Patents
Literature
50 results about "Tomoxetine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Orally disintegrating tablets of atomoxetine
InactiveUS20060057199A1Low incidenceHighly irritateCosmetic preparationsToilet preparationsOral medicationPopulation
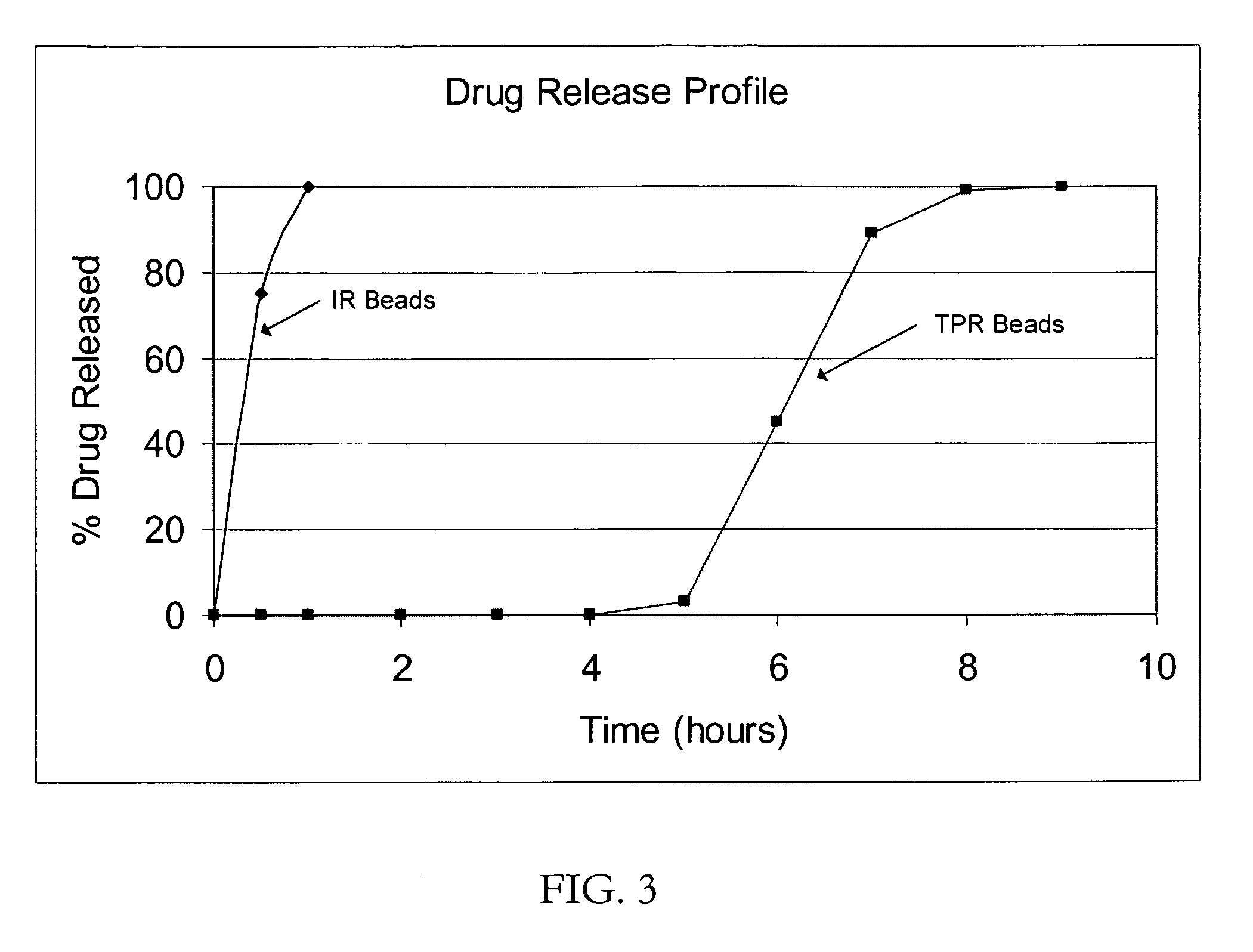
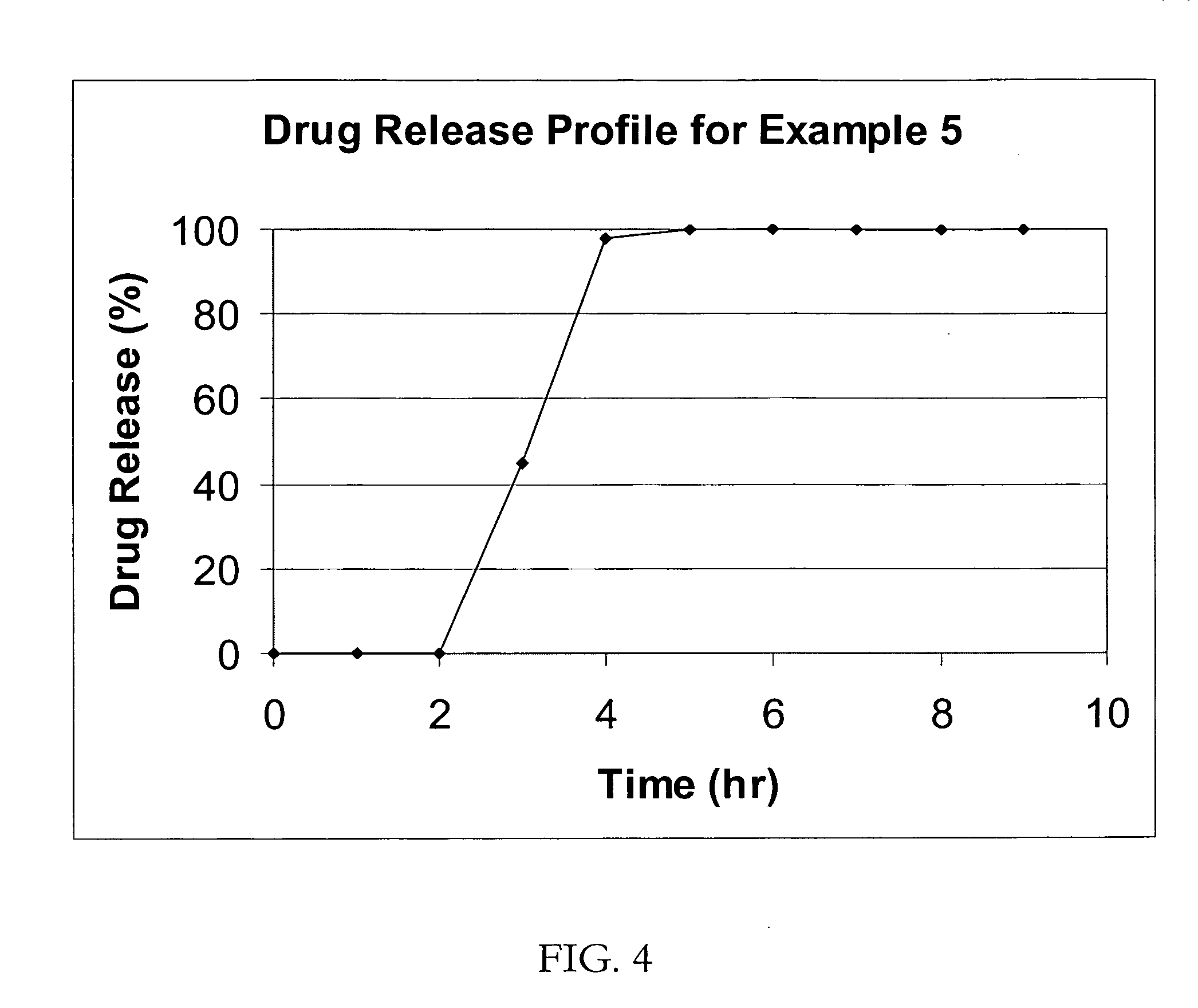
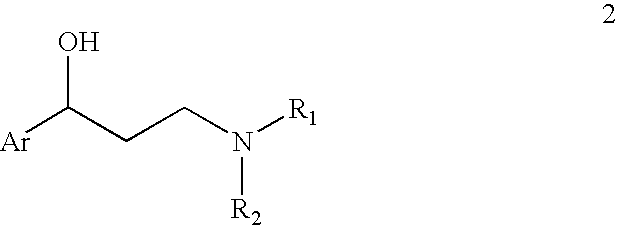
A coated multi-particulate pharmaceutical dosage form such as an orally disintegrating tablet (ODT) presentation for delivering atomoxetine or a pharmaceutically acceptable salt thereof, a selective norepinephrine reuptake inhibitor indicated for the treatment of ADHD, into the body to maintain a therapeutically effective amount of atomoxetine in the plasm. The dosage form may comprise one or more populations of coated atomoxetine-containing particles (beads, pellets, granules etc.) providing a pre-designed rapid release profile after a predesigned lag-time of about 0 to 6 hours following oral administration.
