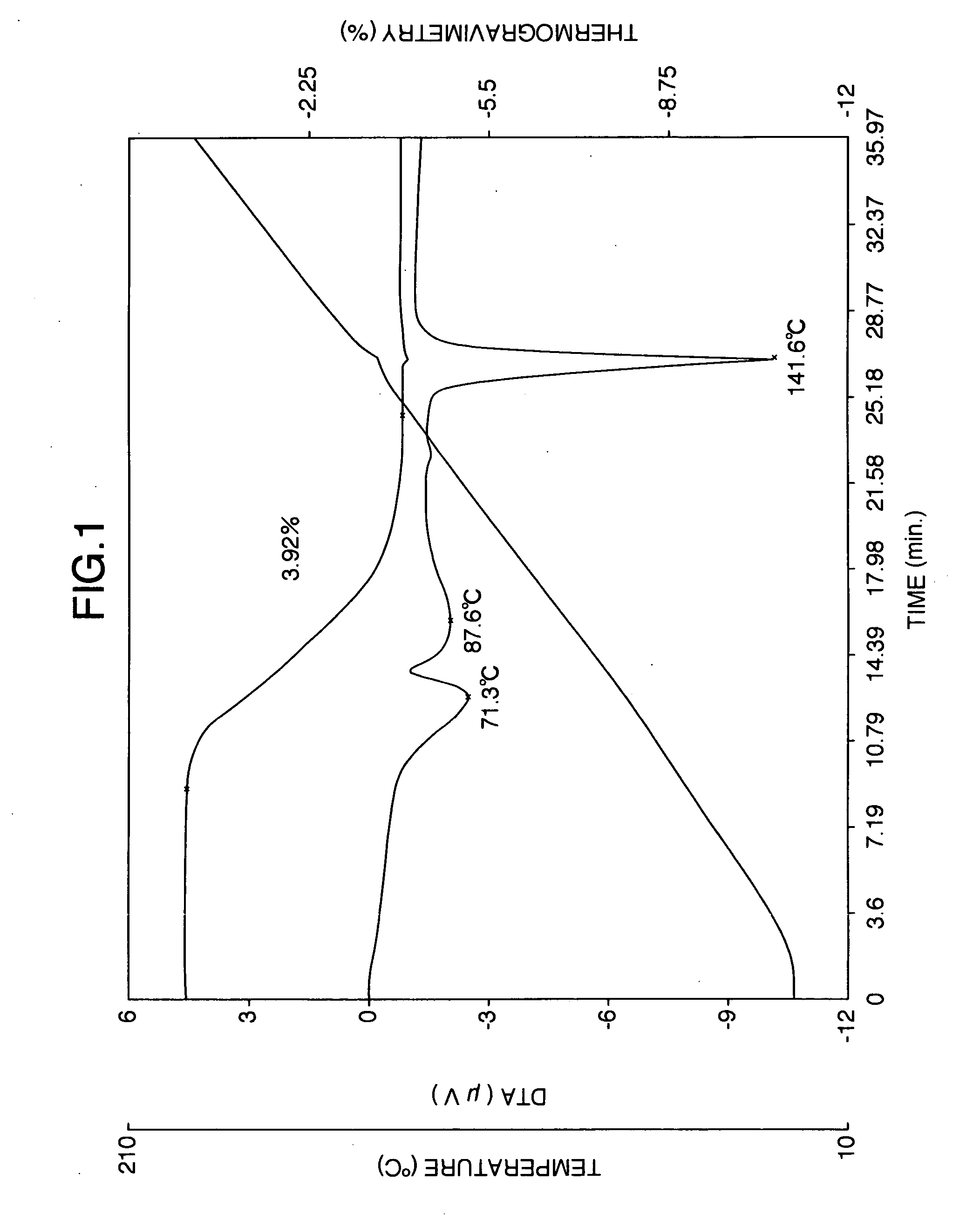
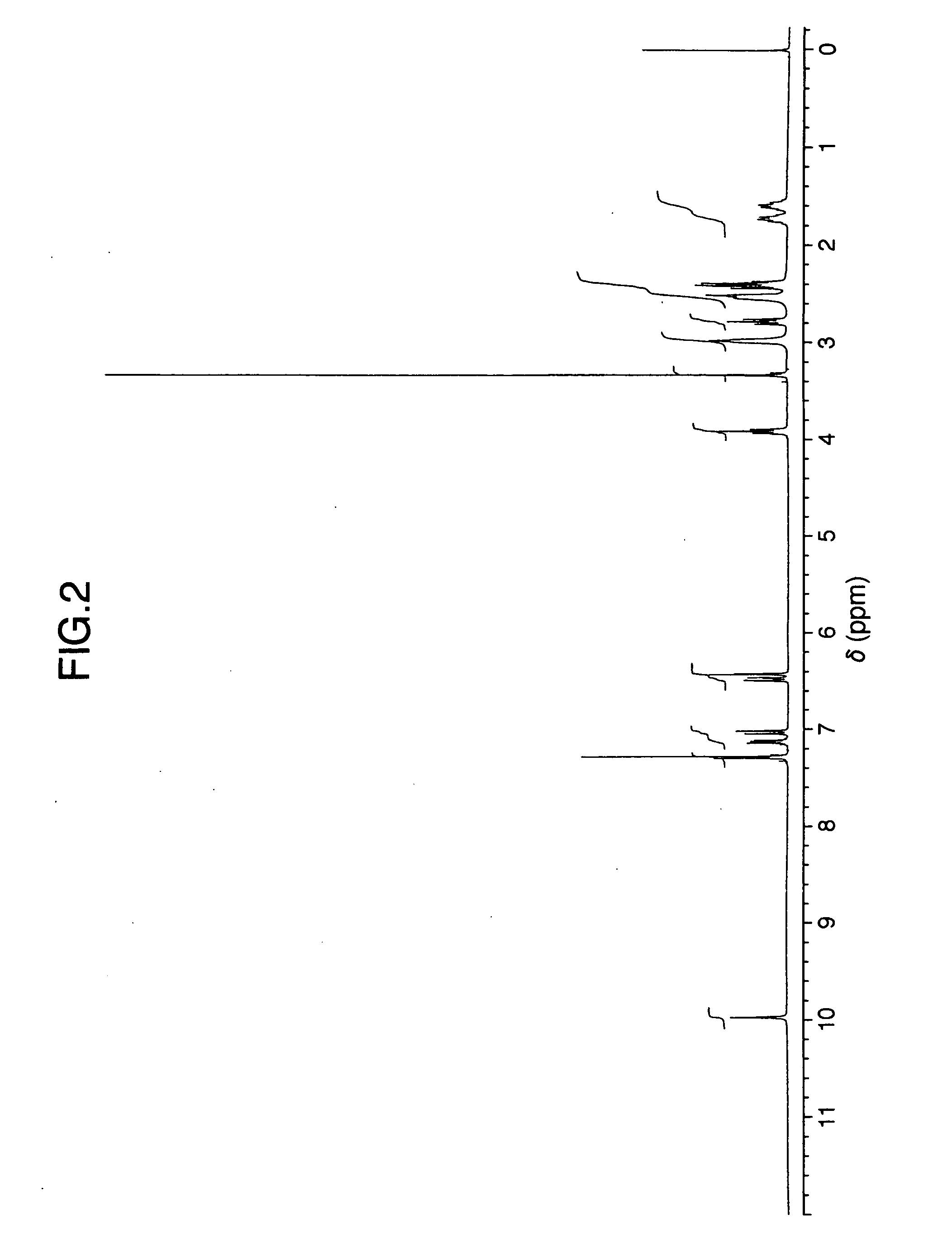
Patents
Literature
136 results about "Duloxetine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
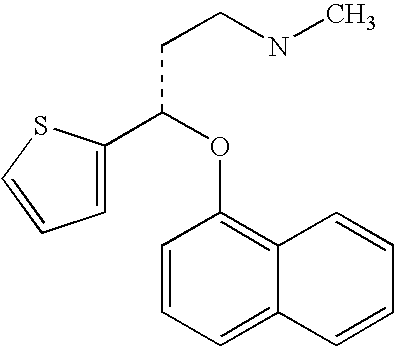
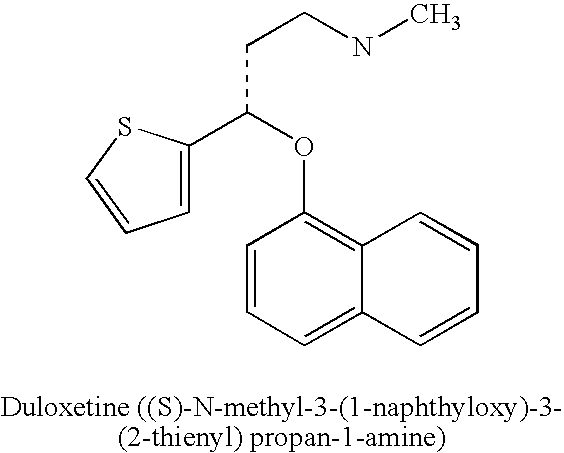
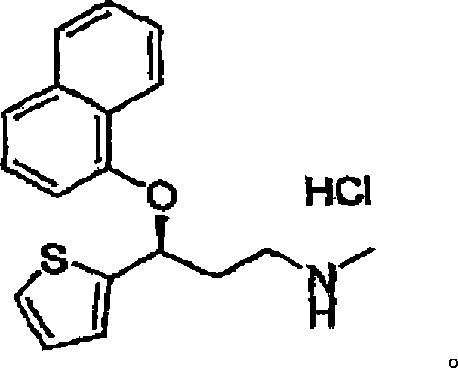
Duloxetine is used to treat depression and anxiety. In addition, duloxetine is used to help relieve nerve pain (peripheral neuropathy) in people with diabetes or ongoing pain due to medical conditions such as arthritis, chronic back pain, or fibromyalgia (a condition that causes widespread pain).
