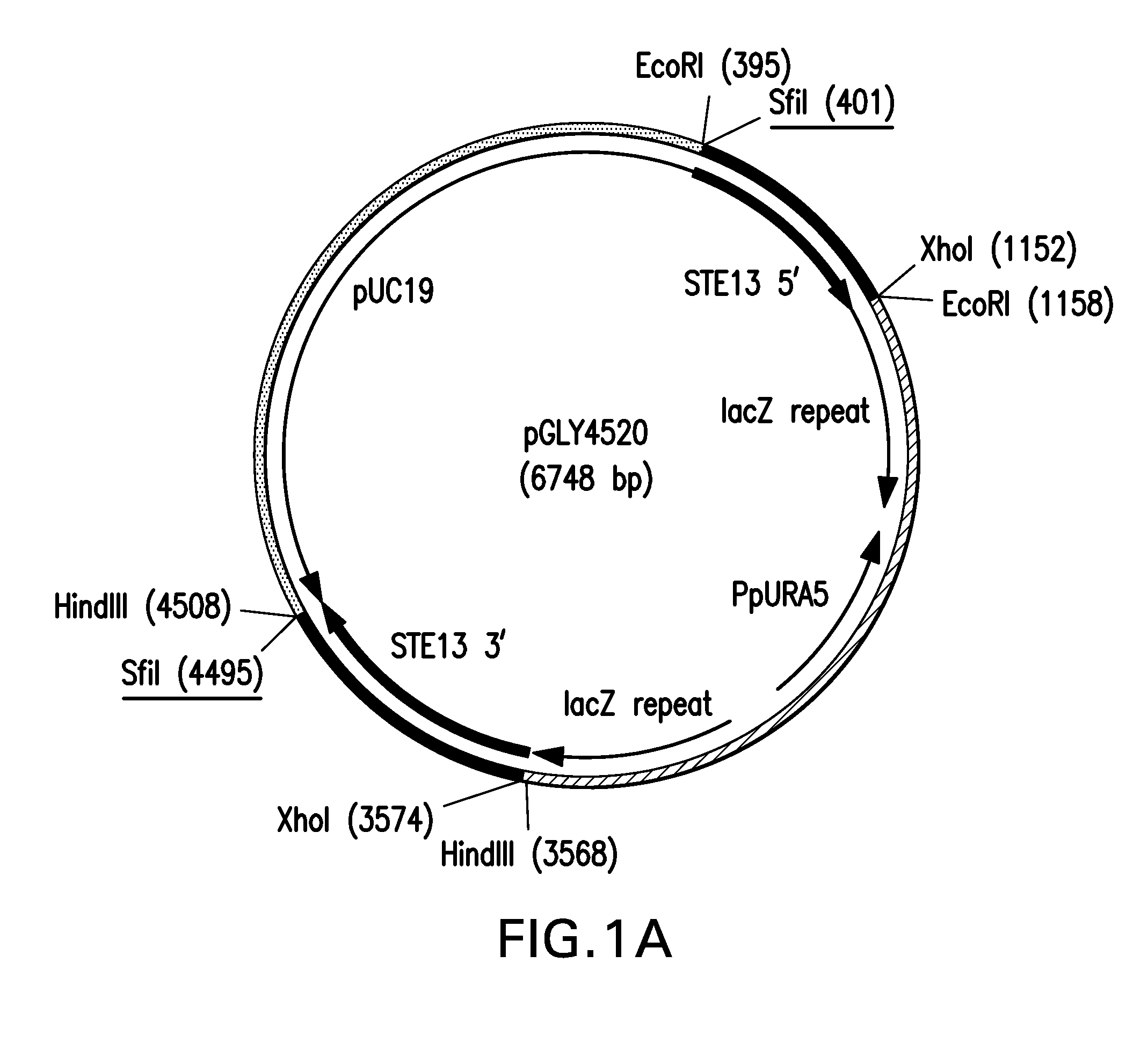
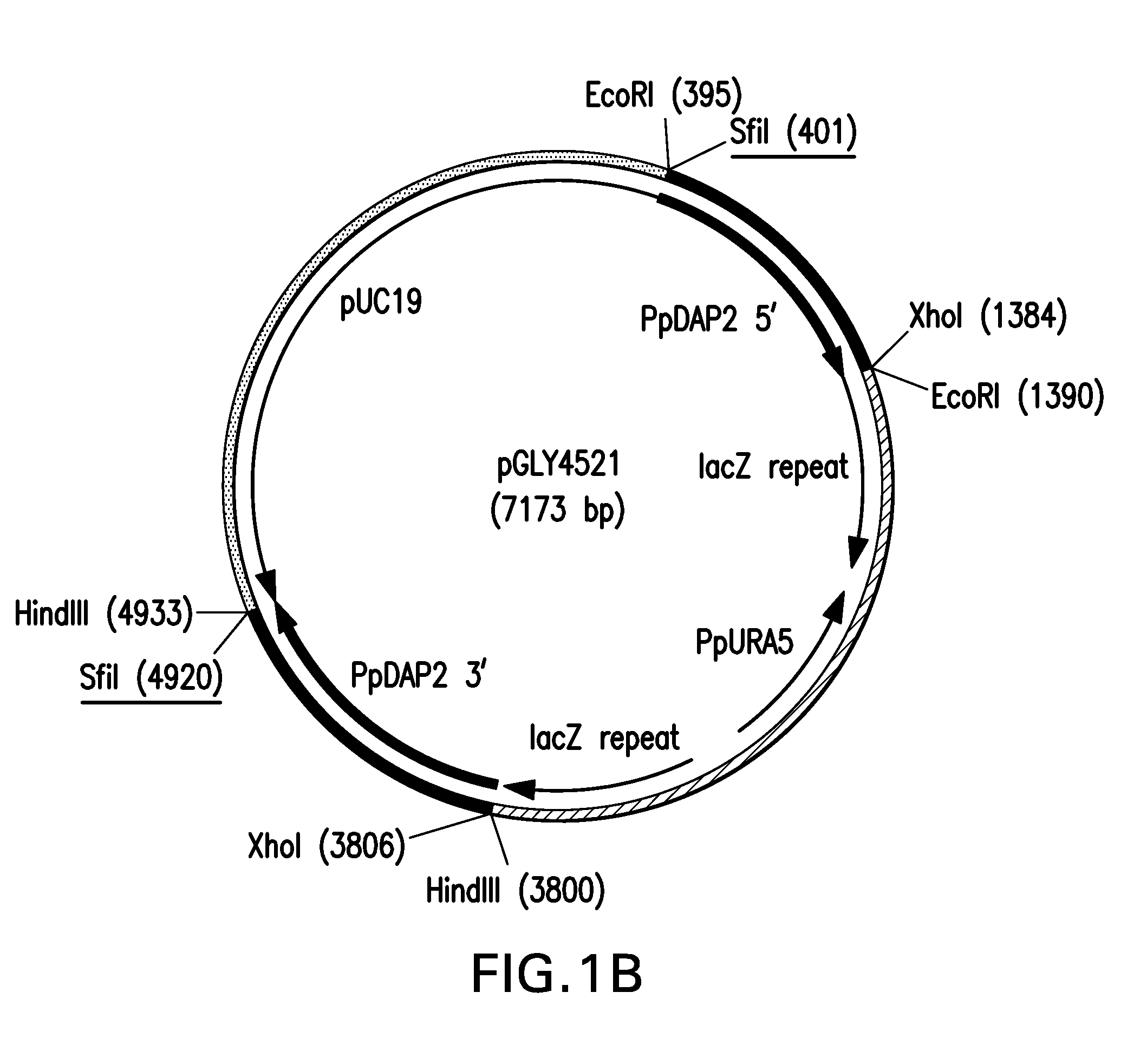
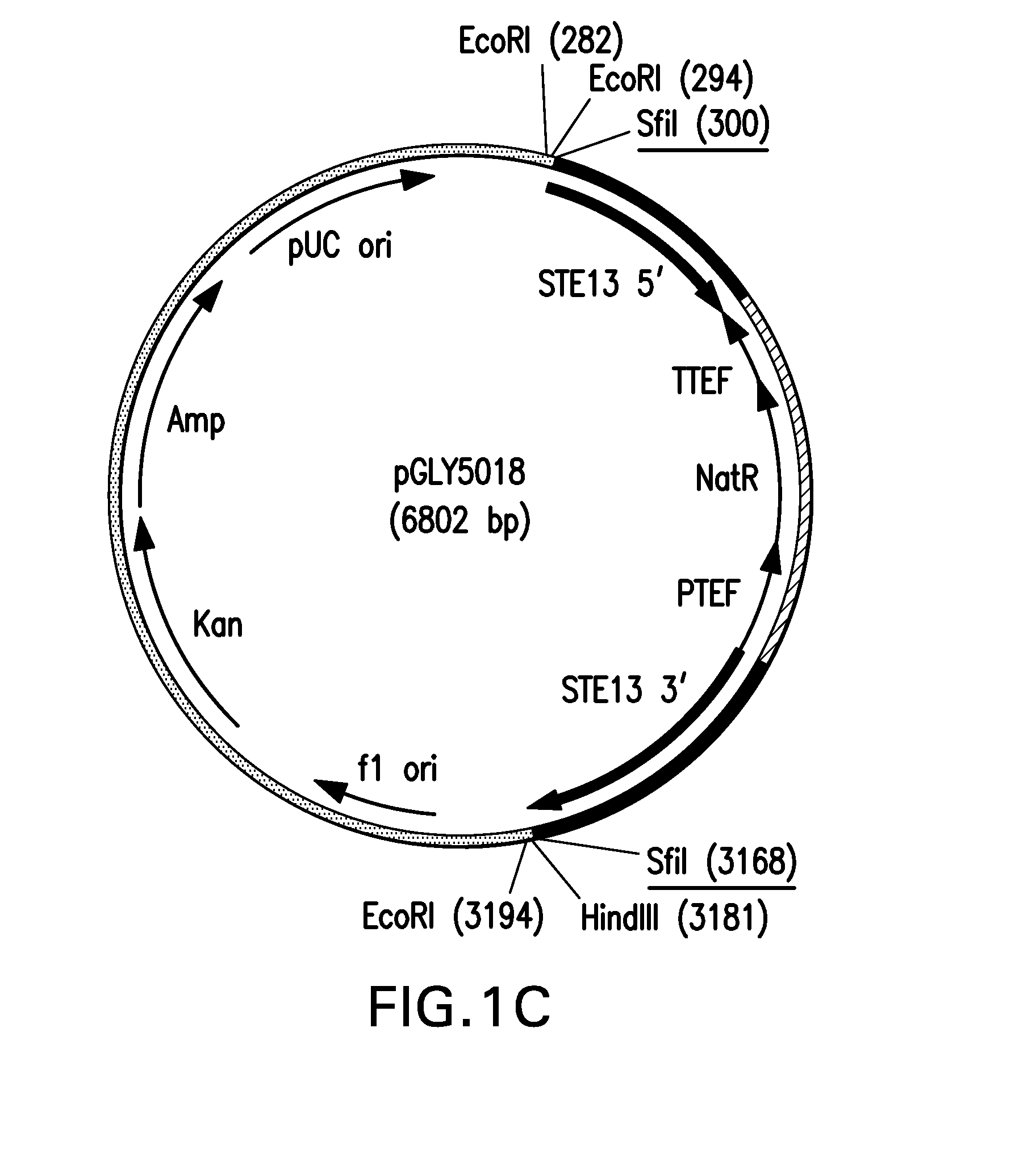
Method for producing therapeutic proteins in pichia pastoris lacking dipeptidyl aminopeptidase activity
The present invention related to methods and compositions for producing therapeutic proteins in yeast cell lines, and in particular Pichia pastoris, lacking dipeptidyl aminopeptidase (DAP) activity. DAP activity has been eliminated by genetically modifying a Pichia pastoris cell line such that STE13 and DAP2 have been deleted.