Owner:ADARE PHARM INC
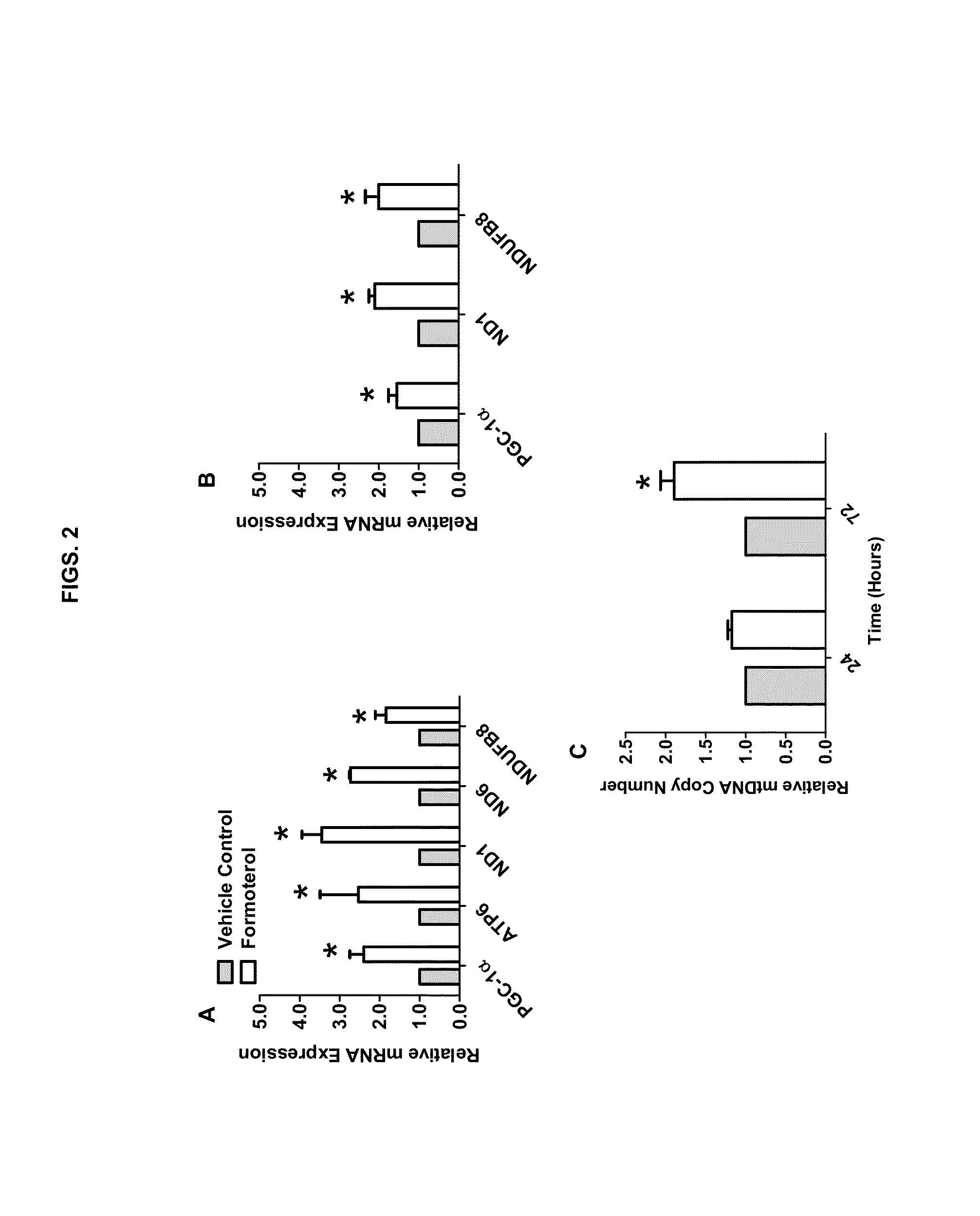
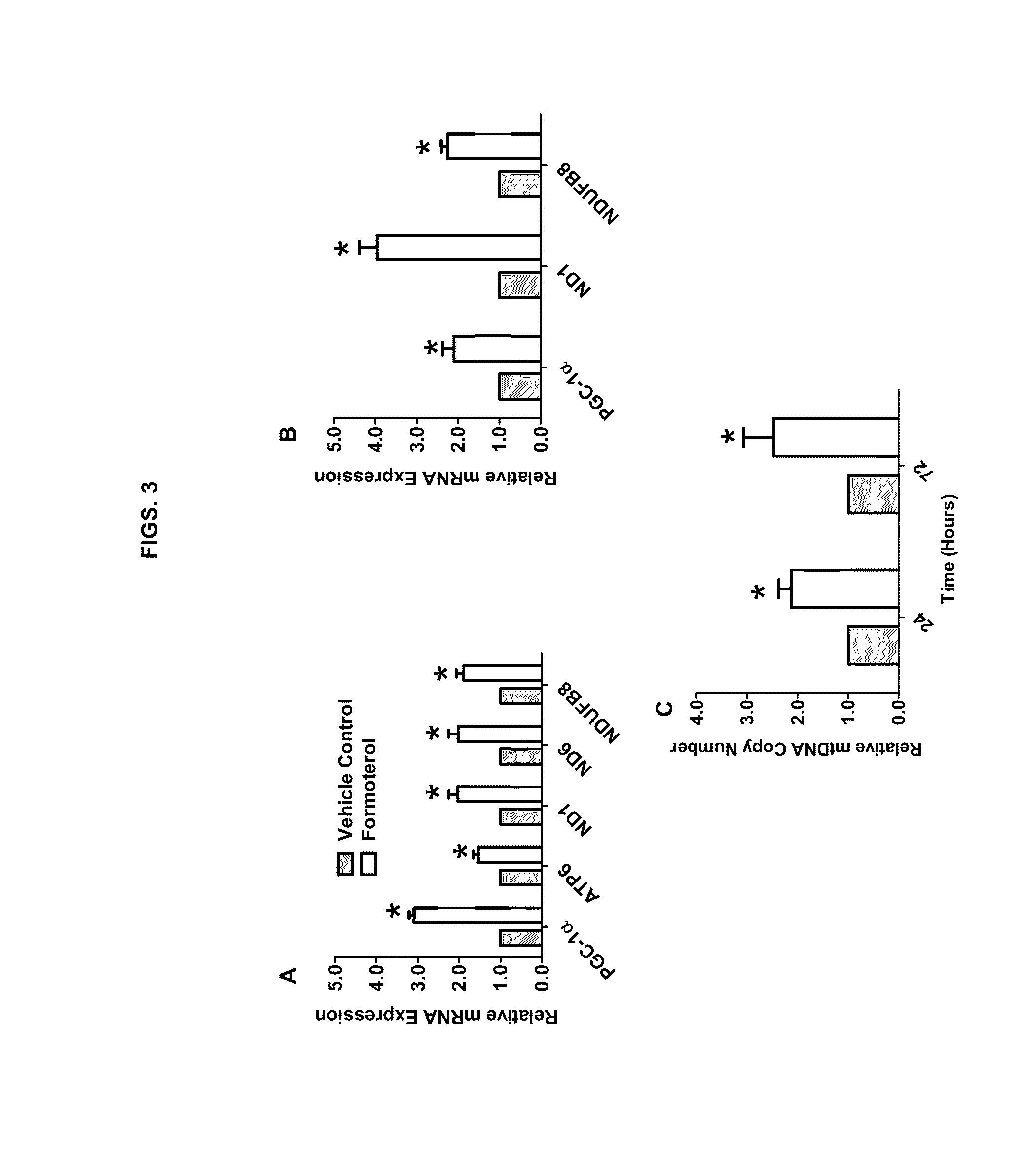
Methods for inducing mitochondrial biogenesis
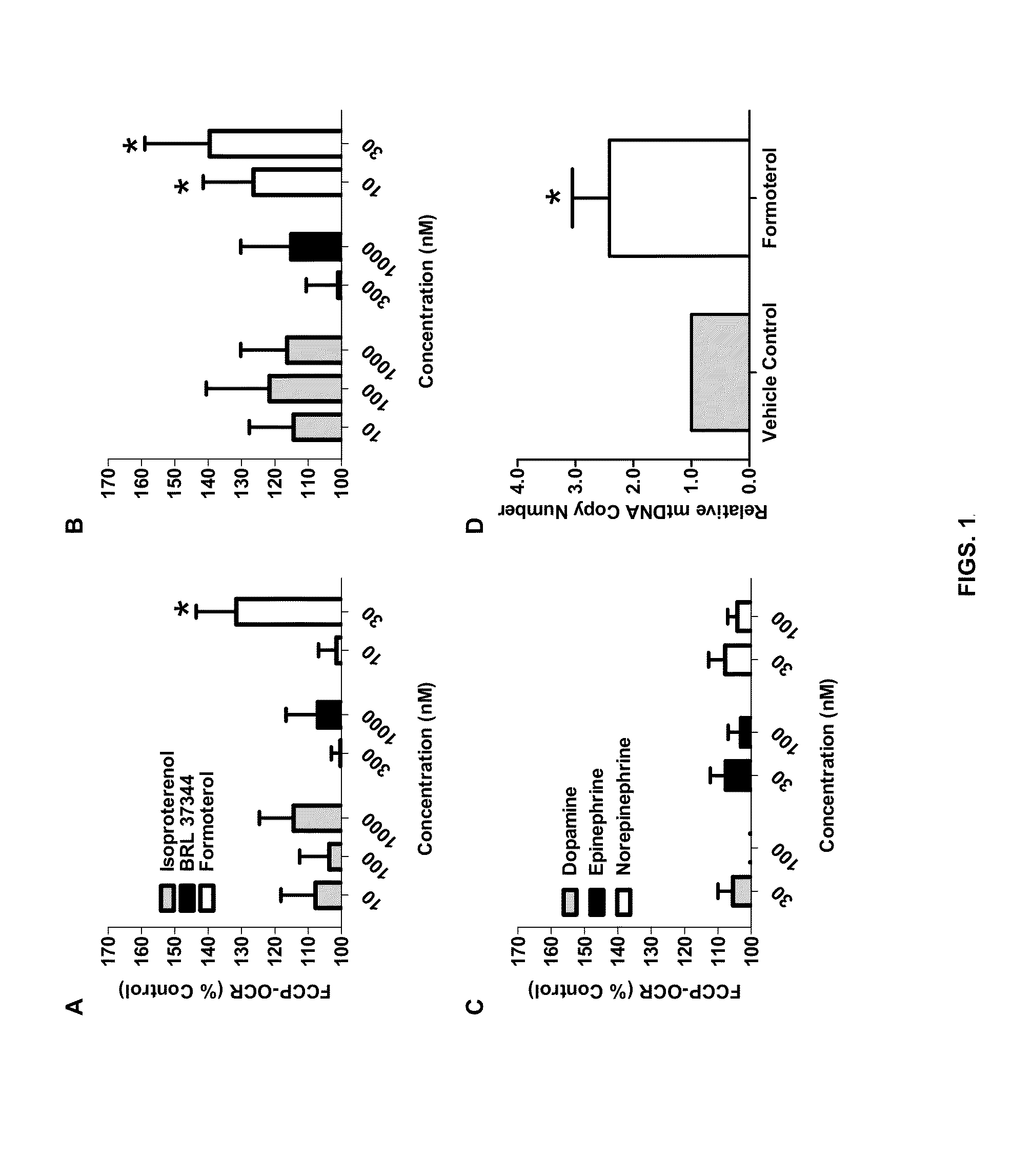
Methods and compositions for inducing mitochondrial biogenesis are provided. In some aspects, methods for the treatment of diseases such as acute kidney disease (AKI) or a muscle wasting disease by administering tomoxetine, nisoxetine, fenoterol, formoterol, or procaterol to an individual are provided.
Owner:MUSC FOUND FOR RES DEV
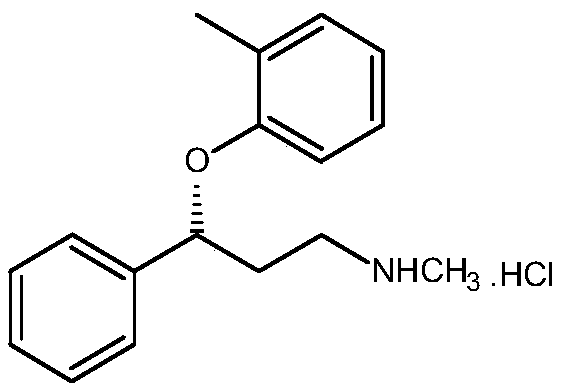
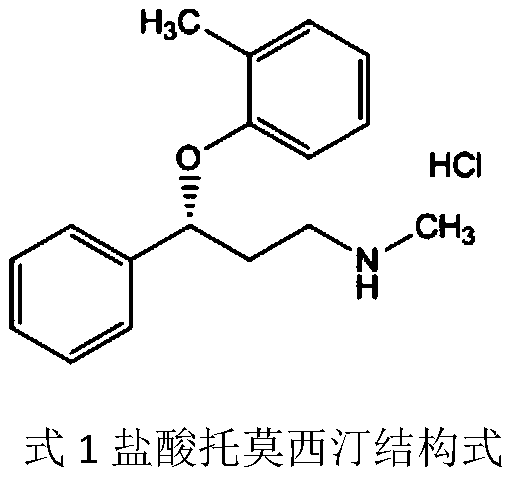
R-(-)-atomoxetine hydrochloride preparation method
ActiveCN110194719AEliminate the refining processFew reaction stepsOrganic compound preparationOrganic chemistry methods1-PropanolSolvent
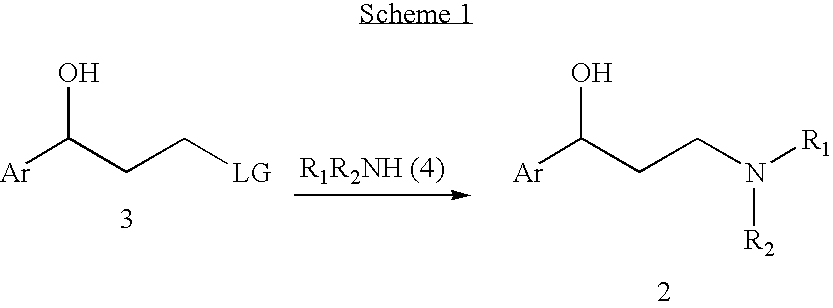
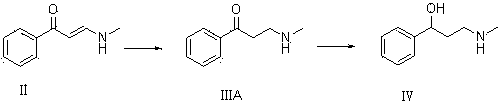
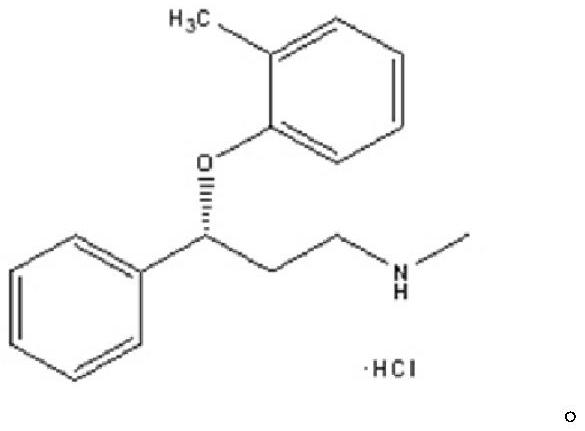
The invention provides an R-(-)-atomoxetine hydrochloride preparation method, which comprises: preparing 3-methylamino-1-phenyl-1-propanol by using 1-phenyl-2-propenyl-1-one as a starting raw material, carrying out etherification on the 3-methylamino-1-phenyl-1-propanol and o-halo toluene in an inorganic alkali environment, splitting with L-(+)-mandelic acid to obtain R-(-)-tomoxetine-S-(+)-mandelate, refining the R-(-)-tomoxetine-S-(+)-mandelate, and carrying out hydrochloride forming to obtain the R-(-)-atomoxetine hydrochloride. According to the present invention, the method eliminates theoxalate refining step so as to reduce the reaction step, has advantages of cheap and easily available raw materials, less side reactions, low toxicity of the reaction solvent, high yield, high purity,low cost and the like, and is suitable for industrial production.
Owner:SHANDONG UNIV
Process for the preparation of atomoxetine hydrochloride
InactiveUS20060009489A1Easy to optimizeHigh reaction yieldBiocideNervous disorderAtomoxetine hydrochlorideOrganic solvent
The present invention provides improved processes for the preparation of atomoxetine hydrochloride under reaction conditions that improve reaction yields and facilitate commercial synthesis. In particular, the invention is directed to the synthesis of atomoxetine HCl by adding HCl to a mixture of (R)-(−)-tomoxetine (S)-(+)-mandelate with an organic solvent, with or without a base and water.