Antidepressant oral pharmaceutical compositions
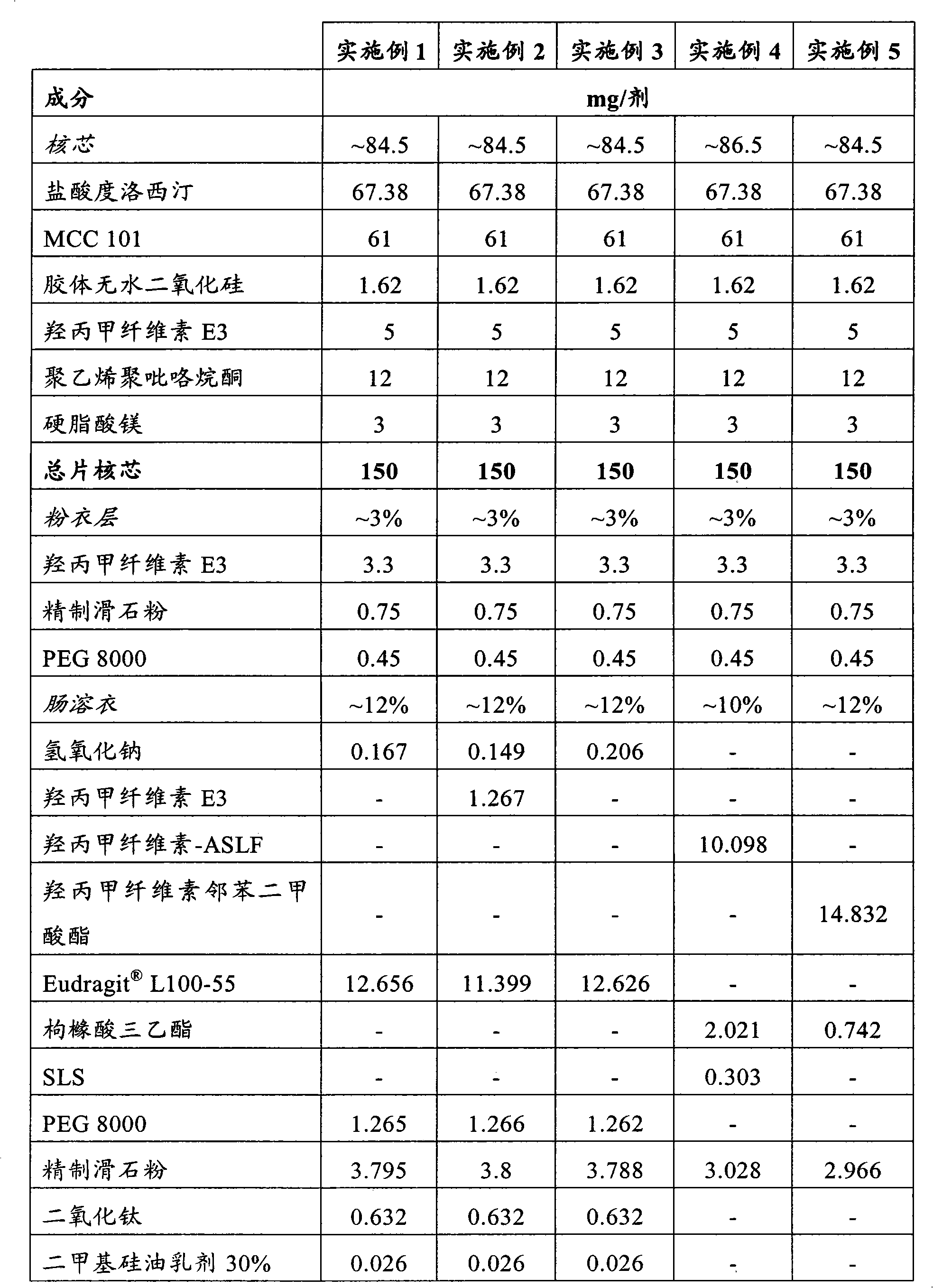
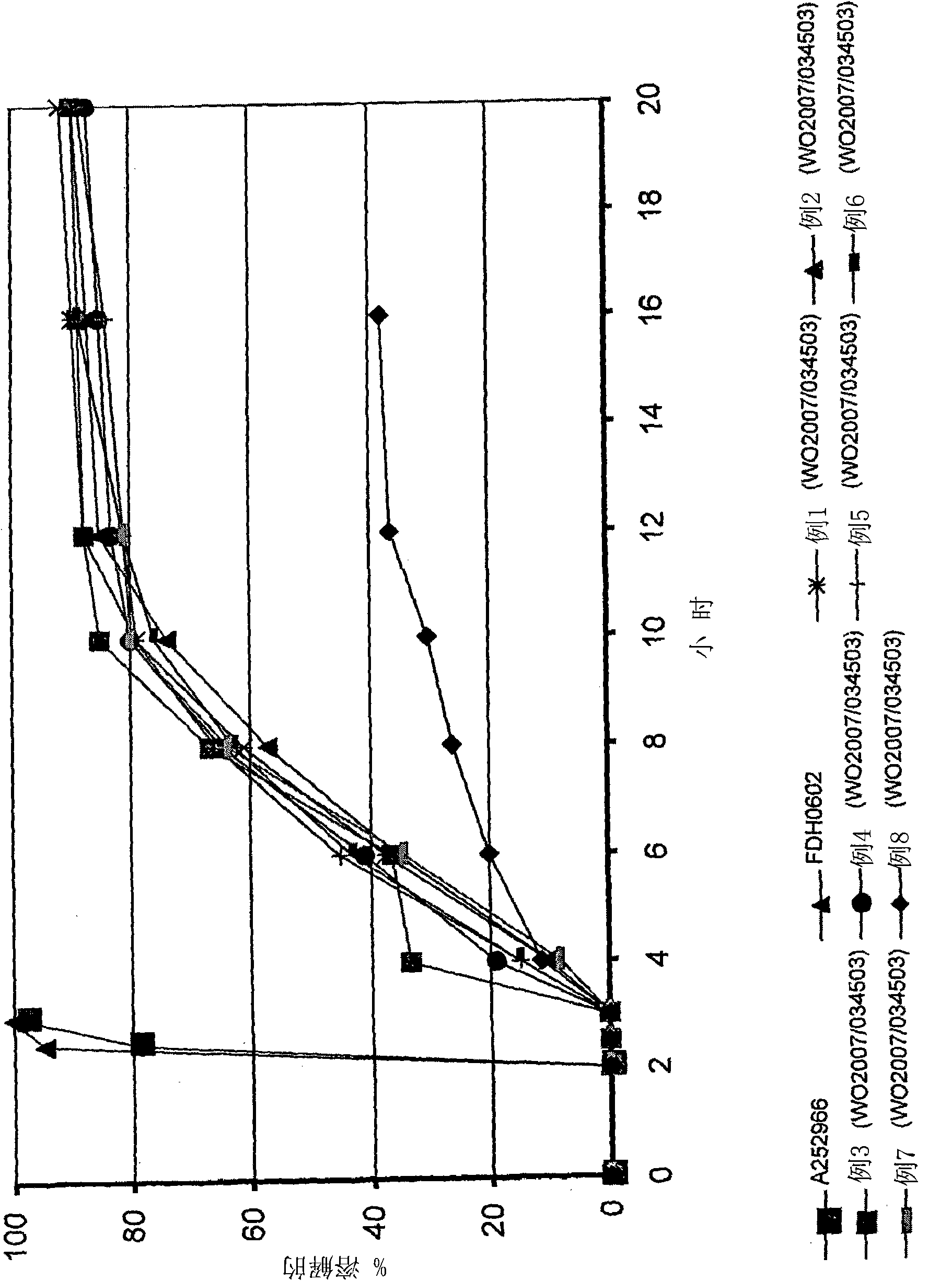
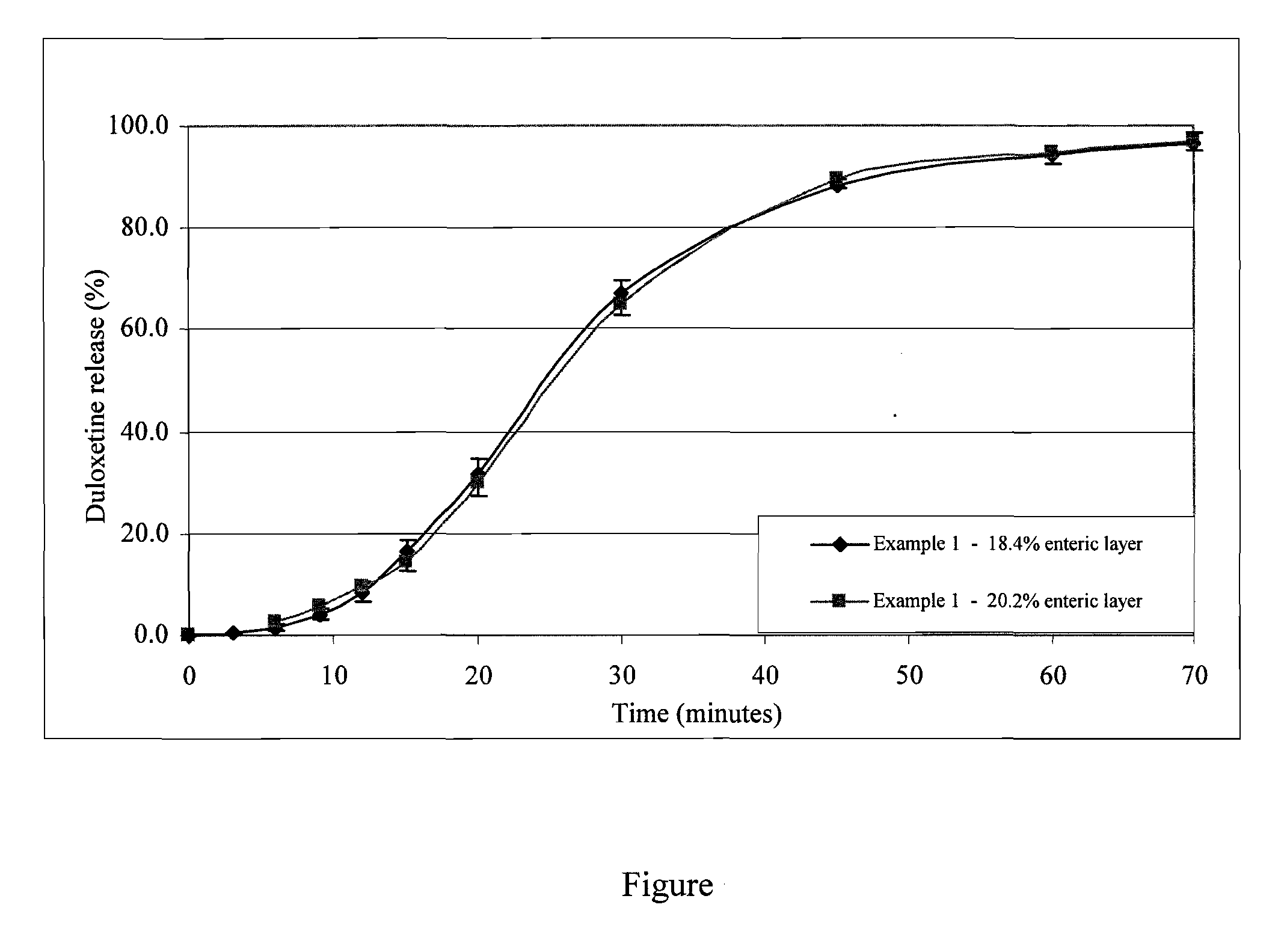
Novel enteric compositions suitable for oral administration comprising Duloxetine or its pharmaceutical derivatives thereof and methods for preparing such compositions are disclosed. Such compositions contain a core consisting of a Duloxetine or its pharmaceutical derivatives thereof, the said core comprised of a pharmaceutically inert nuclei and the Duloxetine or its pharmaceutical derivatives thereof compressed together, an intermediate and an enteric layer. Duloxetine or its pharmaceutical derivatives thereof may be any pharmaceutically acceptable prodrug, salt, solvate or derivative of Duloxetine. The novel compositions prepared according to the present invention have enhanced stability and bioavailability.
Owner:COMPANY WOCKHARDT THE
Duloxetine enteric coated tiny pill capsule, and preparation method
ActiveCN1759829AImprove solubilityReduced bioavailabilityNervous disorderGranular deliveryDuloxetineMedicine
An enteric micropill (microsoftgel) of Duluoxiting and its preparing process are disclosed. Said micropill is composed of an empty core pill and a coated external layer consisting of a principal medicine layer, an isolating layer and an enteric layer. It features that its principal medicine can be quickly released only in small intestine.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Treatment of chronic pain associated with drug or radiation therapy
Methods for treating chronic widespread pain associated with drug therapy or radiation therapy are described. The method generally involves administering a therapeutically effective amount of a dual or tri reuptake inhibitor of a specific type or a pharmaceutically acceptable salt thereof. Preferably the compound is a non-tricyclic dual reuptake inhibitor. The most preferred compound is milnacipran or a bioequivalent or pharmaceutically acceptable salt thereof. Other preferred compounds are duloxetine and venlafaxine or a bioequivalent or pharmaceutically acceptable salt thereof. In yet another embodiment, a therapeutically effective amount of a non-tricyclic triple reuptake inhibitor (“TRI”) compound of a specific type, or a pharmaceutically acceptable salt thereof, is administered. The TRI compounds are characterized by their ability to block the reuptake (and, hence, increase central concentrations of) the three primary brain monoamines: serotonin, noradrenaline, and dopamine.
Owner:CYPRESS BIOSCI
Antidepressant oral pharmaceutical compositions
InactiveUS20070004795A1Easy to prepareInhibition of reuptakeAntibacterial agentsBiocideDuloxetinePeripheral neuropathic pain
The invention provides a pharmaceutical composition of duloxetine or its pharmaceutically equivalent derivatives like salts, isomers, complexes, polymorphs, hydrates or esters thereof and at least one buffering agent. The duloxetine or its pharmaceutically equivalent derivative is present from about 2 mg to approximately 200 mg; and the buffering agent is present in an amount of approximately 0.1 mEq to approximately 2.5 mEq per mg of duloxetine. Also provided is a method for treating of major depressive disorder and or diabetic peripheral neuropathic pain comprising administering to a mammal in need of such treatment a therapeutically effective amount of a composition.
Owner:COMPANY WOCKHARDT THE +1
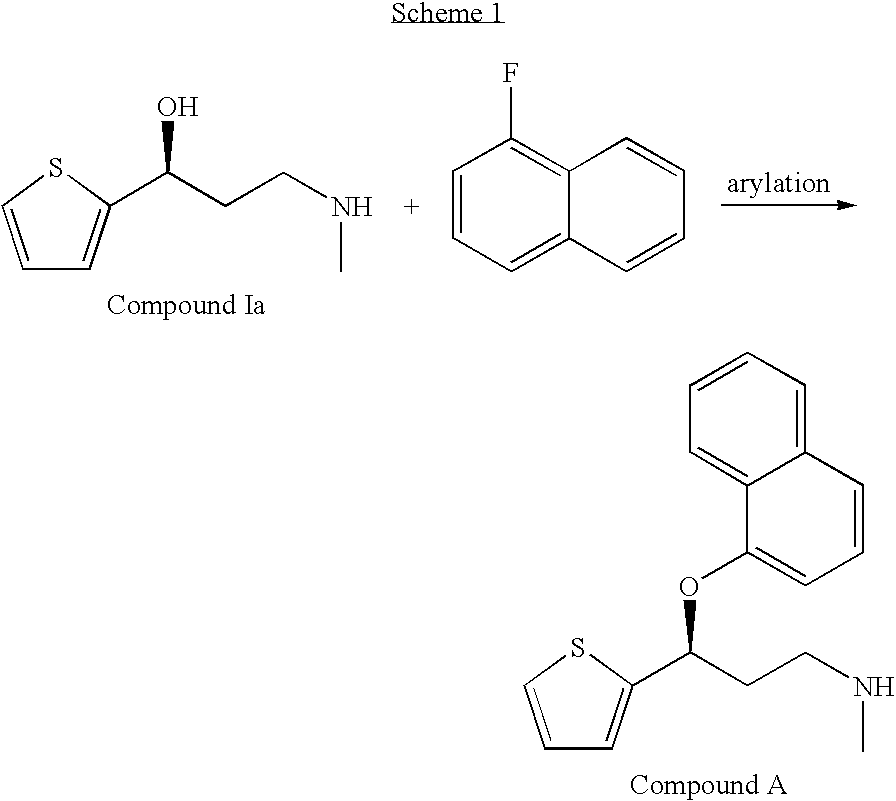
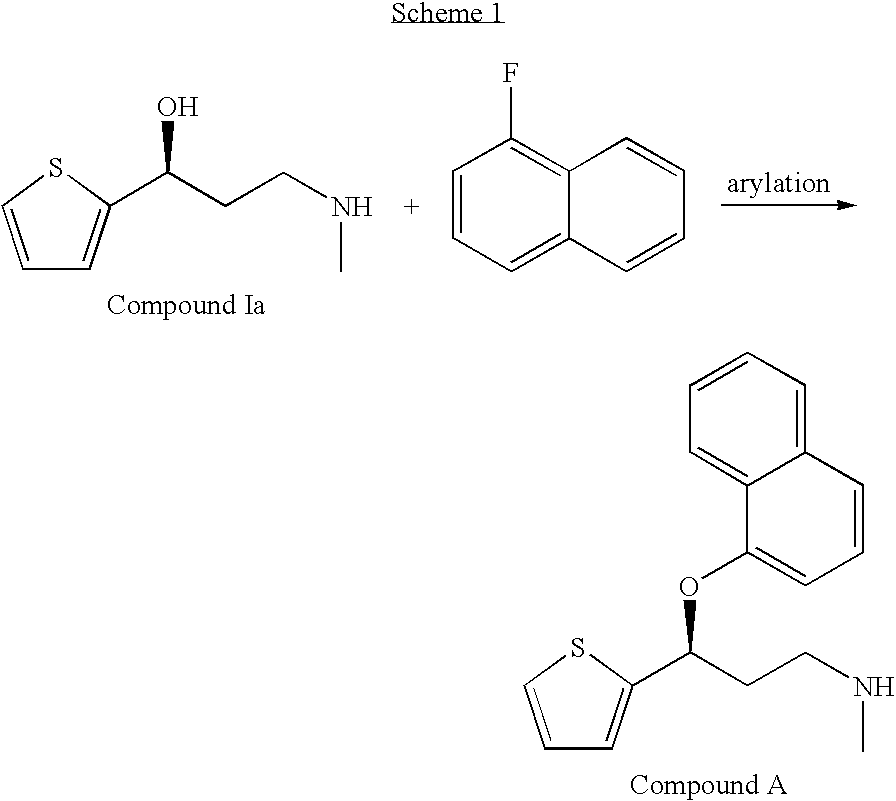
Improved process for the asymmetric synthesis of duloxetine
InactiveUS20070167636A1Reduce racemizationHigh amount of racemizationOrganic chemistryChemistryDuloxetine
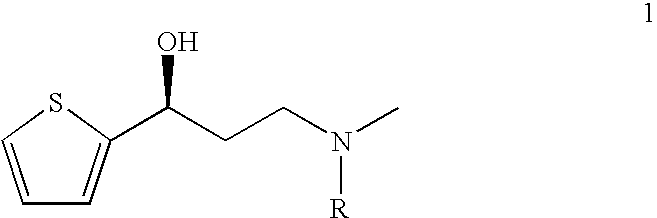
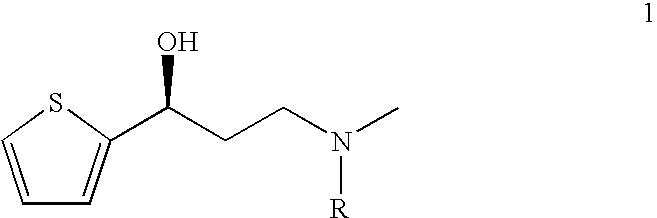
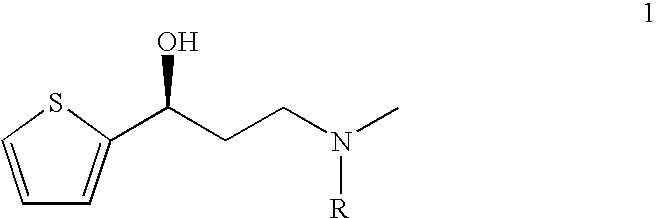
This invention provides an improved asymmetric process for the synthesis of duloxetine involving arylation of Compounds of Formula I.