Owner:MERCK SHARP & DOHME CORP
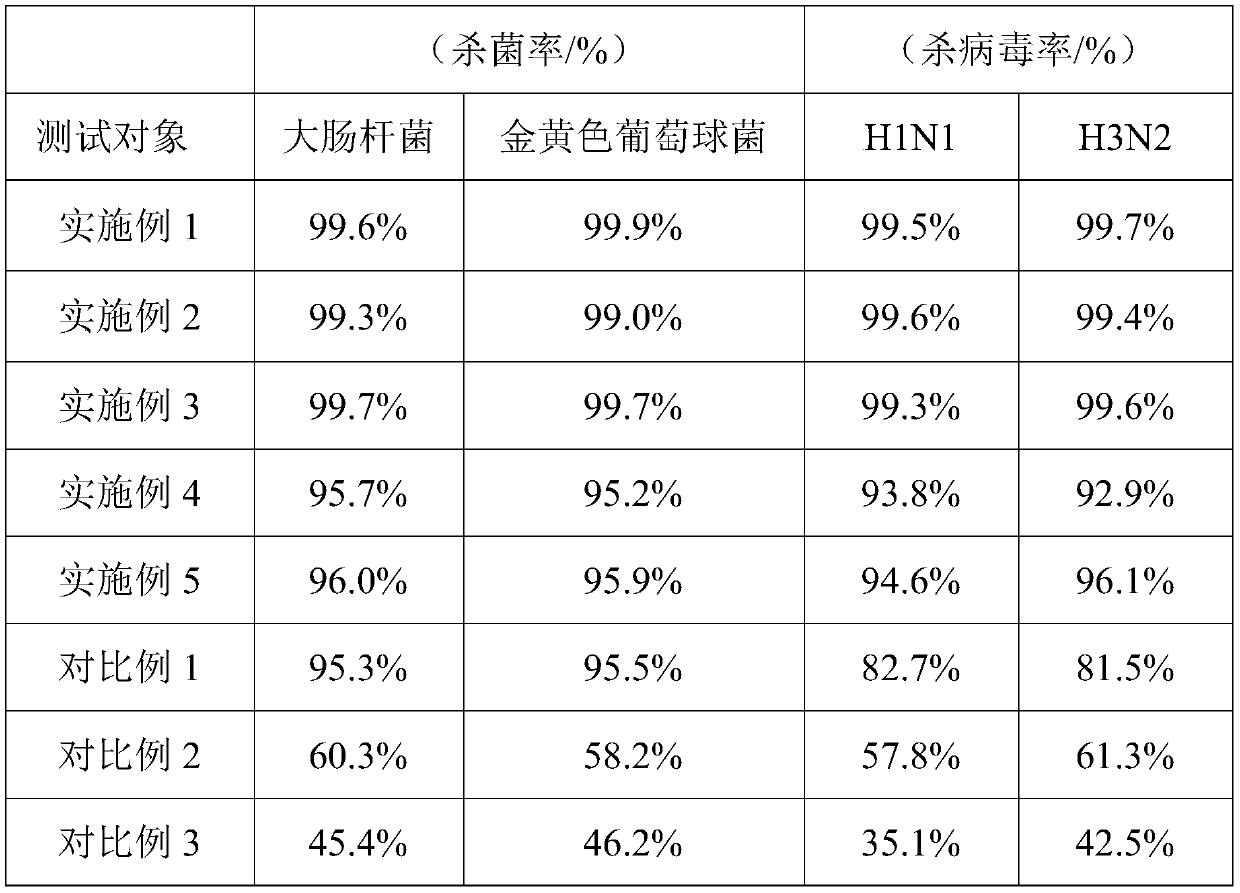
Antibacterial and antiviral mask and preparation method thereof
The invention relates to an antibacterial and antiviral mask and a preparation method thereof. The antibacterial and antiviral mask sequentially comprises an antibacterial layer, a bacteria dissolvinglayer and an antiviral layer from outside to inside, wherein the antiviral layer comprises aminopeptidase, and the aminopeptidase is derived from streptomyces nitro. According to the antibacterial and antiviral mask, aminopeptidase from streptomyces nitro is selected as an antiviral agent, so that the antibacterial and antiviral mask has the advantages of being high in antiviral property, safe and environmentally friendly; the antibacterial and antiviral mask has lasting antibacterial and antiviral capacity, the antibacterial and antiviral effects are thorough, and secondary infection of bacteria and viruses is effectively avoided; and the preparation method of the antibacterial and antiviral mask has the advantages of simplicity in operation, low cost, easiness in implementation, environmental friendliness and the like.
Owner:南京同曦大圣健康科技有限公司
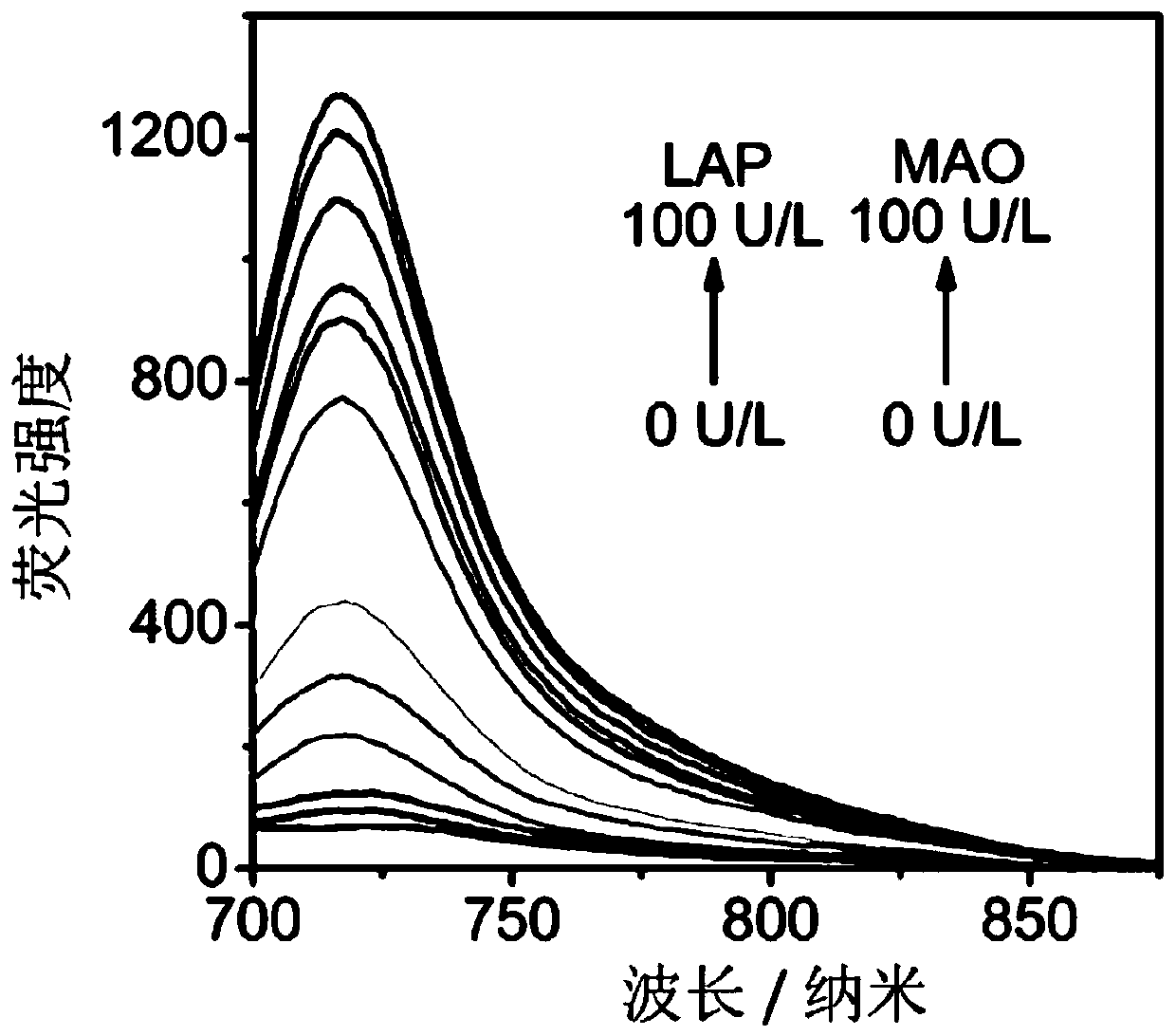
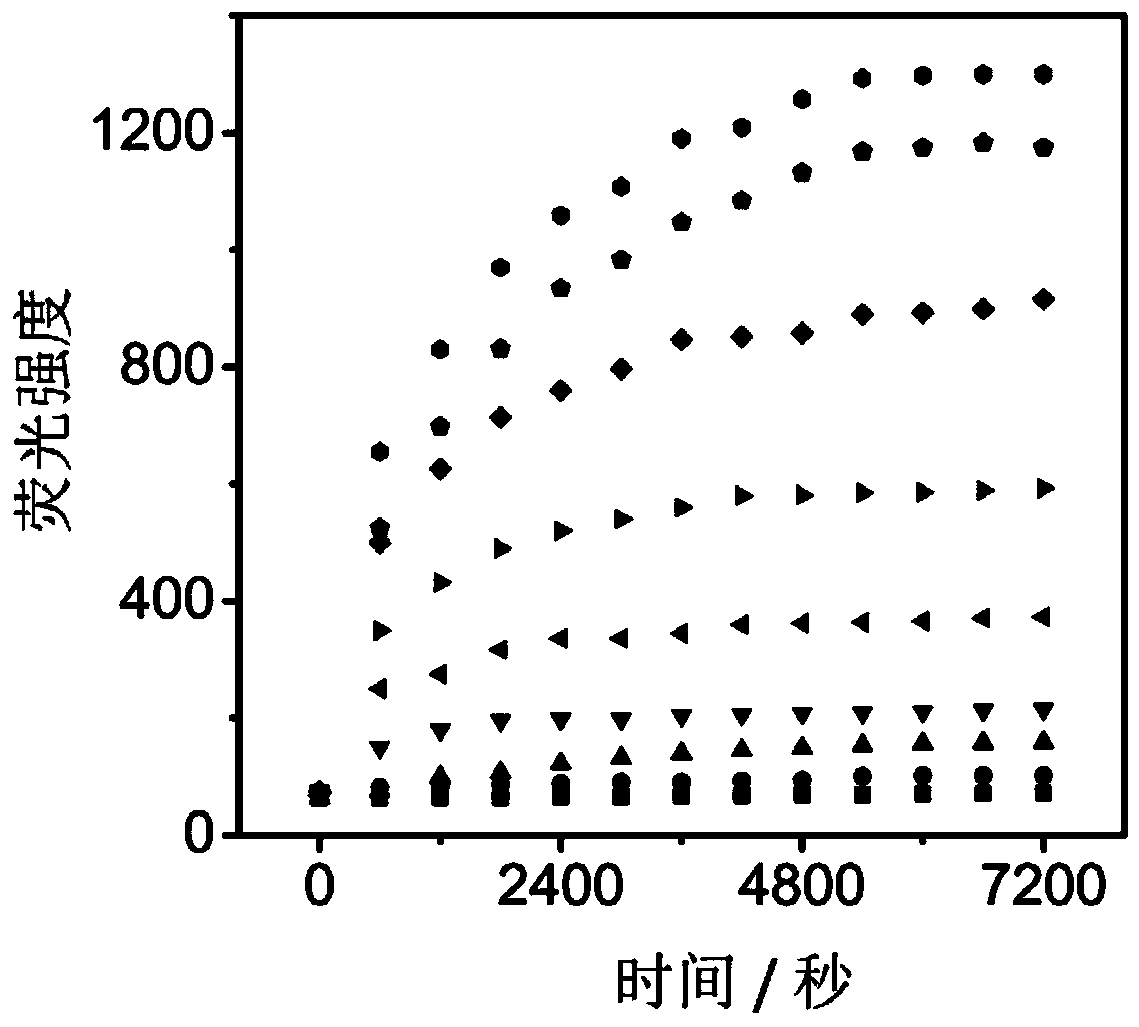
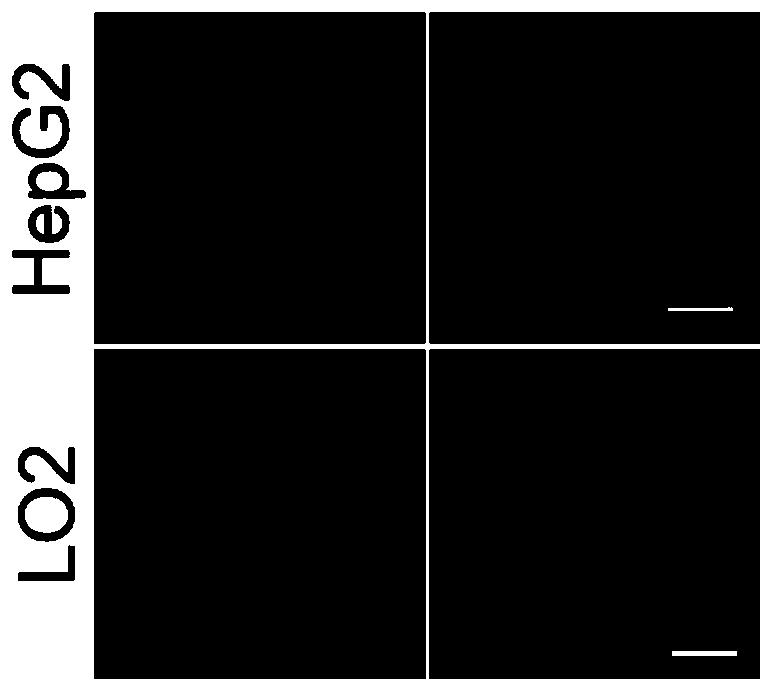
Leucine aminopeptidase and monoamine oxidase-activated near-infrared fluorescent probe as well as synthetic method and biological application thereof
ActiveCN110746410AHas near-infrared luminescent propertiesHigh sensitivityOrganic chemistryFluorescence/phosphorescenceFluoProbesStaining
The invention discloses a leucine aminopeptidase and monoamine oxidase-activated near-infrared fluorescent probe as well as a synthetic method and biological application thereof. The adopted syntheticmethod includes the following step: (1) synthesizing the novel leucine aminopeptidase and monoamine oxidase-activated near-infrared fluorescent probe (NML); and the biological application comprises the following step: (1) applying the probe to biological imaging and serum detection. The leucine aminopeptidase and monoamine oxidase-responsive fluorescent probe is synthesized for the first time, and the problem of low specificity of a traditional single-enzyme fluorescent probe is overcome; the fluorescent probe has near-infrared fluorescence properties, the background fluorescence signal can be effectively reduced, and the sensitivity of the probe can be improved; and the fluorescent probe has a good staining effect on living cells and high staining efficiency, and can detect cell endogenous leucine aminopeptidase and monoamine oxidase.
