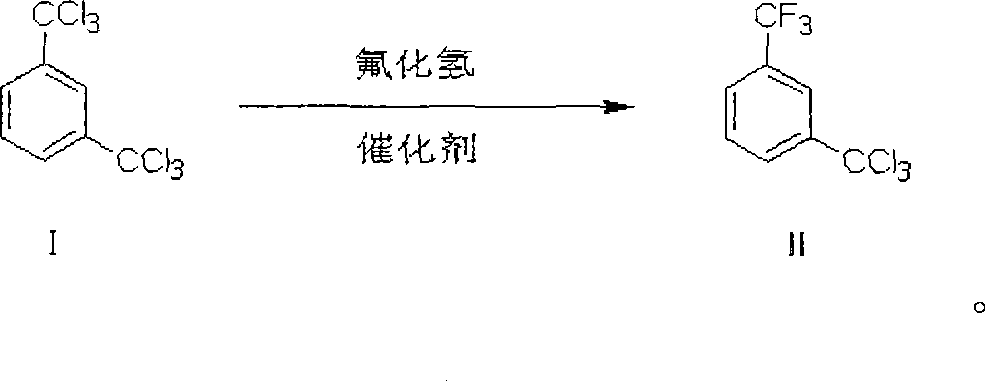
Process of preparing 3-trifluoromethyl benzoic acid
A technology of trifluoromethylbenzoic acid and trichloromethyltrifluoromethylbenzene, which is applied in the field of preparation of 3-trifluoromethylbenzoic acid, can solve problems affecting product purity, high cost, and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
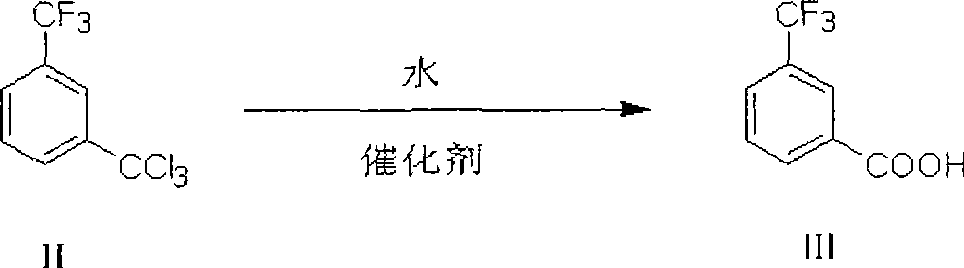
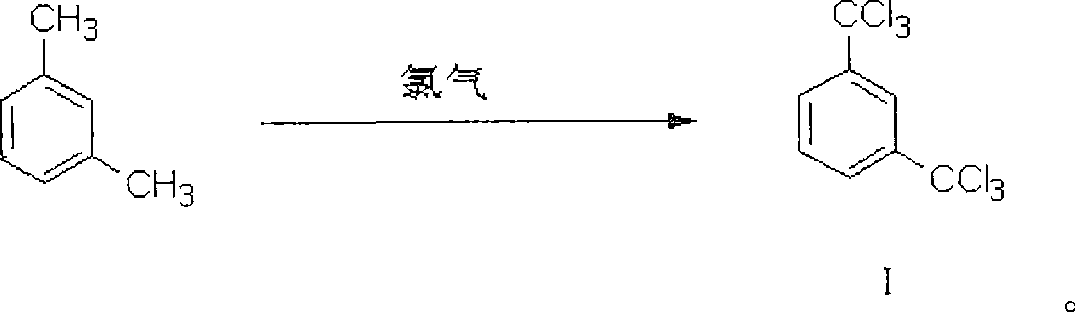
[0064] Preparation of m-ditrichloromethylbenzene
[0065] After mixing 563 mole parts of m-ditrichloromethylbenzene and 1689 mole parts of m-xylene, the temperature inside the reactor was raised to 120°C, then the ultraviolet lamp was turned on, chlorine gas was introduced, and the temperature inside the reactor was kept at 120-140°C. The reaction was carried out for 30 hours. According to gas chromatography analysis, the content of m-dichloromethyltrichloromethylbenzene, an intermediate of the chlorination reaction, is lower than 0.5%. Measured by GC-MS method, the reaction product is m-ditrichloromethylbenzene.
[0066] Degassing under reduced pressure, distilling the reaction solution under reduced pressure, collecting fractions at 154-158°C / 10mmHg to obtain 2068 parts of m-ditrichloromethylbenzene, the yield of chlorination reaction was 89.3%, m-ditrichloromethyl The purity of benzene is 97.2%.
Embodiment 2-9
[0068] Preparation of m-ditrichloromethylbenzene
[0069] Proceed in the same manner as in Example 1, except that the reaction temperature, raw material ratio and reaction time are as shown in Table 1. Table 1 also shows the yield, the purity and the high boiler content of the obtained product of m-bistrichloromethylbenzene.
[0070] In addition, Example 8 uses 2% by weight of azobisisobutyronitrile (based on the weight of m-xylene) as an initiator instead of UV initiation.
Embodiment 9
[0071] Example 9 uses 2% by weight of benzoyl peroxide (based on the weight of m-xylene) as the initiator instead of UV initiation.
[0072] Table 1
[0073] implement
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com