Owner:ELI LILLY & CO
Process for the asymmetric synthesis of duloxetine
InactiveUS7538232B2Reduce racemizationReduce the amount of solutionOrganic chemistryDuloxetineBiochemistry
This invention provides an improved asymmetric process for the synthesis of duloxetine involving arylation of Compounds of Formula I.
Owner:ELI LILLY & CO
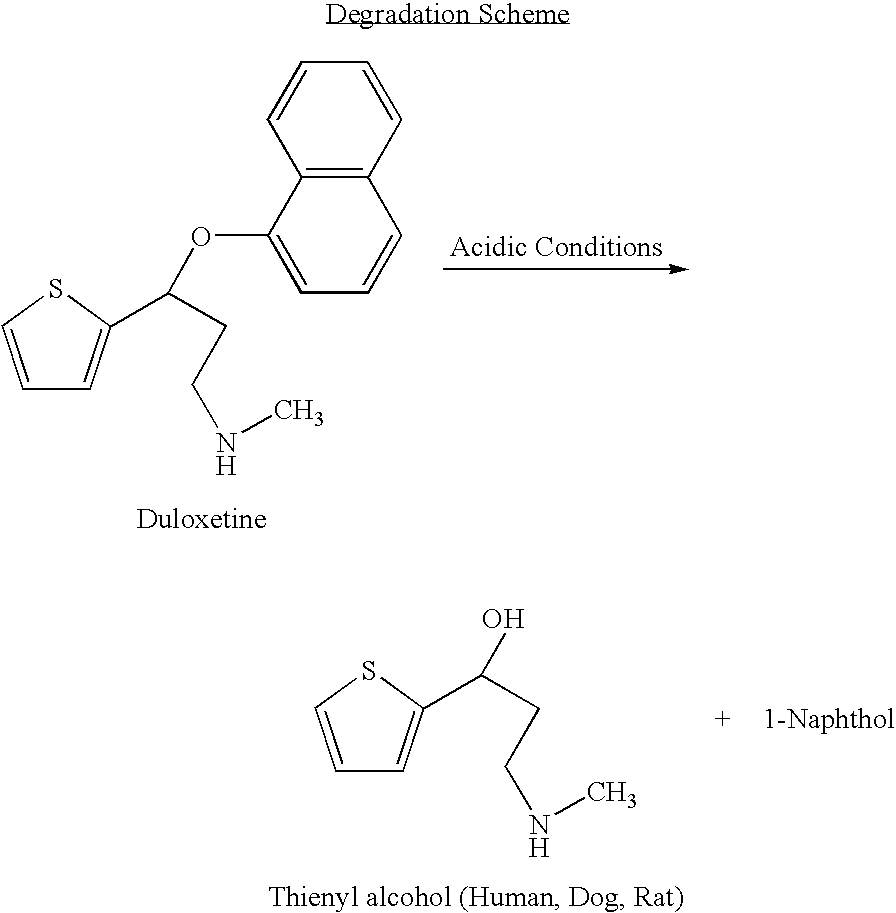
Antidepressant oral liquid compositions
InactiveUS8153824B2Inhibition of reuptakeImprove concentrationBiocideNervous disorderDuloxetinePeripheral neuropathic pain
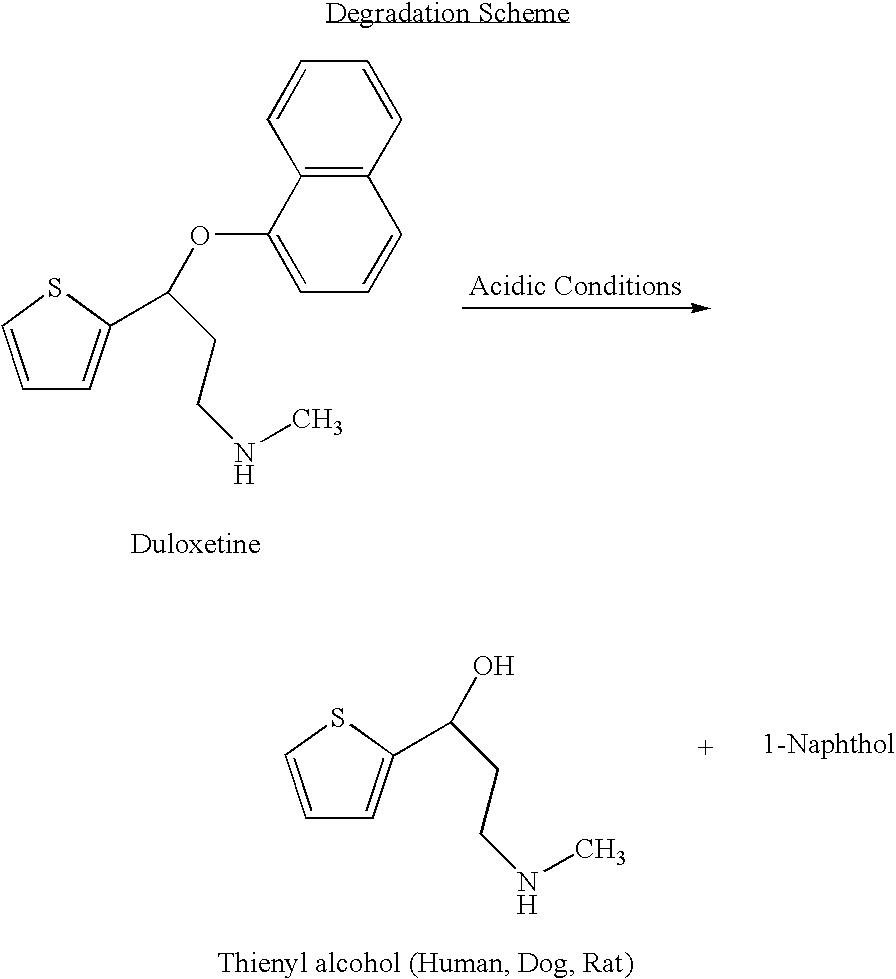
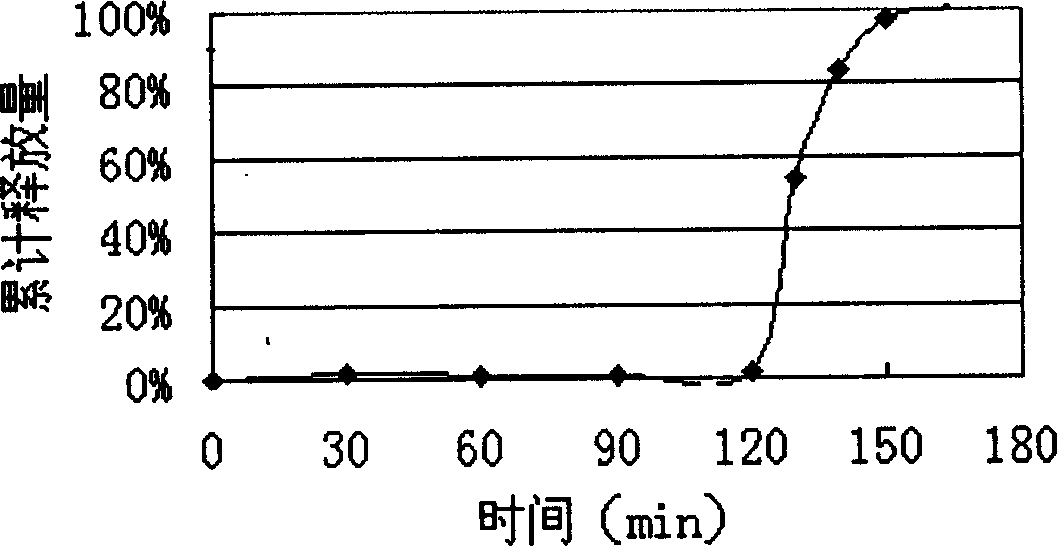
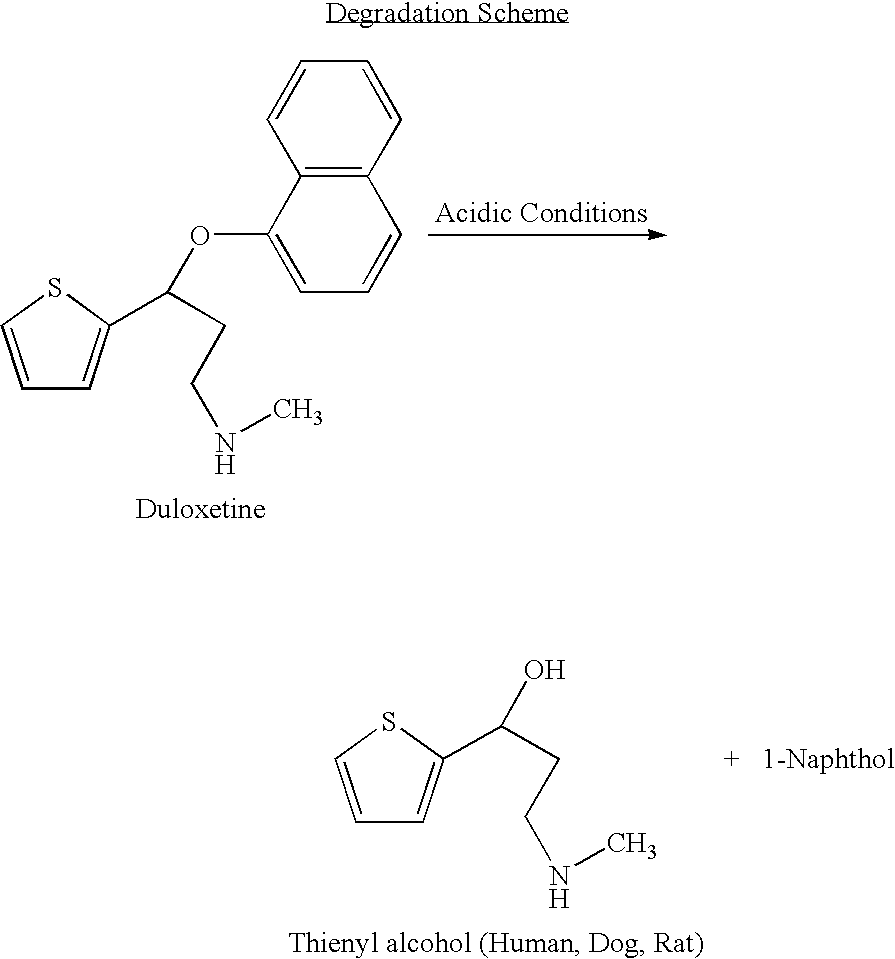
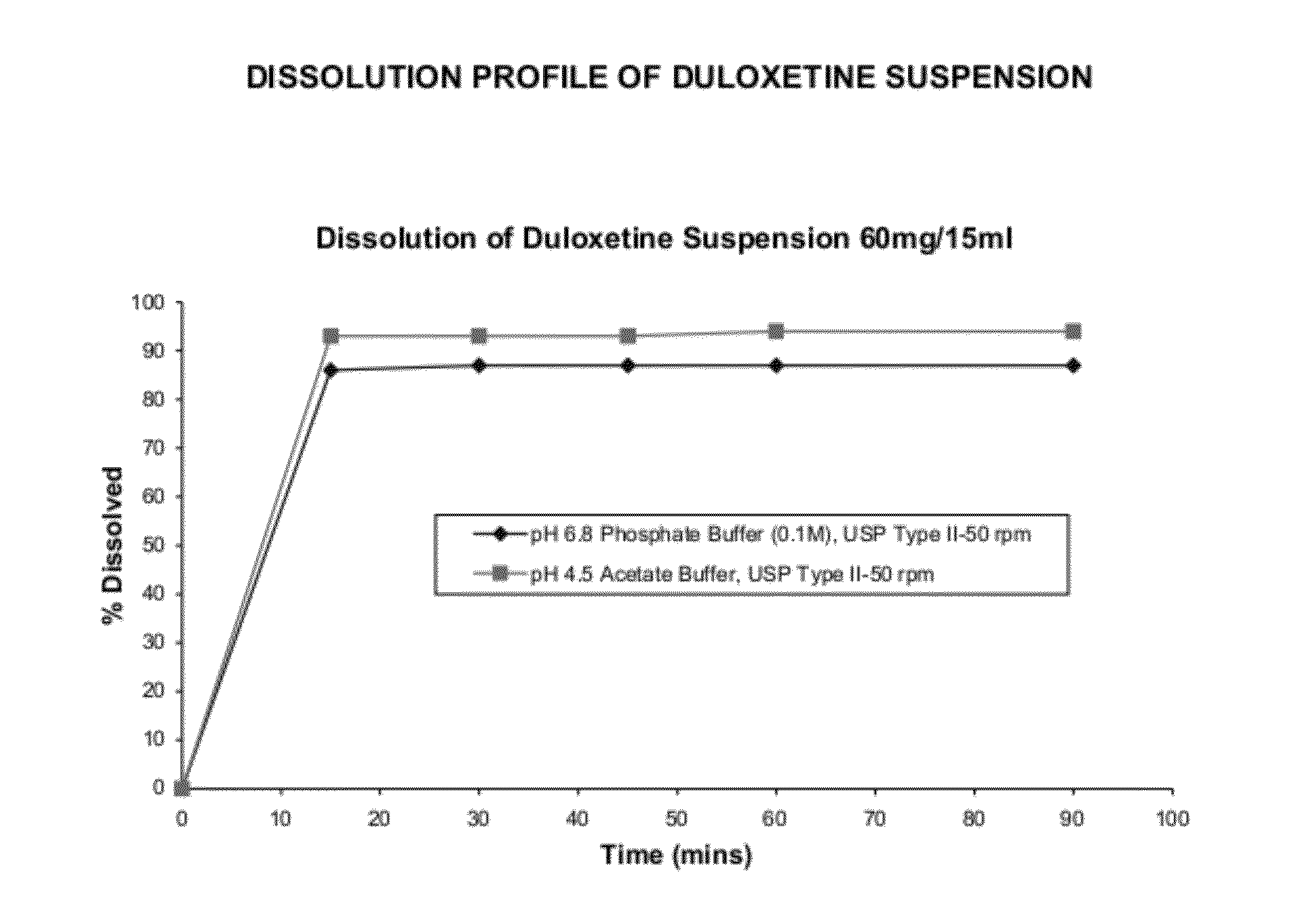
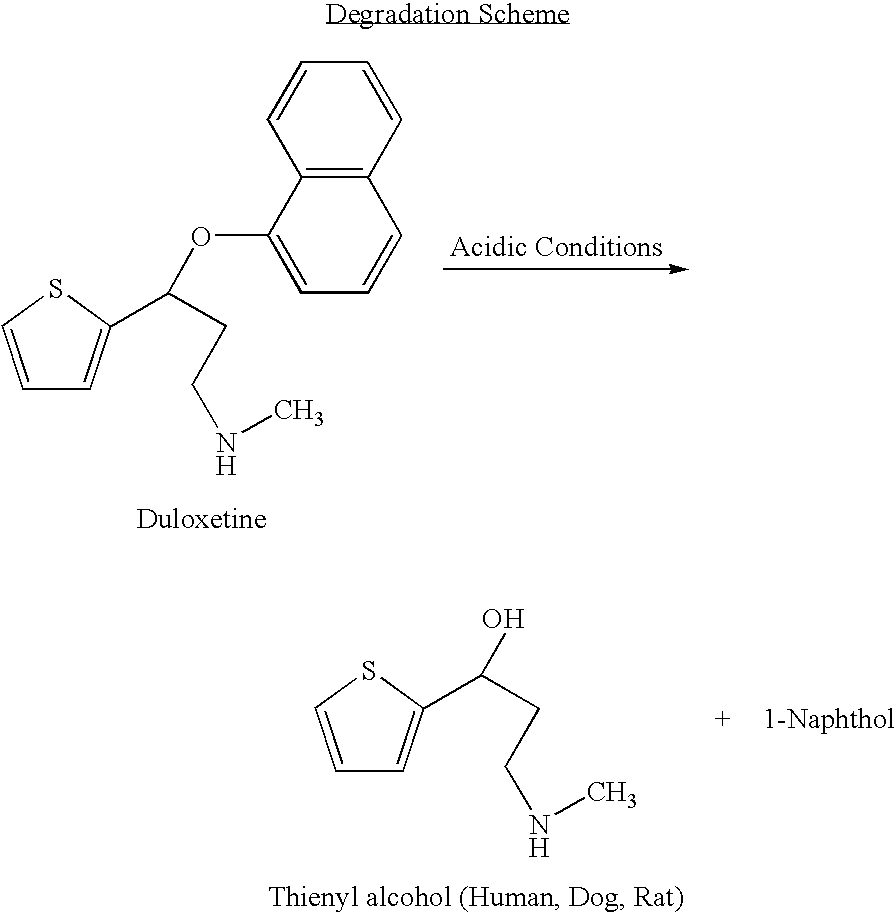
The invention provides for the first time an oral liquid composition of duloxetine or its pharmaceutically equivalent derivatives like salts, isomers, complexes, polymorphs, hydrates or esters thereof. The duloxetine or its pharmaceutically equivalent derivative is present from about 2 mg to approximately 200 mg; and a buffering agent was used to stabilize the acid sensitive duloxetine. The composition has duloxetine from about 0.1 meq to about 2.5 mEq per mg of duloxetine. The invention further discloses an oral liquid composition of duloxetine or its pharmaceutically equivalent derivative wherein the degradation product 1-Naphthol is less than 0.01%. Also provided is a method for treating of major depressive disorder and or diabetic peripheral neuropathic pain comprising administering to a mammal in need of such treatment a therapeutically effective amount of a composition.
Owner:COMPANY WOCKHARDT THE