Owner:HUNAN UNIV
Aspergillus oryzae ZA184 and use thereof
The invention relates to the technical field of food processing and microbial fermentation, in particular to aspergillus oryzae ZA184 and use thereof in soy sauce brewing. The aspergillus oryzae ZA184can increase the pH of koji, enhance the activity of the koji enzymes (such as neutral protease, alkaline protease, leucine aminopeptidase and glutaminase), and improve the contents of amino nitrogen, total nitrogen and glutamic acid in soy sauce, so that the flavor and quality of the soy sauce is improved, the fermentation cycle can be significantly shortened, and the production efficiency is improved.
Owner:GUANGDONG HAITIAN INNOVATION TECH CO LTD +2
Novel fluorescence dyes and their applications for whole-cell fluorescence screening assays for caspases, peptidases, proteases and other enzymes and the use thereof
The present invention relates to novel fluorescent dyes, novel fluorogenic and fluorescent reporter molecules and new enzyme assay processes that can be used to detect the activity of caspases and other enzymes involved in apoptosis in whole cells, cell lines and tissue samples derived from any living organism or organ. The reporter molecules and assay processes can be used in drug screening procedures to identify compounds which act as inhibitors or inducers of the caspase cascade in whole cells or tissues. The reagents and assays described herein are also useful for determining the chemosensitivity of human cancer cells to treatment with chemotherapeutic drugs. The present invention also relates to novel fluorogenic and fluorescent reporter molecules and new enzyme assay processes that can be used to detect the activity of type 2 methionine aminopeptidase, HIV protease, adenovirus protease, HSV-1 protease, HCMV protease and HCV protease.
Owner:CYTOVIA INC
Aminoacid derivatives containing a disulfanyl group in the form of mixed disulfanyl and aminopeptidase n inhibitors
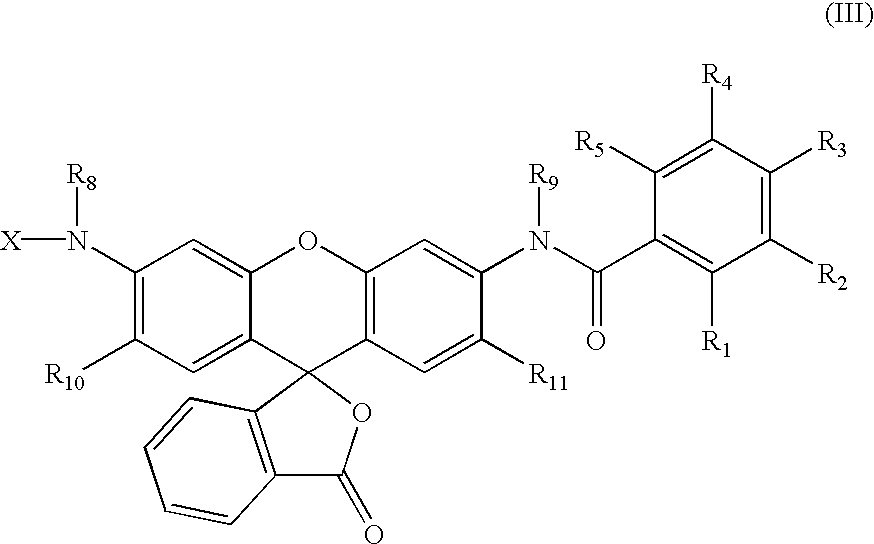
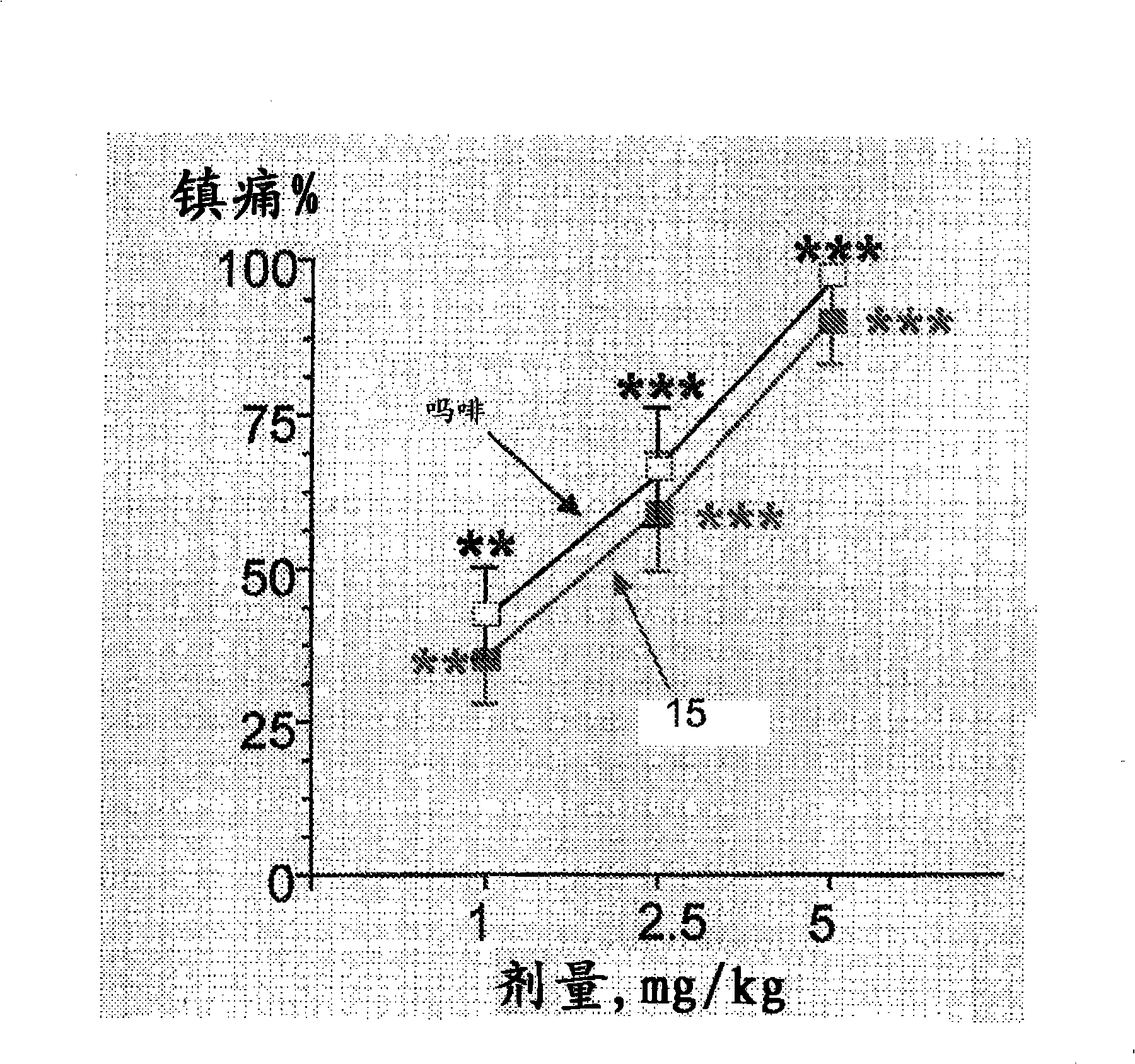
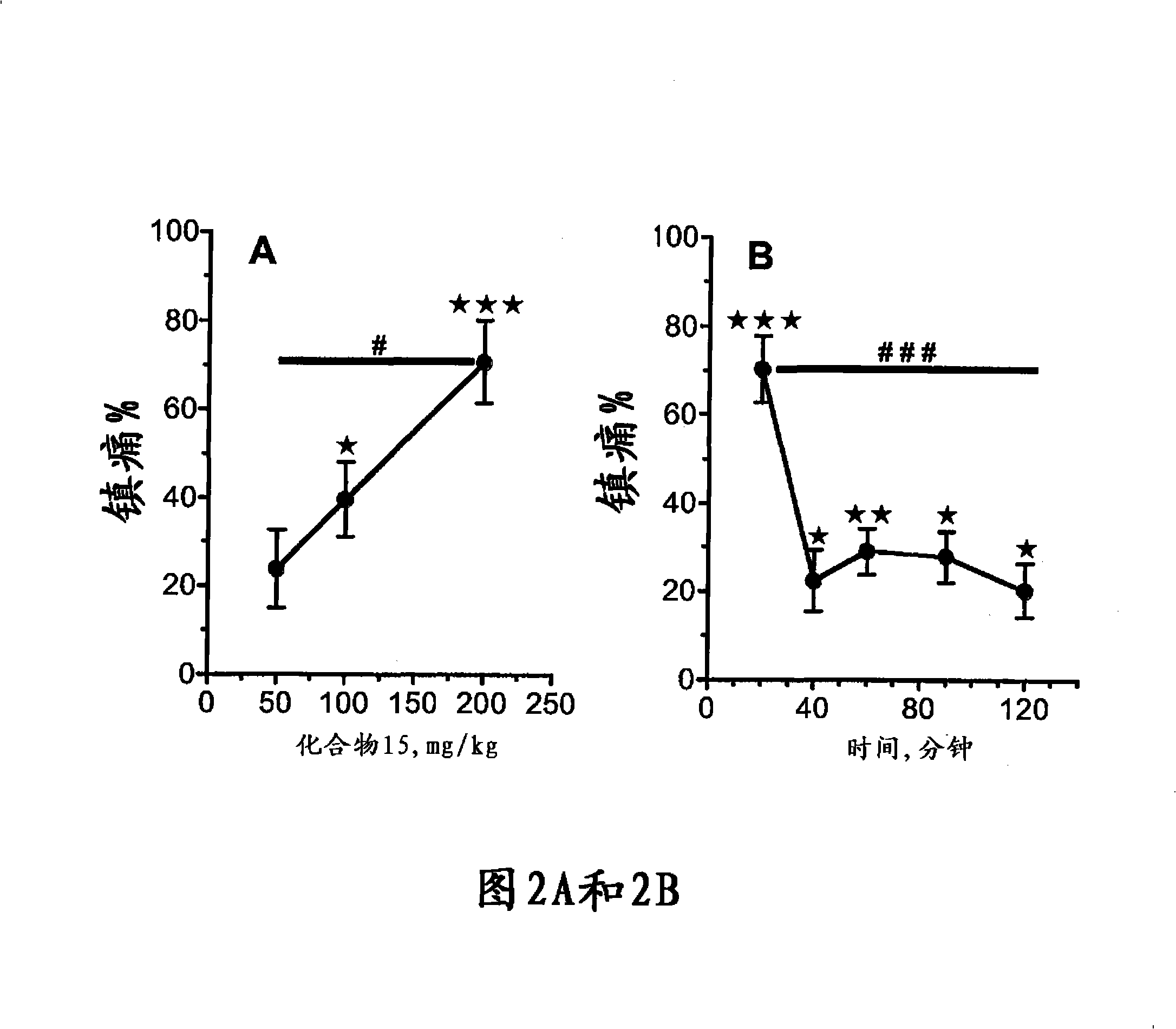
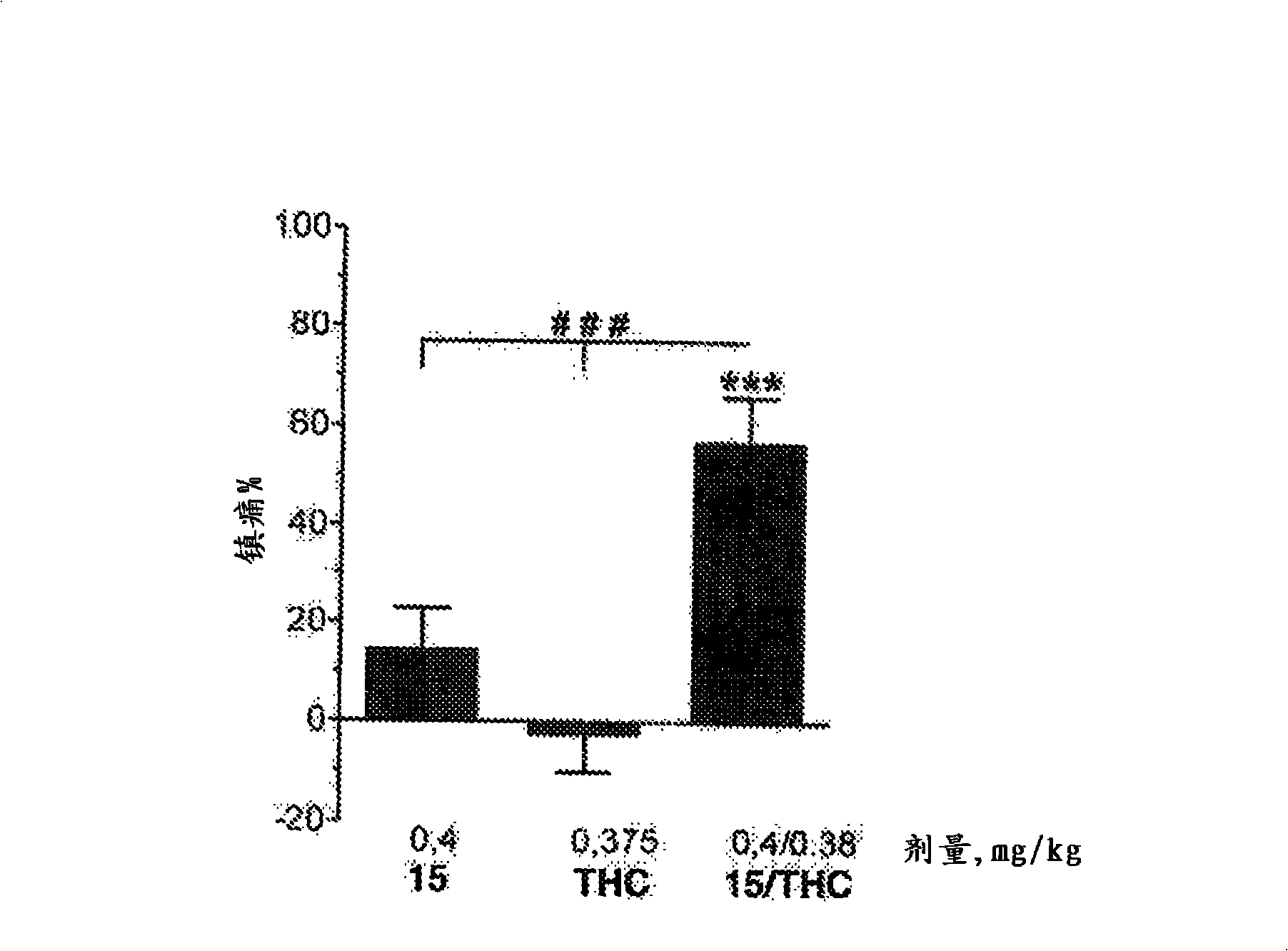
The invention relates to novel compounds of formula (I): H2N-CH(R1)-CH2-S-S- CH2-CH(R2)-CONH-R5, wherein R1 is a hydrocarbon chain, phenyl or benzyl radical, methylene radical substituted by a 5 or 6 atom heterocycle; R2 is a phenyl or benzyl radical, a 5 or 6 atom aromatic heterocycle, methylene group substituted by a 5 or 6 atom heterocycle; R5 is a CH(R3)-COOR4 radical, wherein R3 is hydrogen, an OH or OR group, a saturated hydrocarbon group, a phenyl or benzyl