Cyclic NGR polypeptide, radionuclide labeled molecular probe and application thereof
A radionuclide and molecular probe technology, applied in the fields of radioactive carrier, peptide, peptide preparation, etc., can solve the problems of kidney side effects, inability to achieve tumor treatment, etc., and achieve the effect of improving targeting and retention time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
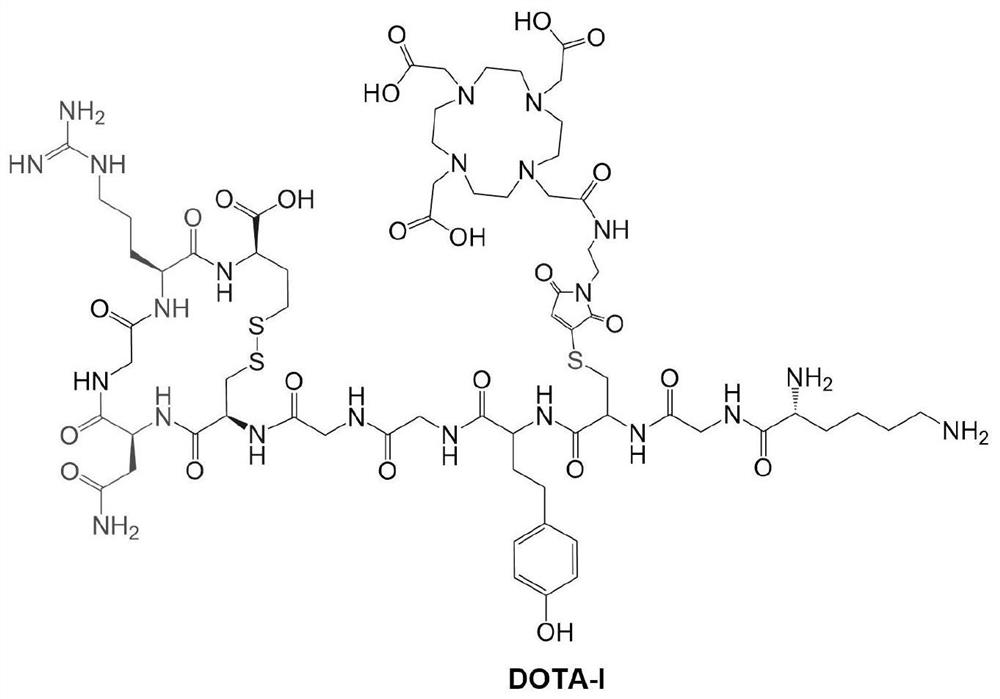
[0036] Synthesis of a DOTA-NGR Cyclic Polypeptide DOTA-I
[0037] Its chemical structure is as figure 1 Shown:
[0038] The preparation method is as follows: take 10 μmol of NGR polypeptide I solution, dissolve it in 80 μL N,N-dimethylformamide (DMF), add 11 μmol of bifunctional chelating group (Mal-DOTA), 30 μmol of diisopropylethylamine Carry out coupling, stir and react at room temperature for 1 to 2 hours, use high performance liquid chromatography (HPLC) to separate and purify the obtained product, freeze-dry to obtain the coupling product, and test the chemical composition of the obtained coupling product by high performance liquid chromatography (HPLC). Purity>95%, recorded as DOTA-I.
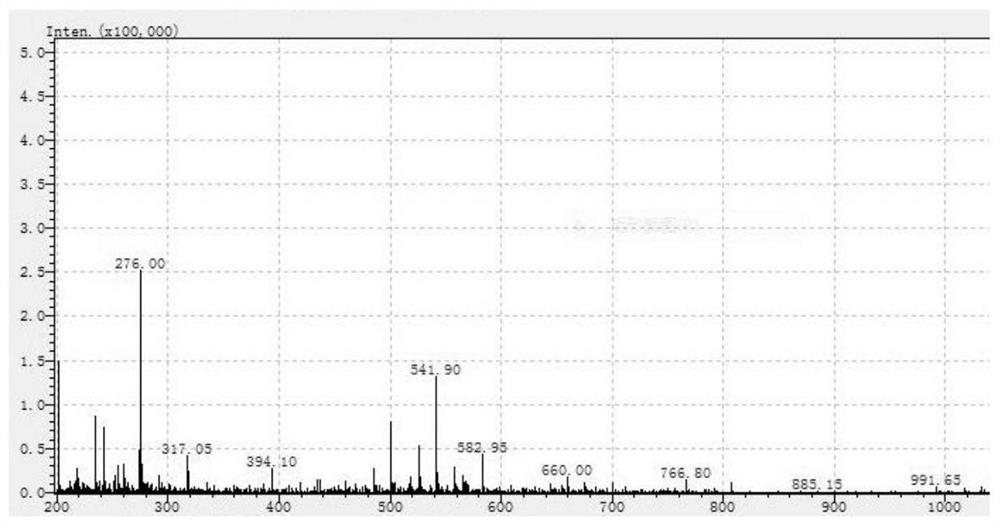
[0039] The mass spectrogram of above-mentioned DOTA-I polypeptide is as follows figure 2 shown.
