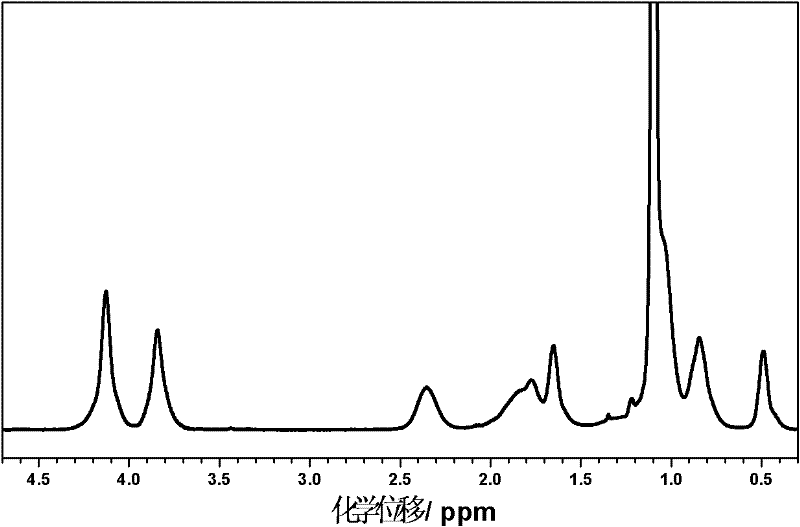
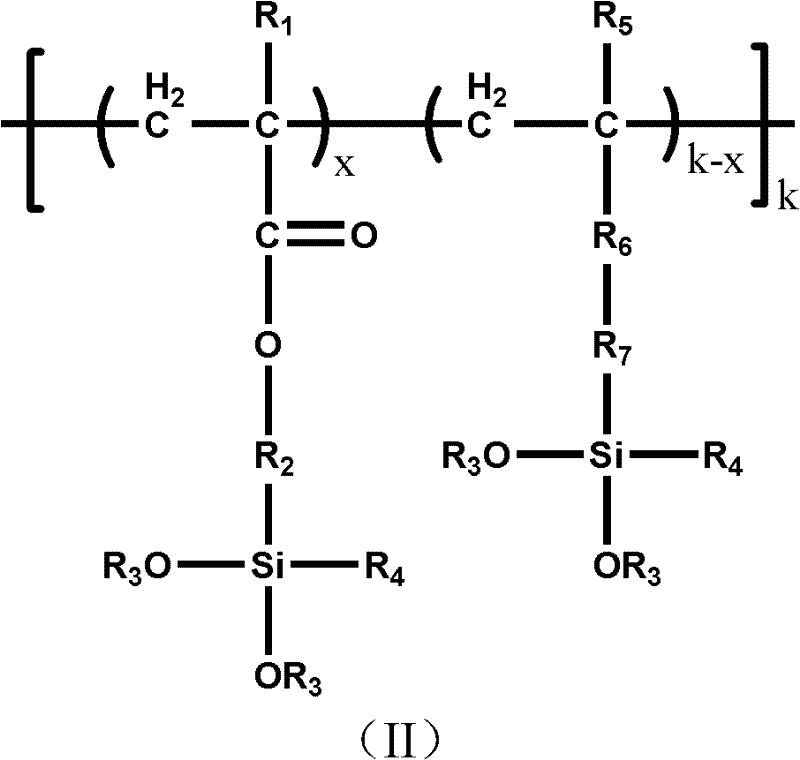
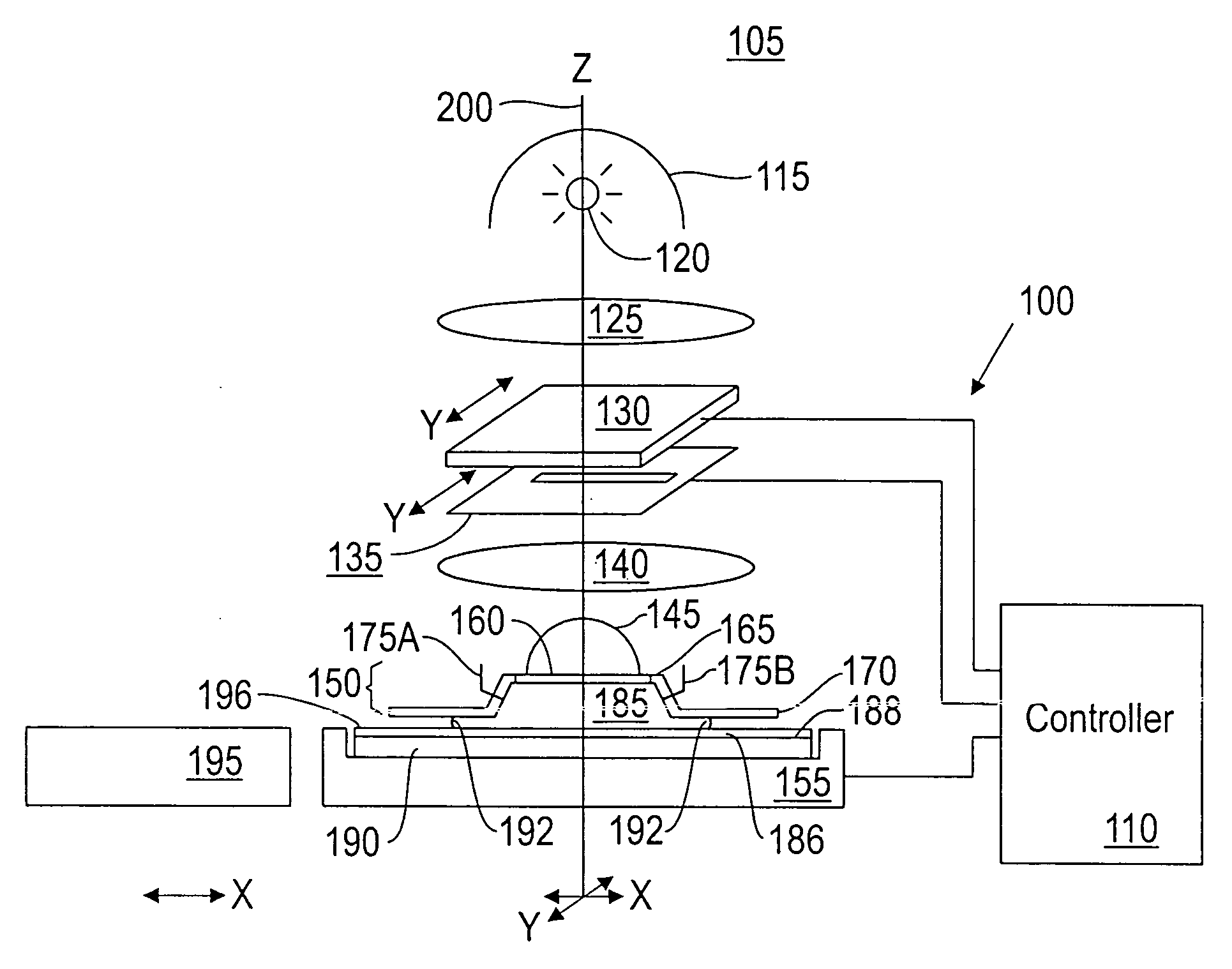
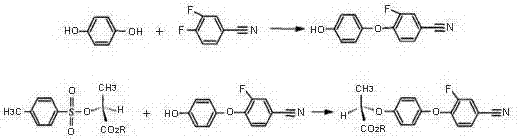
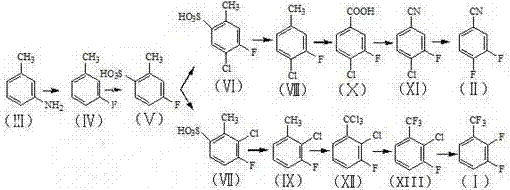
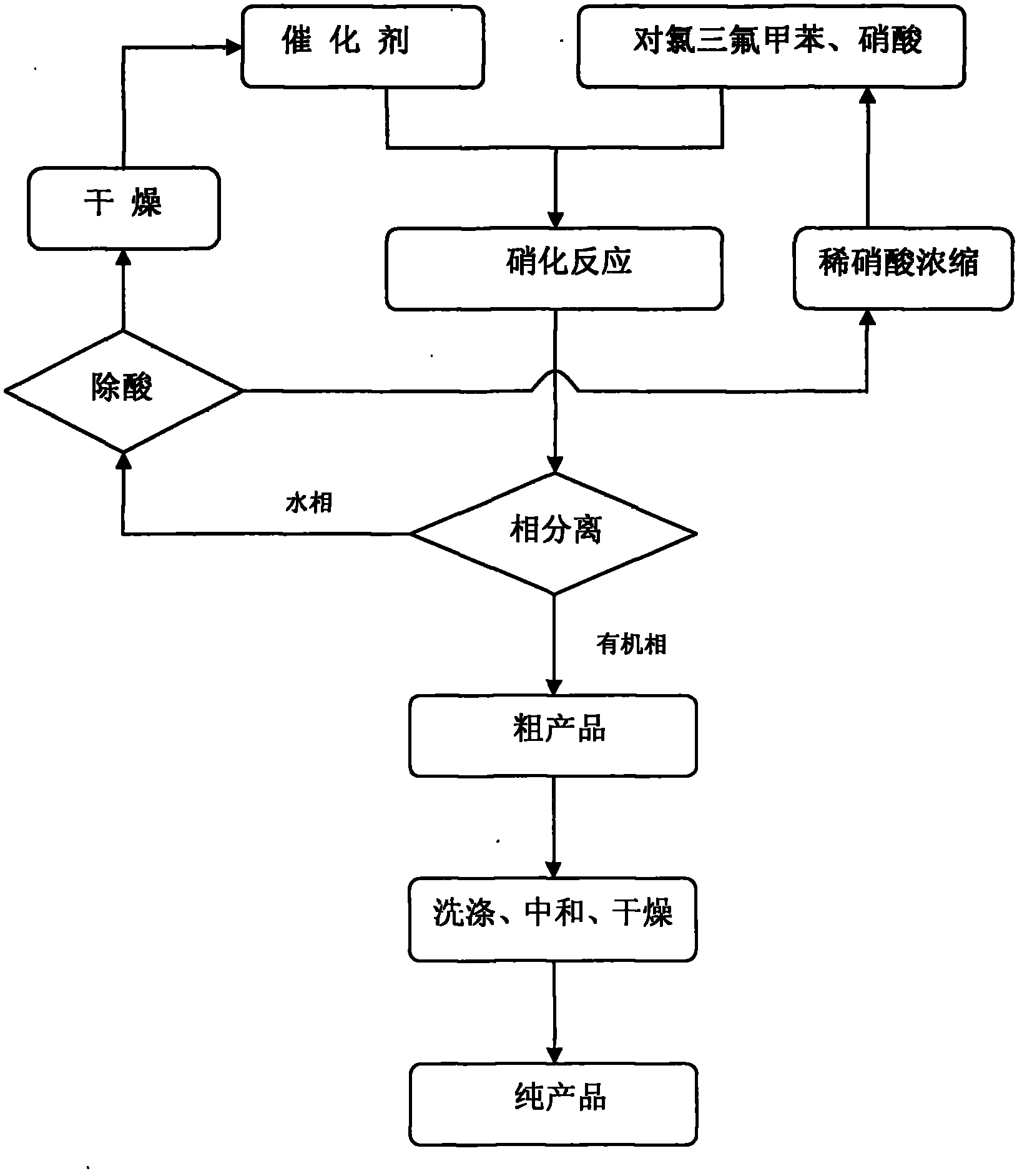
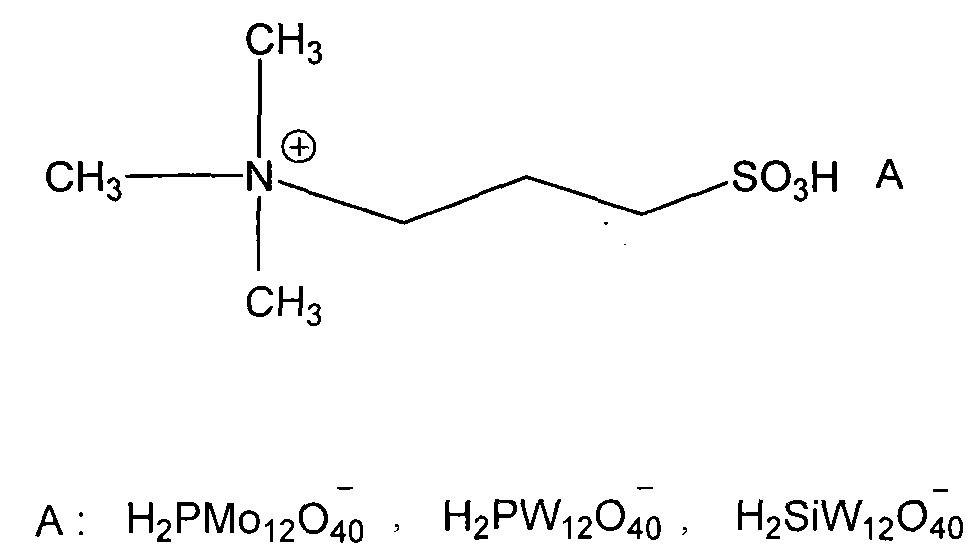
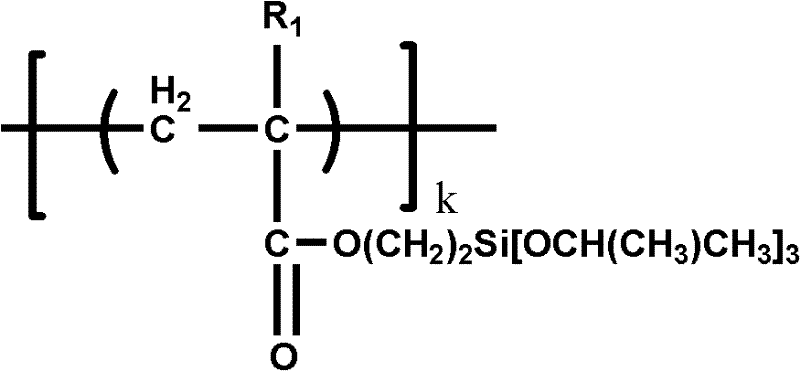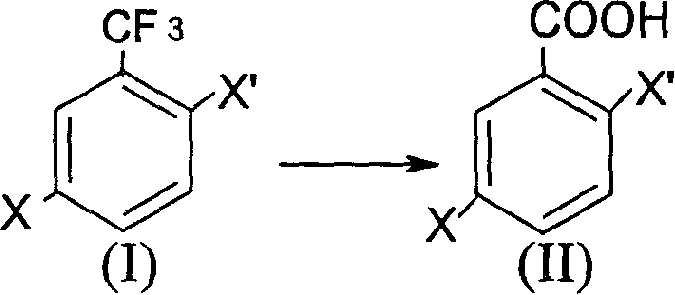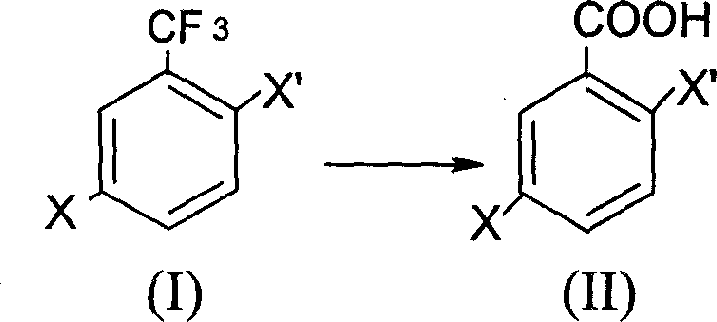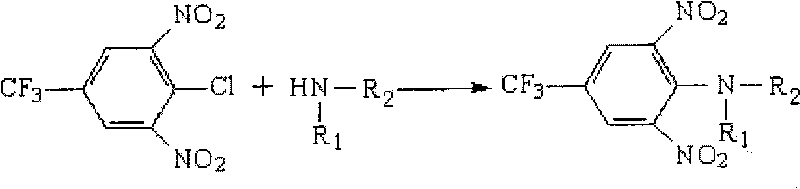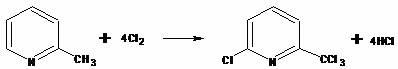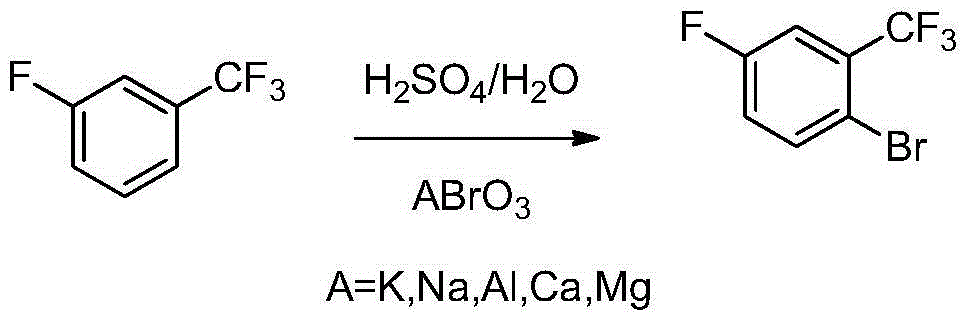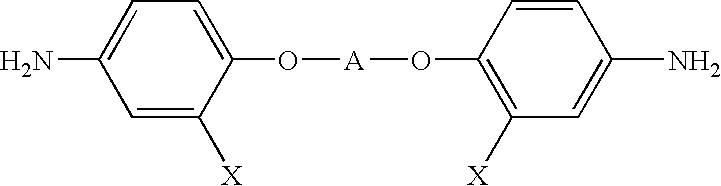Patents
Literature
158 results about "Trifluorotoluene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
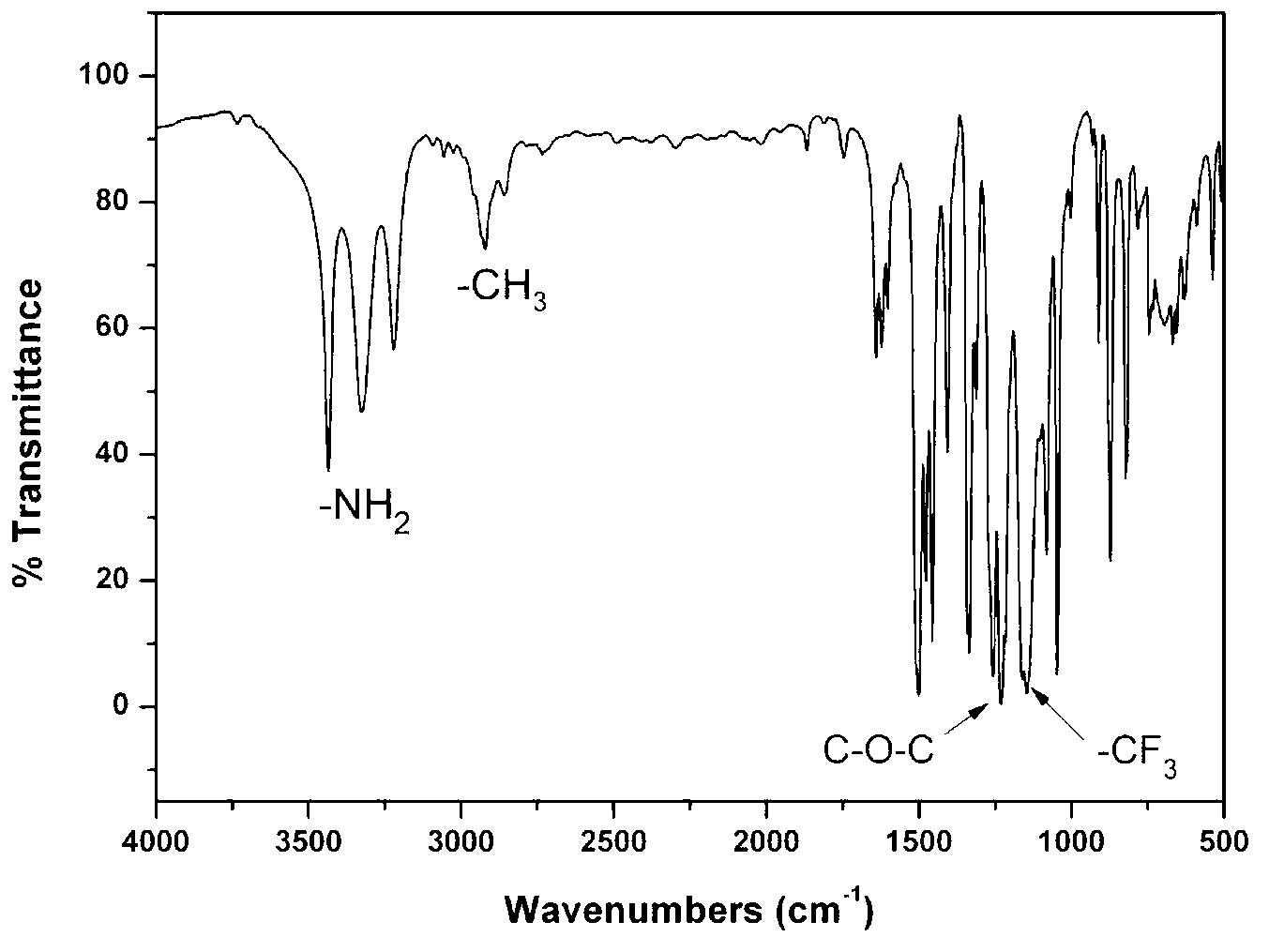
Trifluorotoluene is an organic compound with the formula of C₆H₅CF₃. This colorless fluorocarbon is used as a specialty solvent in organic synthesis and an intermediate in the production of pesticides and pharmaceuticals.
Amphiphobic fluoro-containing crosslinkable block copolymer and preparation method and application thereof
InactiveCN102199263AGood superhydrophobic and oleophobic propertiesPrecise structurePaper coatingCoatingsNano siliconPolymer science
The invention discloses amphiphobic fluoro-containing crosslinkable block copolymer and a preparation method and application thereof. The block copolymer can be used for preparing a super-amphiphobic coating on the surface of glass or printing paper, and the super-amphiphobic coating is prepared by the following steps of: (1) putting silica nano spheres into benzotrifluoride to obtain solution ofsilica nano spheres; (2) adding solution of amphiphobic fluoro-containing crosslinkable block copolymer, tetrahydrofuran, hydochloric acid solution and water into the solution of silica nano spheres,and reacting for 7 to 12 hours to obtain a crude product of modified silica nano spheres; (3) washing the crude product of modified silica nano spheres to obtain modified silica nano spheres; and (4)dispersing the modified silica nano spheres into the benzotrifluoride to obtain solution, dripping the solution onto a glass sheet, and after the solvent is volatilized, forming the super-amphiphobiccoating on the surface of the glass sheet, or soaking the printing paper in the solution, taking the paper out, and drying the paper to obtain the super-amphiphobic coating on the surface of the paper. The amphiphobic fluoro-containing crosslinkable block copolymer can endow a material with good superhydrophobic and oleophobic properties; and the coating has high stability and hardly falls off and denatures.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
Topcoat compositions and methods of use thereof