radical and OR4 is hydrophile ester, or 5 or 6 membered heterocycle comprising several heteroatoms selected from a group consisting of nitrogen, sulphur and oxygen, with at least two nitrogene atoms, wherein said heterocycle is substitutable by an alkyl C1-C6, phenyl or benzyl radical.; The use of the inventive compounds in the form of drugs, a pharmaceutical composition comprising said compounds, a pharmaceutically acceptable excipient, the use in conjunction of at least one type of cannabinoid derivative for potentiating the analgesic and antidepressant effect of the novel compounds of formula (I) and / or morphine or the derivatives thereof are also disclosed.
Owner:智腾大中华区有限公司
Method of improving taste and/or flavour of foods and beverages
InactiveUS20060068056A1Increased free Glu contentFree contentBacteriaTea extractionProteinase activityProtein materials
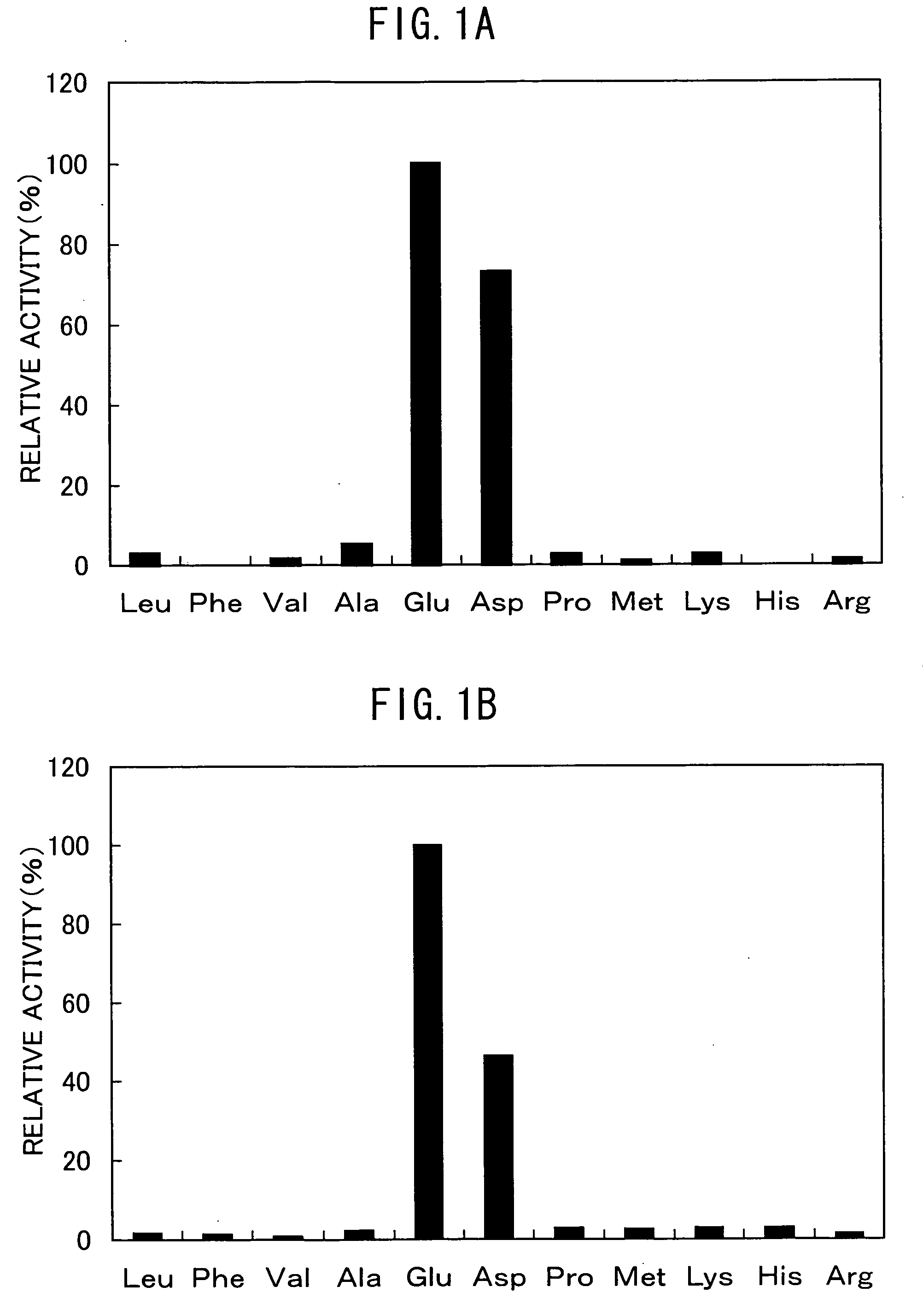
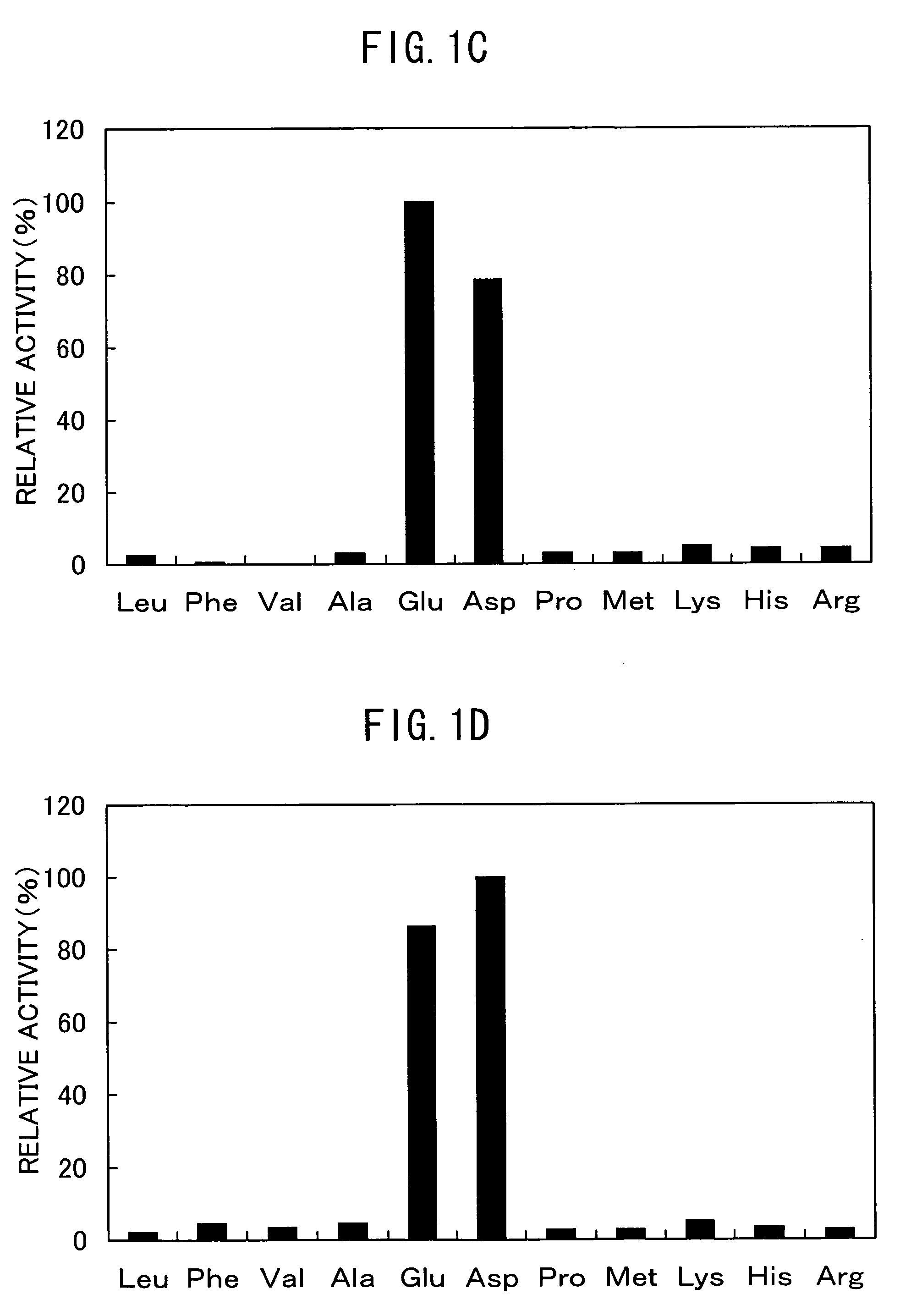
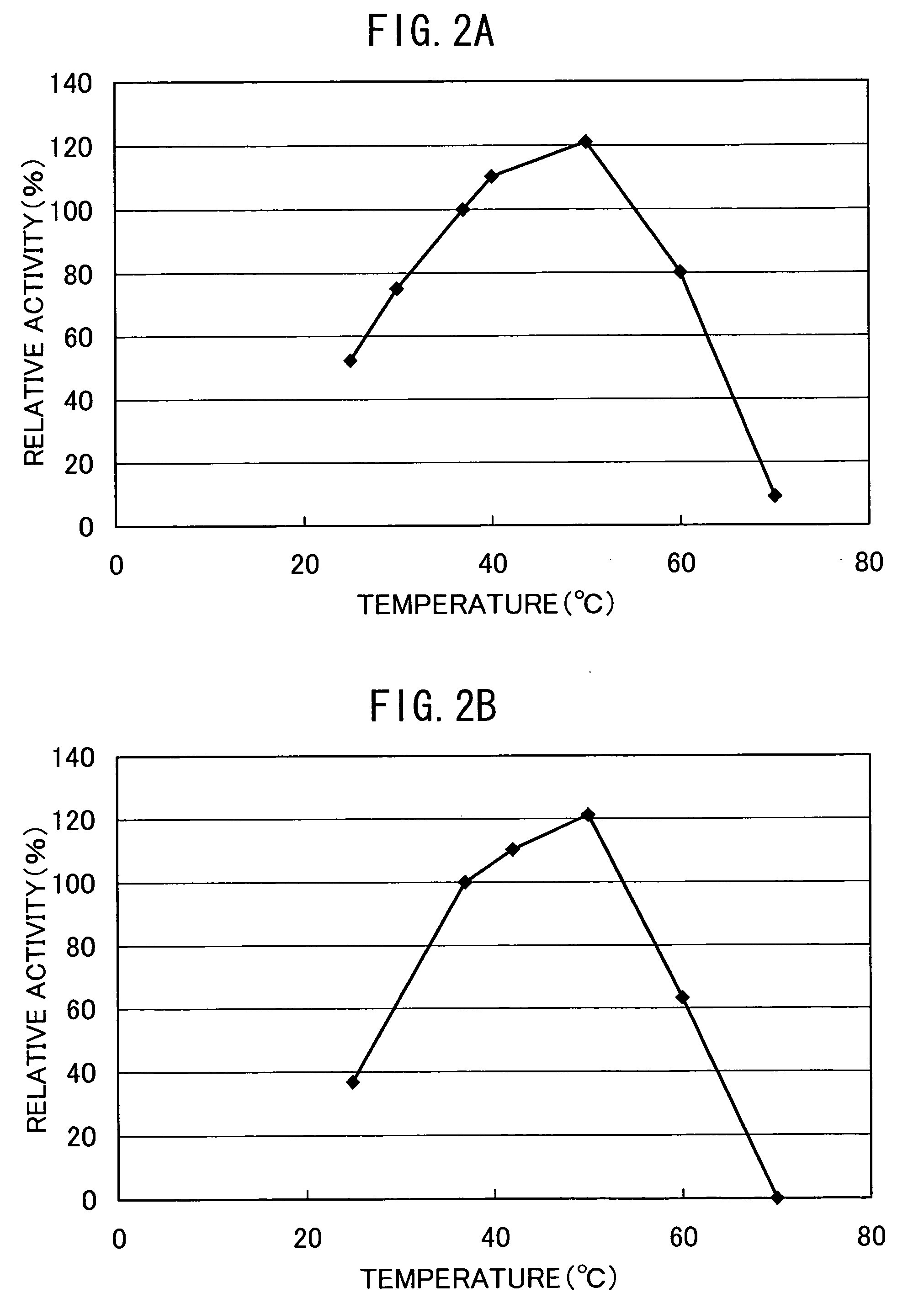
The present invention discloses a method for producing foods and / or beverage having improved taste and / or flavour, comprising reacting a microbial aminopeptidase on a protein material optionally under the co-existence of a protease, wherein said aminopeptidase has the properties of: (a) having an activity of catalyzing the reaction of specifically releasing a glutamic acid and an aspartic acid from the N-terminal of a peptide and / or a protein; (b) having 50% or more activity at pH6.0-9.0 as compared with the activity at the optimum pH; (c) having 40% or more activity after heating at 25-60° C., pH7.5 for 30 minutes as compared with the activity of the non-heated enzyme; (d) having a molecular weight of about 40-60 kD as measured by SDS-PAGE and about 300-480 kD as measured by native-PAGE; (e) having a hydrolyzing activity of the aminopeptidase toward Glu-Glu dipeptide is 5 U / mg or more, preferably 10 U / mg or more.
Owner:AJINOMOTO CO INC
Preparation method of low-biogenic amine soy sauce
The invention provides a preparation method of low-biogenic amine soy sauce. The preparation method comprises the following steps: adding glycine and citric acid in starter propagation process, fermenting for 60-90 days by adopting a high-salt diluted-state fermented soy sauce brewing method, then squeezing and filtering, thereby being capable of obviously reducing the number of microorganisms in the yeast of the soy sauce. With the adoption of the method disclosed by the invention, the total content of biogenic amine in the crude soy sauce can be reduced by more than 60%, the activity of neutral protease and acid protease in the yeast of the soy sauce can be increased by more than 10%, the aminopeptidase activity can be increased by more than 20%, and the recovery rate of the protein can be increased by more than 3%.
