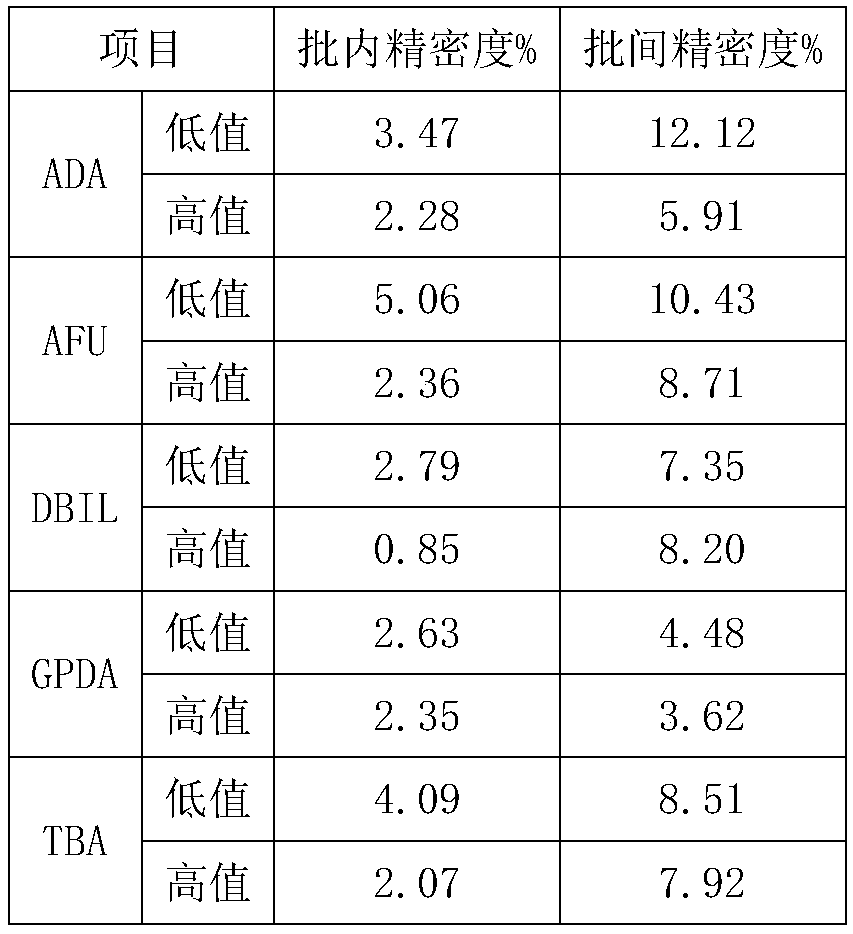
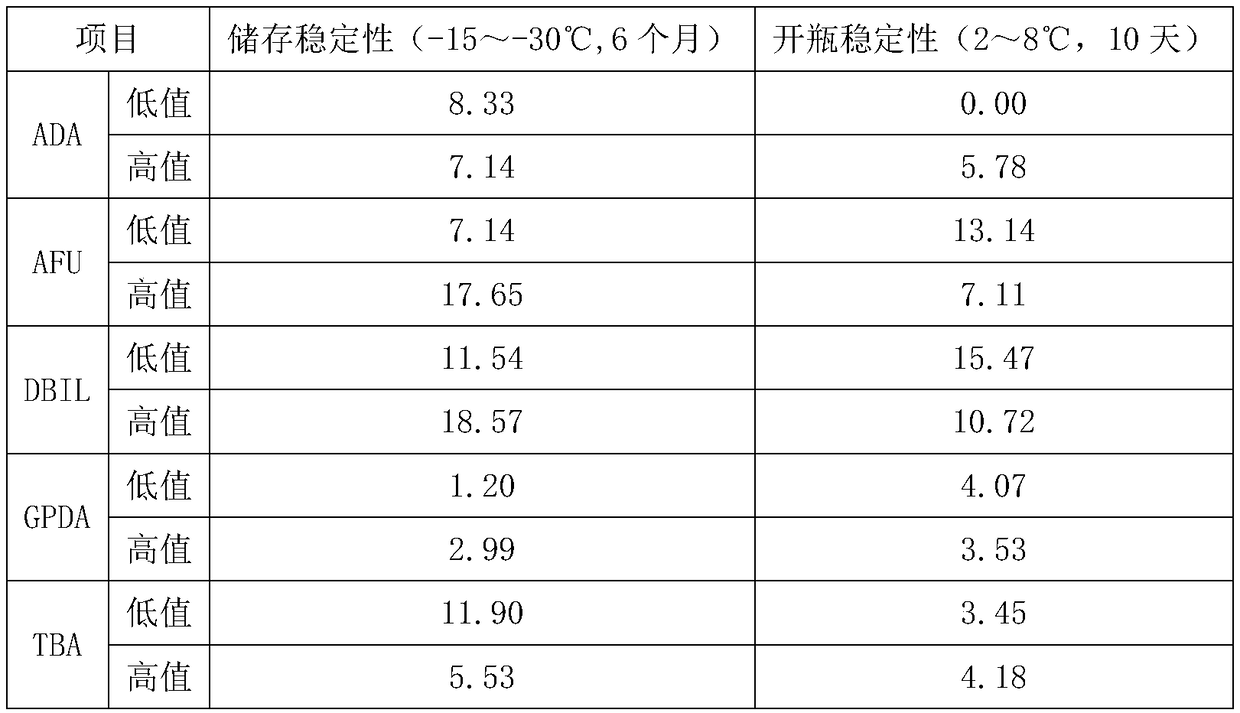
Quality control product for biochemical detection and quality control method
A technology of biochemical testing and quality control products, applied in the field of medical testing, can solve the problems of lack, waste, and nowhere to use serum, etc., and achieve the effects of long precision between batches, avoiding waste, and avoiding matrix effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0034] 1) Configure reagents for backup
[0035] Prepare 10mmol / L sodium bicarbonate solution, 10mmol / L Na 2 - EDTA solution (pH=8.0), 50vol% ethanol solution and 0.2wt% sodium azide solution.
[0036] 2) Dialysis bag processing
[0037] a) Cut the dialysis bag into small pieces (choose the size according to your needs). Since the dialysis bag is easily contaminated by cellulose-decomposing microorganisms, gloves must be worn during operation;
[0038] b) wet the dialysis membrane in excess 10mmol / L sodium bicarbonate solution, and boil for 10 minutes;
[0039] c) Put the dialysis membrane in 10mmol / L Na 2 -Boil in EDTA solution (pH=8.0) for 10 minutes;
[0040] d) repeat the operations in b) and c) once;
[0041] e) wash several times with distilled water;
[0042] f) Store at 4°C in 20%-50% ethanol to prevent the growth of cellulolytic microorganisms.
[0043] 3) Preparation of low-value quality control products
[0044] S1. From the remaining serum samples of clini...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com