Owner:TEVA PHARM USA INC
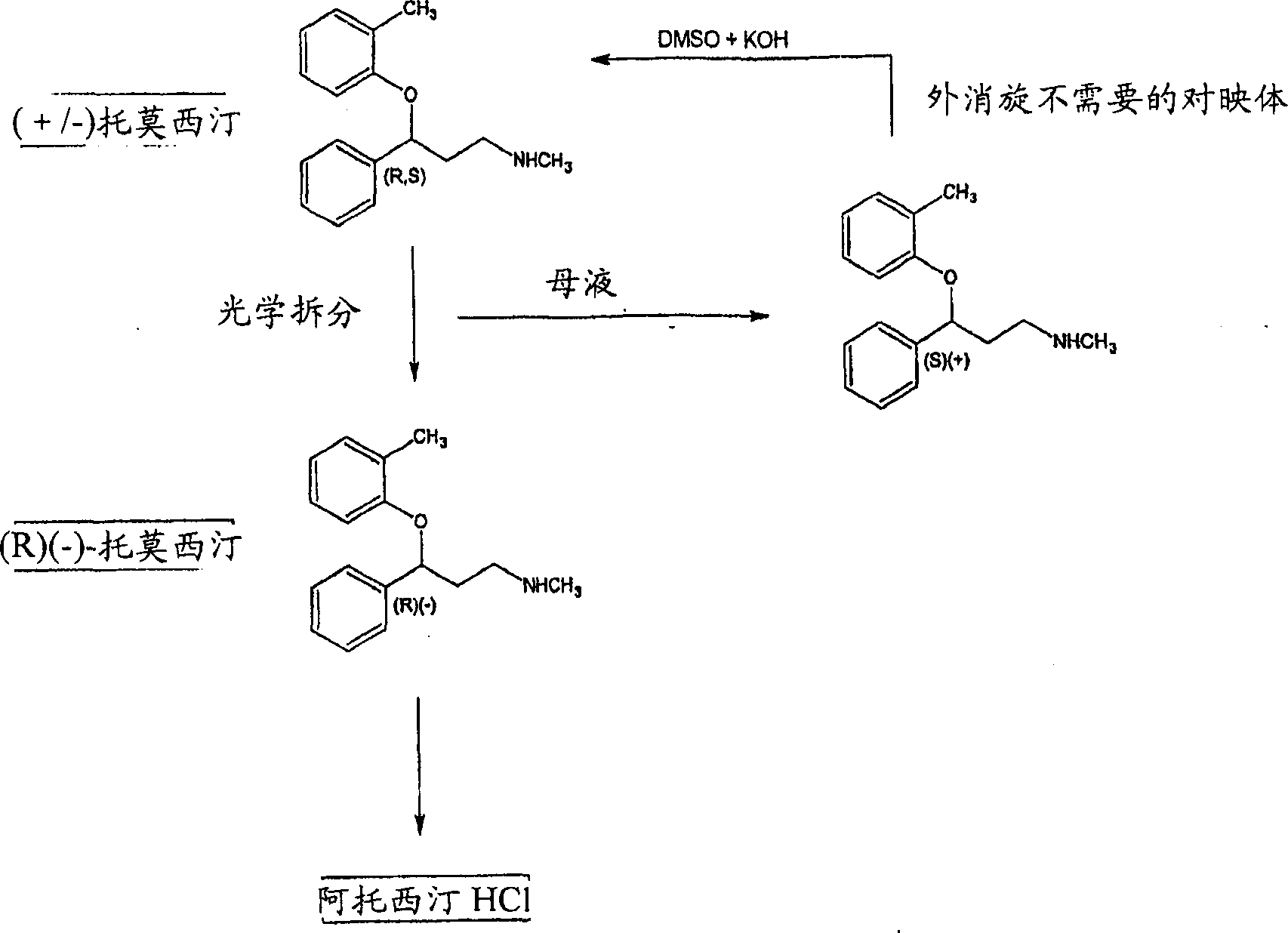
Process for the optical resolution and recycling of tomoxetine
InactiveCN1950326AFacilitate large-scale commercial productionOrganic compound preparationAmino-hyroxy compound preparationEnantiomerOptical resolution
The present invention provides a process for the optical resolution of racemic tomoxetine under reaction conditions that improve reaction yields and optical purity. The invention also provides an epimerization process for the (S)-(+) enantiomer. The invention further provides the conversion of the enantiomer obtained from the optical resolution into atomoxetine or a pharmaceutically acceptable salt thereof.
Owner:TEVA PHARMA FINE CHEMI
Processes for the preparation of tomoxetine
InactiveCN1942429AOrganic compound preparationAmino-hyroxy compound preparation3-phenylpropylamineAtomoxetine
Provided are processes for preparing tomoxetine comprising reacting N-methyl-3-hydroxy-3-phenylpropylamine with dimethylsulfoxide (DMSO) and 2-fluorotoluene in the presence of an alkali base to form tomoxetine. Also provided is the conversion of said tomoxetine into atomoxetine or a pharmaceutically acceptable salt thereof.
Owner:TEVA PHARMA FINE CHEMI
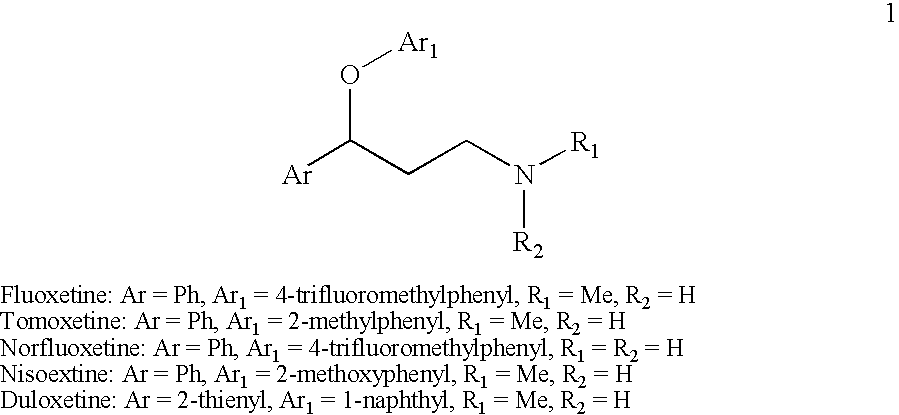
Efficient method for preparing 3-aryloxy-3-arylpropylamines and their optical stereoisomers
Provided is an efficient method for the preparation of 3-aryloxy-3-arylpropylamines, their optical stereoisomers, and pharmaceutically acceptable salts thereof. The process allows for the isolation of 3-aryloxy-3-arylpropylamines in high yield and purity. The present invention further relates to a process for producing fluoxetine, tomoxetine, norfluoxetine, duloxetine, nisoxetine, and their optically enriched (R)- and (S)-enantiomers.
Owner:APOTEX PHARMACHEN INC
Synthetic method of tomoxetine
ActiveCN103664658AStable in natureSimple stepsOrganic compound preparationAmino-hyroxy compound preparationCombinatorial chemistryKetone
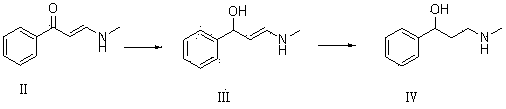
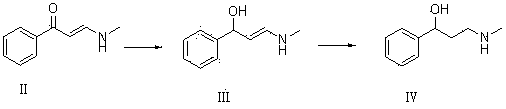
The invention relates to a synthetic method of tomoxetine. The tomoxetine is generated by taking (E)-3-(N-methyl amino)-1-phenyl-2-propylene-1-ketone as the material through reduction and 2-benzyl halide etherification reaction. The synthetic method disclosed by the invention is simple in step, easy to operate, less in byproducts, better in yield, lower in cost and suitable for large-scale industrial production.
Owner:BEIJING WINSUNNY PHARMA CO LTD
Pharmaceutical composition containing tomoxetine and preparation method thereof
InactiveCN102114009APromote dissolutionGood content uniformityOrganic active ingredientsNervous disorderPharmacologyTomoxetine
The invention relates to a pharmaceutical composition containing tomoxetine and a preparation method thereof. The pharmaceutical composition contains tomoxetine and carrier auxiliary materials which are pharmaceutically acceptable, and the pharmaceutical composition can exist in solid or liquid form, wherein the essential ingredient is added by being dispersed in a solution.