Pharmaceutical compositions of duloxetine
The present invention relates to solid oral pharmaceutical compositions of duloxetine, process for preparing such compositions and method of using such compositions. Preferably, the invention relates to a delayed release composition of duloxetine comprising a core comprising duloxetine, optional separating coat and an enteric coat, wherein the enteric coat comprises methacrylic acid copolymer.
Owner:TORRENT PHARMA LTD
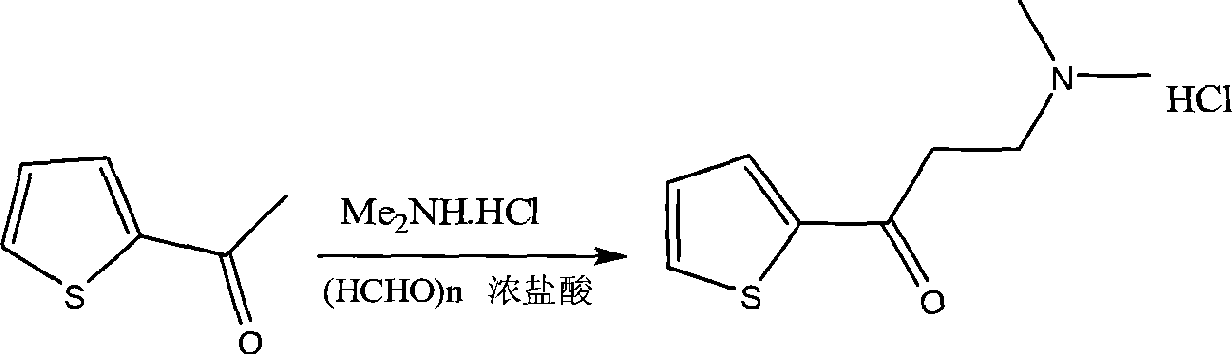
Method for preparing duloxetine
InactiveCN101391991AHigh yieldReduce manufacturing costNervous disorderOrganic chemistryDuloxetineMannich reaction
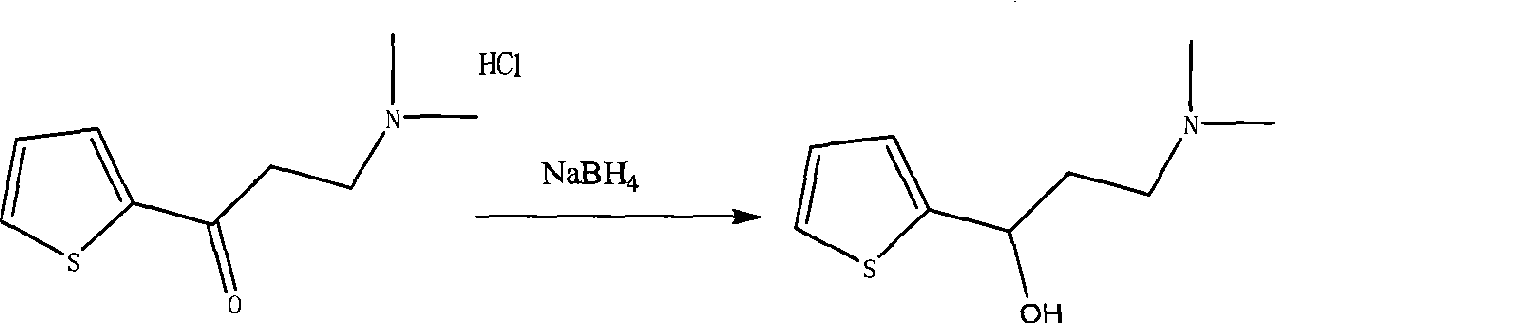
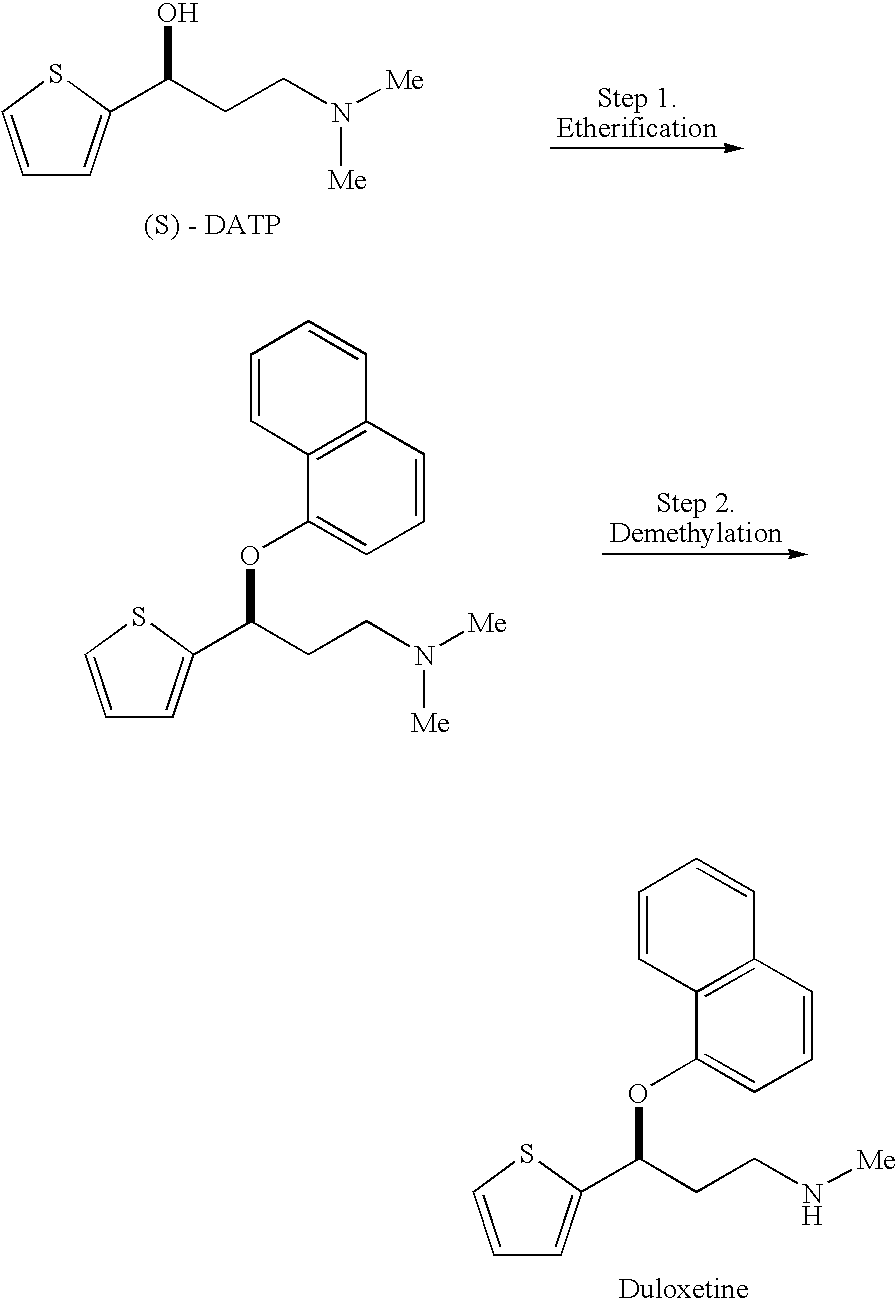
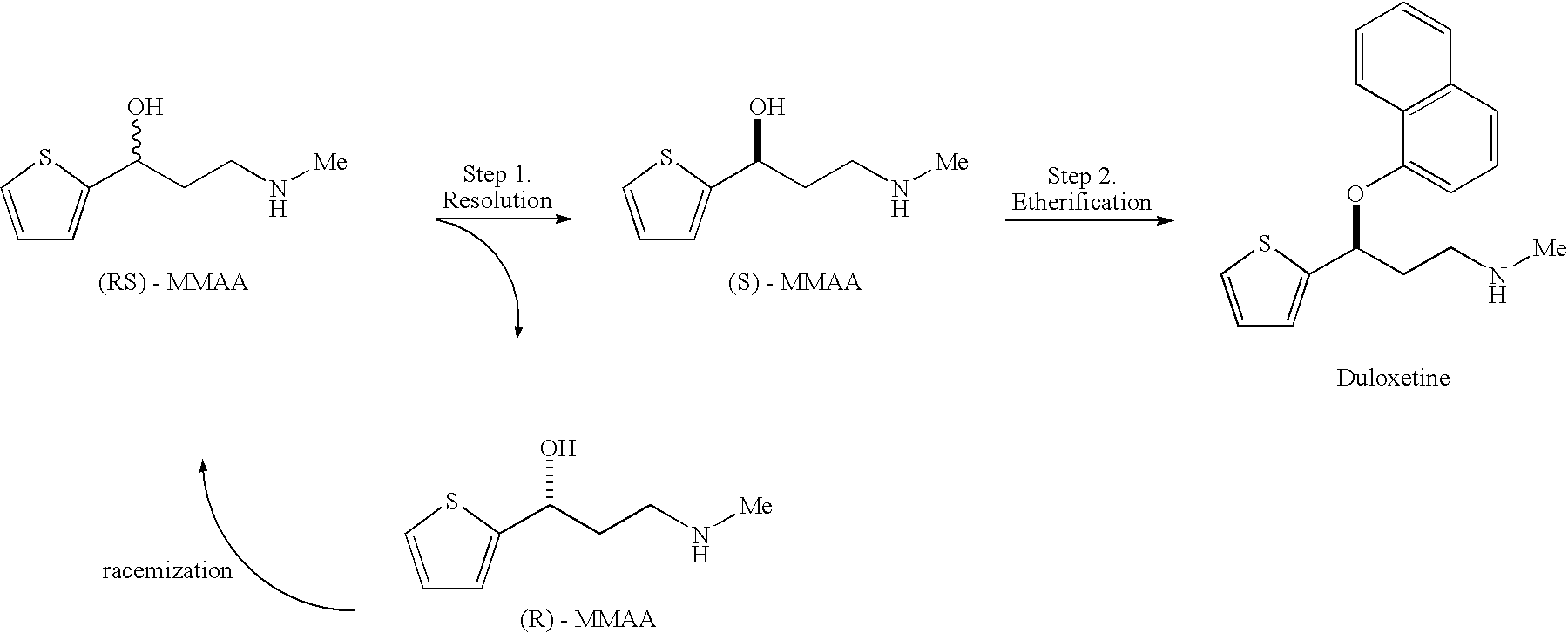
The invention relates to a preparation method of duloxetine, which takes 2-acetyl phenol, paraformaldehyde and dimethylamine hydrochloride as raw materials to obtain the target product by five steps of Mannich reaction, reduction, resolution, etherification and demethylation, and optimizes the reaction conditions of resolution and etherification, wherein, the resolution reaction comprises the steps of one-time resolution, racemization-resolution and secondary racemization-resolution. The invention can improve the yield of the duloxetine and reduce the production cost, and is easy for industrial production.
Owner:SUZHOU LEADER CHEM
Pharmaceutical compositions of duloxetine
The present invention relates to solid oral pharmaceutical compositions of duloxetine, process for preparing such compositions and method of using such compositions. Preferably, the invention relates to a delayed release composition of duloxetine comprising a core comprising duloxetine, optional separating coat and an enteric coat.