A topcoat composition that includes a fluorine-containing polymer and a casting solvent selected from the group consisting of α,α,α-trifluorotoluene, 2,2,3,3,4,4,5,5-octafluoropentyl-1,1,2,2-tetrafluoroethyl ether (OFP-TFEE), and a mixture consisting of a hydrophobic alkane and an alcohol is provided. Also provided is method of forming an image on a photoresist that includes forming a photoresist over a substrate; applying a topcoat composition, the topcoat composition comprising at least one fluorine-containing polymer and a casting solvent, onto the photoresist; removing the casting solvent of the topcoat composition resulting in the formation of a topcoat material over the photoresist; exposing the photoresist to radiation, the radiation changing a chemical composition of the regions of the photoresist exposed to the radiation, forming exposed and unexposed regions in the photoresist; and removing i) the topcoat material and ii) the exposed regions of the photoresist or the unexposed regions of the photoresist.
Owner:TAIWAN SEMICON MFG CO LTD
Unsymmetrical fragrant diamine containing fluorine, preparation and application in synthesizing polyimide thereof
InactiveCN101270059AHigh purityImprove performanceOrganic chemistryOrganic compound preparationSolubilityOptical transparency
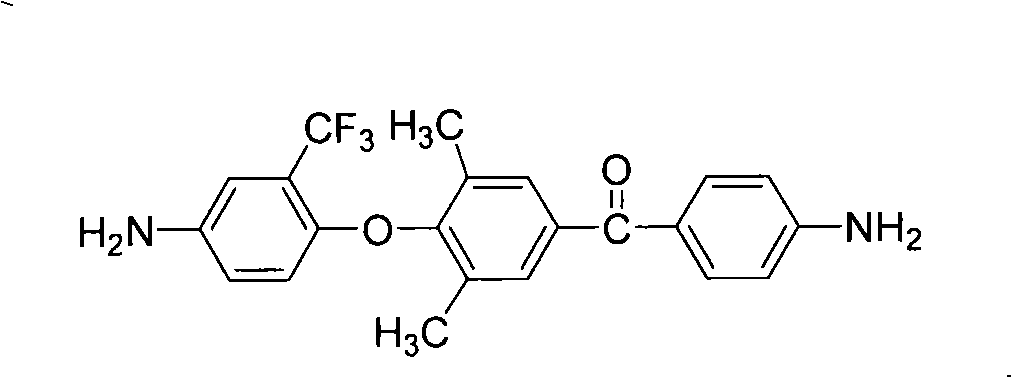
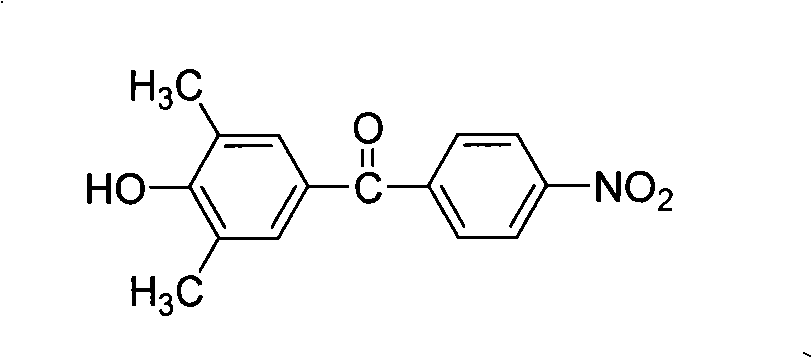
The present invention relates to fluorine-containing asymmetric aromatic diamine, a preparation method and an application thereof in the synthesis of polyimide. The compound has a constitutional formula as shown in the right. The preparation method comprises the following steps: (1) 2, 6-dimethyl phenol, paranitrobensoyl chloride and alchlor have catalytic reaction in an organic solvent to prepare (4'-hydroxy-3', 5'-dimethyl benzene)-(4-nitrophenyl) ketone; (2) the (4'-hydroxy-3', 5'-dimethyl benzene)-(4-nitrophenyl) ketone reacts with 2-chlorine-5-nitro trifluorotoluene with alkaline, to prepare (3', 5'-dimethyl-4'-(4''-nitro-2''-trifluoromethyl phenoxy) benzene)-(4-nitrophenyl) ketone; (3) the prepared product in the second step is reduced by reducing agent with organic solvents and catalysts; thus the fluorine-containing asymmetric aromatic diamine can be prepared. The fluorine-containing asymmetric aromatic diamine can be used for preparing fluorine-containing polyimide. The fluorine-containing asymmetric aromatic diamine prepared in the method has high purity and can keep stable at the room temperature; the polyimide made of the fluorine-containing asymmetric aromatic diamine has excellent solubility, film forming capability, optical transparency, mechanical properties and heat resistance.