Owner:BEIJING D VENTUREPHARM TECH DEV
Atomoxetine hydrochloride oral solution and preparation method thereof
InactiveCN112451476AMask bad tasteImprove stabilityOrganic active ingredientsNervous disorderO-Phosphoric AcidCyclodextrin
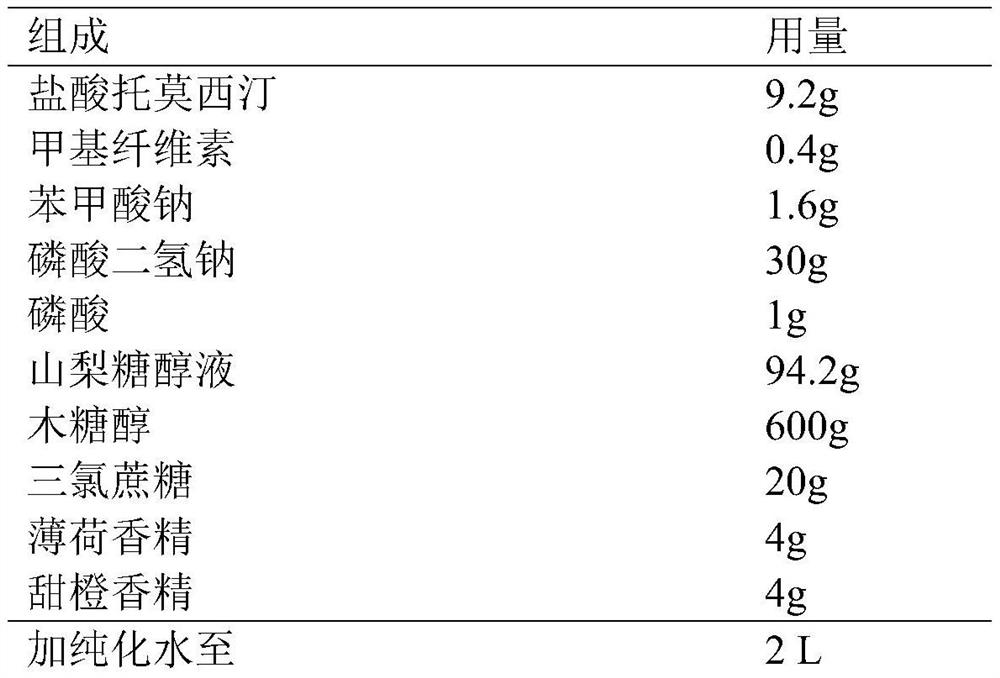
The invention discloses an atomoxetine hydrochloride oral solution for treating the attention deficit hyperactivity disorder, and a preparation method of the atomoxetine hydrochloride oral solution. The preparation comprises atomoxetine hydrochloride, hydroxypropyl-[beta]-cyclodextrin, pH regulating agent, preservative, essence, 50% ethyl alcohol and water. The preparation method comprises the following steps of: putting the water into a jar 1, carrying out stirring, adding the cyclodextrin, and carrying out dissolving to obtain a solution 1; putting 50% ethyl alcohol into a jar 2, carrying out stirring, adding the atomoxetine hydrochloride, and carrying out dissolving to obtain a solution 2; evenly mixing the solution 1 and the solution 2, transferring an obtained mixture into a grindingjar, carrying out grinding, adding the cyclodextrin, and after one hour, carrying out drying in an oven to obtain an intermediate 1; and putting the water in a preparation jar, adding the converted intermediate 1, sodium dihydrogen phosphate, phosphoric acid and sodium benzoate, carrying out stirring and dissolving, adding the essence, after the obtained mixture is even, measuring the pH value, controlling the pH value to 3.7-4.3, and obtaining 4.6mg / ml of atomoxetine hydrochloride oral solution. The oral aqueous solution improves the stability of the atomoxetine hydrochloride to a high degree, and a higher social value is brought.
Owner:BEIJING DO-PHARMA TECH CO LTD
Pharmaceutical composition for treating children's hyperactivity
ActiveCN102416012AAddress neurodevelopmental issuesTo achieve the goal of "treating both symptoms and root causes"Organic active ingredientsNervous disorderVitamin b6Hydrochloride
The invention relates to the field of medicines and specifically to a pharmaceutical composition for treating children's hyperactivity. The pharmaceutical composition for treating children's hyperactivity in the invention comprises tomoxetine hydrochloride and vitamin B6, and the mass ratio of tomoxetine hydrochloride to vitamin B6 is 1: 0.5 to 1:4; tomoxetine hydrochloride and vitamin B6 can be compounded with normal accessories to prepare a medicine for treating children's hyperactivity. According to the invention, composition of tomoxetine hydrochloride and vitamin B6 which weighs 0.5 to 4times of tomoxetine hydrochloride has a synergistic effect, can substantially enhance the upturning effect of NA, promote the development of brain nerves and stabilizing the functions of brain cells,and produces an effect of simultaneous treatment of principal and subordinate symptoms on children's hyperactivity.
Owner:CHONGQING TECH & BUSINESS UNIV
Tomoxetine hydrochloride oral liquid and preparation method thereof
PendingCN112076154AGreat tasteDoes not affect the efficacyOrganic active ingredientsNervous disorderDrug efficiencyDrugs stability
The invention belongs to the technical field of medicines, particularly relates to the field of pharmacy, and more particularly relates to a tomoxetine hydrochloride oral liquid and a preparation method thereof. The tomoxetine hydrochloride oral liquid is mainly prepared from tomoxetine hydrochloride, a preservative, a pH regulator, a sweetening agent and a taste improver, wherein the taste improver is prepared from a thickening agent, mint essence and sweet orange essence. The taste improver prepared from the thickening agent, the mint essence and the sweet orange essence can significantly improve the taste of the tomoxetine hydrochloride oral liquid in the taking process, and significantly reduces the bitter taste during taking and the bitter taste of the oral cavity after taking. Meanwhile, a test verifies that the drug effect and the drug stability of an active drug are not affected by adding of the taste improver, and the quality of the tomoxetine hydrochloride oral liquid can beconsistent with that of an original drug.
Owner:JIANGSU ALPHA PHARM CO LTD
Method for treating neurasthenia or somatic form disorders and medicinal composition
ActiveCN1850271AOrganic active ingredientsSolution deliveryNorepinephrine reuptake inhibitorSertraline
The present invention relates to a method for curing and preventing neurosism or somatic formal disturbance and its medicine composition. Said invention belongs to the field of pharmacy. Said invention provides a medicine composition containing norepinephrine reuptake inhibitor with medicinal dose or its medicinal salt and selective 5-hydroxytryptamine reuptake inhibitor with medicinal dose or its medicinal salt for preventing and curing neurosism or somatic formal disturbance. The norepinephrine reuptake inhibitor is selected from reboxetine, tomoxetine, amfebutamone, nortriptyline, norimipramine, maprotiline and protiline, and the selective 5-hydroxytryptamine reuptake inhibitor is selected from sertraline, citalopram, s-citalopram, fluoroxeline, paroxetine and fluvoxamine.
Owner:SHENZHEN AUSA PHARM CO LTD +2
Process for the optical resolution and recycling of tomoxetine
InactiveUS20060009530A1High purityHigh yieldBiocideOrganic active ingredientsEnantiomerOptical resolution
The present invention provides a process for the optical resolution of racemic tomoxetine under reaction conditions that improve reaction yields and optical purity. The invention also provides an epimerization process for the (S)-(+) enantiomer. The invention further provides the conversion of the enantiomer obtained from the optical resolution into atomoxetine or a pharmaceutically acceptable salt thereof.