Owner:TORRENT PHARMA LTD
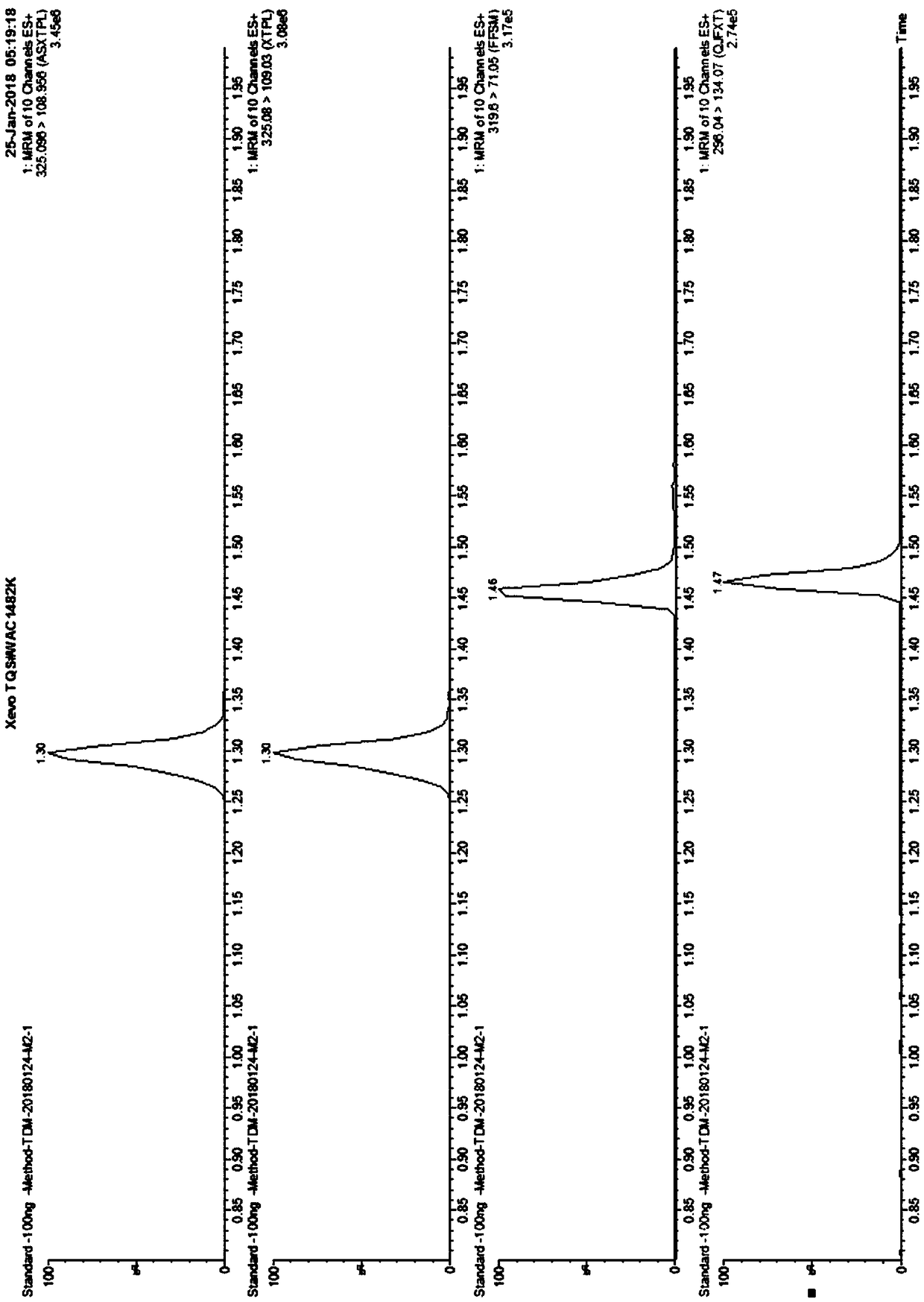
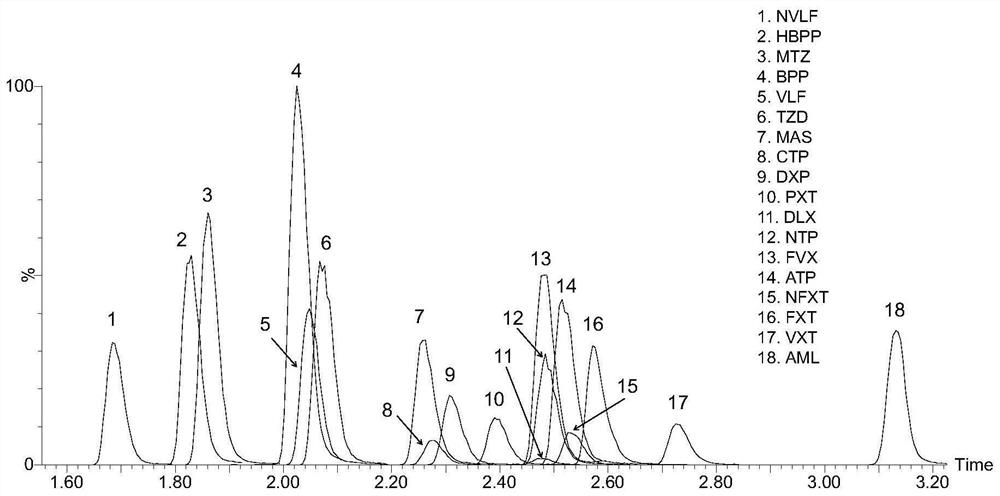
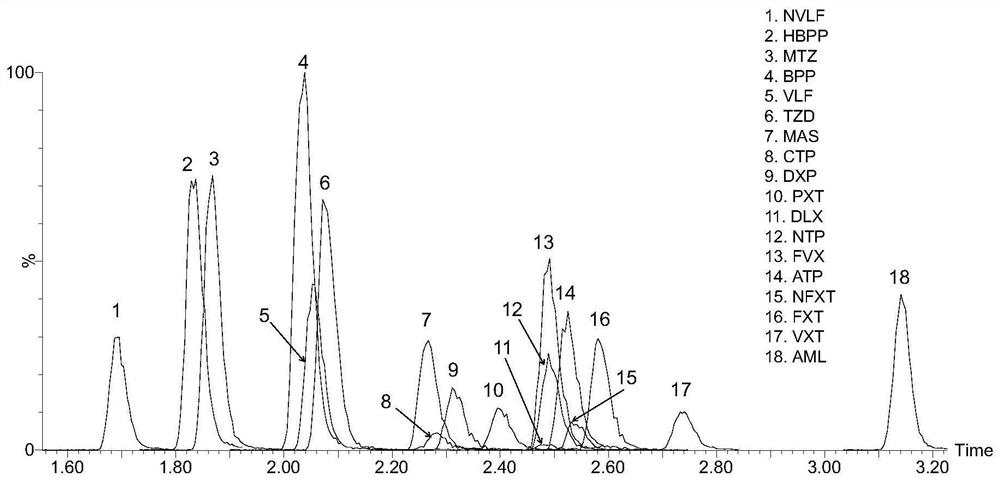
Kit for detecting anti-depressant drugs in serum and plasma by liquid chromatography tandem mass spectrometry and application thereof
InactiveCN109085264APharmacologically activeInterpret blood levelsComponent separationSertralineTandem mass spectrometry
The invention provides a kit for detecting anti-depressant drugs in serum and plasma by liquid chromatography tandem mass spectrometry. The kit comprises drug standard substances, drug internal standardization compounds, drug extraction compositions, negative plasma and a diluent. The drug standard substances comprise amfebutamone, oxybupropion, citalopram, Escitalopram, venlafaxine, O-desmethylvenlafaxine, duloxetine, fluoxetine, norfloxetine, fluvoxamine, mirtazapine, paroxetine, sertraline and trazodone. The drug internal standardization compounds comprise amfebutamone-d9, oxybupropion-d6,citalopram-d6, venlafaxine-d6, O-desmethylvenlafaxine-d6, duloxetine-d3, fluoxetine-d6, norfloxetine-d6, fluvoxamine-d4, mirtazapine-d3, paroxetine-d6, sertraline-d3 and trazodone-d6. The drug extraction compositions comprise, by volume, 60% of methanol solution, 20% of acetonitrile solution, 10% of isopropyl alcohol solution and 10% of purified water. The diluent comprises 50 % of methanol waterfluid. The kit can be used for simultaneous detection of the anti-depressant drugs and active metabolites, the detection time is short, and flux is high.