Owner:DONGHUA UNIV
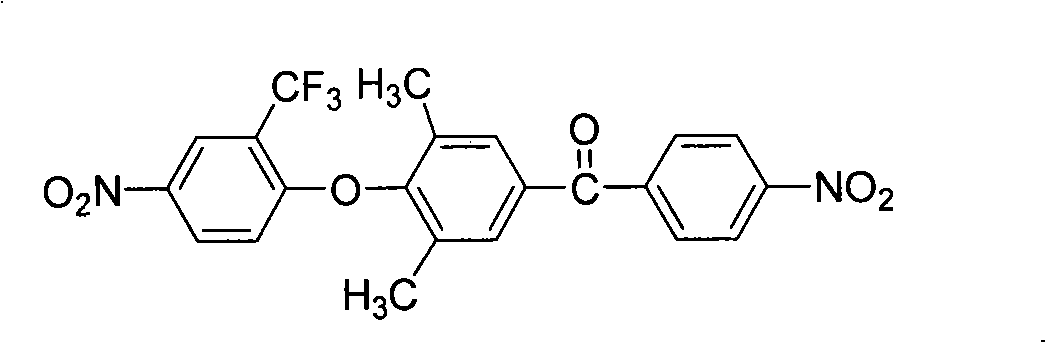
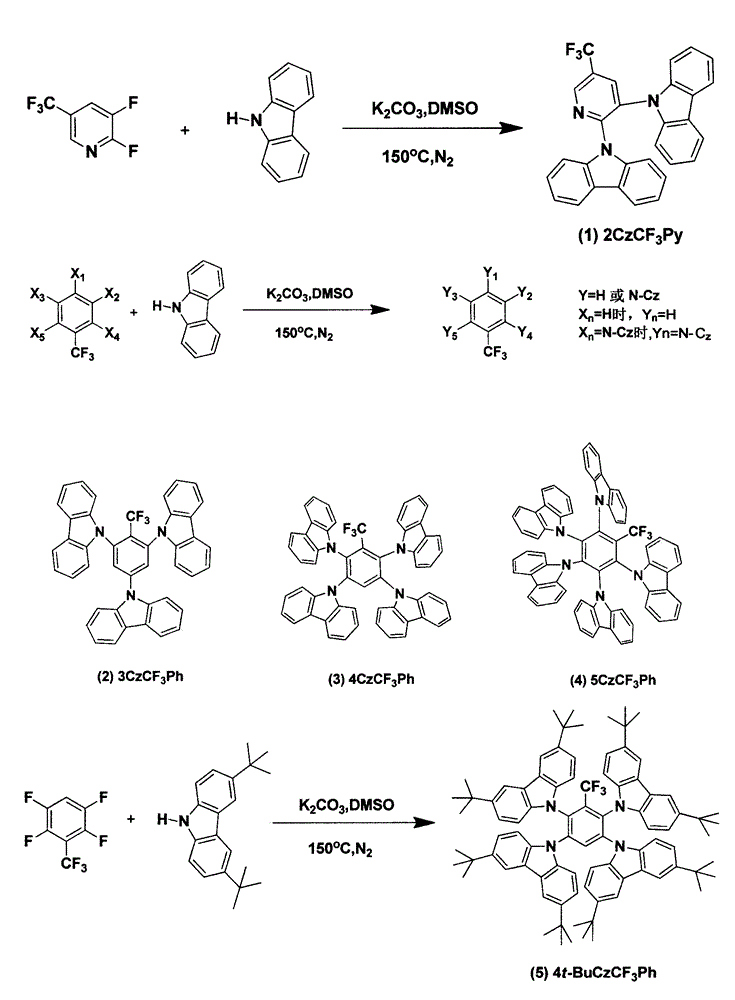
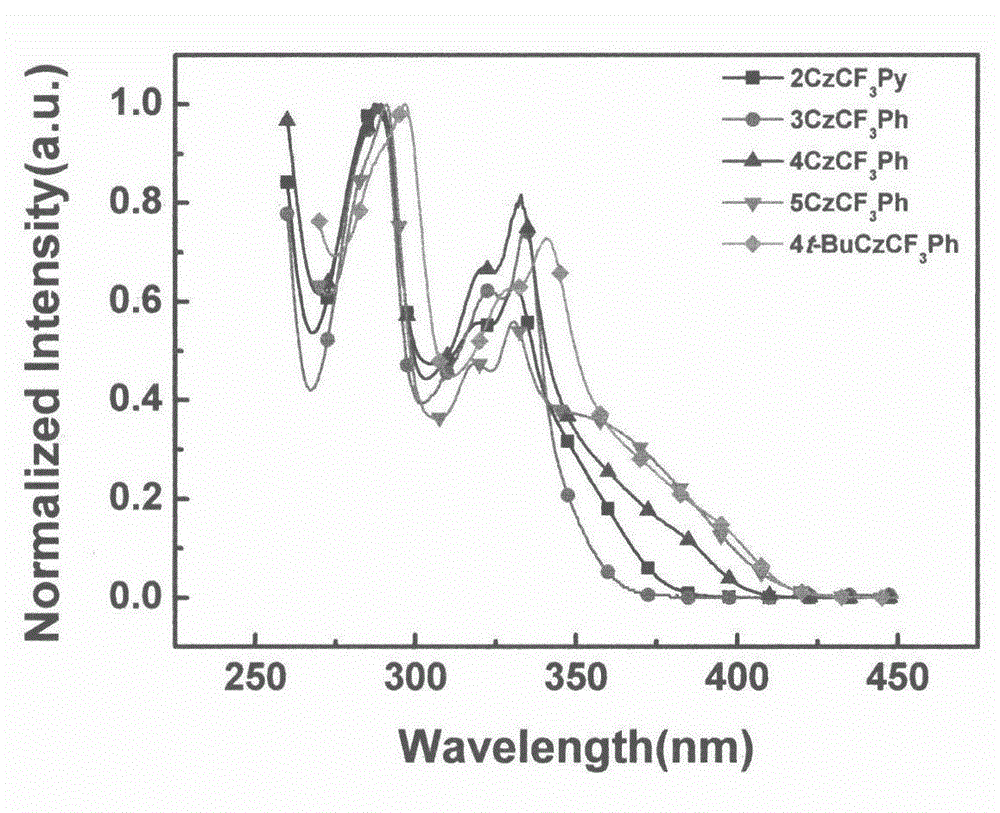
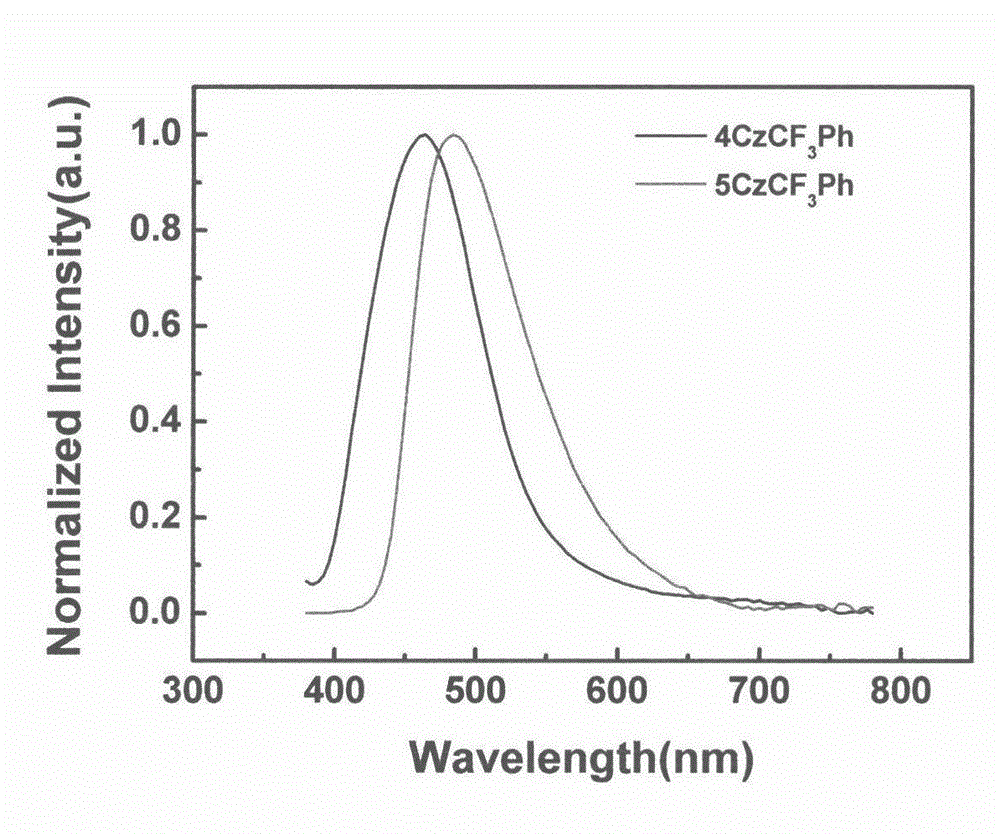
Synthesis of trifluoromethyl derivative and application of synthesis of trifluoromethyl derivative in organic electroluminescence
The invention relates to synthesis of trifluoromethyl derivative and an application of the synthesis of trifluoromethyl derivative in organic electroluminescence. According to the method for synthesizing a series of trifluoromethyl compound in one step, raw materials are cheap and easy to obtain, noble metal catalysts are not needed, operation is simple and convenient, the reaction conditions are mild, the production cost is low, the yield is high, and commercialization is facilitated. The invention relates to the field of organic electroluminescence materials, in particular to materials which are synthesized in one step, have bipolar carrier transportation performance and serve as an electrophosphorescence subject or a delay fluorescent object to prepare an organic electroluminescence element. The materials with the bipolar carrier transportation performance comprise a carbazole unit with hole transport performance and a benzotrifluoride or 2-(trifluoromethyl) pyridine unit with electronic transport performance, namely CzCF3Ph and CzCF3Py class compound.
Owner:NANJING UNIV OF TECH
Novel preparation method of cinacalcet hydrochloride
ActiveCN101993379AUnique reaction lineGood choiceOrganic compound preparationAmino compound preparationSolventReaction step
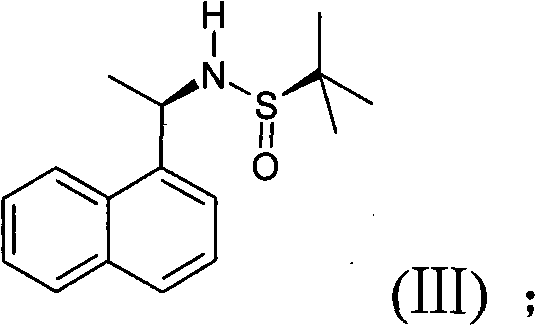
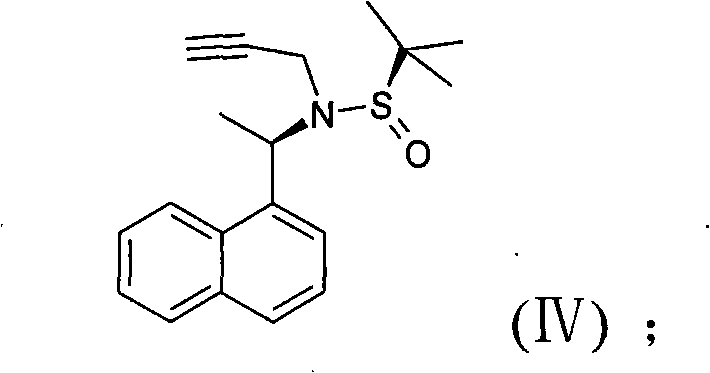
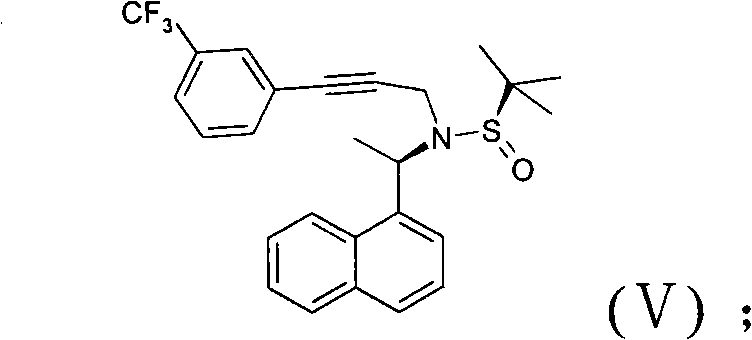
The invention discloses a novel preparation method of cinacalcet hydrochloride, belonging to the technical filed of preparation of cinacalcet hydrochloride and comprising the steps of: firstly, with 1-acetonaphthone (II) as a raw material, introducing a chiral reagent, and reducing with sodium borohydride to obtain a compound (III); secondly, dissolving the compound (III) into a proper solvent, and reacting with 3-propiolic halide to obtain a compound (IV); thirdly, adding a proper catalyst into the obtained compound (IV) in a solvent at a certain temperature, and carrying out a coupling reaction with meta-chlorobenzotrifluoride to generate a compound (V); and fourthly, reducing the obtained compound (V) with a proper reducing agent and acidizing with hydrochloric acid to obtain the cinacalcet hydrochloride (I). The cinacalcet hydrochloride is synthesized chirally, and has the advantages of special reaction course, good selectivity, high yield, less reaction steps, safe and simple operation and little environment pollution. The raw material used in the reaction course is easy to obtain, has lower production cost and high optical purity, and is suitable for industrialized production.
Owner:NENTER & CO
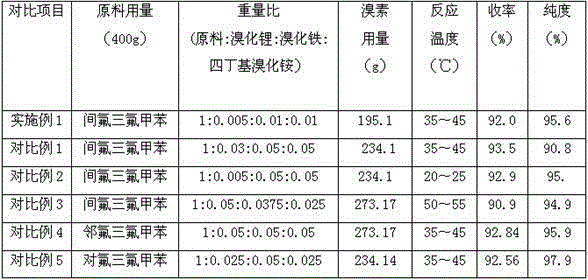
Method for preparing 2-bromine-5-trifluorotoluene chloride
ActiveCN104447183AIncrease profitHigh reaction conversion rateHalogenated hydrocarbon preparationBulk chemical productionOrganic solventBromine
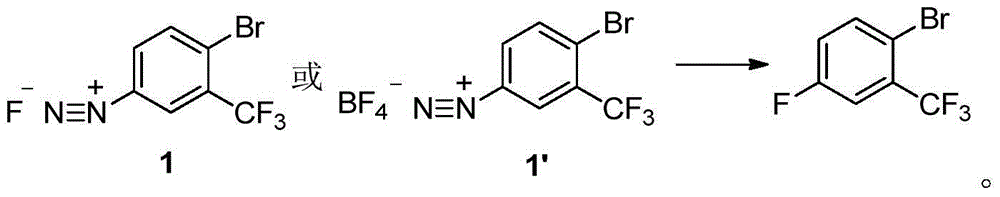
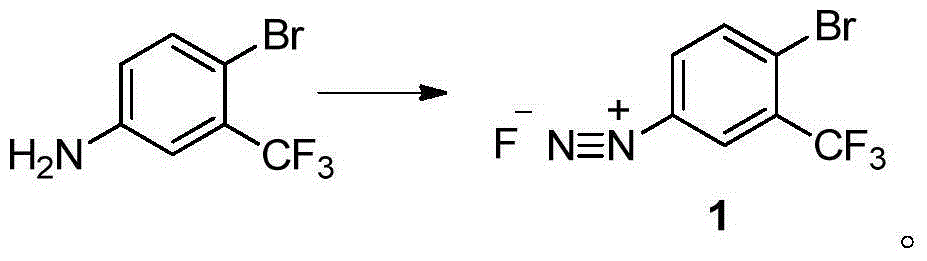
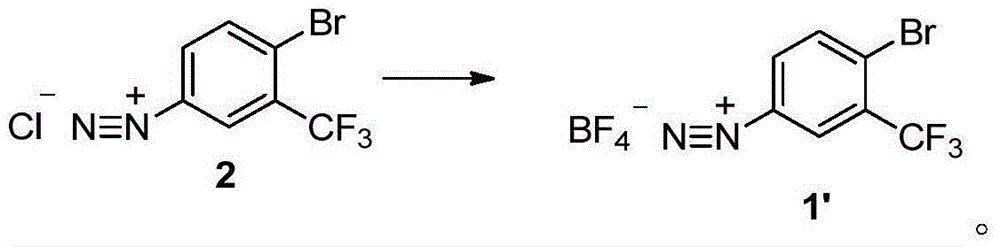
The invention discloses a method for preparing 2-bromine-5-trifluorotoluene chloride. The method comprises the following step: under an anhydrous condition and in an organic solvent, performing cracking reaction on an anhydrous compound 1 or a compound 1', thereby preparing 2-bromine-5-trifluorotoluene chloride. According to the method, the reaction of an upper amino protecting group of m-trifluoromethyl phenylamine, the bromination reaction and the reaction of removing the amino protecting group can be all performed in one same reaction kettle without transferring or storing materials. The raw materials used in the method disclosed by the invention are cheap and easy to obtain, the reaction step is short, the reaction condition is gentle, the utilization rate of bromine is high, and the positioning selectivity of bromine feeding is high, so that a final product is low in isomeride impurity, high in reaction conversion rate, high in yield, high in product purity, low in production cost is low and applicable to industrialization production. The compound 1 and compound 1' are as shown in the specification.
Owner:JIANGSU LIANHE CHEM TECH +4
Prepn process of 2,5-dihalogeno benzoic acid
InactiveCN1740135ASimple processLow costOrganic compound preparationCarboxylic compound preparationBenzoic acidToluene
The present invention discloses the preparation process of 2, 5-dihalogeno benzoic acid with the general expression as shown. The preparation process includes hydrolysis of 2, 5-dihalogeno trifluoro toluene under acid condition to obtain 2, 5-dihalogeno benzoic acid. The preparation process can realize the synthesis of the target compound in low cost and high yield.
Owner:LYNCHEM
Continuous production method for industrially preparing 2,3-difluorobenzotrifluoride and 3,4-difluorobenzonitrile
ActiveCN107488098ALow application costAvoid it happening againOrganic compound preparationPreparation by halogen replacementTolueneRaw material
The invention discloses a continuous production method for industrially preparing 2,3-difluorobenzotrifluoride and 3,4-difluorobenzonitrile. The preparation process of the 2,3-difluorobenzotrifluoride comprises the preparation step of an intermediate raw material for benzene sulfonamide and benzenesulfonylurea herbicides, and the preparation process of the 3,4-difluorobenzonitrile comprises the preparation step of a cthalofop-butyl intermediate raw material. The method is suitable for industrial production; and compared with the prior art, the method has the advantages of low cost, high production method and less pollution.