Owner:SOUTH CHINA UNIV OF TECH
Novel fungal proteins and nucleic acids encoding same
Disclosed herein are fungal nucleic acid sequences that encode novel polypeptides. Also disclosed are polypeptides encoded by these nucleic acid sequences, as well as derivatives, variants, mutants, or fragments of the aforementioned polypeptide, polynucleotide, or antibody. The novel leucine aminopeptidase (LAP) and other amino- and carboxypeptidases polypeptides, referred to herein as EXOX nucleic acids and proteins of the invention are useful in a variety of medical, research, and commercial applications.
Owner:AMYRA BIOTECH
Bacillus subtilis and application thereof
ActiveCN109306329AIncreased chance of stickingIncrease enzyme activityBacteriaFood processingMicrobiologyBacillus subtilis
Owner:广州大峰收技术服务有限公司
Quality control product for biochemical detection and quality control method
InactiveCN108802355AAvoid matrix effectsAvoid wastingBiological testingQuality controlDirect bilirubin
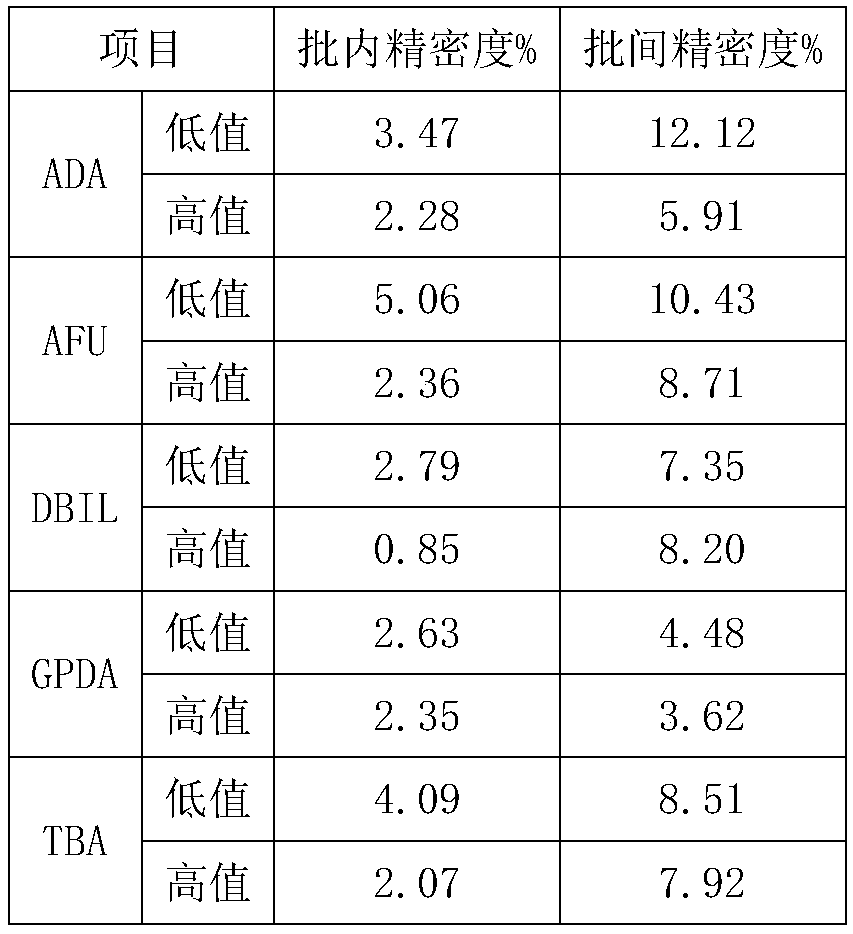
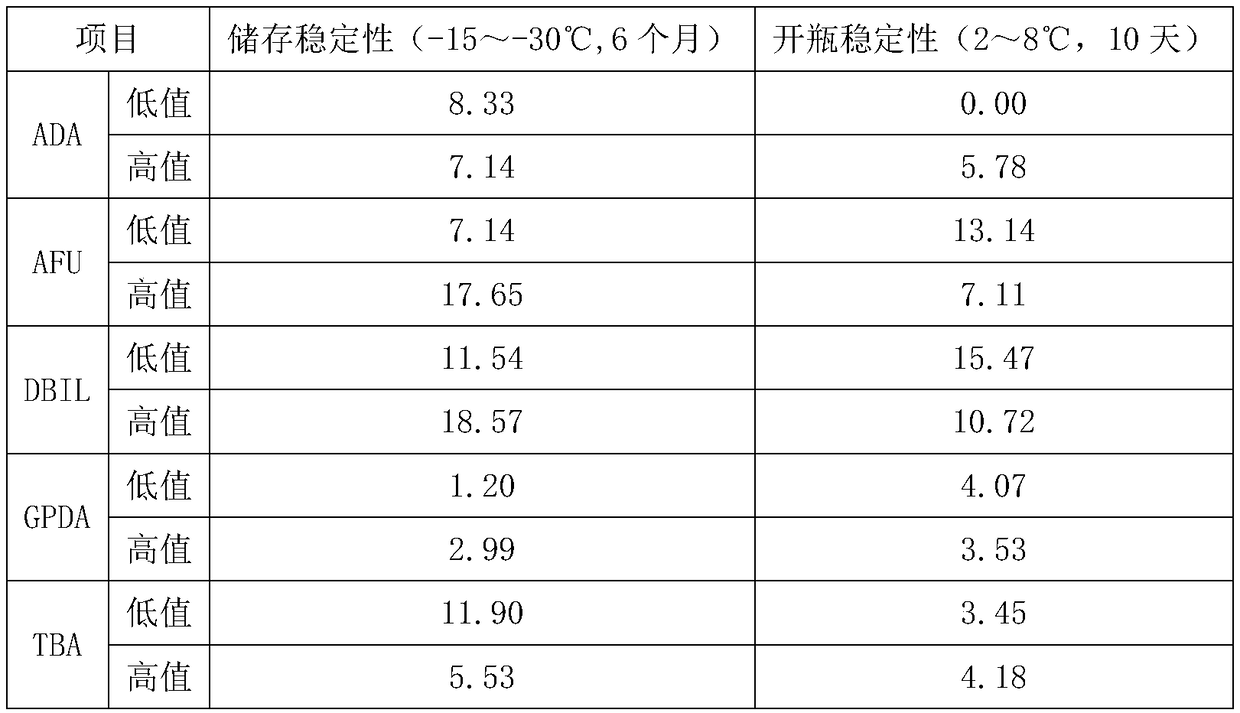
The invention relates to a quality control product for biochemical detection and a quality control method. The quality control product has the beneficial technical effects that (1) the quality controlproduct for biochemical detection is prepared by using the serum of a healthy person as the raw material, and is consistent with a clinical detection sample, so that while the matrix effect is furthest avoided, the qualifies of the five biochemical detection indexes (ADA (adenosine deaminase), AFU (alpha-fucosidase), DBIL (direct bilirubin), GPDA (glycyl-proline-dipeptidyl aminopeptidase) and TBA(total bile acid)) are effectively controlled, and the defect of lack of part or all of five substances in the existing quality control product is overcome; (2) by using the serum of the healthy person as the raw material, the waste due to no use of a large amount of remaining serum in the clinical biochemical inspection is avoided, and a large amount of original wasted serum resources is saved;(3) the quality control product for biochemical detection is prepared by scientific design, so that the good within-run accuracy and between-run accuracy and the longer stability are realized under the reasonable preservation condition, and the quality control product is suitable for being applied in clinics.