Owner:TEVA PHARM USA INC
Atomoxetine hydrochloride oral solution and preparation method thereof
InactiveCN108785248AGreat tasteAvoid side effectsOrganic active ingredientsNervous disorderAtomoxetine hydrochlorideFlavor
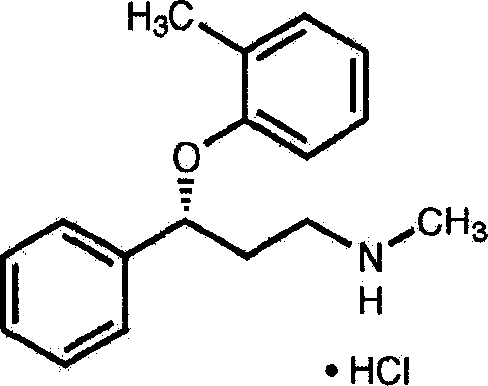
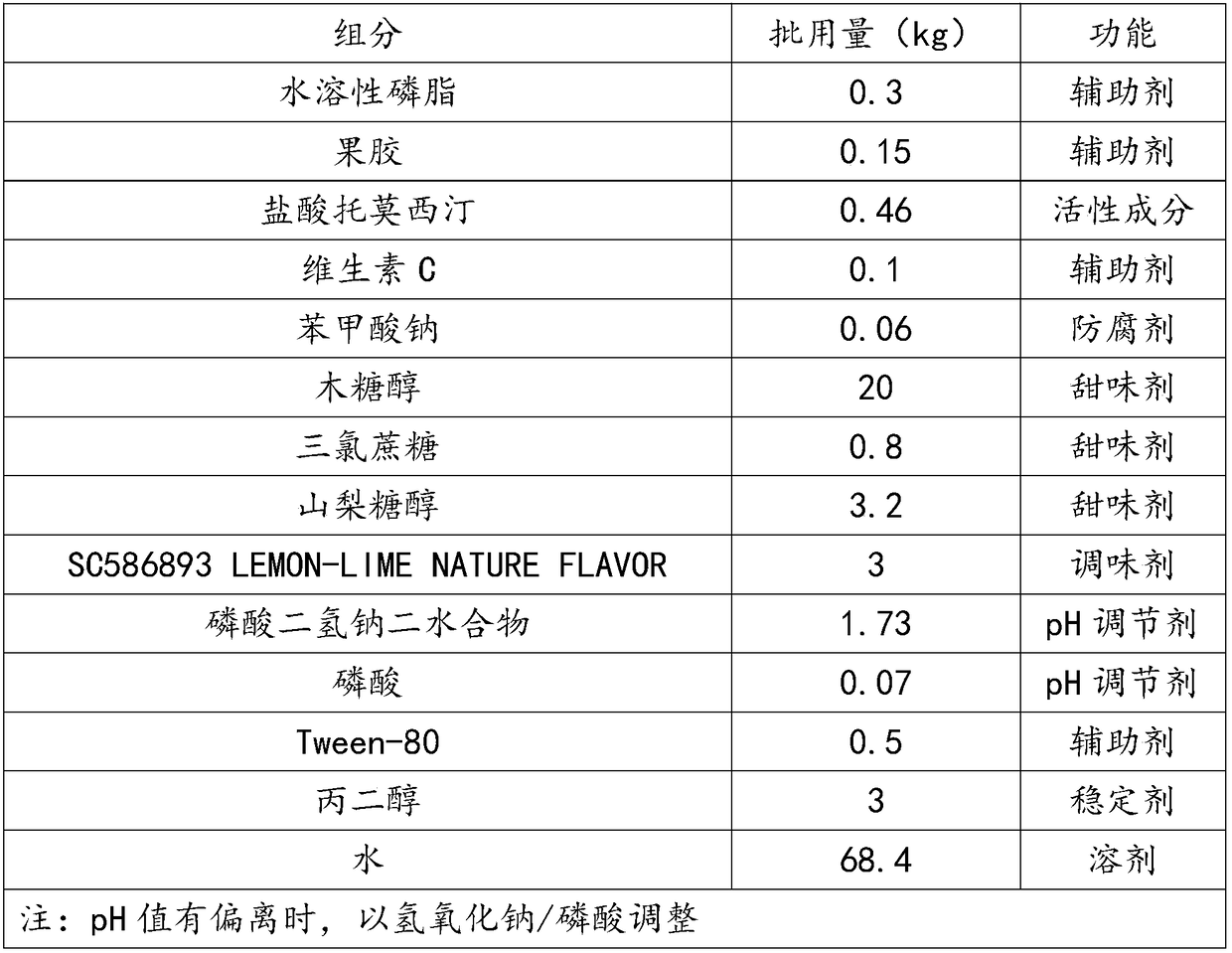
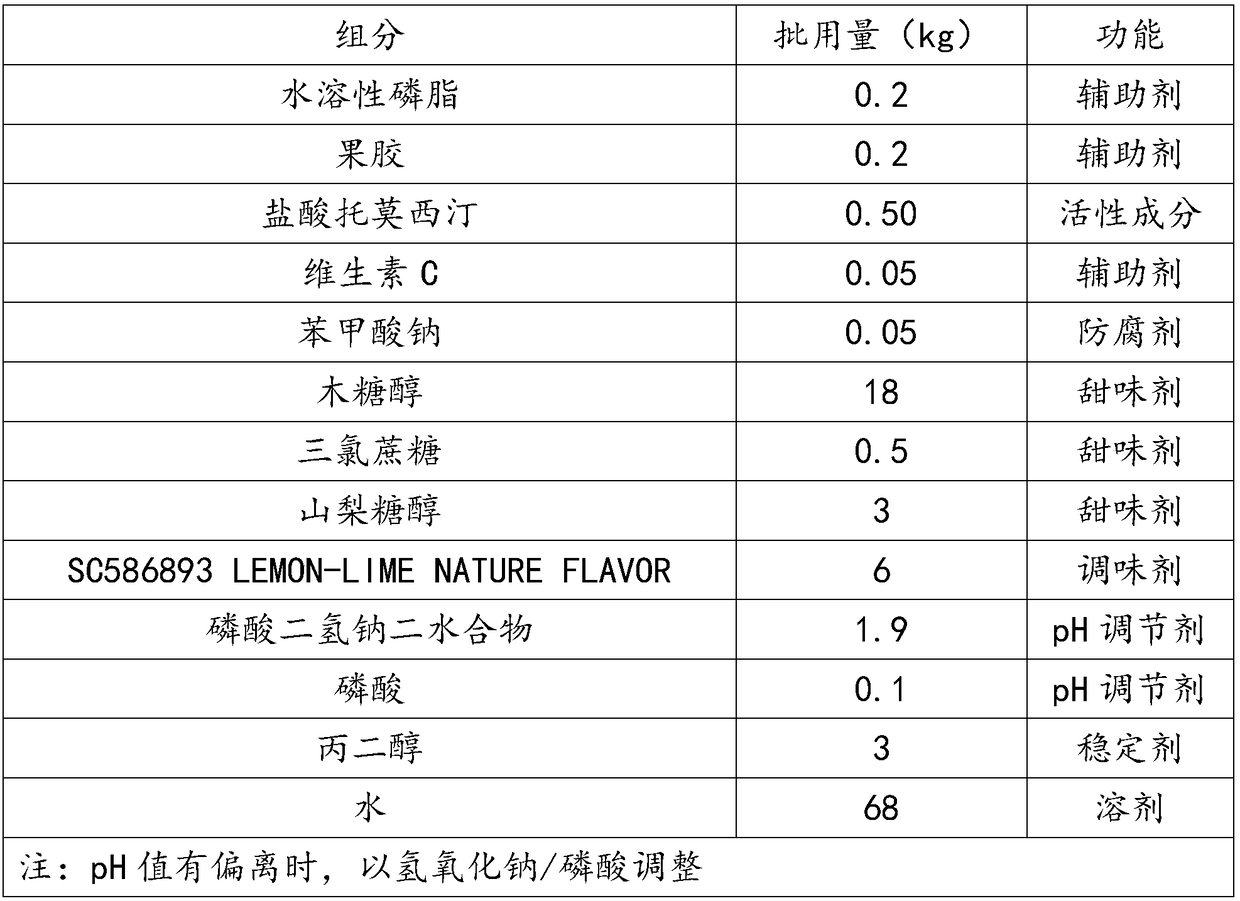
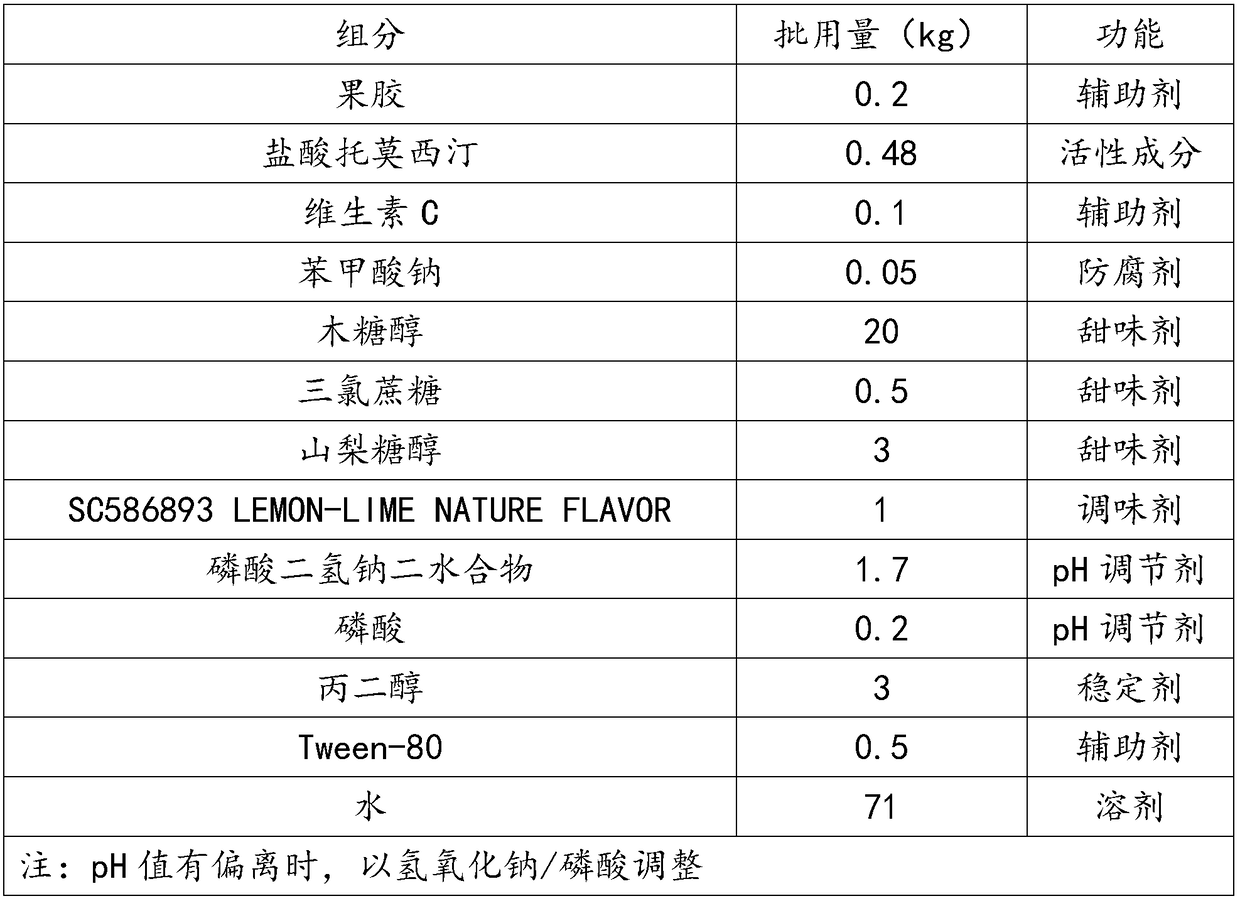
The invention discloses an atomoxetine hydrochloride oral solution which contains 2.0-4.0mg / mL of water-soluble phospholipid, 1.5-2.0mg / mL of pectin, 4.0-5.0mg / mL of atomoxetine hydrochloride, 0.5-1.0mg / mL of vitamin C, 0.5-0.6mg / L of preservative, 200-250mg / mL of sweetening agent, 0.1-60mg / mL of SC586893 LEMON-LIME NATURE FLAWOR, 16-20mg / mL of pH regulator, 4-6mg / mL of Tween, 30mg / mL of FLAVOR, and the balance of water. In the preparation process, high pressure homogenization, microfiltration and high temperature instantaneous sterilization technologies are adopted. The atomoxetine hydrochloride oral solution is good in taking compliance, has a function of assisting in lead detoxification of the body, and can effectively alleviate the pain of the patients and increase the treatment efficiency.