Owner:ZIBO FEIYUAN CHEM CO LTD
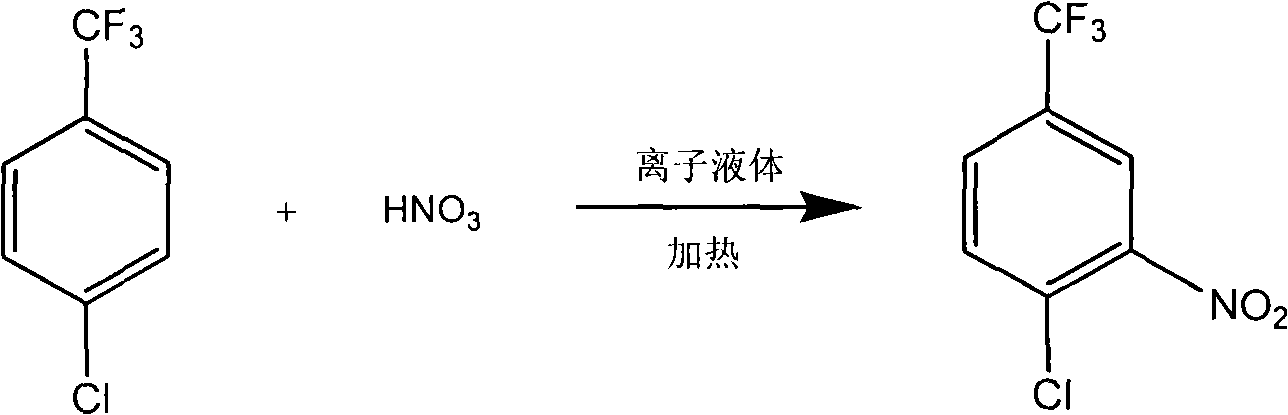
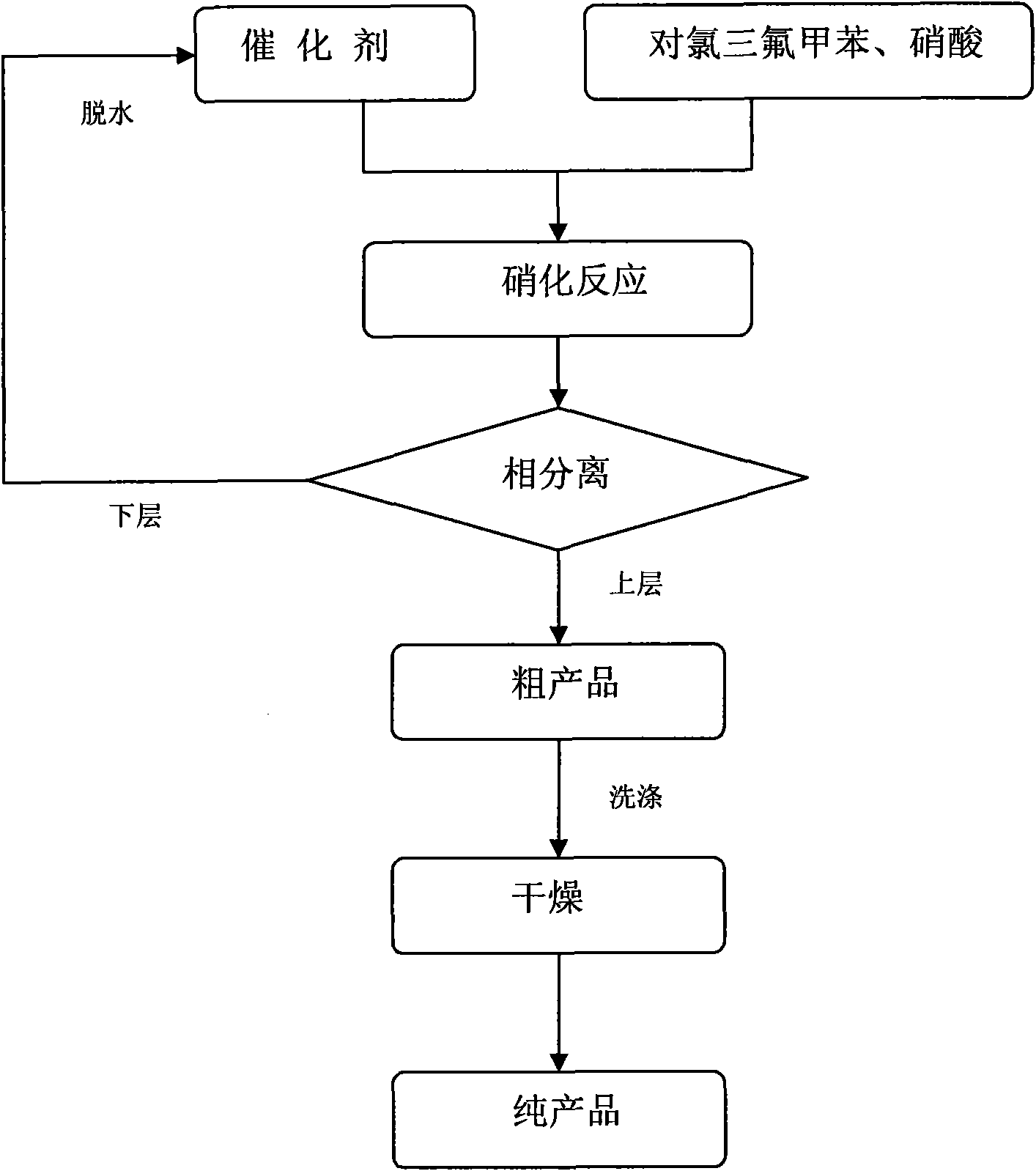
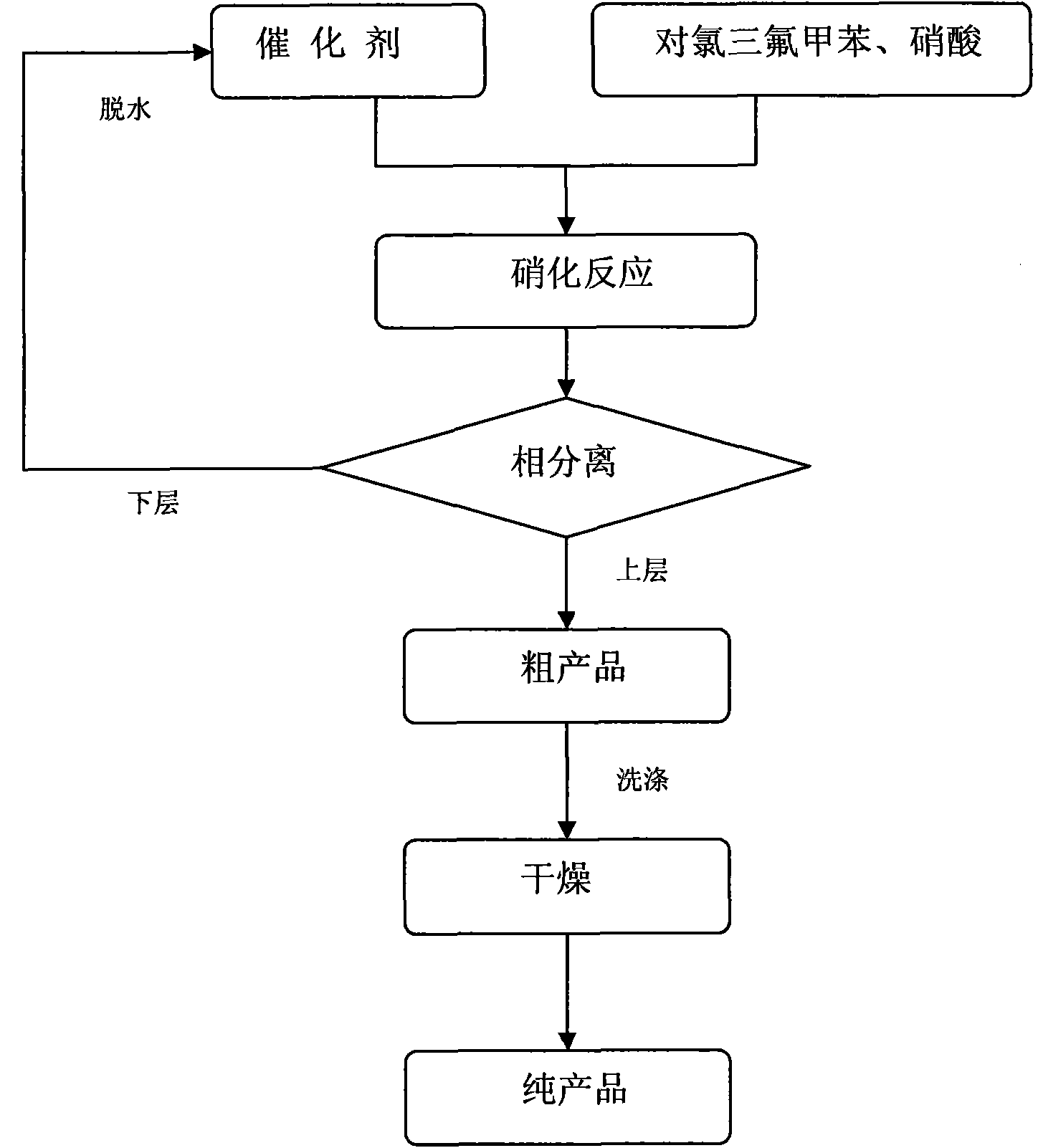
P-chlorobenzotrifluoride clean nitration reaction catalyzed by heteropoly acid ionic liquid
InactiveCN102417457AReduce dosageWide variety of sourcesOrganic-compounds/hydrides/coordination-complexes catalystsNitro compound preparationP-chlorobenzotrifluorideHeteropoly acid
The invention discloses a novel method for carrying out p-chlorobenzotrifluoride clean nitration reaction catalyzed by a heteropoly acid ionic liquid. The used catalyst is an ionic liquid having a heteropoly acid anionic structure, and p-chlorobenzotrifluoride and nitric acid used as raw materials are subjected to nitration reaction under the action of the catalyst, thus obtaining 4-chloro-3-nitrobenzotrifluoride. Compared with the prior art, the invention has the following advantages: (1) the used ionic liquid having a heteropoly acid anionic structure has a wide source of raw materials and is convenient to prepare; and the catalyst is stable in water, can not be inactivated and can be used circularly; (2) compared with corresponding inorganic acid, the heteropoly acid forming the anionic structure has higher strength, higher catalytic activity, less consumption and better environment friendliness; and (3) by using the ionic liquid instead of concentrated sulfuric acid, the nitration process is environment-friendly and has industrial application prospects.
Owner:YANCHENG TEACHERS UNIV
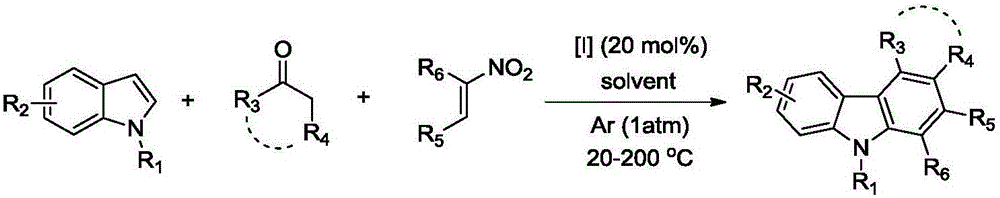
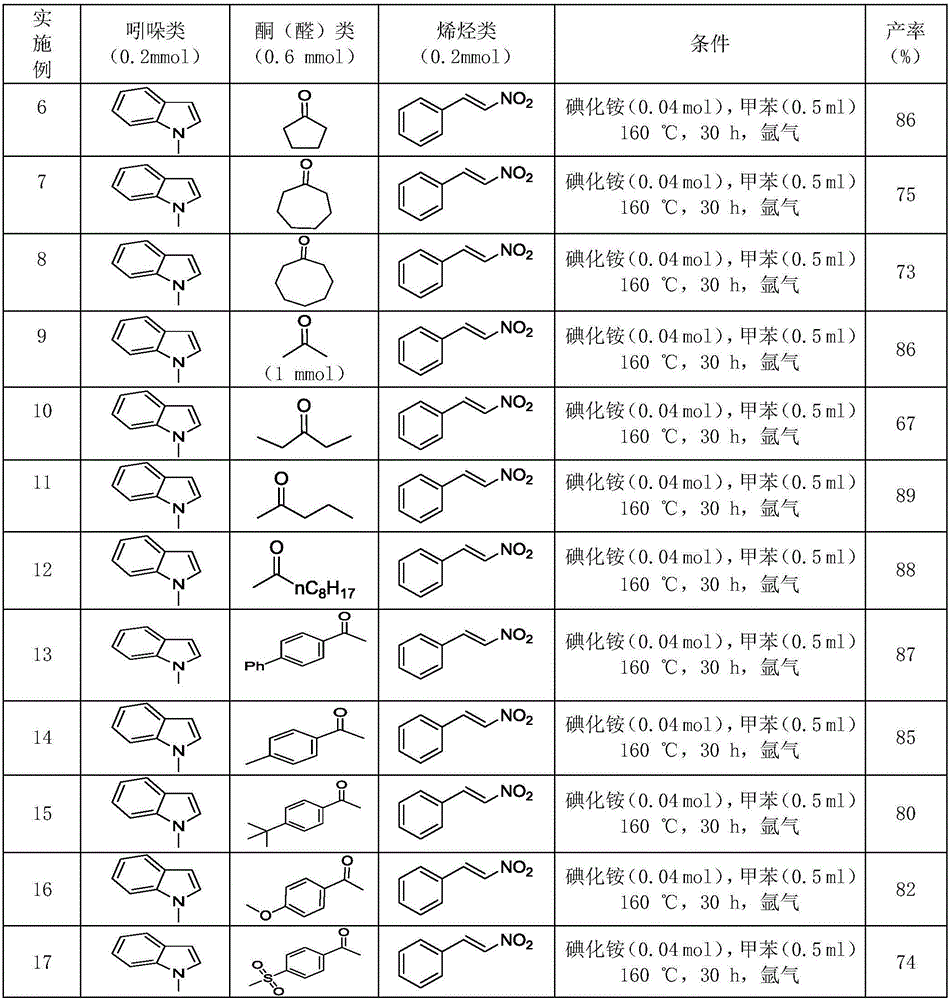
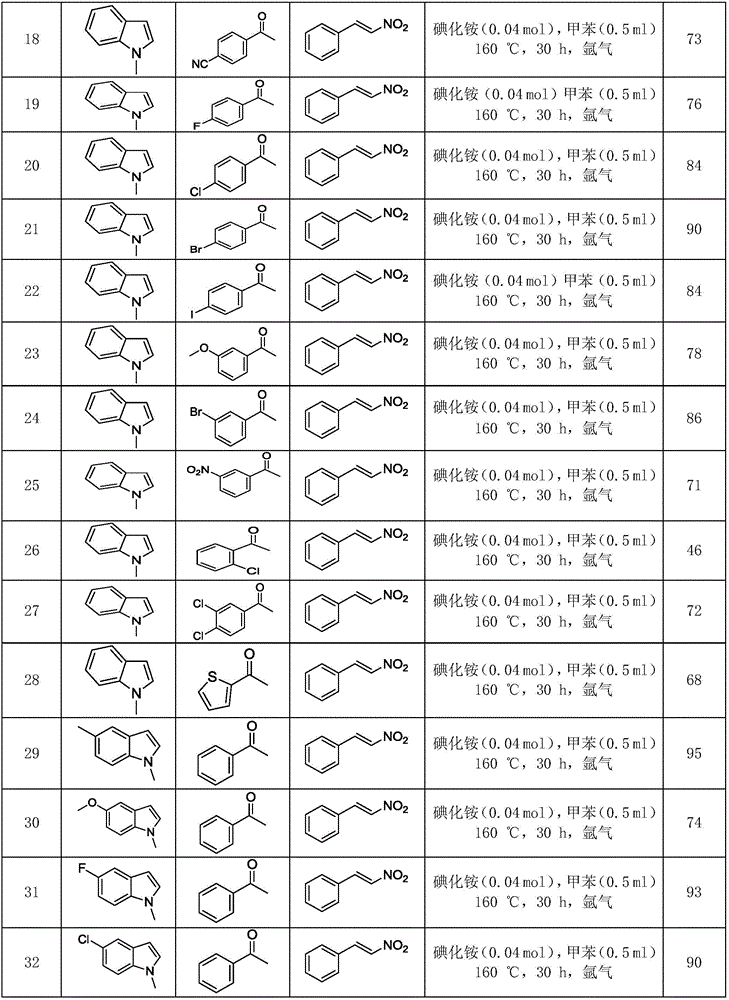
Polysubstituted carbazole, derivative and synthesis method thereof
ActiveCN106117113AReduce pollutionStable molecular structureOrganic chemistryKetoneMolecular recognition
The invention discloses polysubstituted carbazole, a derivative and a synthesis method thereof. The technical scheme includes that under inert gas shielding, three simple components including indole, alkene, and ketone (aldehyde) are adopted for selective synthesis of the polysubstituted carbazole and the derivative for the first time by adoption of ammonium iodide as a catalyst for the first time and adoption of acetonitrile, dichloroethane, tetrahydrofuran, N,N-dimethyl acetyl, benzene, chlorobenzene, orthodichlorobenzene, cyclohexane, 1,4-dioxane, anisole, benzonitrile, dimethylbenzene, trifluorotoluene, methylbenzene, trimethylbenzene and the like as organic solvents. The defect that an existing synthesis method is complex in synthesis steps and requires multi-step synthesis, tradition metal catalysts, chemical equivalent metal oxidizing agents and the like is overcome. The polysubstituted carbazole, the derivative and the synthesis method are widely applicable to various fields of photoelectricity, printing and dyeing, medicines, molecular recognition and the like and is especially suitable for research and development of metal-catalysis-free multi-component one-pot selective synthesis of polysubstituted carbazole compounds.