Owner:ZHEJIANG PROVINCIAL PEOPLES HOSPITAL
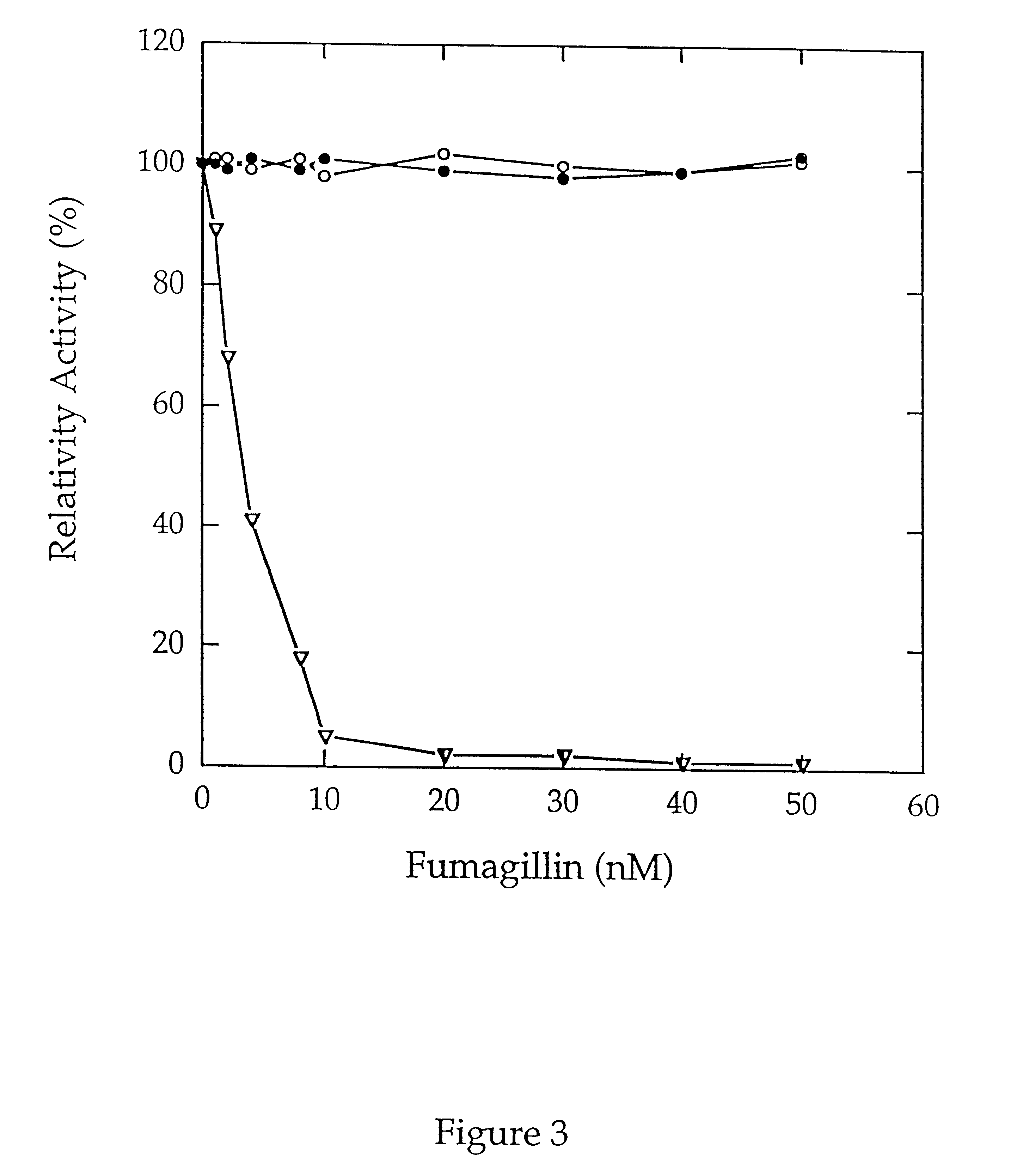
Substituted beta-amino acid inhibitors of methionine aminopeptidase-2
A class of substituted beta -amino acids are potent inhibitors of methionine aminopeptidase type 2 (MetAP2) and are thus useful in inhibiting angiogenesis and disease conditions which depend upon angiogenesis for their development such as diabetic retinopathy, tumor growth, and conditions of inflammation. Pharmaceutical compounds containing the compounds and methods of inhibiting methionine aminopeptidase-2, and angiogenesis are also disclosed.
Owner:ABBOTT LAB INC
Methods for diagnosis of chronic prostatitis/chronic pelvic pain syndrome
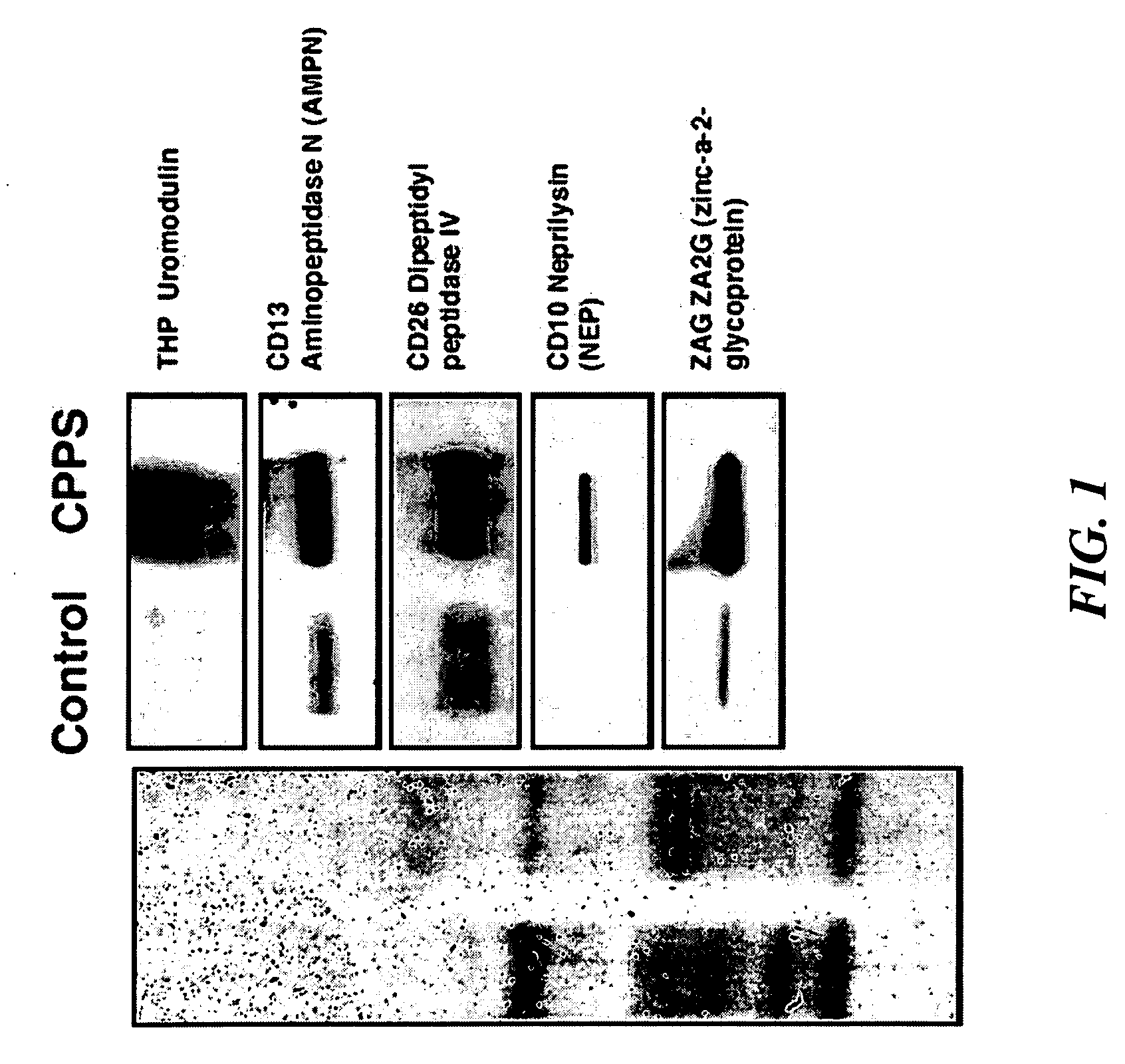
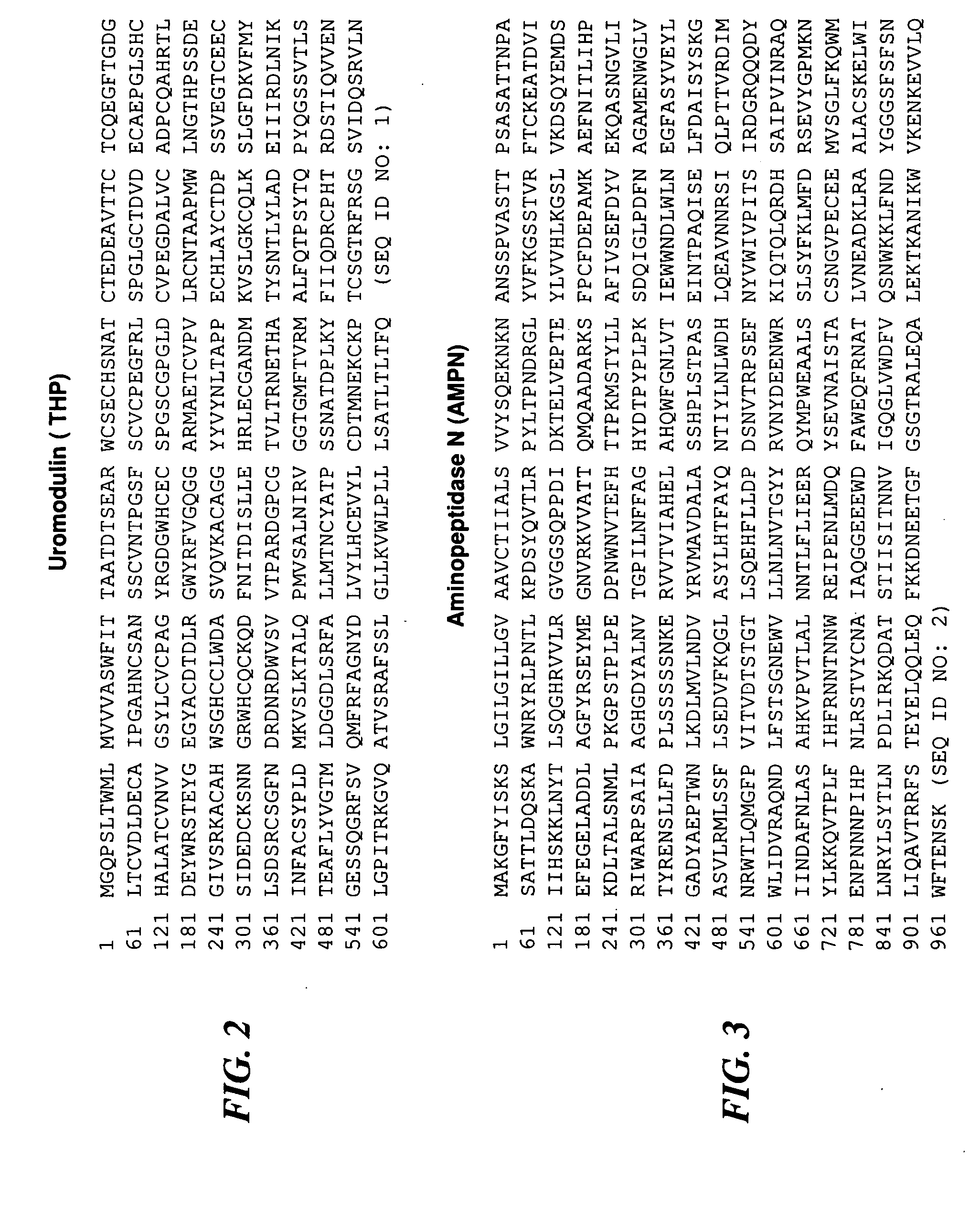
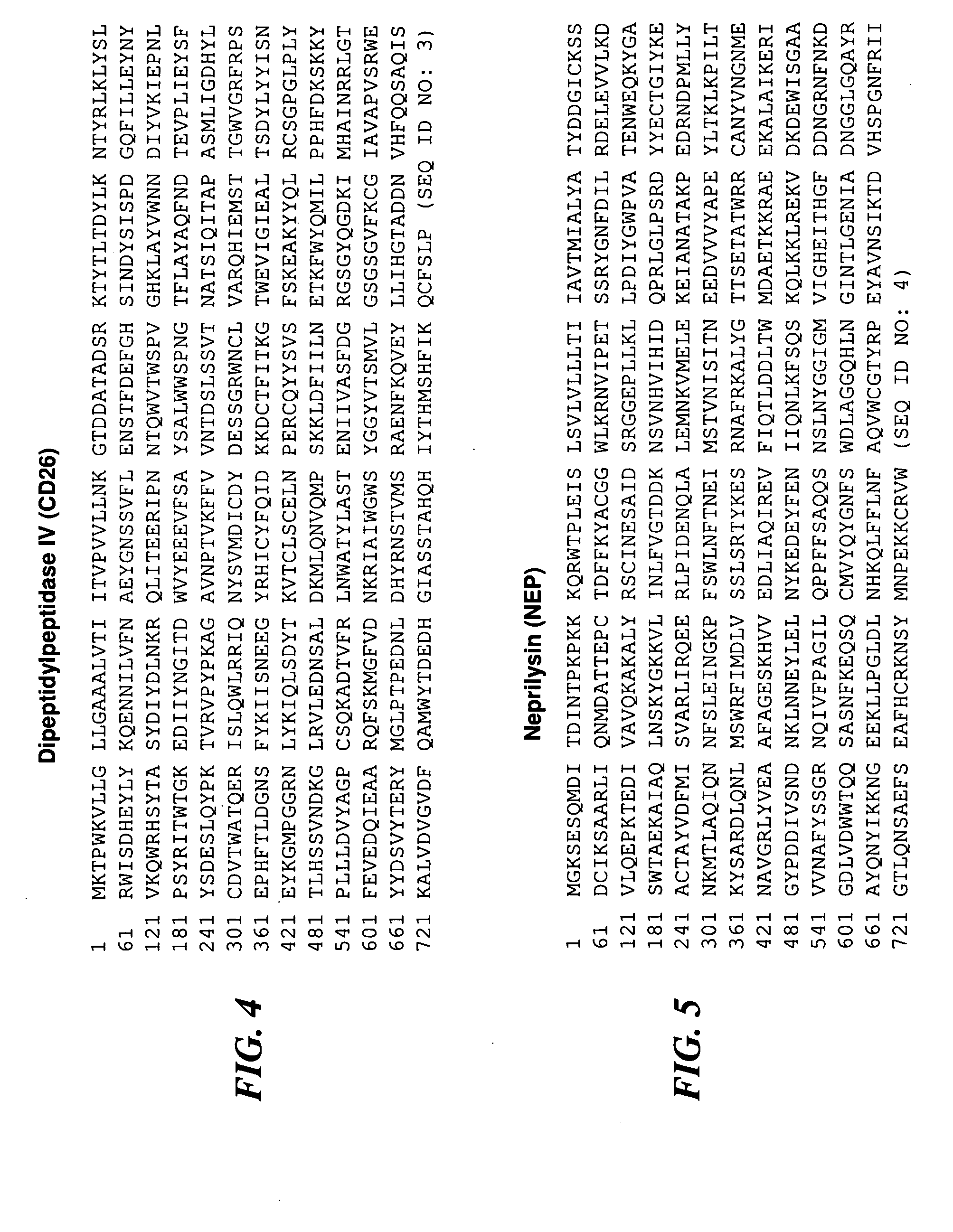
The present invention relates to methods for diagnosis of chronic prostatitis / chronic pain pelvic syndrome (CP / CPPS). We have found specific biomarkers that are present in higher concentrations in patients that have chronic prostatitis / chronic pain pelvic syndrome (CP / CPPS) as compared to subjects that have no symptoms of CP / CPPS. In particular, uromodulin (THP), aminopeptidase N (AMPN), dipeptidylpeptidase IV (CD26), neprilysin (NEP), zinc-α-2-glycoprotein (ZA2G) and alkaline phosphatase (ALP) were found to be present at higher concentrations in CP / CPPS patient urine that is voided after prostatic