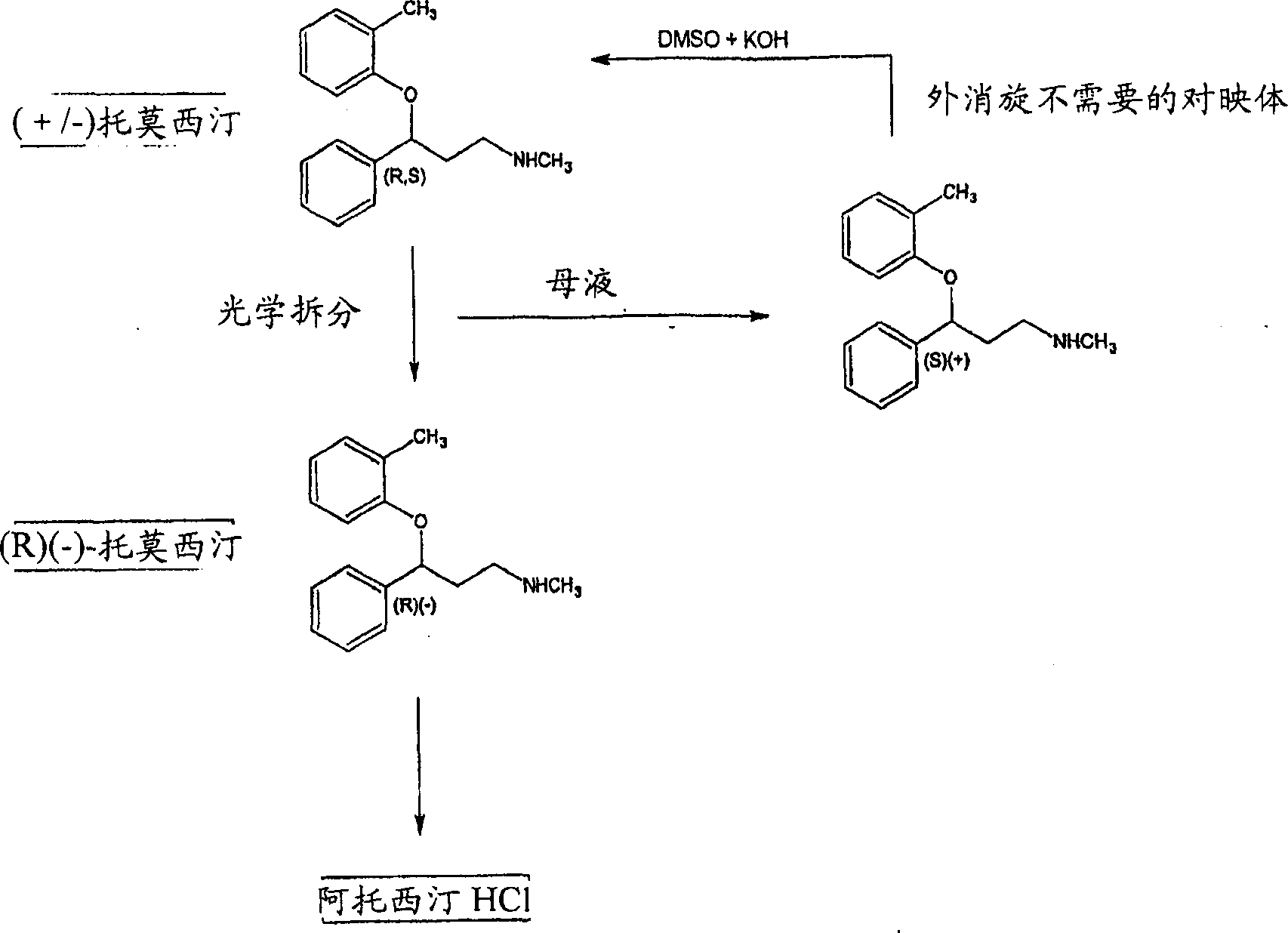
Process for the optical resolution and recycling of tomoxetine
A atomoxetine, pharmaceutical technology, applied in the field of optical resolution and reuse of atomoxetine, can solve problems such as dangerous solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
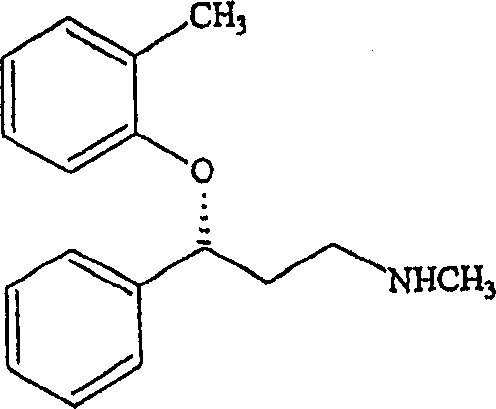
[0064] (R,S)-N-Methyl-3-(2-methylphenoxy)-3-phenylpropylamine (tomoxetine synthesis)
[0065] 1100g (14.1mol) dimethyl sulfoxide, 200g (1.21mol) N-methyl-3-hydroxy-3-phenylpropylamine, 221g (3.63mol) KOH (batch industrial grade, 92.1% detection) at 110°C Heat and stir, then concentrate the mixture by vacuum distillation until about 130 g of solvent is removed. After the mixture had cooled to 80°C, 400 g (3.63 mol) of 2-fluorotoluene were added. The mixture was heated to reflux (145°C-147°C) for one hour and then allowed to cool to 90°C. 1000ml of water and 1000ml of toluene were added. The mixture was stirred for several minutes, at which point the phases separated. The aqueous phase was extracted with 2 x 200 ml toluene. The organic phase was collected and washed with 3 x 200ml water. Final organic phase weight: 1700 g. Atomoxetine content: 16.83% (weight ratio) (HPLC detection).
[0066] Yield: 92.7%.
Embodiment 2
[0068] (R)-(-)-tomoxetine (S)-(+)-mandelate (atomoxetine optical resolution)