Owner:XIANGTAN UNIV
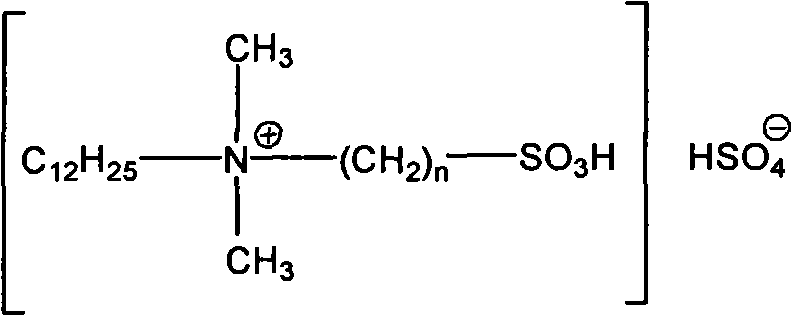
Clean nitration reaction of p-chlorobenzotrifluoride under catalysis of degradable functional ionic liquid
ActiveCN102249927AWide variety of sourcesEasy to manufactureOrganic-compounds/hydrides/coordination-complexes catalystsNitro compound preparationQuaternary ammonium cationP-chlorobenzotrifluoride
The invention discloses a new method of clean nitration reaction of p-chlorobenzotrifluoride under catalysis of degradable functional ionic liquid. Biodegradable ionic liquid of quaternary ammonium cation structure is used as a catalyst, p-chlorobenzotrifluoride and nitric acid are used as raw materials, and 4-chloro-3-nitrobenzotrifluoride is obtained by nitration reaction under the action of the catalyst. Compared with the prior art, the method has the advantages that: (1) because the degradable ionic liquid of quaternary ammonium cation structure is adopted, the raw material source is extensive, and the preparation is convenient; the catalyst has high activity, low consumption and water stability, is not inactivated and can be recycled; (2) the ionic liquid is biodegradable and environment-friendly; and (3) because the ionic liquid is used for substituting concentrated sulfuric acid, the method is an environment-friendly chemical process, and has good industrialized application prospect.
Owner:JIANGSU DAHUA CHEM IND
High-efficiency liquid phase chromatographic pre-column derivatization reagent for amino compound and detection method of amino compound
InactiveCN101726552AAccurate detectionSensitive detectionComponent separationColor/spectral properties measurementsRepeatabilityChromatography column
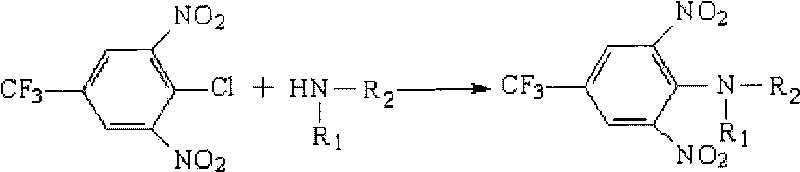
The invention discloses a high-efficiency liquid phase chromatographic pre-column derivatization reagent for an amino compound and a derivation condition thereof, which belong to the field of analysis and detection. In the invention, a derivatization reaction is carried out between 3,5-dinitro-4-chloro-trifluorotoluene(CNBF) derivatization reagent and the amino compound undergo derivatization, the detectability and sensitivity of the amino compound in a high-efficiency liquid phase chromatograph are improved, so that the purpose of rapid, accurate and sensitive detection of the amino compound is achieved. In the detection method of the invention, the derivatization is performed under mild conditions and at a high speed and avoids interference peaks, and the detection is accurate, flexible and good in repeatability. The detection method can effectively detect the amino compound and other medicinal or chemical amino compounds in food, feed and environment samples and has an actual promotion and application prospect.
Owner:CHINA AGRI UNIV
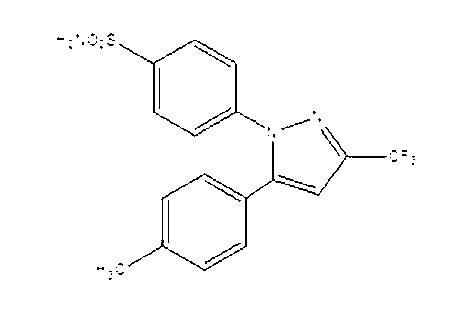
Preparation method for celecoxib
The invention relates to a preparation method for celecoxib, and belongs to the field of chemical pharmaceuticals. The method comprises the step of performing cyclization reaction on 4, 4, 4-trifloro-1-(4-tolyl)-1, 3-butanedione and p-sulfamine phenylhydrazine or 4-sulfamine phenylhydrazine hydrochloride in a solvent to obtain a celecoxib coarse product, wherein the solvent for cyclization reaction is a low molecular organic acid or a low molecular organic acid aqueous liquor. According to the method, the product is high in yield, good in purity, easy to purity, good in quality and low in cost; and the method is environment-friendly in condensation process, and is suitable for large-scale industrial production.
Owner:HENAN DONGTAI PHARM
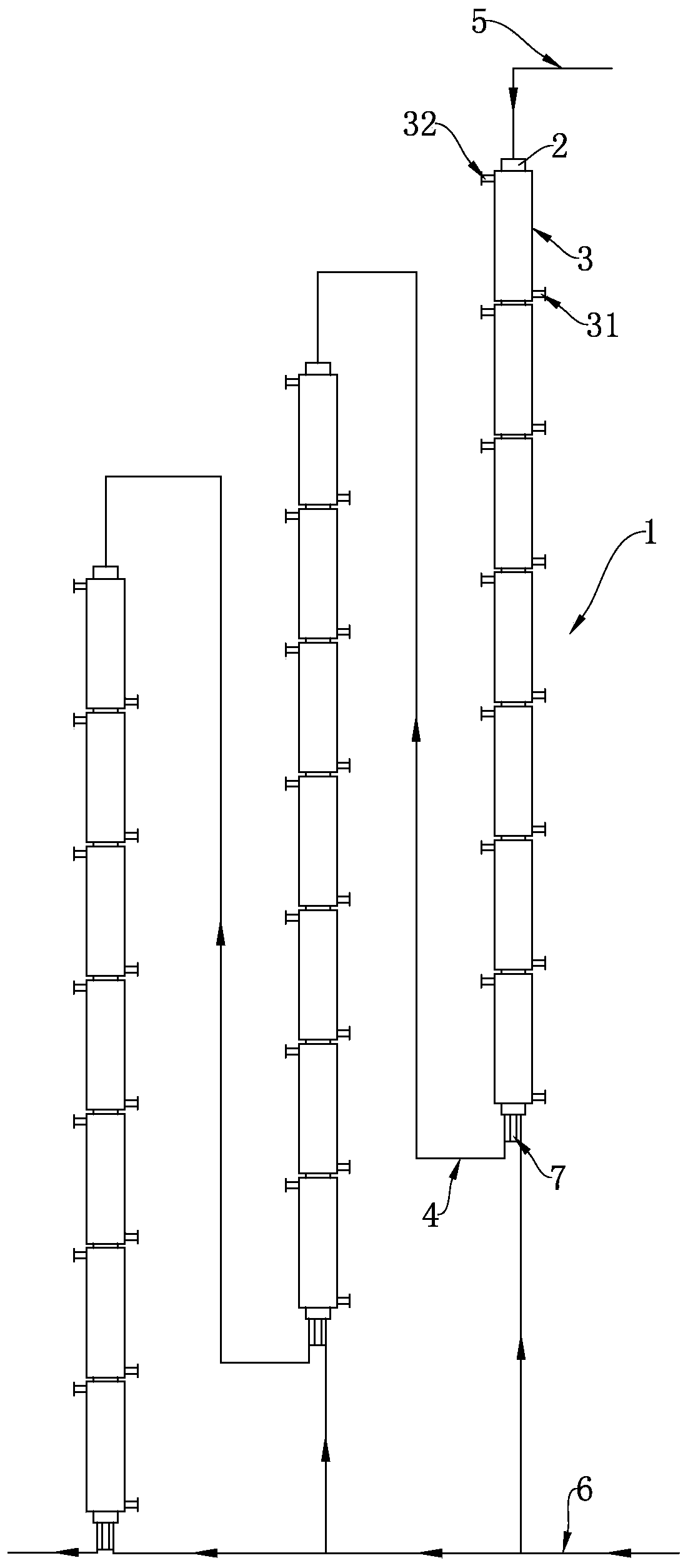
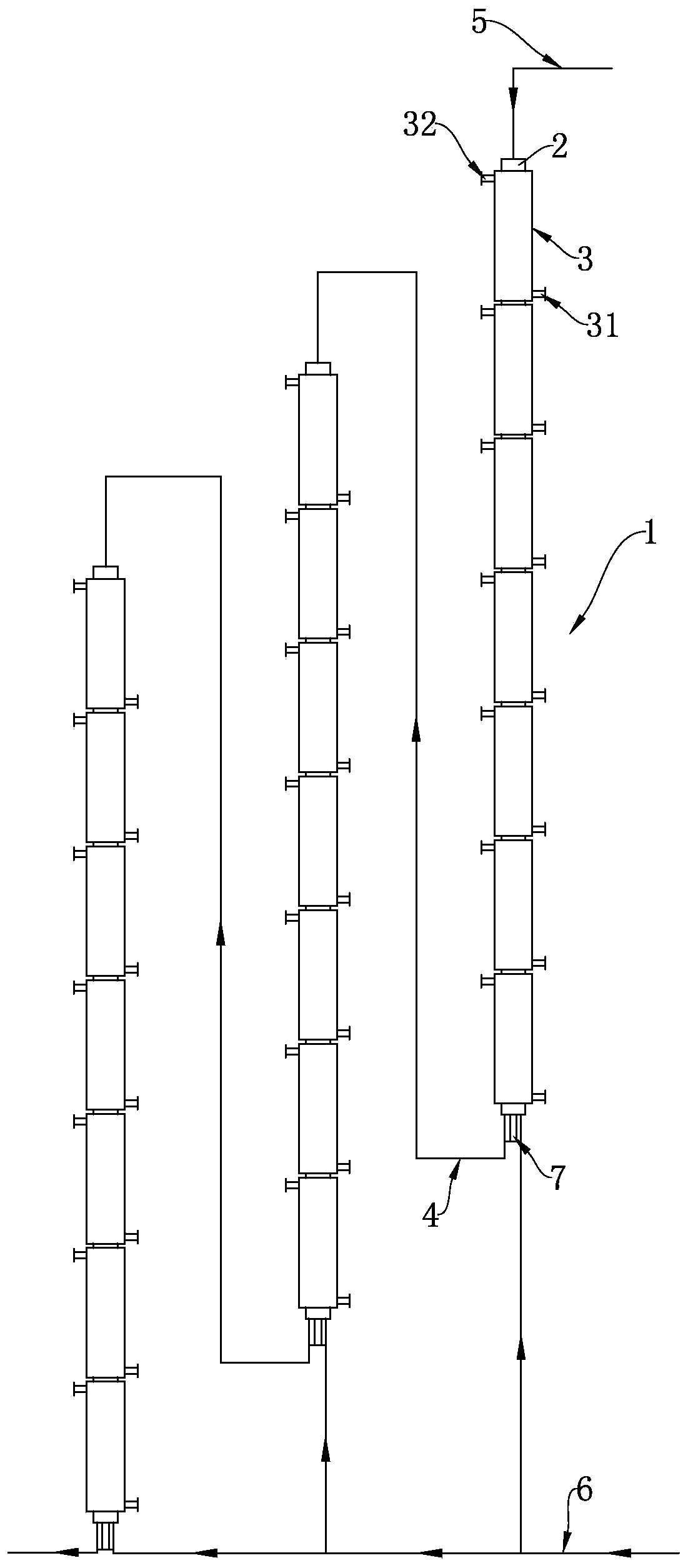
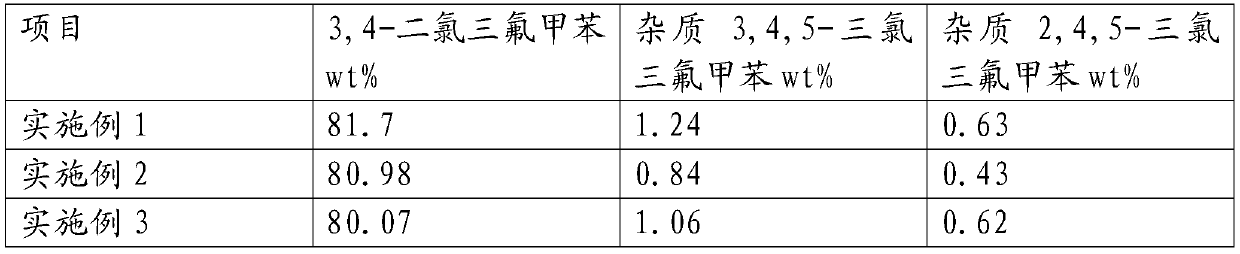
Continuous production method and continuous production equipment for 3, 4-dichloro-trifluorotoluene
ActiveCN109970507AAvoid heatingRealize continuous productionChemical/physical/physico-chemical stationary reactorsHalogenated hydrocarbon preparationHeight differenceToluene
The invention discloses a continuous production method and continuous production equipment for 3, 4-dichloro-trifluorotoluene. The production method comprises the steps that, chlorine and p-chloro-trifluorotoluene are used as raw materials for multi-stage continuous reaction, and the p-chloro-trifluorotoluene enters from the top of a first-stage tubular reactor; the chlorine enters from the bottomof a tubular reactor of each stage for countercurrent reaction; materials in the tubular reactor of each stage overflow from the bottom of the tubular reactor into a tubular reactor of next stage forcontinuous countercurrent reaction with the chlorine entering from the bottom of the tubular reactor; and the multi-stage continuous reaction adopts a step-by-step gradient cooling reaction mode. Theproduction equipment comprises multi-stage tubular reactors which are connected in series, wherein the multi-stage tubular reactors connected in series are arranged in a ladder shape with certain height differences; and an overflow pipeline is arranged between the bottom of an upper-stage tubular reactor and the top of a lower-stage tubular reactor. According to the production method and production equipment, continuous production can be achieved, moreover, reaction energy consumption is reduced, the reaction effect is good, reaction with less byproduct, and the product yield is high.
Owner:SHANDONG DOCRIS CHEM +1
Production method of 6-chloro-2-(trichloromethyl)pyridine
The invention relates to a production method of a nitrogen fertilizer synergist-6-chloro-2-(trichloromethyl)pyridine, belonging to the crop production field. The production method comprises the following steps: taking 2-methylpyridine as a staring material; in the presence of solvents such as chlorobenzene, dichlorobenzene, chloro-trifluorotoluene, nitrobenzene and the like, introducing excessive chlorine at the temperature of 130-205 DEG C for chlorination to obtain a crude product of the 6-chloro-2-(trichloromethyl)pyridine; and purifying the crude product through rectification to obtain the high-purity product of the 6-chloro-2-(trichloromethyl)pyridine. The production method can overcome the defects of low yield, easy generation of a tar polymer, pipe blockage caused by materials and the like of the existing production method, thus achieving the purpose of improving reaction selectivity and product yield, reducing waste discharge and being easy to be industrialized.
Owner:SHANDONG SHENGBANG LUNAN PESTICIDE
Topcoat compositions and methods of use thereof
A topcoat composition that includes a fluorine-containing polymer and a casting solvent selected from the group consisting of α,α,α-trifluorotoluene, 2,2,3,3,4,4,5,5-octafluoropentyl-1,1,2,2-tetrafluoroethyl ether (OFP-TFEE), and a mixture consisting of a hydrophobic alkane and an alcohol is provided. Also provided is method of forming an image on a photoresist that includes forming a photoresist over a substrate; applying a topcoat composition, the topcoat composition comprising at least one fluorine-containing polymer and a casting solvent, onto the photoresist; removing the casting solvent of the topcoat composition resulting in the formation of a topcoat material over the photoresist; exposing the photoresist to radiation, the radiation changing a chemical composition of the regions of the photoresist exposed to the radiation, forming exposed and unexposed regions in the photoresist; and removing i) the topcoat material and ii) the exposed regions of the photoresist or the unexposed regions of the photoresist.
Owner:TAIWAN SEMICON MFG CO LTD
Preparation method of 3,5-dihalobenzotrifluoride and 3'-chloro-5'-(trifluoromethyl)phenyltrifluoroethanone
ActiveCN112110790AImprove economyReduce manufacturing costOrganic compound preparationMagnesium organic compoundsTrifluoromethylationMethyl benzene
The invention relates to the technical field of chemical pharmacy, particularly to a preparation method of 3,5-dihalobenzotrifluoride. According to the preparation method, 3,5-dihalogen-4-amino trifluorotoluene is used as a raw material and is subjected to diazotization deamination reaction to obtain the 3,5-dihalobenzotrifluoride, so that the production cost is relatively low, and the economic effect is relatively good. The invention further relates to a preparation method of 3'-chloro-5'-(trifluoromethyl)phenyltrifluoroethanone. According to the preparation method, 3,5-dihalobenzotrifluorideis used as a raw material, and is subjected to a Grignard reagent reaction, then a nucleophilic addition reaction is carried out with and a trifluoromethylation reagent, and a good economic effect isalso achieved.
