A kind of atomoxetine hydrochloride oral solution and preparation method thereof
A technology of atomoxetine hydrochloride and oral solution, which is applied in the field of atomoxetine hydrochloride oral solution and preparation thereof, can solve the problems of difficult taking by patients, poor taste and the like, achieves high product stability, simple preparation method, and eliminates fear sense of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
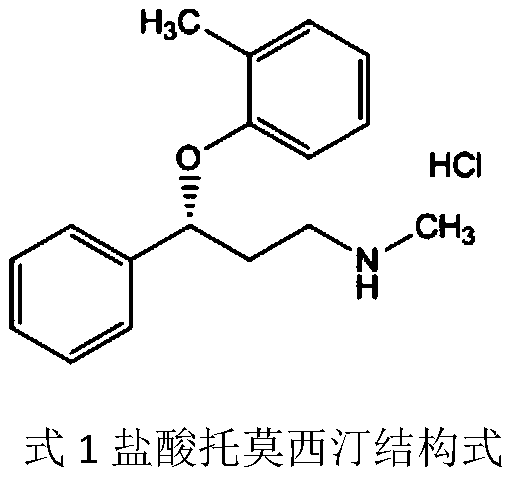
[0030] formula:
[0031]
[0032] Preparation Process:
[0033] 1) Preparation of the drug-containing solution: Dissolve the prescribed amount of β-cyclodextrin in 75ml of water at 40°C, add the prescribed amount of atomoxetine hydrochloride, tartaric acid, and sorbitol, stir to dissolve, and cool down to room temperature;
[0034] 2) Preparation of sucralose and preservative solution: dissolve the prescribed amount of sucralose, sodium methylparaben and sodium propylparaben in 5ml of water at room temperature;
[0035] 3) Add 2) into 1), stir evenly, add sodium citrate to adjust the pH value to neutral, add cherry essence, and dilute to 100ml with purified water to obtain the product.
Embodiment 2
[0037] formula:
[0038]
[0039] Preparation Process:
[0040] 1) Preparation of the drug-containing solution: Dissolve the prescribed amount of β-cyclodextrin in 75ml of water at 40°C, add the prescribed amount of atomoxetine hydrochloride, tartaric acid, and sorbitol, stir to dissolve, and cool down to room temperature;
[0041] 2) Preparation of sucralose and preservative solution: dissolve the prescribed amount of sucralose, sodium methylparaben and sodium propylparaben in 5ml of water at room temperature;
[0042] 3) Add 2) into 1), stir evenly, add sodium citrate to adjust the pH value to neutral, add cherry essence, and dilute to 100ml with purified water to obtain the product.
Embodiment 3
[0044] formula:
[0045]
[0046] Preparation Process:
[0047] 1) Preparation of the drug-containing solution: Dissolve the prescribed amount of β-cyclodextrin in 75ml of water at 40°C, add the prescribed amount of atomoxetine hydrochloride, tartaric acid, and sorbitol, stir to dissolve, and cool down to room temperature;
[0048] 2) Preparation of sucralose and preservative solution: dissolve the prescribed amount of sucralose, sodium methylparaben and sodium propylparaben in 5ml of water at room temperature;
[0049] 3) Add 2) into 1), stir evenly, add sodium citrate to adjust the pH value to neutral, add cherry essence, and dilute to 100ml with purified water to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com