[0069] A toluene solution of crude racemic atomoxetine prepared as described in Example 1 (376.13 g, 1.081 mL, detected by HPLC) was concentrated in vacuo to remove water. The residue was taken up in 2025 ml of toluene and 26 ml of methanol. To the resulting solution was added 94 g (0.618 mol) of (S)-(+)-mandelic acid at 25°C. Heat to 60°C-70°C to dissolve all solids, then cool the crude mandelate to crystallize. The solid was isolated by filtration at 5°C-10°C, washed with about 300 ml of toluene and dried under vacuum. Weight: 178g. Atomoxetine content: 63.2% (weight ratio) (HPLC detection). Yield: 43.15%. The ratio of (R)-(-)-atomoxetine enantiomers of the crude mandelate: R / S is about 95 / 5 (by chiral HPLC).
[0070] The crude mandelate obtained by recrystallizing 163 g from 489 ml of toluene and 49 ml of methanol is as follows: heat to 65°C-70°C to dissolve the salt, (R)-(-)-atomoxetine (S...
Embodiment 3
[0072] (R)-(-)-tomoxetine (S)-(+)-mandelate (atomoxetine optical resolution)
[0073] To a toluene solution (derived from Example 2) of 26.5 g of racemic atomoxetine crude product (0.104 mol, detected by HPLC) at 25° C., 1.6 ml of methanol and 9.6 g (0.063 mol) of (S)-(+ )-mandelic acid. Heating to 65°C-70°C dissolves all solids, and the crude mandelic acid salt crystallizes upon cooling. The salt was isolated by filtration at 5°C-10°C, washed with about 30 ml of toluene and dried in vacuo. Weight: 16.4g. Atomoxetine content: 64.35% (weight ratio) (HPLC detection). Yield: 40% (from racemic atomoxetine). The ratio of (R)-(-)-atomoxetine enantiomers of the crude mandelate: R / S is about 97 / 3 (by chiral HPLC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com