Owner:TAIZHOU ABSOBIOTEC CO LTD
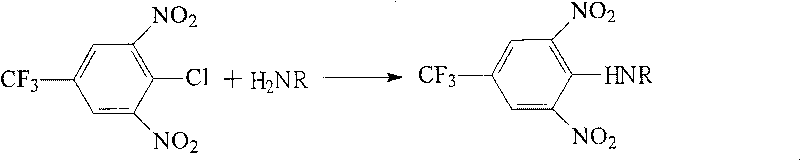
Production process of p-trifluoromethyl aniline
ActiveCN1847214AOrganic-compounds/hydrides/coordination-complexes catalystsChemical recyclingPotassium fluorideAniline
The present invention discloses the production process of p-trifluoromethyl aniline. P-trifluorotoluene and liquid ammonia are reacted in the presence of alcohol and catalyst to obtain p-trifluoromethyl aniline. The catalyst may consist of ferrocene, triphenyl phosphor, potassium fluoride, ammonium tetrabutyl bromide. The present invention has high efficient composite catalyst used, single pass conversion rate up to 66 % and yield up to 90 %. The organic solvent may be used circularly, the excessive liquid ammonia may be prepared into ammonia water, the catalyst may be regenerated and reused, and the process has no waste water exhausted.
Owner:江苏优普生物化学科技股份有限公司
Method for preparing trifluoromethoxybenzene
ActiveCN103553884AAddress toxicityLow toxicityOrganic chemistryOrganic compound preparationHydrogen fluorideParylene
The invention discloses a method for preparing trifluoromethoxybenzene. The method comprises the following steps: (1) introducing chlorine into the raw material of benzaldehyde or a mixture of benzaldehyde and parylene for chlorination; (2) carrying out fluoridation on the chlorination product prepared in the step (1) with anhydrous hydrogen fluoride to obtain trifluoromethoxybenzene and paradibenzenyl. The method has the advantage that trifluoromethoxybenzene and paradibenzenyl are prepared by using benzaldehyde or the mixture of benzaldehyde and parylene as the raw material and paradibenzenyl as a solvent, and reacting under the condition of initiator without illumination. The bi-product of paradibenzenyl can be recycled as a solvent for chlorination or sold as a product. As the paradibenzenyl is low in toxicity and environment-friendly, the problem that carbon tetrachloride is high in toxicity can be completely solved. Besides, the paradibenzenyl is better in symmetry than trifluorotoluene, chlorobenzotrifluoride and chlorobenzotrifluoride, so that the paradibenzenyl is weaker in polarity and can be easily separated from target products.
Owner:KINGCHEM LIAONING CHEMICAL CO LTD
Method for preparing 2-bromine-5-fluorobenzotrifluoride
ActiveCN105152853AReduce dosageEasy to buyHalogenated hydrocarbon preparationSocial benefitsPtru catalyst
The invention discloses a method for preparing 2-bromine-5-fluorobenzotrifluoride. According to the preparation method, fluorobenzotrifluoride serves as a raw material, under the condition of concentrated sulfuric acid and composite catalysts, bromine is stirred and added dropwise, and 2-bromine-5-fluorobenzotrifluoride is obtained after separation and purification. The yield reaches over 91.1%, the reaction conversion rate is larger than 99%, and selectivity is 92-95%. The raw material applied in the reaction process is convenient to purchase, production cost is low, waste acid obtained in the reaction process can be recycled, post-processing is easy, and the whole technology does not generate a large amount of wastewater containing salt and organic matter. Economic benefits and social benefits are good.
Owner:JIANGSU YONGCHUANG PHARMA TECH CO LTD +1
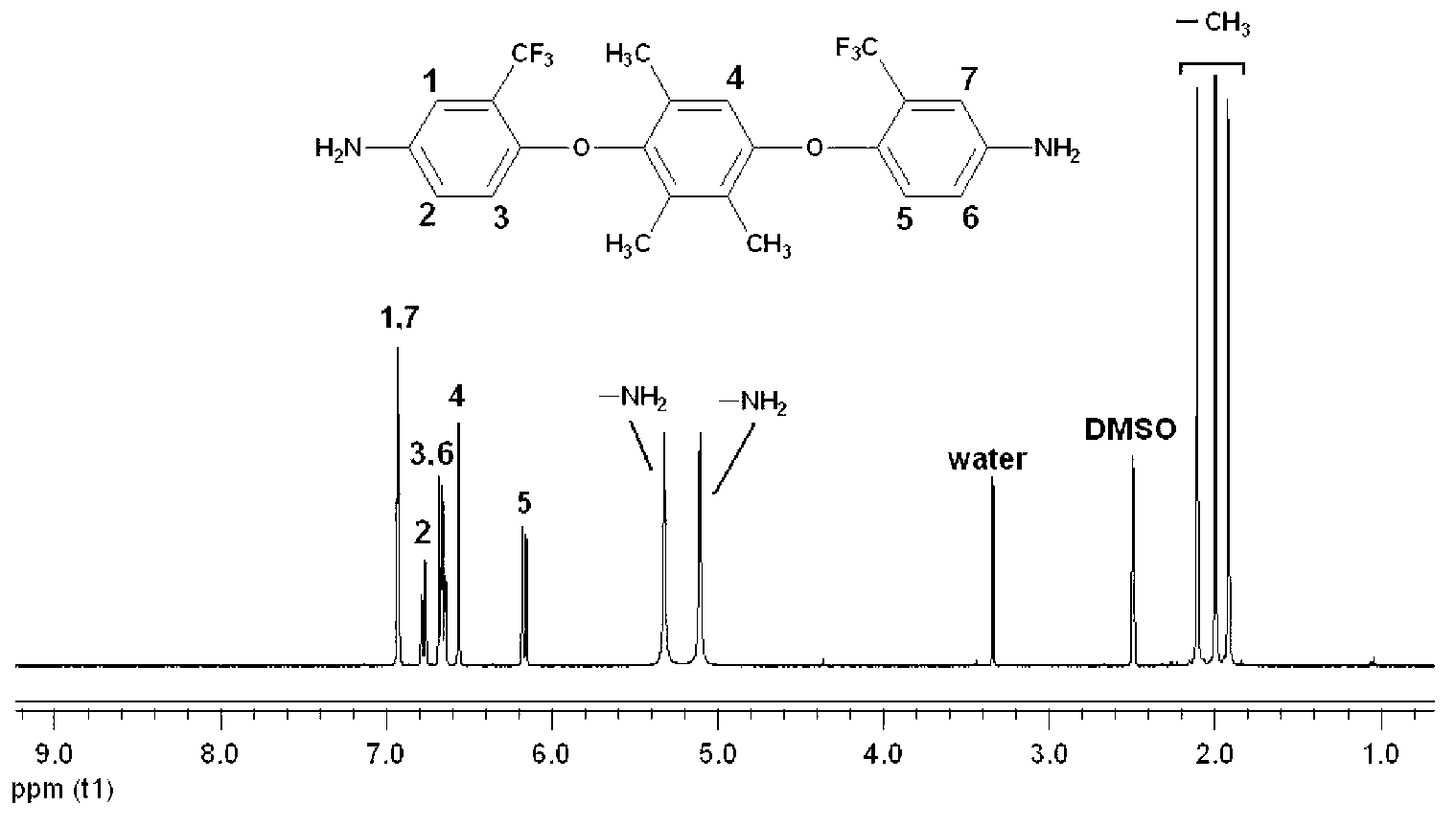
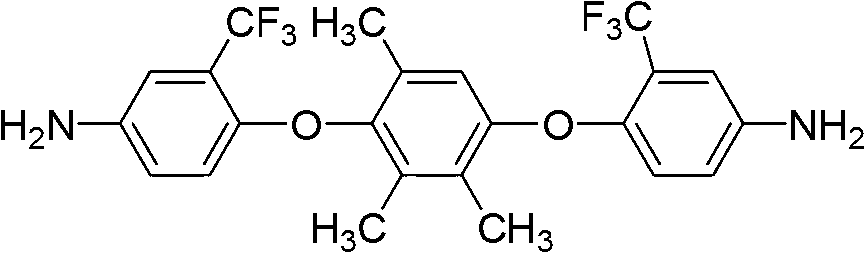
Double trifluoromethyl substituent-containing asymmetric aromatic diamine monomer and preparation method thereof
InactiveCN102796015AThe synthetic route is simpleHigh yieldOrganic compound preparationAmino-hyroxy compound preparationNitro compoundEthyl Chloride
A double trifluoromethyl substituent-containing asymmetric aromatic diamine monomer is 1,4-bis(4-amino-2-trifluoromethylphenoxy)-2,3,5-trimethylbenzene. The synthetic route of the monomer is simple, the yield is high, the purification is easy, and the monomer is stable at room temperature and can be used for the preparation of soluble fluorine-containing polyimide polymers. The preparation method of the double trifluoromethyl substituent-containing asymmetric aromatic diamine monomer includes the following steps: reacting 2,3,5-trimethylhydroquinone with 2-chloro-5-nitrobenzotrifluoride under alkaline conditions with the protection of nitrogen, sufficiently stirring, precipitating and leaching, washing the product with hot water, further recrystallizing to get a fluorine-containing dinitrocompound which is 1,4-bis(4-nitro-2-trifluoromethylphenoxy)-2,3,5-trimethylbenzene, and reacting the fluorine-containing dinitrocompound with a catalyst and a reducing agent in the presence of an organic solvent to get 1,4-bis(4-amino-2-trifluoromethylphenoxy)-2,3,5-trimethylbenzene by reduction.
Owner:CHANGZHOU UNIV +1
Preparation method of 2-bromine-5-fluorine trifluorotoluene
ActiveCN104610015ALow priceFew reaction stepsHalogenated hydrocarbon preparationOrganic synthesisBromine
The invention discloses a method for preparing 2-bromine-5-fluoro-benzo-trifluoride, and belongs to the field of organic synthesis. The method comprises the following steps: firstly, mixing sulfuric acid with fluorobenzotrifluoride, and further adding bromated into the obtained mixture at 0-80 DEG C to react, thereby obtaining 2-bromine-5-fluorobenzotrifluoride. Through the adoption of the method, the yield can be greater than 90.0%, the purity is greater than 99.0%, the raw materials are easy to obtain, and the reaction condition is mild and easy to control.
Owner:ADAMA HUIFENG (JIANGSU) LTD
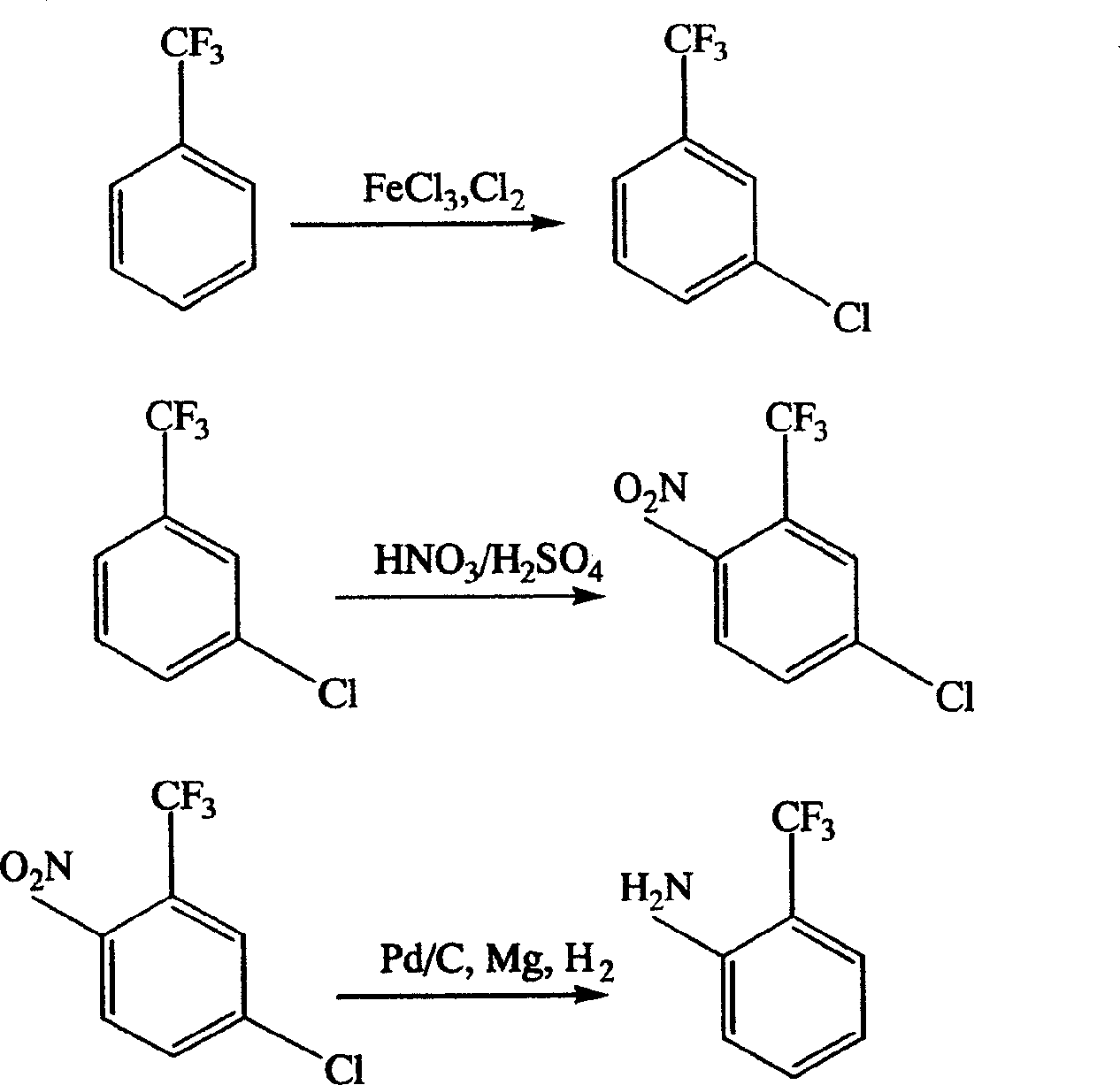
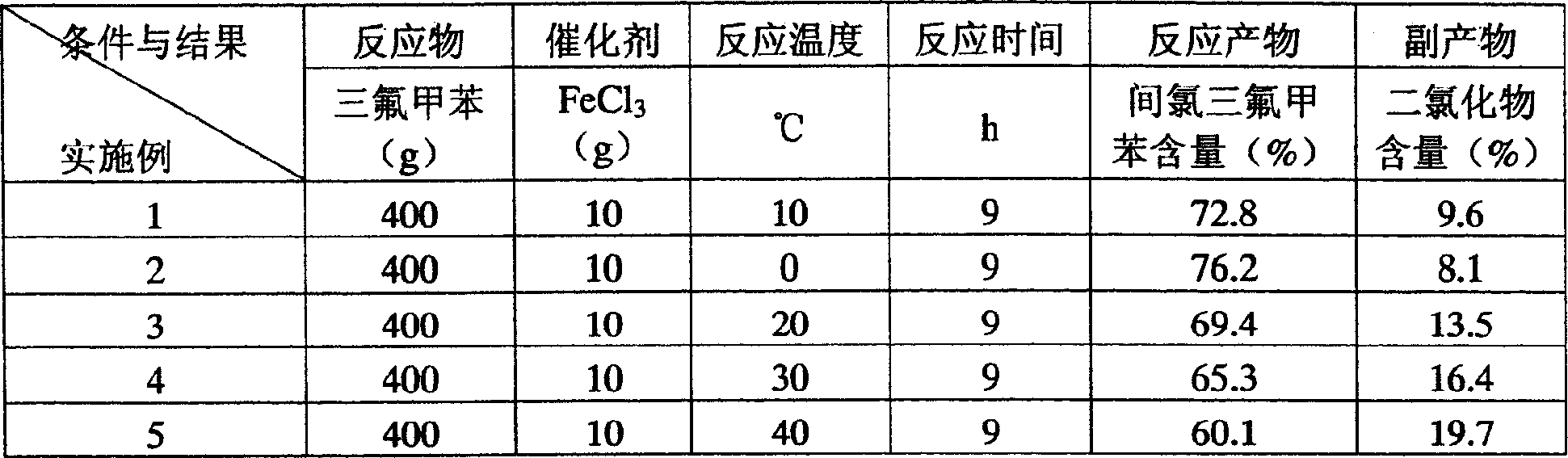
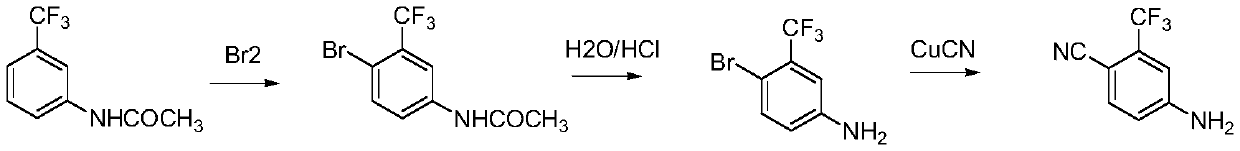
Process for preparing o-trifluoromethyl aniline
ActiveCN1666974AAdvanced technologyHigh yieldOrganic compound preparationAmino compound preparationNitrationAniline
This invention is a preparation method of near trifluoromethyl aniline, the material is benzotrifluoride, and it is generated into compound of between chlorine benzotrifluoride and its isomer through circle chlorination reaction. Then the got compound is generated into compound of 2-nitryl-5-chlorine benzotrifluoride and its isomer through nitration. Finally the compound after nitration is proceeded by catalytic hydrogenation-hydrogenolysis dechlorination reaction to generate three isomers compound which is mainly near trifluoromethyl aniline, then pure near trifluoromethyl aniline is got after rectification. The synthesis line is short, technique is advanced, and result is good and easy to be industrial generated.