Owner:HANGZHOU BAICHEN MEDICAL INSTR CO LTD +1
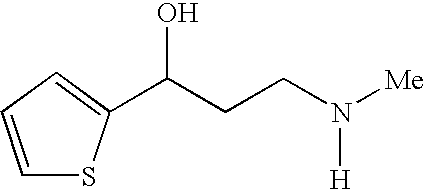
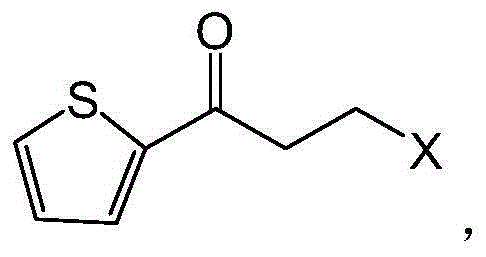
Process for preparing optically active 3-(methylamino)-1-(2-thienyl) propan-1-ol and intermediates for preparation
InactiveUS20060063943A1Ease of industrial applicationHigh optical purityOrganic chemistryDuloxetineMandelic acid
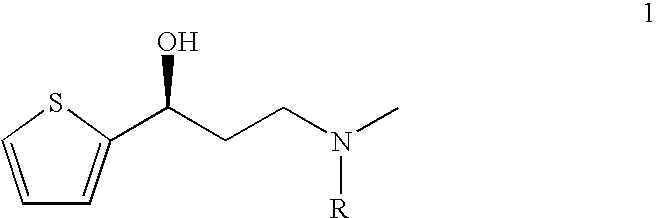
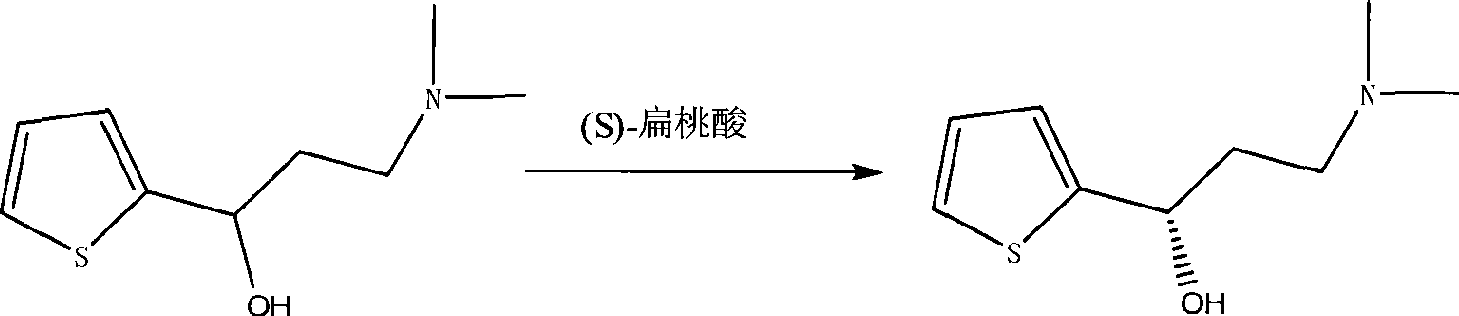
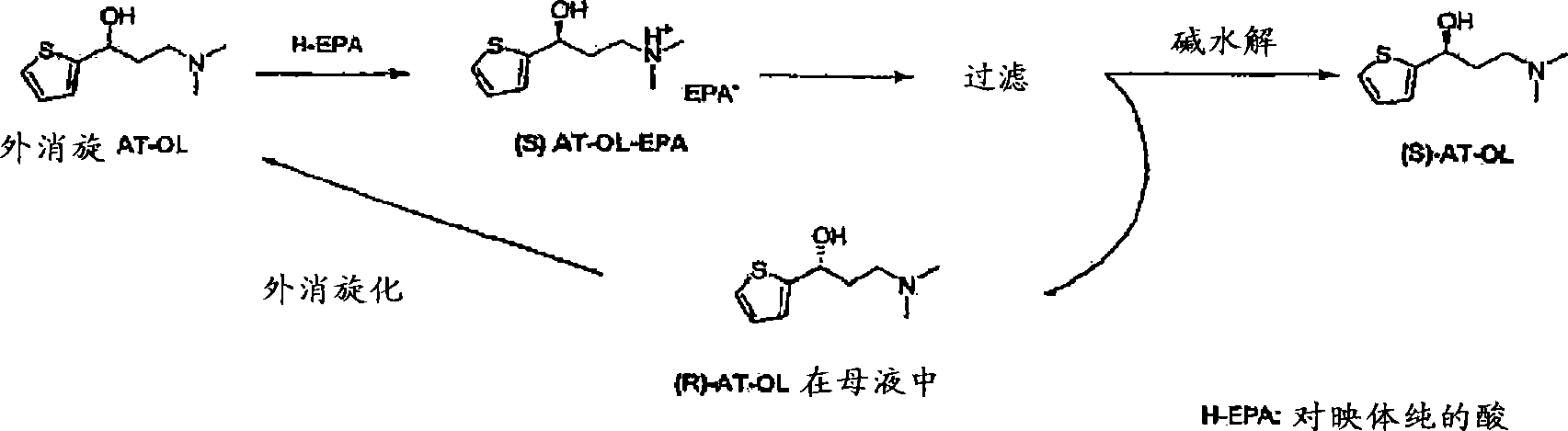
Disclosed is a process for commercial preparation of 3-(methylamino)-1-(2-thienyl)propan-1-ol (hereinafter abbreviated as “MMAA”) of the formula below: The process is carried out by the diastereomeric salt formation method using optically active mandelic acid or its derivatives, or an optically active tartaric acid derivative as the resolving agent. The product compound, diastereomeric satls, is useful as the intermediate for producing pharmaceuticals, such as duloxetine.
Owner:YAMAKAWA CHEM IND
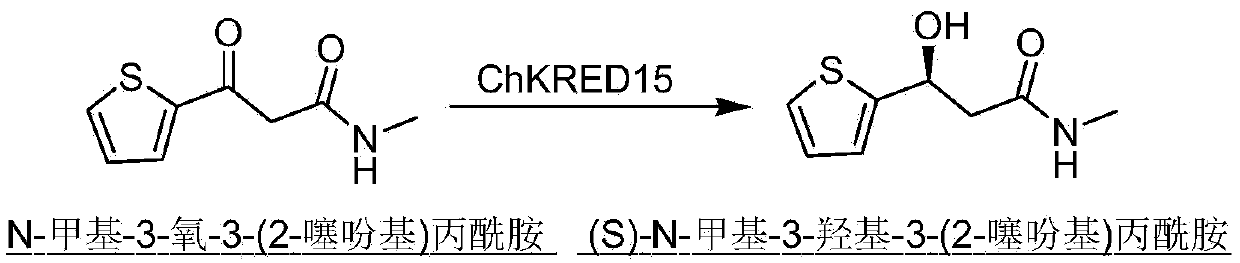
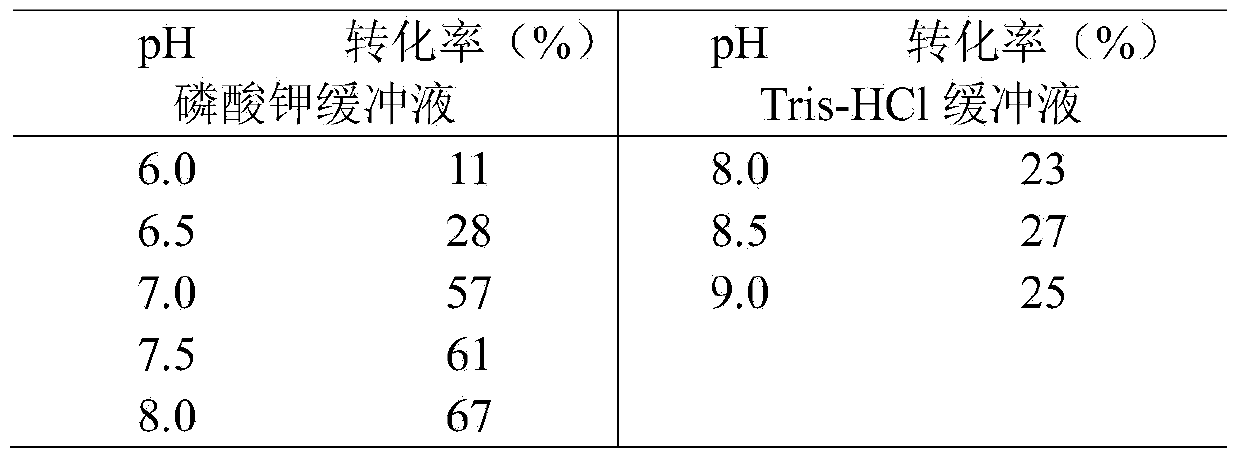
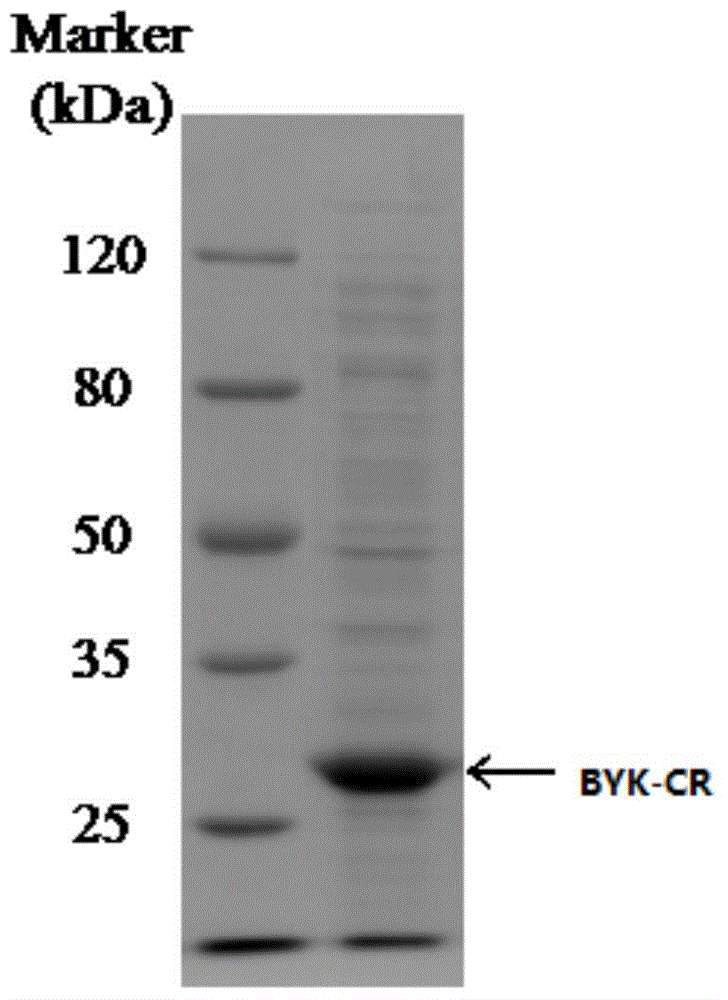
Carbonyl reductase, gene thereof, and application thereof in preparing Duloxetine chiral intermediates
The invention discloses a carbonyl reductase (i)Ch ( / i) KRED15 derived from Chryseobacterium ((i) Chryseobacterium ( / i) (i) ( / i) sp. CA49), and a coding gene thereof; the invention also discloses application of the carbonyl reductase as a biocatalyst in preparing a Duloxetine chiral intermediate ((i) S ( / i))-N-methyl-3-hydroxy-3-(2-thienyl) propanamide. The enantiomer excess of products is greater than 99.9%. 20 g / L of substrate can be catalyzed by Ch( / i) KRED15 crude enzyme (2U / mL), and the conversion rate of over 99% can be obtained in 2 hours; a coenzyme circulation system is simple, and has large industrial application prospect.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
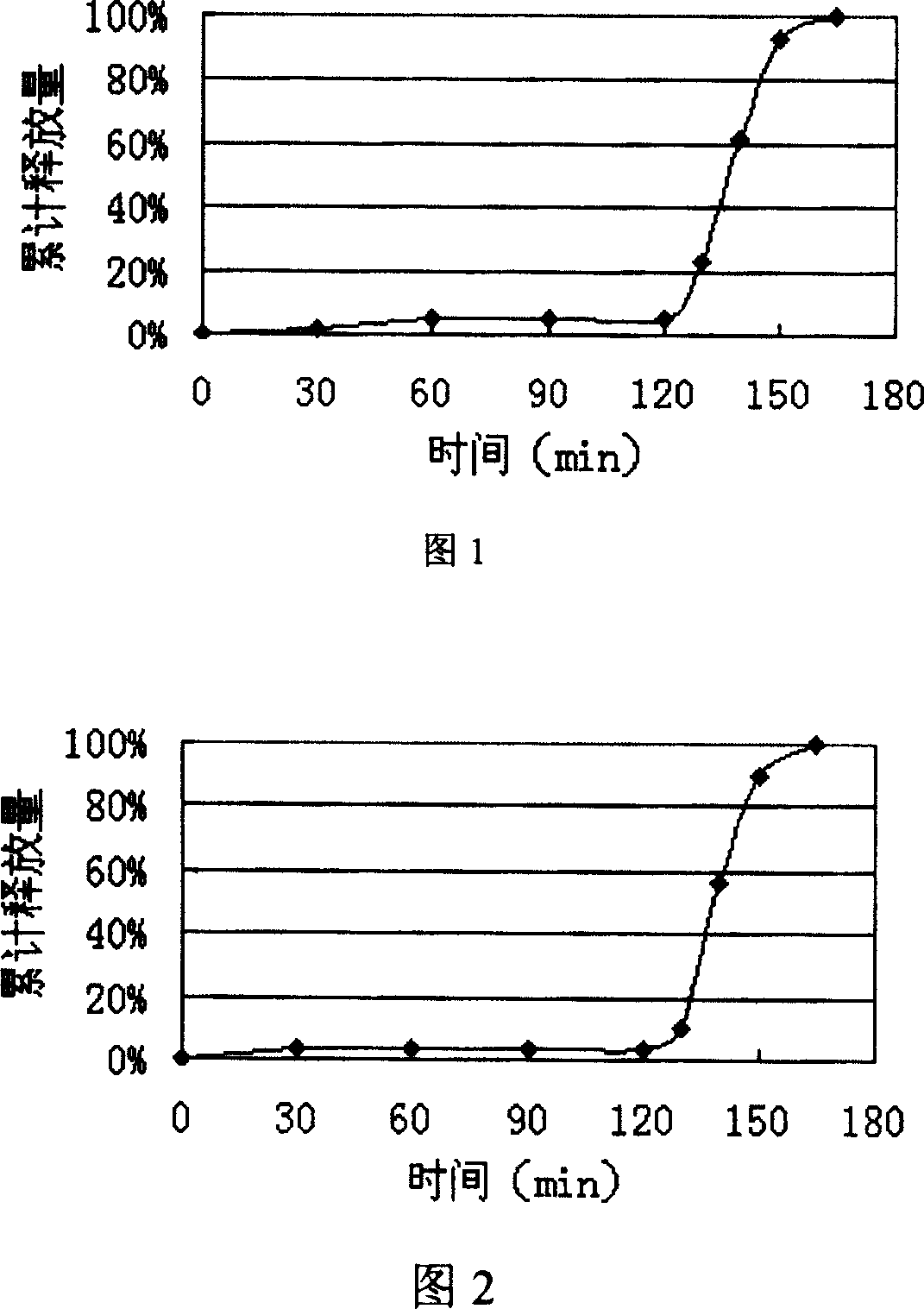
Delayed release pharmaceutical composition of duloxetine
InactiveCN101939004AOvercoming Concomitant and Anticipated ProblemsOrganic active ingredientsNervous disorderDuloxetinePharmaceutical drug
A pharmaceutical composition comprising duloxetine or a pharmaceutically acceptable salt thereof and one or more pharmaceutically acceptable excipient(s) characterised in that the duloxetine has a D90 particle size of 2 to 40 [mu]m.