Owner:YANTAI JUXIAN PHARMA
Processes for the preparation of atomoxetine hydrochloride
InactiveUS20060211772A1High reaction yieldEasy to synthesizeBiocideOrganic active ingredientsAtomoxetine hydrochlorideOrganic solvent
The present invention provides improved processes for the preparation of atomoxetine hydrochloride under reaction conditions that improve reaction yields and facilitate commercial synthesis. In particular, the invention is directed to the synthesis of atomoxetine HCl by adding HCl to a mixture of (R)-(−)-tomoxetine (S)-(+)-mandelate with an organic solvent, with or without a base and water. In preferred embodiments, the atomoxetine hydrochloride produced is Form A.
Owner:TEVA PHARM USA INC
Chemical-enzyme synthesis method of atomoxetine
PendingCN112708641AEasy to operateLow costOrganic compound preparationOxidoreductasesChlorobenzeneCarbonyl Reductase
The invention provides a chemical-enzyme synthesis method of atomoxetine. A preparation method comprises the following steps: by using 3-chloropropiophenone as a raw material, performing asymmetric reduction reaction under the catalysis of a carbonyl reductase and a coenzyme to obtain (S)-3-chlorophenylpropanol with chiral purity ee value of 99.9%, performing light delay reaction and methylation reaction, and performing salifying with hydrochloric acid to obtain the atomoxetine. The preparation method can significantly reduce the production cost, is green and environment-friendly, and has potential industrial application value.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Preparation method of optically pure (R)-N-methyl-3-phenyl-3-(o-tolyloxy)-propylamine hydrochloride
ActiveCN111302958ALow costOrganic compound preparationOrganic chemistry methodsPropylaminePhenyl group
The invention relates to a preparation method of (R)-N-methyl-3-phenyl-3-(o-tolyloxy)-propylamine hydrochloride, belonging to the technical field of chemistry. According to the method, high-optical-purity atomoxetine hydrochloride can be prepared by salifying and crystallizing atomoxetine racemate and diluted hydrochloric acid and then performing recrystallizing with water. According to the invention, steps are simple, a chemical resolving agent and an organic solvent are not used, and the obtained finished product is high in purity and suitable for popularization.
Owner:HEFEI IND PHARMA INST CO LTD +2
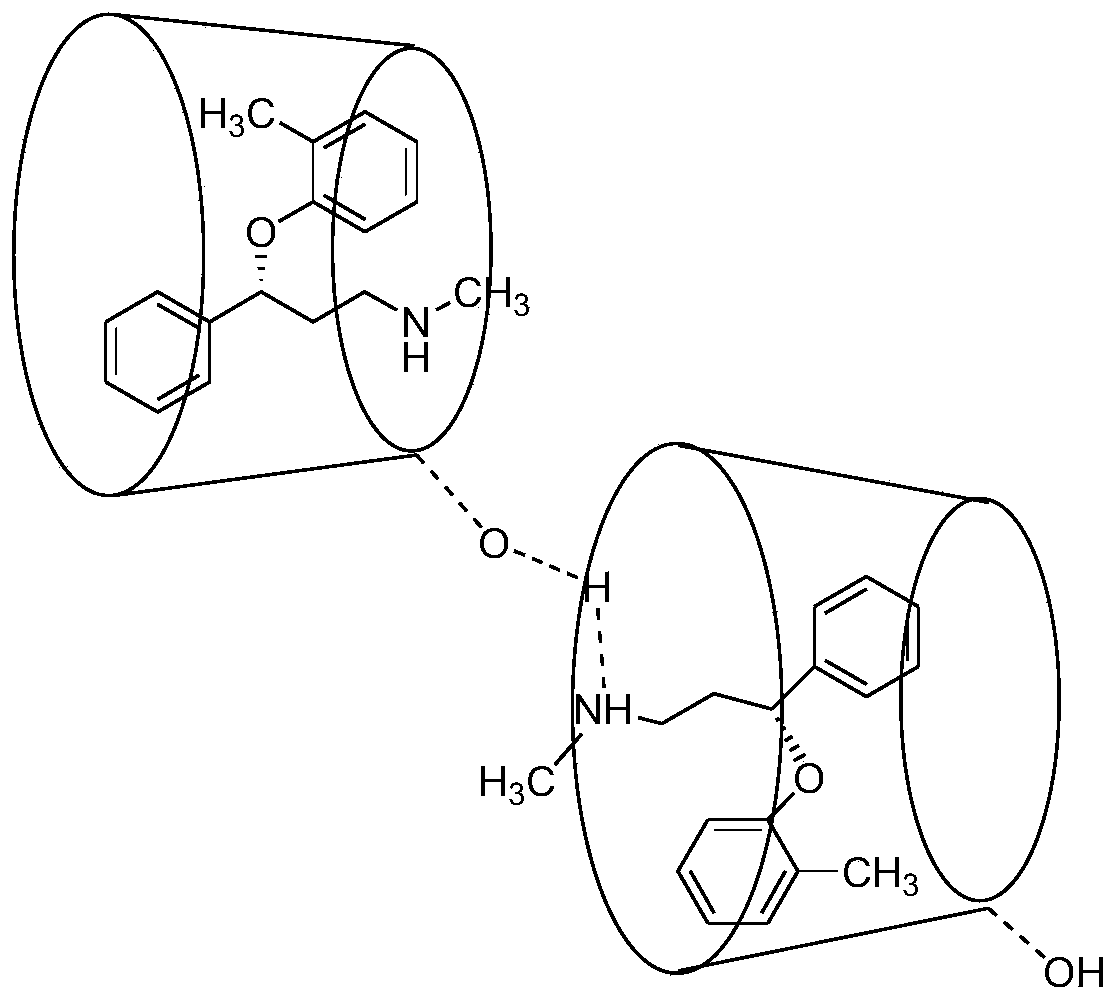
Enantiomerically pure atomoxetine and tomoxetine mandelate
The present invention provides enantiomerically pure (R)-(−)-tomoxetine (S)-(+)-mandelate and atomoxetine HCl. The present invention further provides enantiomerically pure (R)-(−)-tomoxetine (S)-(+)-mandelate prepared from racemic tomoxetine. The present invention also provides enantiomerically pure atomoxetine HCl prepared from (R)-(−)-tomoxetine (S)-(+)-mandelate.