Owner:ZHEJIANG WEIHUA NEW MATERIAL CO LTD
Synthetic method of beflubutamid
InactiveCN102766067ARaw materials are cheap and easy to getMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationEthyl butyrateNitration
The invention discloses a synthetic method of beflubutamid. The synthetic method includes that fluorobenzotrifluoride and the like are used as initial raw materials, 4-fluorine-3-trifluoromethylphenol is obtained through nitration, reduction, diazotization and hydrolysis, the 4-fluorine-3-trifluoromethylphenol is reacted with alkylating agent 2-bromine ethyl butyrate to obtain 2-(4-fluorine-3 trifluoromethyl phenoxyl) ethyl butyrate, and the 2-(4-fluorine-3 trifluoromethyl phenoxyl) ethyl butyrate is reacted with benzylamine to obtain the beflubutamid. Compared with a traditional synthetic method of the beflubutamid, the synthetic method of the beflubutamid is cheap in raw material, easy to obtain the raw material, temperate in reaction condition and relatively high in total yield.
Owner:太仓市运通化工厂
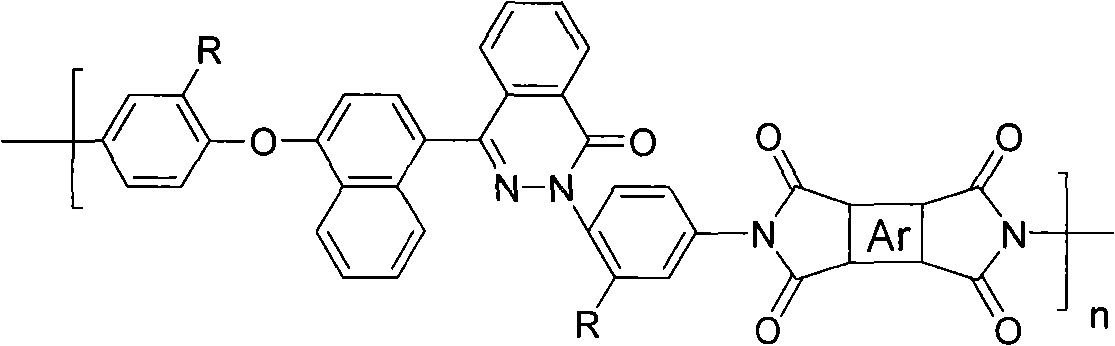
Polysiloxane-polyarylester block copolymer, preparation thereof and applications thereof
ActiveCN103524749ALower surface energyImprove hydrophobicityPharmaceutical containersMedical packagingHemocompatible MaterialsPolymer science
The invention discloses a polysiloxane-polyarylester block copolymer, preparation thereof and applications thereof. The polysiloxane-polyarylester block copolymer is showed in the following formula. The polysiloxane-polyarylester block copolymer has low surface energy, strong hydrophobicity and an obvious microphase separation structure, and has great application value in the field of blood compatible materials. According to a preparation process of the block copolymer, a fluorine-containing / fluorine-free polyarylester having terminal alkenes, and a fluorine-containing / fluorine-free polysiloxanes having terminal hydrogens are used as raw materials, one or over two types of tetrahydrofuran, isopropanol, tolune and benzotrifluoride are used as solvent; and the block polymer is synthesized by hydrosilylation under the catalytic action of a catalyst 1,3-divinyl-1,1,3,3-tetramethyldisiloxane platinum (0) or chloroplatinic acid. The synthetic method is simple with mild and controllable reaction conditions, and is prone to industrial production.
Owner:UNIV OF JINAN
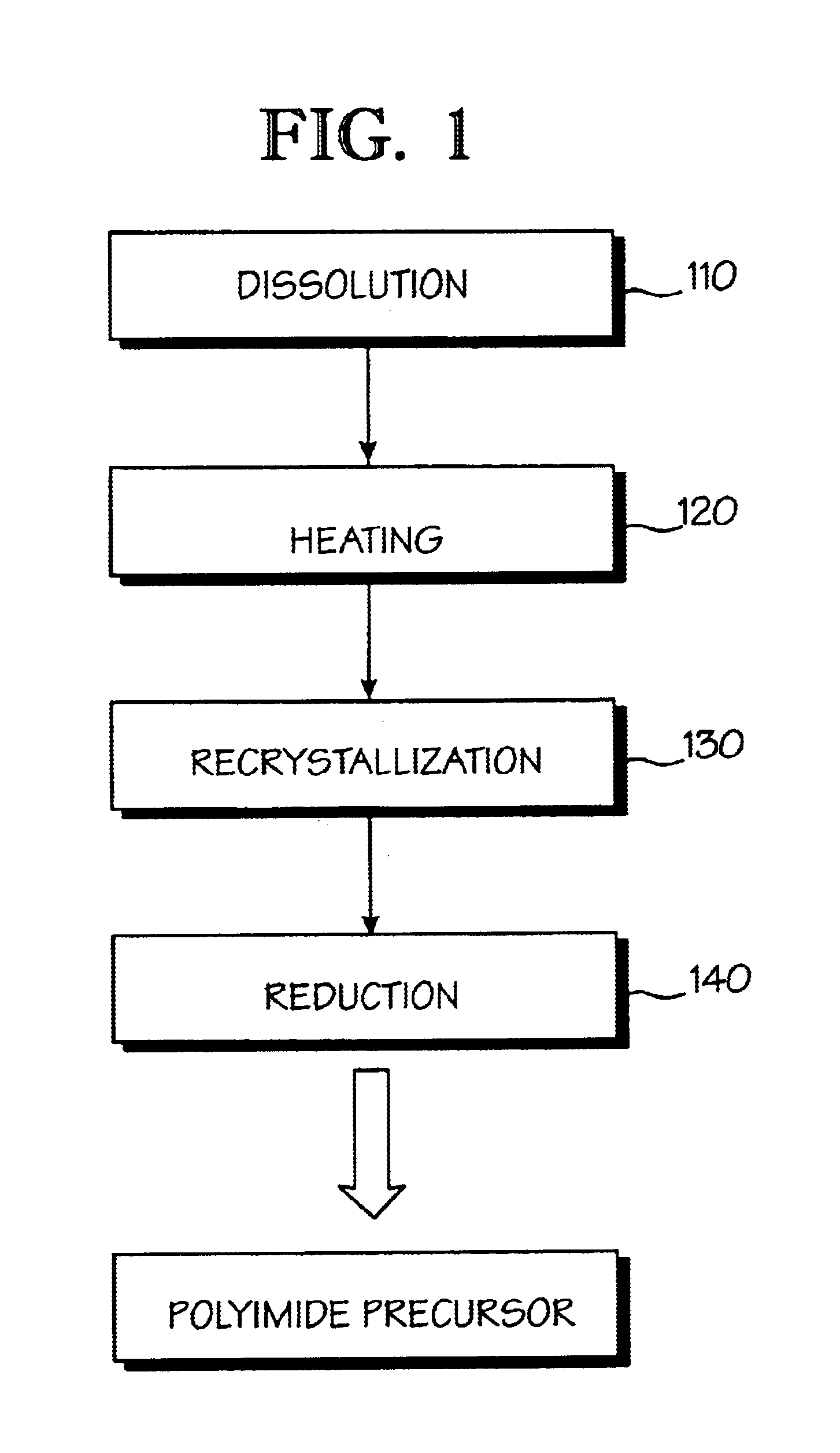
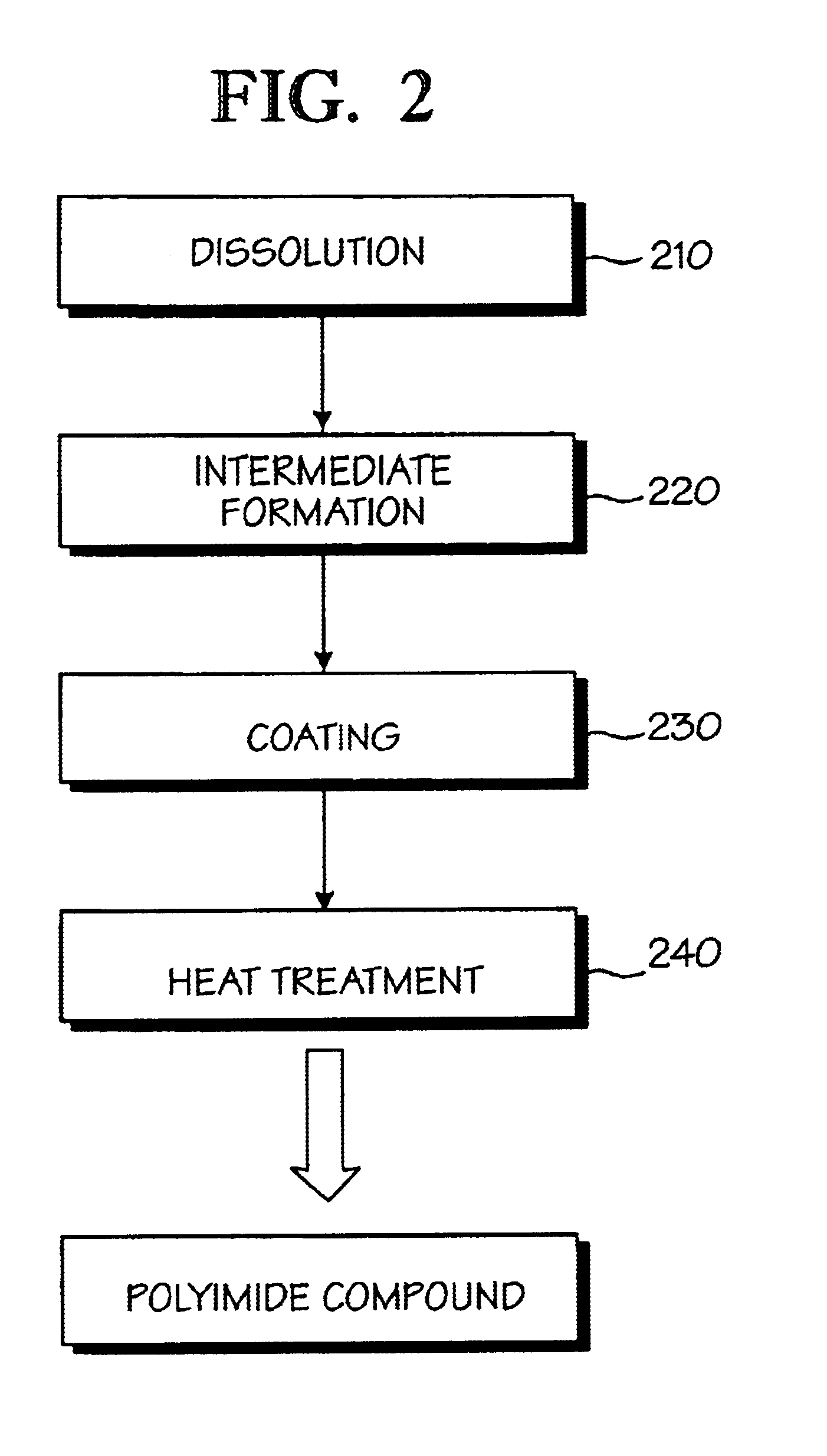
Optical polyimide precursor, optical polyimide compound and fabricating method thereof
InactiveUS6891067B2Avoid crackingReduce absorption lossOrganic compound preparationDiaryl/thriaryl methane dyesNitro compoundAromatic moiety
The present invention provides an optical polyimide precursor for use in making a polyimide. The precursor is defined by the following formula: wherein X is Cl, Br, oxo-halide, or fully halogenated alkyl, and A is a divalent aromatic or halogenated aromatic moiety. The present invention provides a method of preparing a diamine compound for use as an optical polyimide precursor. The method includes the steps of dissolving 2-chloro-5-nitrobenzotrifluoride and a diol in N,N-dimethylacetamide to form a solution, adding potassium carbonate, tert-butylammonium chloride and copper powder to said solution and heating the resulting mixture, removing the copper, precipitating and recrystallizing a dinitro-compound resulting from heating the mixture, and dissolving the dinitro-compound and reducing the dinitro-compound to yield a diamine compound.