Owner:ALPHAPHARM PTY LTD
Pharmaceutical composition of duloxetine
The invention relates to a taste masked pharmaceutical composition comprising duloxetine or pharmaceutically acceptable salts thereof. The invention also relates to processes for the preparation of such compositions. The invention further discloses an inclusion complex comprising duloxetine or pharmaceutically acceptable salts thereof with one or more cyclodextrin or derivatives thereof.
Owner:WOCKHARDT LTD
Alcohol dehydrogenase and uses thereof in synthesis of Duloxetine intermediate
ActiveCN105274069AHigh optical purityMild reaction conditionsBacteriaOxidoreductasesChemical synthesisDuloxetine
The invention provides an alcohol dehydrogenase with high catalytic activity, strong enantiotropic selectivity and good substrate tolerance, and provides an enzymatic synthesis method of employing the alcohol dehydrogenase to catalyze and synthesize (s)-3-substituted amido-1-(thiophenyl-2-yl)-1- propanol and furthermore Duloxetine. The invention also provides a nucleic acid sequence for encoding the alcohol dehydrogenase, a recombinant expression vector including the nucleic acid sequence, and a recombinant expression transformant, and a preparation method of the alcohol dehydrogenase, and uses of the alcohol dehydrogenase in catalyzing asymmetric reduction of carbonyl substrates. The invention has advantages that the product concentration is high, the optical purity of the product is high, the reaction condition is mild and environment-friendly, the operation is convenient and is easy to industrialize, and an extra expensive coenzyme NADP<+> / NAD<+> is not necessary.
Owner:ABIOCHEM BIOTECH CO LTD
Carbostyril derivatives and serotonin reuptake inhibitors for treatment of mood disorders
The pharmaceutical composition of the present invention comprises (1) a carbostyril derivative and (2) a serotonin reuptake inhibitor in a pharmaceutically acceptable carrier. The carbostyril derivative may be aripiprazole or a metabolite thereof, which is a dopamine-serotonin system stabilizer. The serotonin reuptake inhibitor may be fluoxetine, duloxetine, venlafaxine, milnacipran, citalopram, fluvoxamine, paroxetine, sertraline or escitalopram. The pharmaceutical composition of the present invention is useful for treating patients with mood disorders, particularly depression or major depressive disorder.
Owner:OTSUKA PHARM CO LTD
Process for making duloxetine and related compounds
Owner:SYNTHON BV
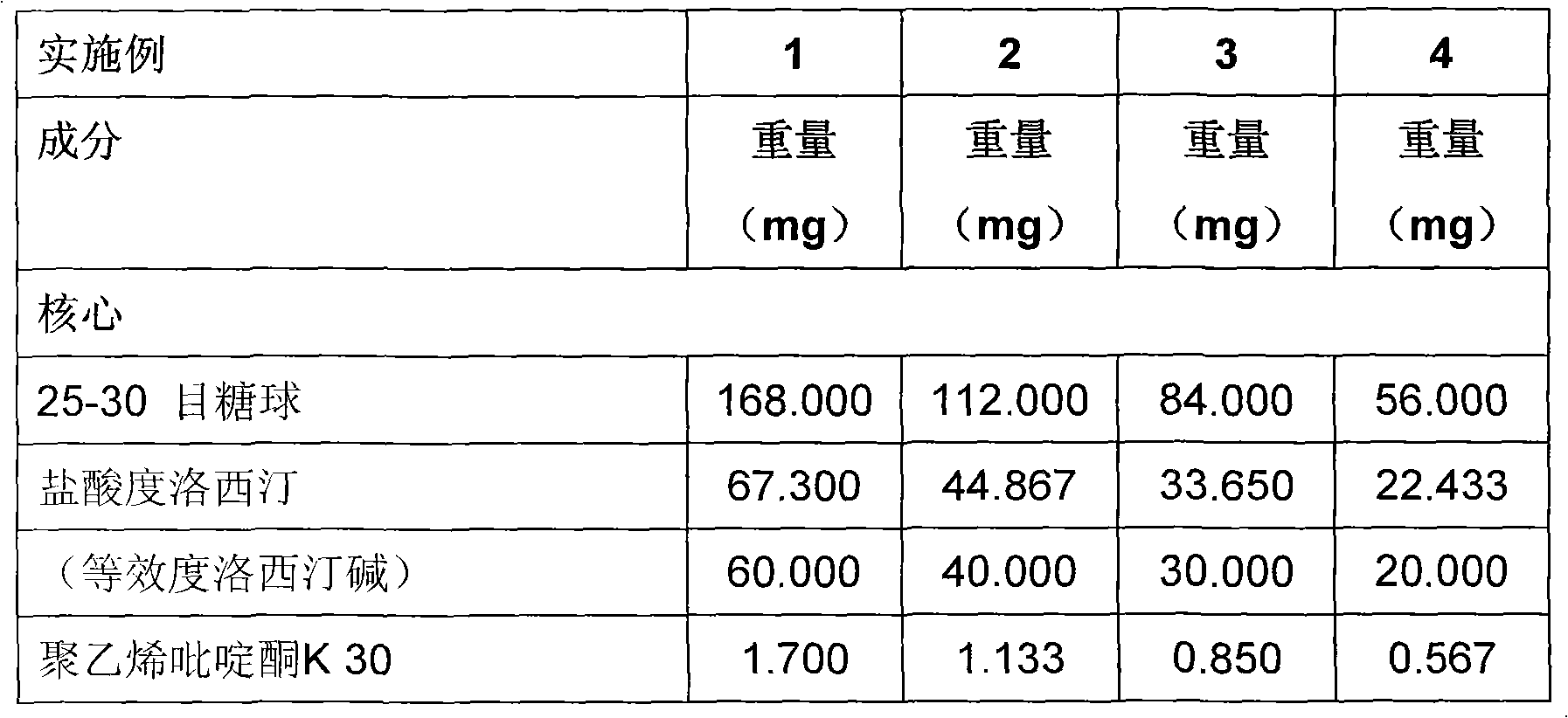
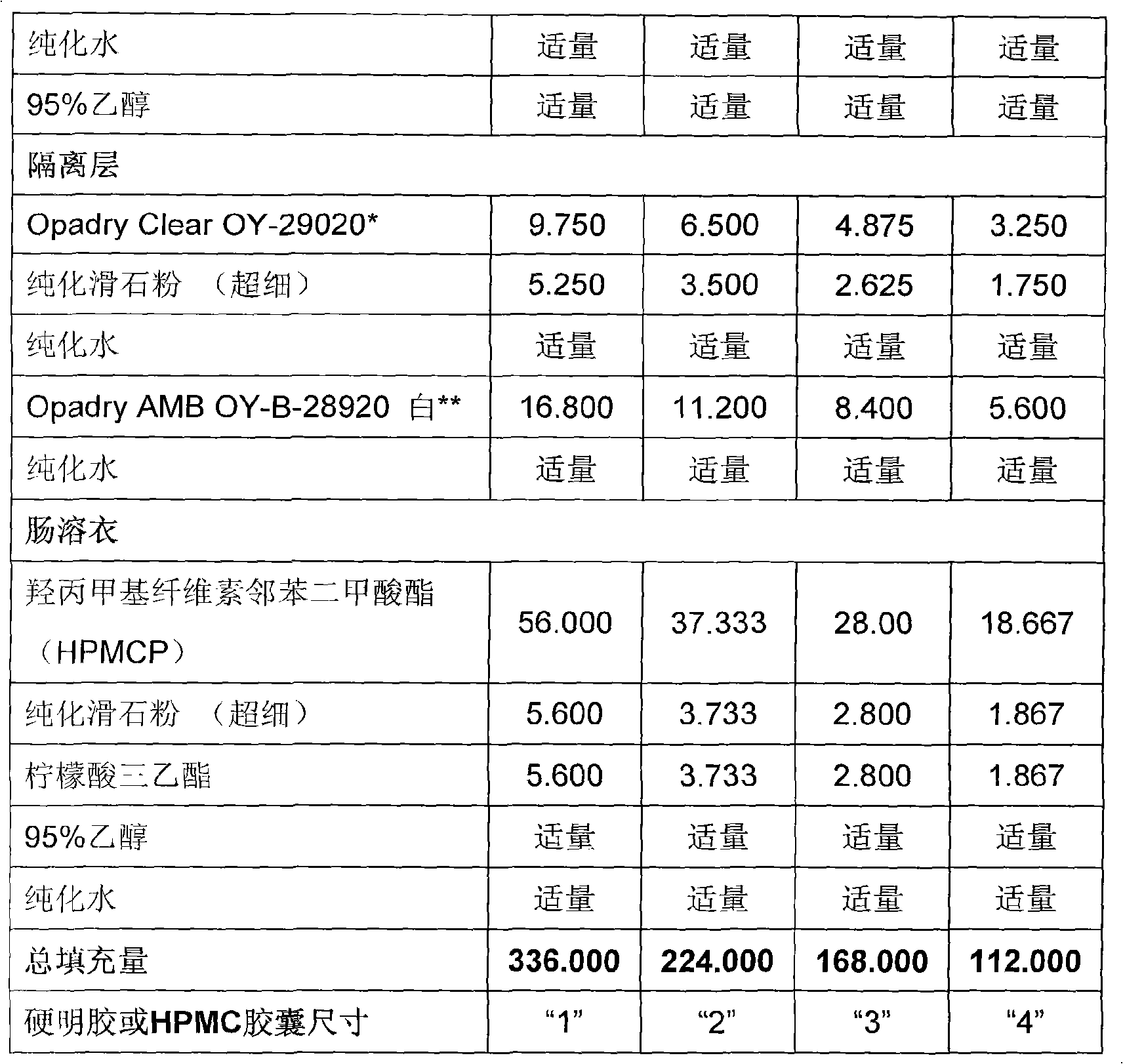
Duloxetine formulation
A duloxetine pellet formulation comprises: (i) a core including a desired amount of duloxetine; (ii) an enteric coating comprising hydroxypropylmethylcellulose phthalate (HPMCP) as an enteric polymer;and, optionally, (iii) a separating layer located between the core and the enteric coating, the separating layer including polyvinyl alcohol and a low molecular weight hydroxypropylmethylcellulose (HPMC).