Embodiment 2
[0041] DOTA-I 177 Lu radiolabeled
[0042] experimental method:
[0043]1) Dissolving DOTA-I in sodium acetate (NaOAc) buffer solution (0.25M, pH=5.5) to prepare a solution with a concentration of 2nmol / μL;
[0044] 2) Take the known activity (100μCi / μL, 3.7MBq / μL) in the lead tank 177 LuCl 3 Solution 400 μCi (14.8 MBq), mixed well with 2 μL of DOTA-I solution, and 20 μl of NaOAc buffer was added to adjust the pH value, and then the reaction mixture was placed in a metal bath at 80° C. for 30 minutes.
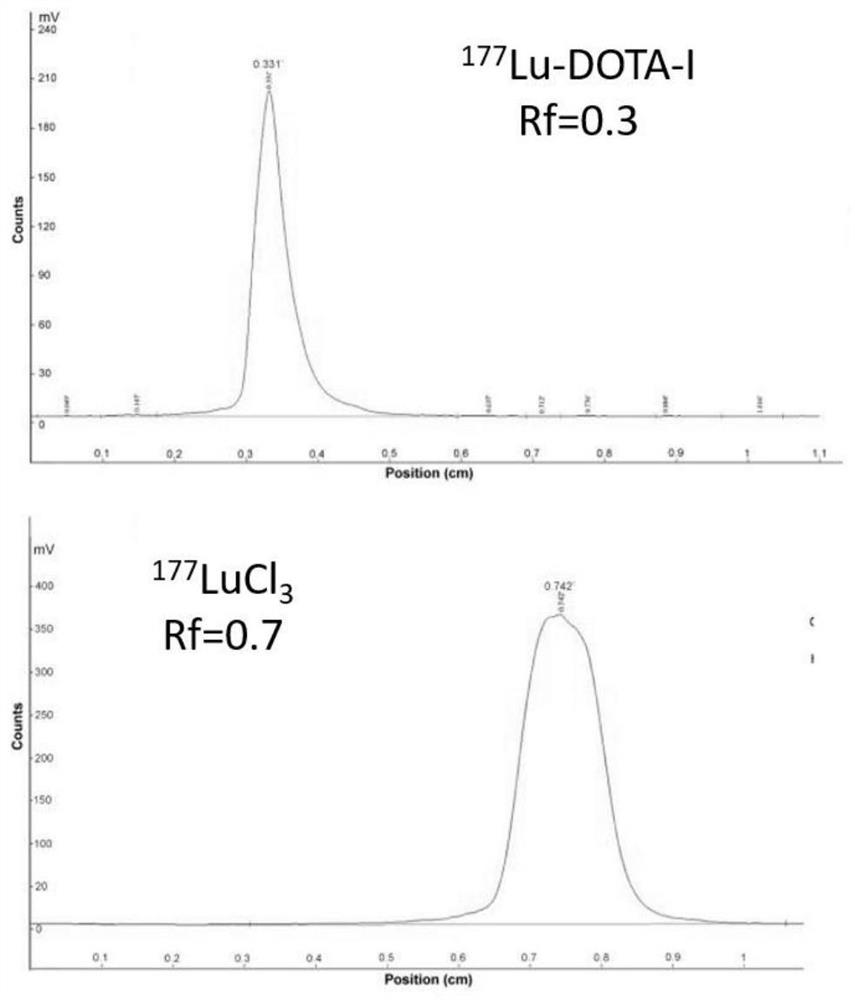
[0045] 3) The radiochemical purity of the marker was determined by instant silica thin layer chromatography (iTLC-SG). Using 0.1M citric acid solution (pH=5.5) as a developing agent to develop, scan with a radiochromatographic scanner (TLC), and calculate the radiolabeling rate of the marker.
[0046] 177 Lu-DOTA-I all remain at the origin of the spectrum (Rf=0), where the free 177 Lu moved to the leading edge together with the developer (Rf=0.62), and the radiolabeling ...
Embodiment 3
[0049] 177 In Vitro Stability Test of Lu-DOTA-I Molecular Probe
[0050] Test method: Take 10μL (30μCi, 1.11MBq) 177 Lu-labeled DOTA-I samples were uniformly mixed with 290 μL of FBS or normal saline, placed in a metal bath at 37°C and 300 rpm, and incubated with shaking, and were analyzed by iTLC-SG at 1, 4, 24, 48, and 72 hours, respectively. The radiochemical purity of the markers was tested to assess the in vitro stability of the prodrugs.
[0051] the above 177 The in vitro stability results of Lu-DOTA-I molecular probe are as follows: Figure 4 shown. The results show that the stability of the molecular probe in physiological saline and FBS solution is greater than 90% within 24 hours, and its stability in physiological saline for 72 hours is still greater than 90%. However, the stability of the molecular probe in FBS solution for 72 hours is greater than 65%. Considering that the serum (FBS) content in the in vivo environment is mostly about 10% during actual use,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com