message. Accordingly, the invention is directed to methods for diagnosis of CP / CPPS by monitoring the levels of at least one of these proteins in post-prostatic massage urine, as well as to diagnostic kits designed for diagnosis of CP / CPPS.
Owner:CHILDRENS MEDICAL CENT CORP
Bacillus subtilis self-induced expression system and application thereof
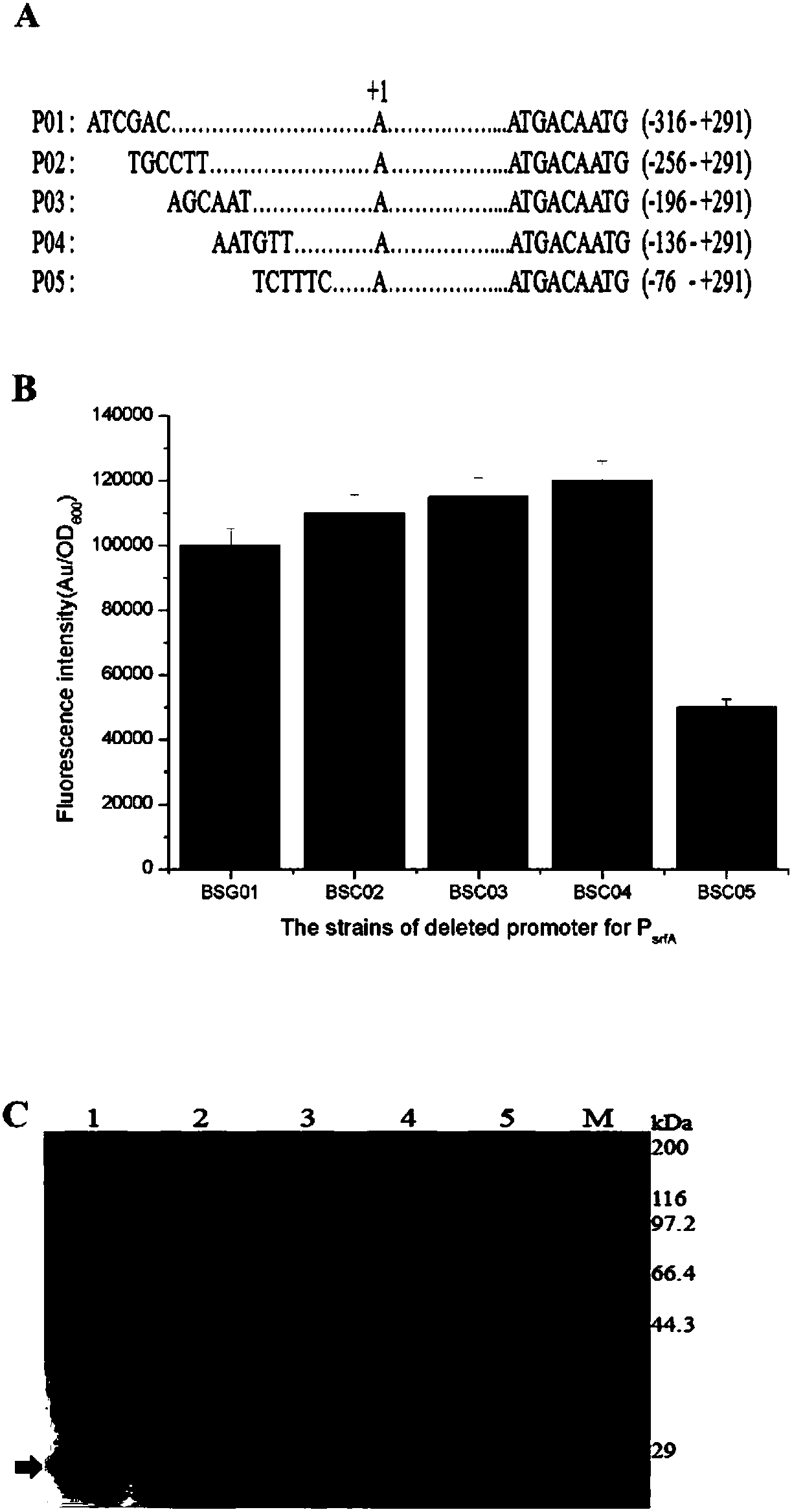
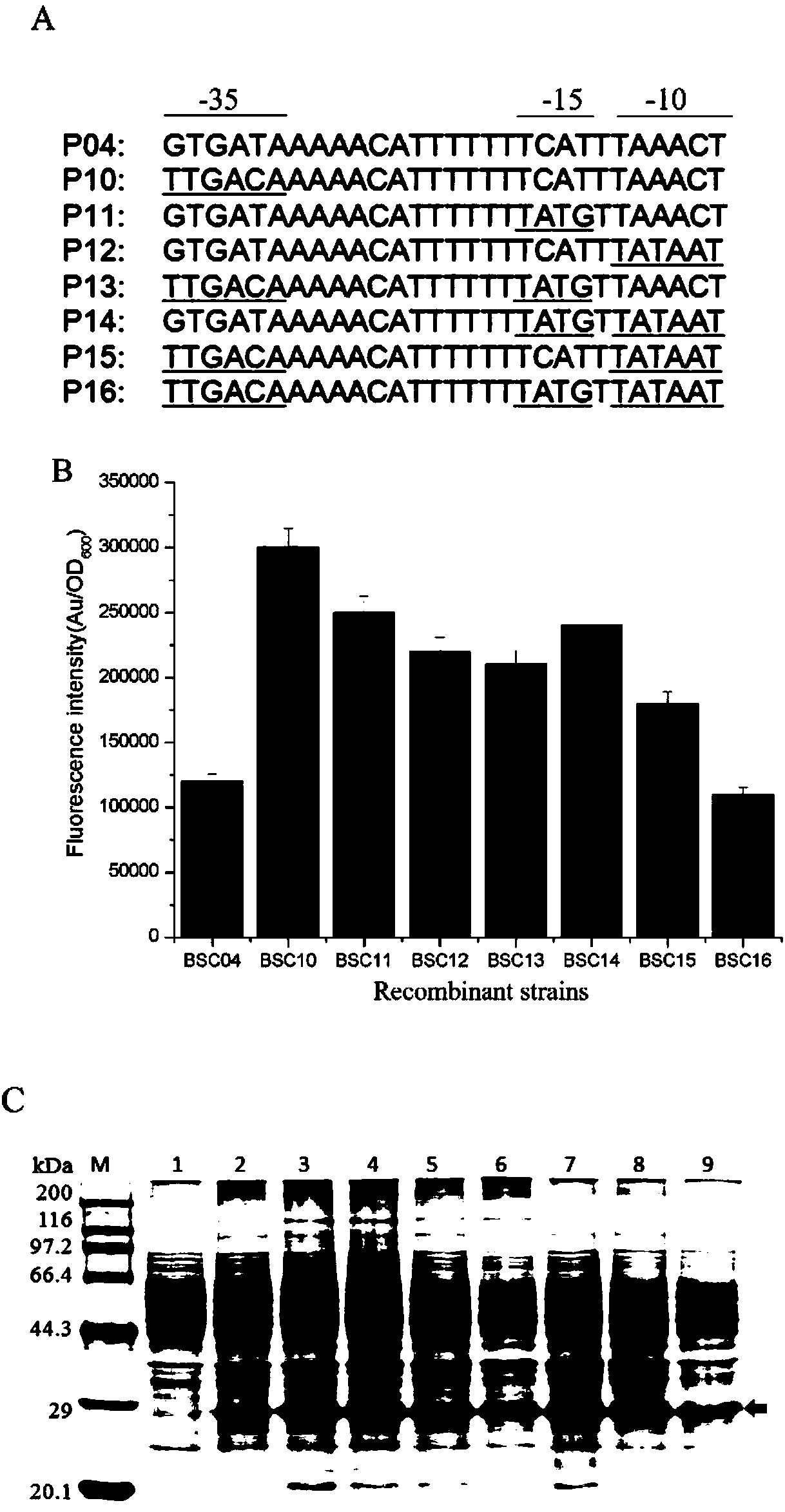
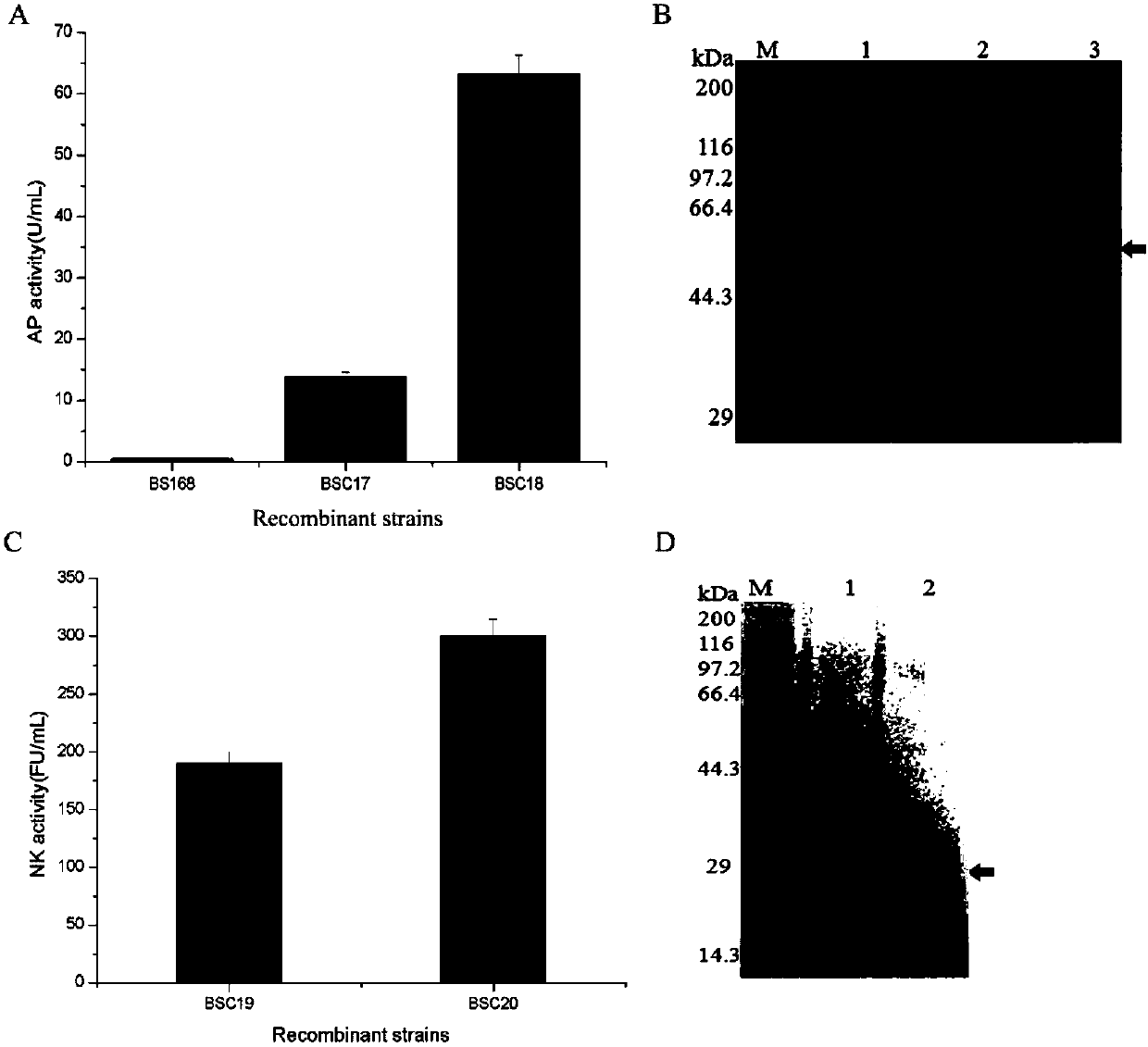
The invention discloses a bacillus subtilis self-induced expression system and an application thereof, and belongs to the field of promoter engineering. On the basis of rational design on a promoter srfA and by mutating core regions, namely No.35 region and No.10 region, of the promoter into a conserved region, some relatively strong promoters are obtained; and with green fluorescent protein GFP as a reporter gene, the mutated promoters are 150% higher than the original promoter in highest expression amount. Subsequently, aminopeptidase AP expression and nattokinase NK expression are conducted, so that mutant expression amounts, in comparison with that of the original promoter, are improved by 360% and 50%. The method provided by the invention, in comparison with random mutation, greatly reduces workload and is effective; and meanwhile, as the strategy is applied to transformation of other promoters, the capacity of the transformed promoters in expressing a target gene is also greatly improved.