Owner:箭锋国际有限公司
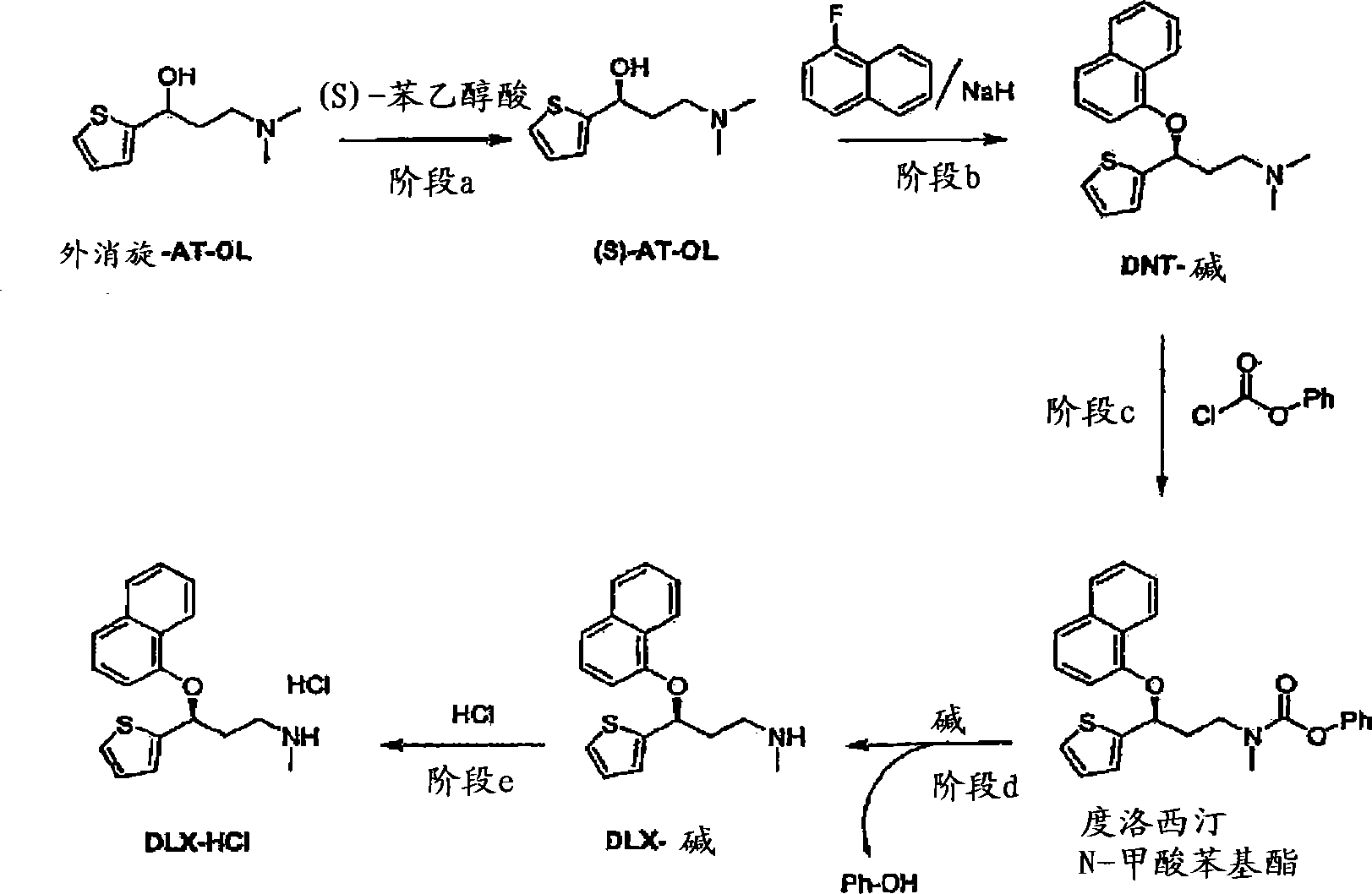
Process for the preparation of (s)-(-)-n,n-dimethyl-3-(2-thienyl)-3-hydroxypropanamine, a duloxetine intermediate
A chiral resolution process for the preparation of (S)-AT-OL, and a process for the racemization of AT-OL are provided. A one pot continuous process for preparing (S)-AT-OL or (S)-AT-OL mandelate comprising: a) converting (R) -AT-OL to (R / S) -AT-OL in a mixture of a Cl-8 alcohols and a C2-8 ether in presence of an acid; b) reacting the (R / S) -AT-OL with (S) - ( + ) -mandelic acid in the mixture to obtain (S)-AT-OL mandelate; and c) optionally converting the (S)-AT-OL mandelate to (S)-AT-OL.
Owner:TEVA PHARMA IND LTD
Antidepressant oral liquid compositions
InactiveUS20060079569A1Easy to prepareInhibition of reuptakeBiocideNervous disorderDuloxetinePeripheral neuropathic pain
The invention provides for the first time an oral liquid composition of duloxetine or its pharmaceutically equivalent derivatives like salts, isomers, complexes, polymorphs, hydrates or esters thereof. The duloxetine or its pharmaceutically equivalent derivative is present from about 2 mg to approximately 200 mg; and a buffering agent was used to stabilize the acid sensitive duloxetine. The composition has duloxetine from about 0.1 meq to about 2.5 mEq per mg of duloxetine. The invention further discloses an oral liquid composition of duloxetine or its pharmaceutically equivalent derivative wherein the degradation product 1-Naphthol is less than 0.01%. Also provided is a method for treating of major depressive disorder and or diabetic peripheral neuropathic pain comprising administering to a mammal in need of such treatment a therapeutically effective amount of a composition.
Owner:COMPANY WOCKHARDT THE
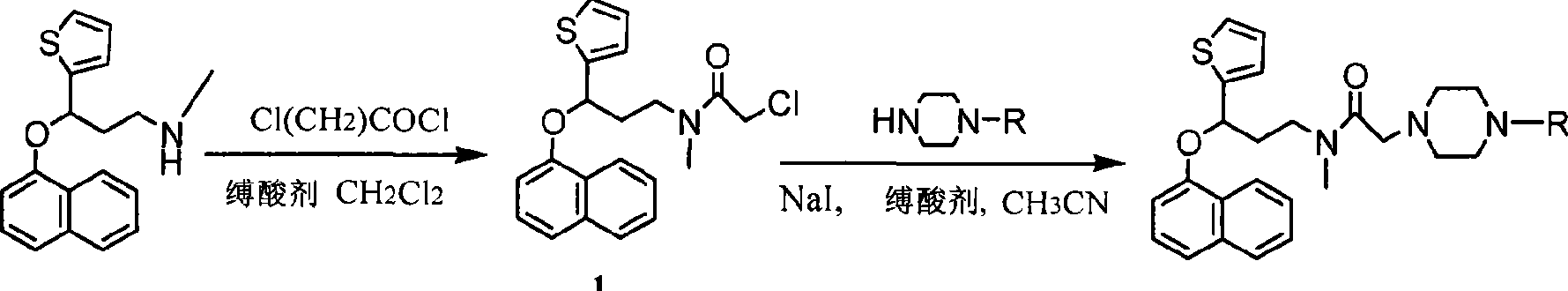
Duloxetine derivative and preparation thereof
InactiveCN101412708AEasy to makeEasy to operateOrganic active ingredientsNervous disorderDuloxetineSodium iodide
Owner:HEBEI UNIV OF TECH
Duloxetine formulations
Duloxetine pellets having an enteric coating containing a polymethacrylate polymer can be formed with desirable release rates / profile and stability.
Owner:SYNTHON BV
Efficient method for preparing 3-aryloxy-3-arylpropylamines and their optical stereoisomers
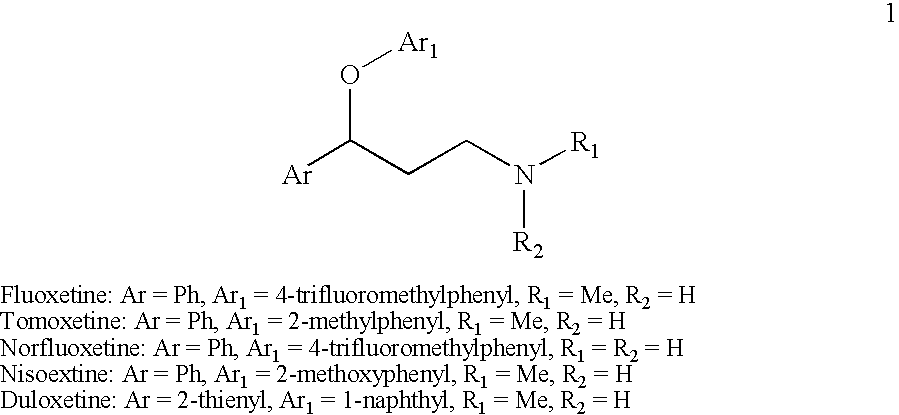
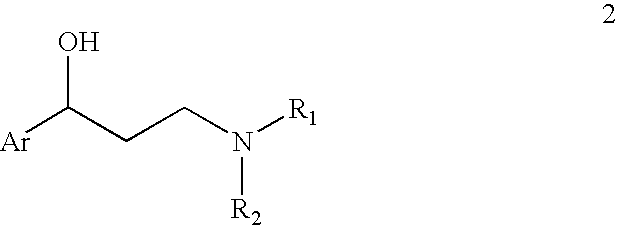
Provided is an efficient method for the preparation of 3-aryloxy-3-arylpropylamines, their optical stereoisomers, and pharmaceutically acceptable salts thereof. The process allows for the isolation of 3-aryloxy-3-arylpropylamines in high yield and purity. The present invention further relates to a process for producing fluoxetine, tomoxetine, norfluoxetine, duloxetine, nisoxetine, and their optically enriched (R)- and (S)-enantiomers.
Owner:APOTEX PHARMACHEN INC
Method for asymmetric synthesis of duloxetine intermediate by carbonyl reductase
The invention belongs to the technical field of biocatalysis and discloses a method for asymmetric synthesis of duloxetine intermediate by carbonyl reductase.In a pure enzyme catalytic system, by control of reaction pH, reaction temperature and metal ions, biocatalysis performance is improved.N,N-dimethyl-3-keto-3-(2-thienyl)-1-propylamine (DKTP) serves as a reaction substrate, a recombinant strain refers to E.coli BL21 / Pet21c-cr2 expressing an aldo-keto reductase gene, a carbonyl reductase gene cr2 is derived from Candida macedoniensis AKU4588 and codes carbonyl reductase CR2, and catalysis of asymmetric reduction of DKTP is realized to obtain (S)-DHTP.By pure enzyme catalytic reaction and optimal control of the reaction pH, the reaction temperature and the metal ions, properties and functions of DKTP catalyzed by CR2 can be known, highly-stereoselective DKTP catalysis is realized, and optically-pure duloxetine key intermediate (S)-DHTP is prepared through asymmetric conversion reaction.
Owner:JIANGNAN UNIV
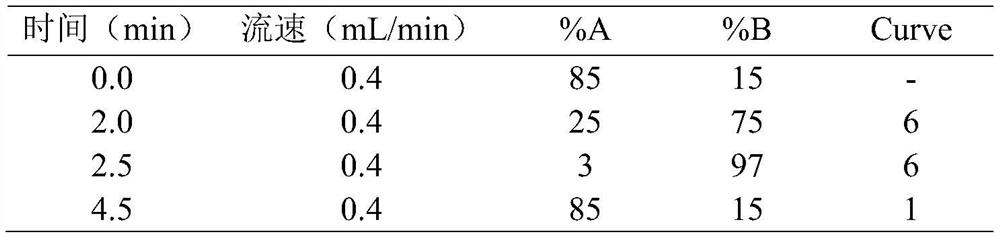
Method for detecting concentration of antidepressant drugs in serum by using ultra-high performance liquid chromatography-tandem mass spectrometry technology
The invention discloses a method for detecting the concentration of antidepressant drugs in serum by an ultra-high performance liquid chromatography-tandem mass spectrometry technology. The antidepressant drugs comprise bupropion, agomelatine, hydroxybupropion, nortriptyline, o-desmethylvenlafaxine, mianserin, mirtazapine, venlafaxine, amitriptyline, doxepin, norfluoxetine hydrochloride, duloxetine, fluoxetine, fluvoxamine, citalopram, paroxetine, trazodone and vortioxetine. After a serum sample is pretreated, a to-be-detected substance is separated from a serum matrix by utilizing ultra-highperformance liquid chromatography, a calibration curve is established by utilizing a mass spectrum isotope internal standard quantitative method and taking a concentration ratio of the standard substance to an internal standard substance as an X axis and a peak area ratio of the standard substance to the internal standard substance as a Y axis, and the content of the drugs in serum is calculated.The method is high in sensitivity, high in specificity, accurate and simple in pretreatment process, separation and detection can be completed within 4.5 min, and the accuracy degree and precision basically meet the requirements.
Owner:南京品生医学检验实验室有限公司
Enteric coated table of duloxetine, and preparation method
ActiveCN100362997CImprove stabilityReduce dosageOrganic active ingredientsNervous disorderDuloxetineMedicine
A coated enteric tablet of Duluoxiding and its preparing process are disclosed. It features that said Duluoxiding can be released only in small intestine, so resulting in high biologic utilization rate.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Duloxetine enteric coated tiny pill capsule, and preparation method
ActiveCN100362996CImprove solubilityReduced bioavailabilityNervous disorderGranular deliveryDuloxetineSmall intestine
An enteric micropill (microsoftgel) of Duluoxiting and its preparing process are disclosed. Said micropill is composed of an empty core pill and a coated external layer consisting of a principal medicine layer, an isolating layer and an enteric layer. It features that its principal medicine can be quickly released only in small intestine.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Duloxetine preparation method
The invention relates to the technical field of chemical synthesis, particularly to a duloxetine preparation method. The duloxetine preparation method comprises the following steps: choosing thiophene and 2-chloroacetyl chloride as raw materials, sealing and storing at 20-25 DEGC for standby application, putting 2-chloroacetyl chloride into a reaction vessel, heating to 60-65 DEGC, adding thiophene, slightly shaking evenly, continuing to heat, stirring for 10 minutes, obtaining 2-chloracetyl-thiophene through a friedel-crafts reaction, heating the produced 2-chloracetyl-thiophene to 80-100 DEGC, adding sodium cyanide into the reaction vessel, continuing to stir, heating for 10 minutes, conducting a cyano substitution reaction, and obtaining 3-hydroxy-3-(2-thiophene)-propionitrile. The cheap thiophene and 2-chloroacetyl chloride are chosen as the raw materials, the preparation is easy, the cost is low, the preparation is conducted through the conventional reactions, and the method has the advantages of low cost, easy preparation and high reaction speed.
Owner:武汉励合生物医药科技有限公司
Controlled Release Dosage Formulation of Duloxetine
InactiveUS20100285123A1Smoothened drug plasma concentration to time profileTight controlBiocideNervous disorderSolubilityDuloxetine
The preset invention provides a controlled release dosage form of duloxetine comprising a homogenous core comprised of duloxetine or its pharmaceutically acceptable salts, pharmaceutically acceptable polymeric carrier, solubility enhancer, a hydrophobic component, a hydrodynamic diffusion enhancer, a viscolyzing agent and pharmaceutically acceptable excipients; a entering coat on said core and a barrier layer between said core and the enteric coat.
Owner:CADILA HEALTHCARE LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com