Owner:TEVA PHARM USA INC
Traditional Chinese medicine for treating attention deficit hyperactivity disorder and preparation method thereof
InactiveCN105998790AReduce adverse reactionsEasy to useNervous disorderDispersion deliverySide effectTherapeutic effect
The invention provides a traditional Chinese medicine for treating an attention deficit hyperactivity disorder and a preparation method thereof. The traditional Chinese medicine is prepared from Nepal rattlesnake plantain roots, Chinese tupistra rhizomes, seeds of ragimillet, dwarf cowlily rhizomes, false-yellowflower milkwort roots or herb, peel of roseapple, giantreed rhizomes, stem leaves or roots of ipomoea cairica (L.) sweet, eastern bracken fern rhizomes, all-grass of filifolium sibiricum (L.) Kitam., acute sida leaves or roots, herb of Japanese phtheirospermum, smallflower seaberry herb, tuber fern, all-grass of aeschynanthus acuminatus Wall., longtube ground ivy herb, common melastoma herb, root of seven-sisters Japanese rose, false Chinese swertia herb, ellpticleaf spurgentian roots, Tonkin tinomiscium stems and henry biondia herb with roots. The traditional Chinese medicine for treating the attention deficit hyperactivity disorder and the preparation method thereof have the advantages that the traditional Chinese medicine can be combined with conventional western medicines (e.g. tomoxetine) for treating the attention deficit hyperactivity disorder, use is safe and convenient, the treatment effect is better, no toxic or side effect exists, the adverse reactions of the western medicines are reduced, and the preparation process is simple.
Owner:王甜甜
A chiral tridentate pnn ligand and its application in asymmetric hydrogenation reactions
ActiveCN105153229BHigh electronegativityGood chiral environmentOrganic compound preparationGroup 5/15 element organic compoundsDuloxetinePhenethyl alcohol
Owner:SHENZHEN CATALYS SCI & TECH CO LTD +2
Propylamine derivative and its application in preparing tomocetin
InactiveCN1948277AThe preparation route is reasonable and feasibleSatisfactory yieldOrganic compound preparationAmino-hyroxy compound preparationState of artChemical structure
The present invention relates to a propylamine derivative and its application in preparation of tomoxetine (atomoxetine raceme). Said invention also provides its chemical structure formula, and the invented preparation method is simple, and is suitable for industrial production.
Owner:EAST CHINA UNIV OF SCI & TECH
Oral solution comprising atomoxetine hydrochloride and methods thereof
ActiveUS9855228B1Effective to tasteEasy to swallowOrganic active ingredientsNervous disorderAttention deficitsPharmaceutical medicine
Disclosed herein is an oral pharmaceutical composition in the form of an aqueous solution of atomoxetine as an active ingredient. The aqueous solution of atomoxetine comprises a taste-masked liquid carrier comprising peppermint, orange flavor and a viscosity agent. The combined flavors successfully masked atomoxetine hydrochloride's bitter smell and / or taste which makes it a novel palatable pharmaceutical composition. The viscosity agent improves the oral pharmaceutical composition's consistency and provides a smooth texture which makes it easy to swallow. More specifically, the oral pharmaceutical composition comprises effective amounts of: (a) atomoxetine or the pharmaceutically acceptable salts thereof; and (b) a taste-masked liquid carrier. Also provided is a method for making the aqueous solution of atomoxetine. The present disclosure also provides methods of using oral pharmaceutical composition for the treatment of a subject having a disorder treatable by the administration of atomoxetine. In one embodiment, the disorder is attention deficit hyperactivity disorder (ADHD).
Owner:TAHO PHARMA
Enzymatic synthesis method of (R)-3-chloro-1-phenyl-propan-1-ol
ActiveCN112980895AReduce generationHigh stereoselectivityBacteriaMicroorganism based processesEnzymatic synthesisPropanol
The invention discloses an enzyme catalytic synthesis method of (R)-3-chloro-1-phenyl-propan-1-ol. According to the method, 3-chloropropiophenone is used as a substrate, optimized carbonyl reductase is used as a catalyst, and the (R)-3-chloro-1-phenyl-propan-1-ol is prepared through biological catalysis. The method for preparing the R-configuration intermediate through the enzyme method is easy and convenient to operate, environmentally friendly, low in cost, few in by-product and high in ee value of the product, and efficient synthesis of the atomoxetine chiral intermediate is achieved.
Owner:宿迁阿尔法科技有限公司
Tomoxetine hydrochloride oral solution and preparation method thereof
InactiveCN111956607AImprove taste effectGreat tasteOrganic active ingredientsNervous disorderSucrosePhosphoric acid
The invention relates to a tomoxetine hydrochloride oral solution. The tomoxetine hydrochloride oral solution comprises the following components in percentage by mass: tomoxetine hydrochloride 4.6 mg / mL, sodium benzoate 0.2-2 mg / mL, sodium dihydrogen phosphate 5-30 mg / mL, phosphoric acid 0.2-2 mg / mL, sorbitol 10-50 mg / mL, xylitol 100-500 mg / mL, sucralose 2-20 mg / mL, strawberry essence 2-20 mg / mL,and the balance being purified water; and the oral solution has pH of 4.5-6.5. The invention also provides a preparation method of the tomoxetine hydrochloride oral solution. The oral solution has advantages of good taste correction effect, good mouthfeel, high comprehensive acceptance and convenience in taking, has an obvious effect on improving medication compliance of children and rationality of clinical medication, has a better treatment effect on attention deficit hyperactivity disorder patients, and effectively solves a problem of poor compliance of tomoxetine hydrochloride due to bittertaste.
Owner:JIANMIN PHARMA GRP CO LTD
Enantiomerically pure atomoxetine and tomoxetine mandelate
InactiveCN1946677AOrganic compound preparationAmino-hyroxy compound preparationMedicinal chemistryAtomoxetine
The present invention provides enantiomerically pure (R)-(-)-tomoxetine (S)-(+)-mandelate and atomoxetine HCI. The present invention further provides enantiomerically pure (R)-(-)-tomoxetine (S)-(+)-mandelate prepared from racemic 5 tomoxetine. The present invention also provides enantiomerically pure atomoxetine HCI prepared from (R)-(-)-tomoxetine (S)-(+)-mandelate.
Owner:TEVA PHARMA FINE CHEMI
A kind of atomoxetine hydrochloride oral solution and preparation method thereof
ActiveCN106727291BGreat tasteSolve the shortcomings of poor complianceOrganic active ingredientsNervous disorderBiologyOral solutions
The invention relates to pharmaceutical composition containing atomoxetine hydrochloride and a preparation method of the pharmaceutical composition, in particular to an atomoxetine hydrochloride oral solution preparation and a preparation method thereof. Beta-cyclodextrin and tartaric acid are combined for taste masking treatment, the atomoxetine hydrochloride oral solution preparation with good taste is prepared, the preparation method is simple, the atomoxetine hydrochloride oral solution preparation has the good taste, large quantities of sweetening agents, flavors and fragrances are not added, the toxic and side effects are reduced, the defects of poor compliance of existing atomoxetine hydrochloride preparations for children and the like are overcome, the medication compliance of children patients is improved, and the clinical medication requirement of children is met.
Owner:SHANDONG DYNE MARINE BIOTECHCAL PHARM HLDG CO LTD
Modulators of zinc activated cation channel
ActiveUS11191743B2Avoid depressionRegulates immune toleranceNervous disorderCell receptors/surface-antigens/surface-determinantsPurinergic EffectsDisease
Various embodiments disclosed herein include methods of modulating the function of Zinc Activated Cation Channel (ZACN) in a subject comprising: administering to the subject a pharmaceutically effective dosage of ATP, a purinergic compound, red or blue dye, heparin or heparin analog, desipramine, reboxetine, and / or tomoxetine; and modulating the function of ZACN in the subject. Various embodiments disclosed herein also include methods of treating a disease in a subject comprising: administering to the subject a pharmaceutically effective dosage of a compound capable of modulating the function of the Zinc Activated Cation Channel (ZACN), and treating the disease.
Owner:DIGNITY HEALTH
Stable Atomoxetine Hydrochloride, a process for the preparation thereof, and an analytical control of its stability
Stable Atomoxetine hydrochloride, a process for the manufacture thereof, the use of stable Atomoxetine Hydrochloride for making a pharmaceutical formulation, a process for the preparation of any form of Atomoxetine Hydrochloride, and an analytical method for analyzing the stability of Atomoxetine Hydrochloride are provided.
Owner:CASTELLI EUGENIO +1
Medicinal composition for treating neurasthenia or somatic form disorders
ActiveCN1850271BOrganic active ingredientsNervous disorderNorepinephrine reuptake inhibitorSertraline
The present invention relates to a method for curing and preventing neurosism or somatic formal disturbance and its medicine composition. Said invention belongs to the field of pharmacy. Said invention provides a medicine composition containing norepinephrine reuptake inhibitor with medicinal dose or its medicinal salt and selective 5-hydroxytryptamine reuptake inhibitor with medicinal dose or its medicinal salt for preventing and curing neurosism or somatic formal disturbance. The norepinephrine reuptake inhibitor is selected from reboxetine, tomoxetine, amfebutamone, nortriptyline, norimipramine, maprotiline and protiline, and the selective 5-hydroxytryptamine reuptake inhibitor is selected from sertraline, citalopram, s-citalopram, fluoroxeline, paroxetine and fluvoxamine.
Owner:SHENZHEN AUSA PHARM CO LTD +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com