Owner:JIANGNAN UNIV
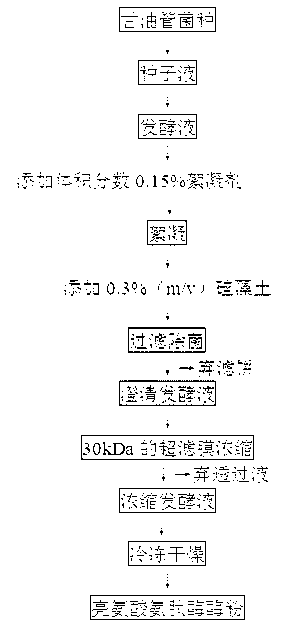
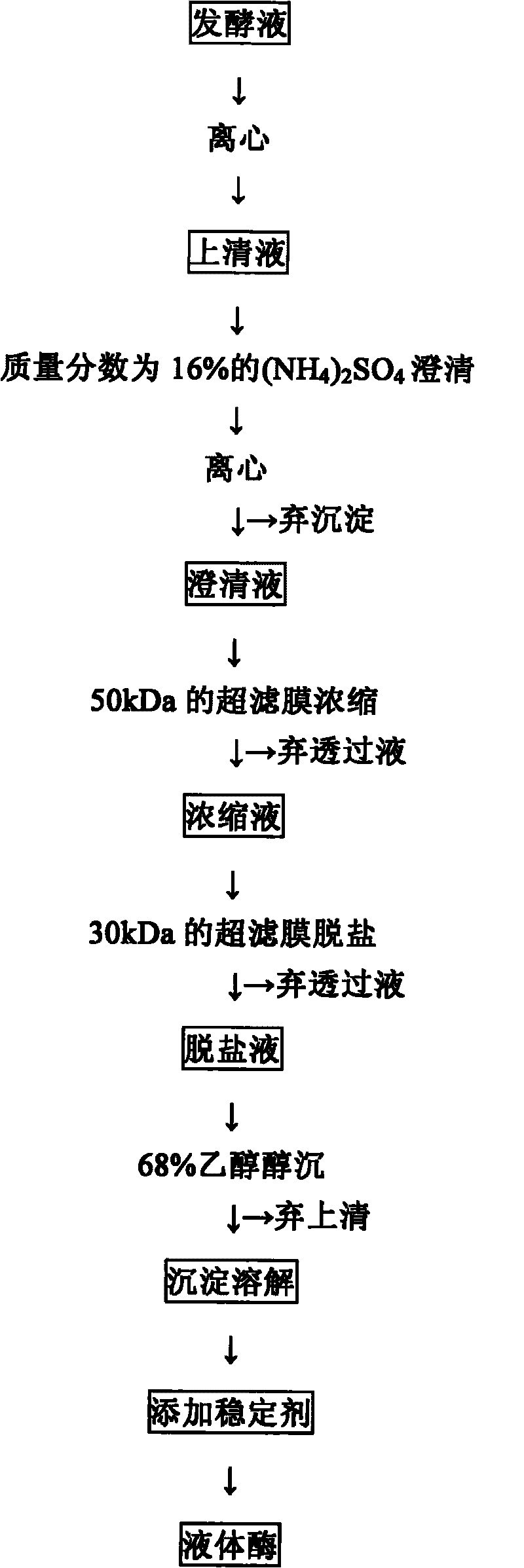
Method for improving stability of liquid aminopeptidase and application of aminopeptidase
InactiveCN101717763AImprove stabilityEasy to expand productionMicroorganism based processesEnzymesFood additiveAlcohol
The invention discloses a method for improving the stability of liquid aminopeptidase and an application of the aminopeptidase, which belong to the technical field of enzymic preparations and food additives. The invention relates to an extracting process of bacillus subtilis Zj016 liquid aminopeptidase to improve the stability of the liquid aminopeptidase and an application of the aminopeptidase in deep hydrolysis of soy isolated protein. The bacillus subtilis Zj016 liquid aminopeptidase is centrifuged, clarified, concentrated, desalinized, deposited by alcohol and redissolved to obtain the liquid aminopeptidase; the liquid aminopeptidase can keep stable by controlling the pH condition and / or the additive; the aminopeptidase can carry out deep hydrolysis on a substrate of the soy isolated protein to generate a small peptide and amino acid with higher content and has a combined application potential with a plurality of incision enzymes, thereby having wide application prospect in food processing of protein raw materials.
Owner:JIANGNAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com