Owner:SAMSUNG ELECTRONICS CO LTD
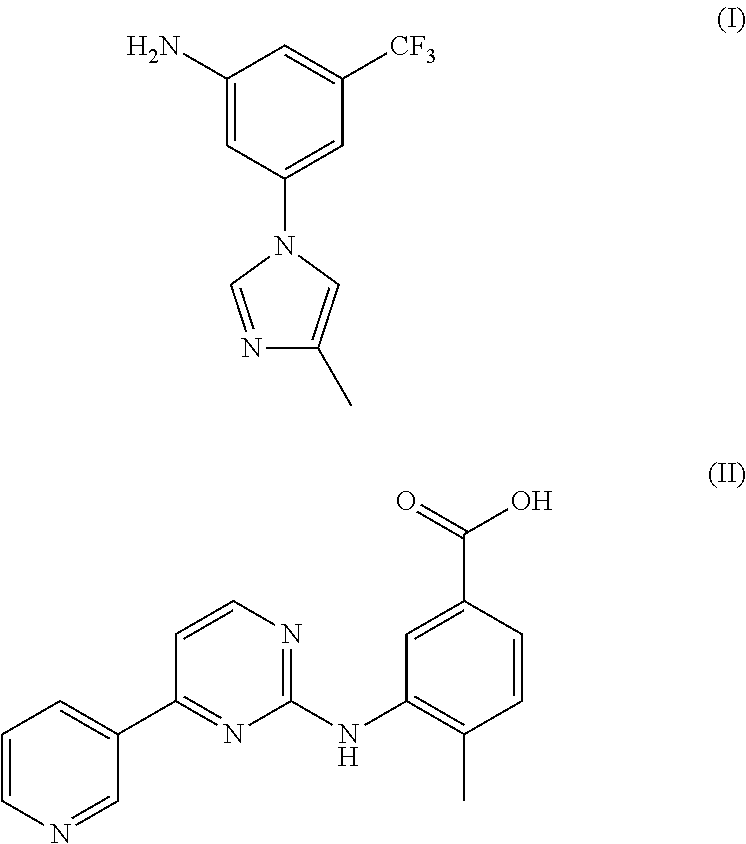
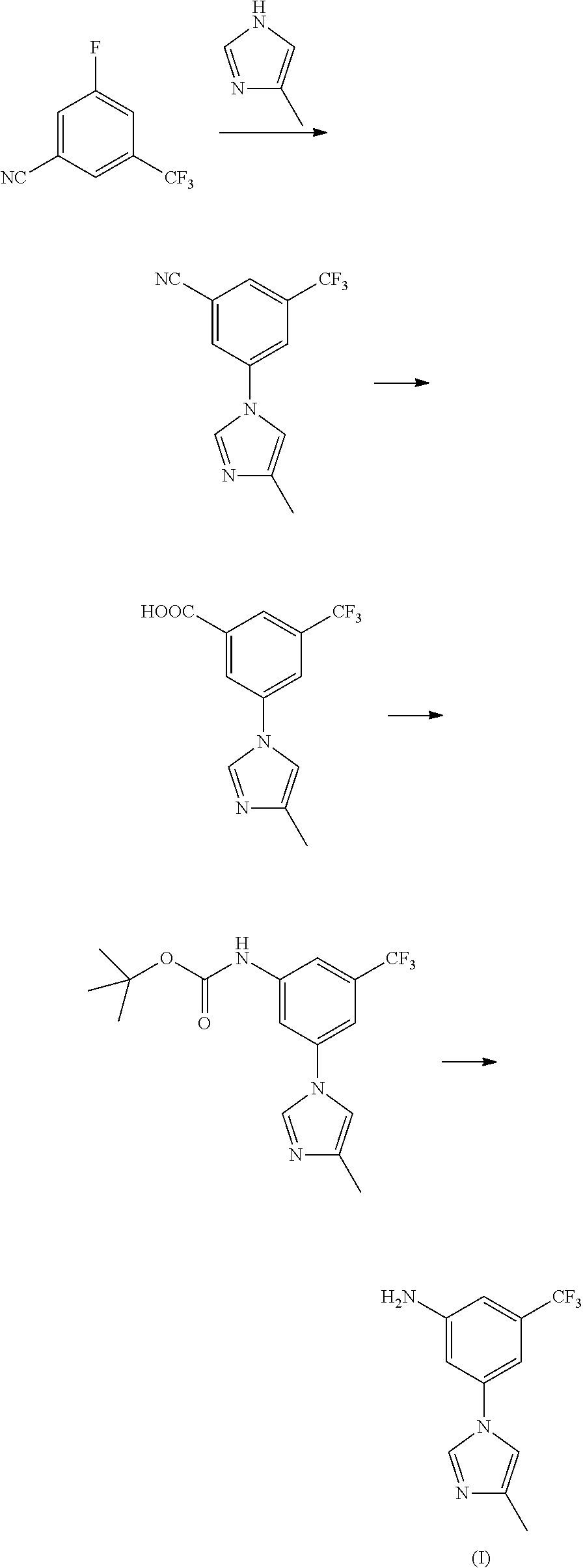
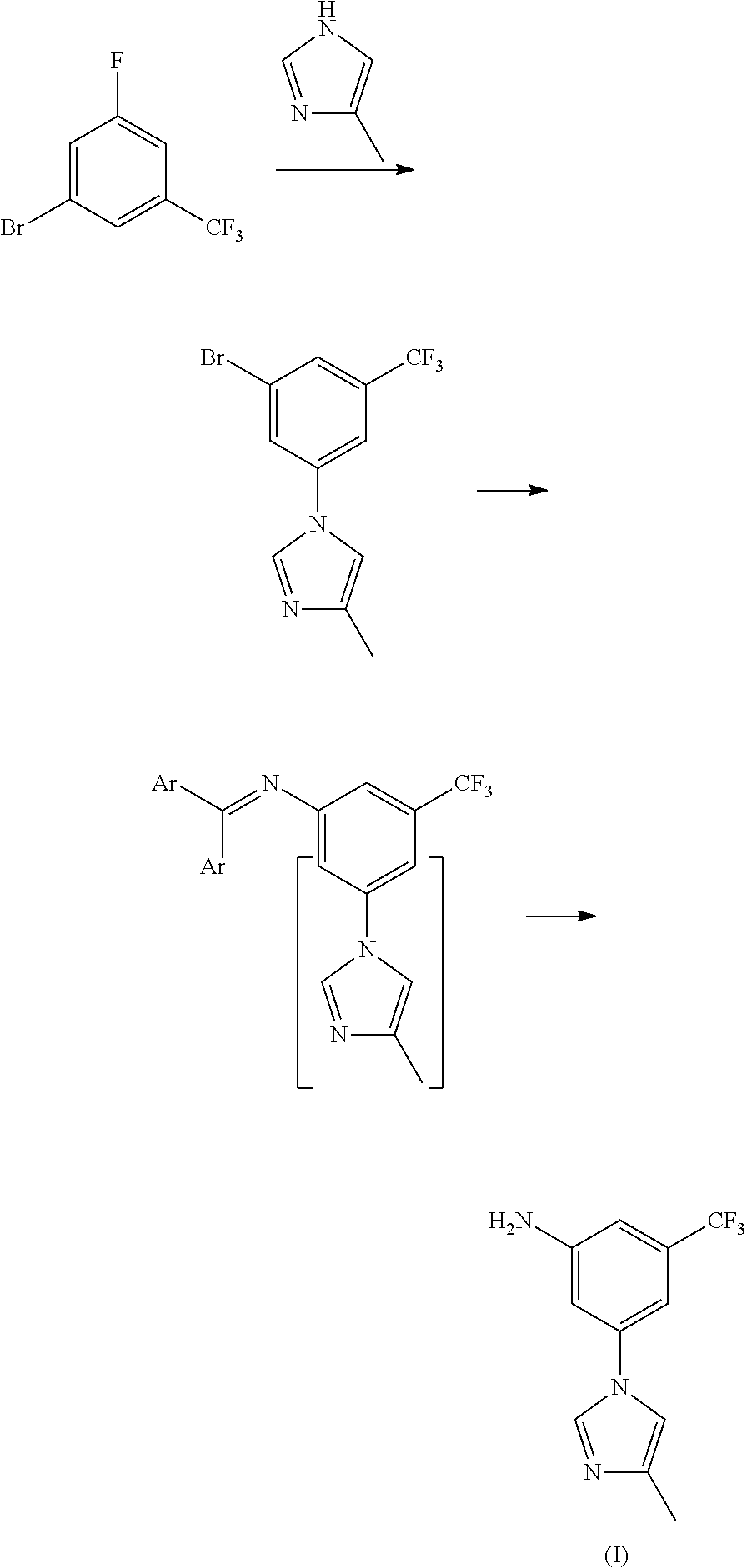
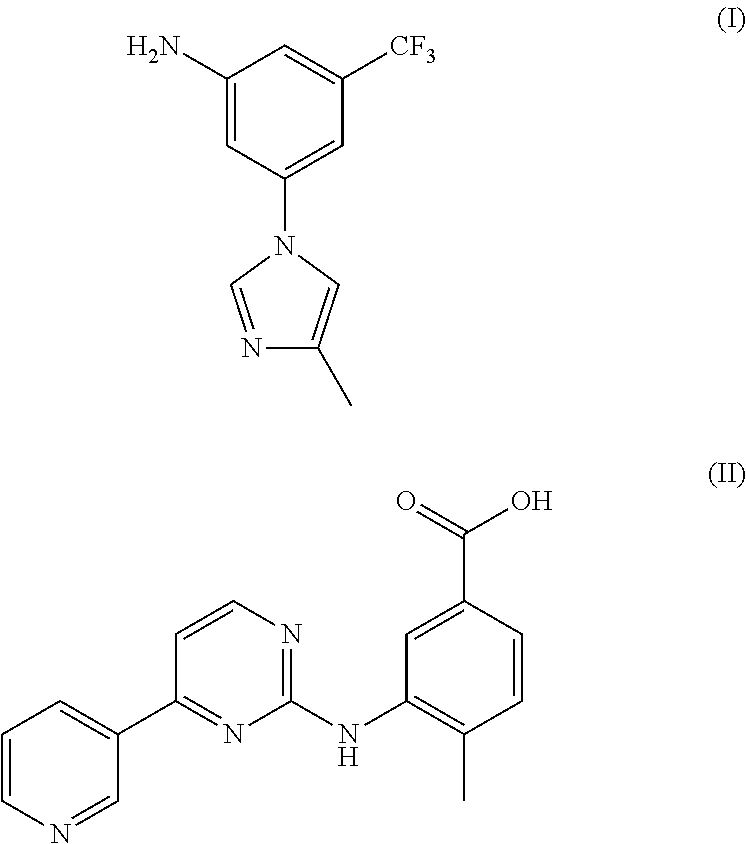
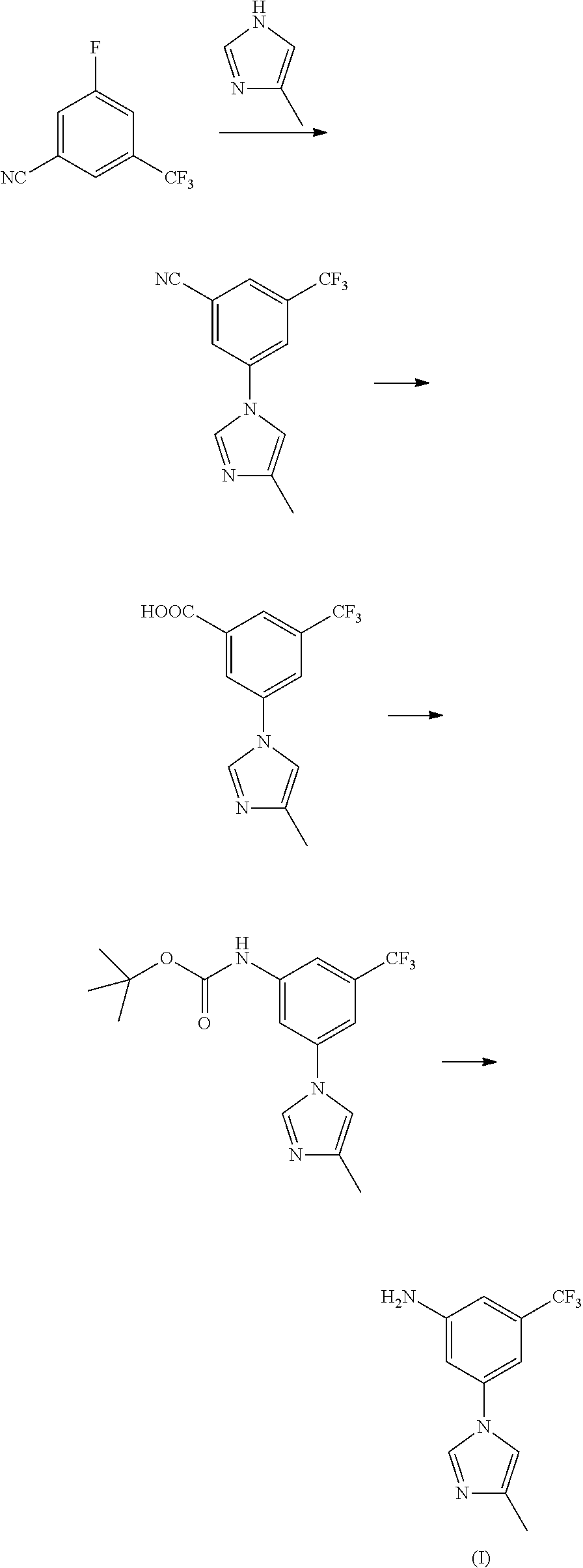
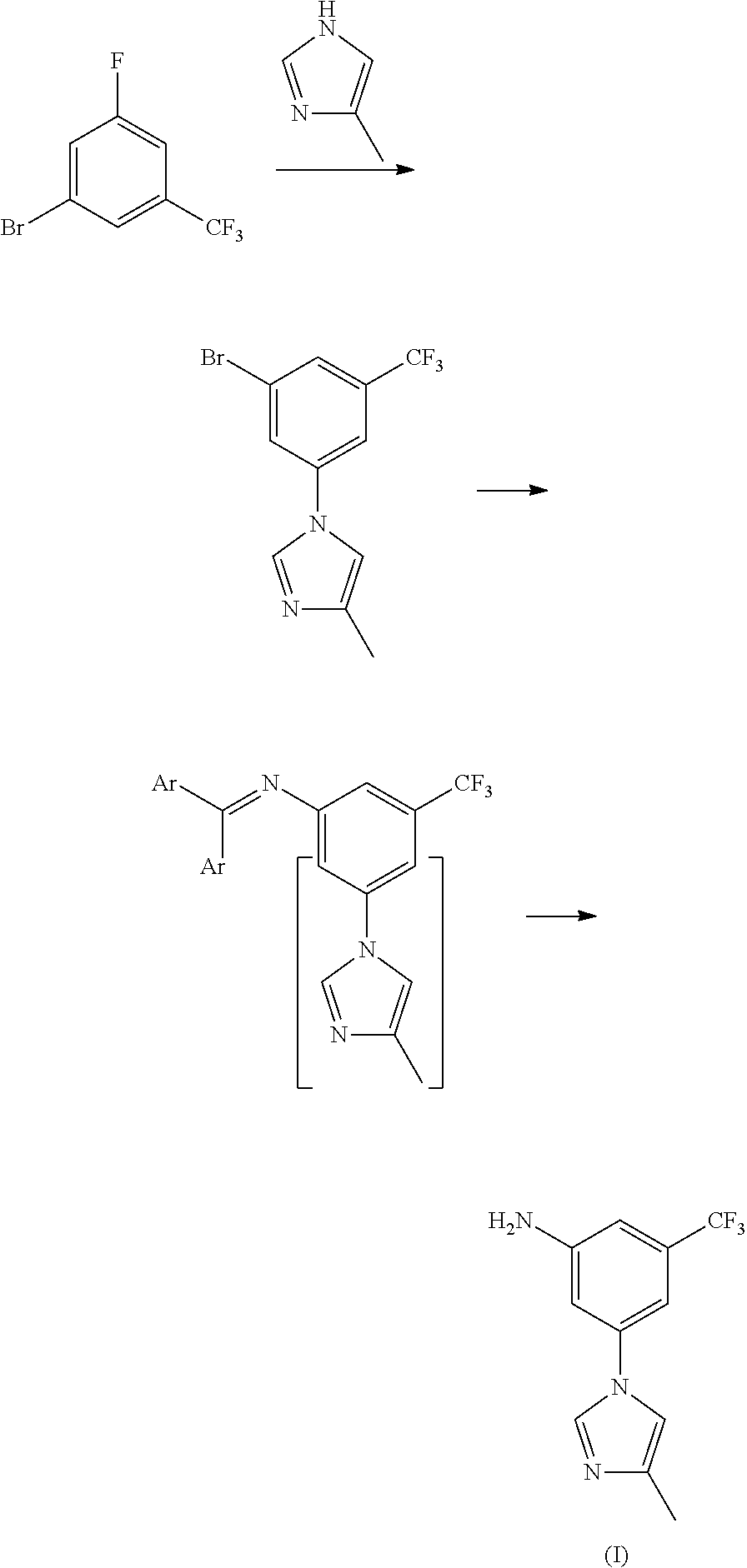
Method for preparing nilotinib intermediate
Disclosed in the present invention is a method for preparing nilotinib intermediate 3-(4-methyl-1H-imidazol-1-yl)-5trifluoromethyl phenylamine (I). The method comprises the following steps: taking trifluorotoluene as an initial material, and preparing the nilotinib intermediate (I) by nitration, bromization, condensation and reduction successively Compared with the prior art, the preparation method has the following advantages: a relatively high yield, the raw materials are easily obtained, a concise process and few side reactions, and is adapted to industrial production, so the development of an economic technology of the bulk drug is pro moted.
Owner:SUZHOU LIXIN PHARMA
Method for preparing nilotinib intermediate
Disclosed in the present invention is a method for preparing nilotinib intermediate 3-(4-methyl-1H-imidazol-1-yl)-5trifluoromethyl phenylamine (I). The method comprises the following steps: taking trifluorotoluene as an initial material, and preparing the nilotinib intermediate (I) by nitration, bromization, condensation and reduction successively Compared with the prior art, the preparation method has the following advantages: a relatively high yield, the raw materials are easily obtained, a concise process and few side reactions, and is adapted to industrial production, so the development of an economic technology of the bulk drug is promoted.
Owner:SUZHOU LIXIN PHARMA
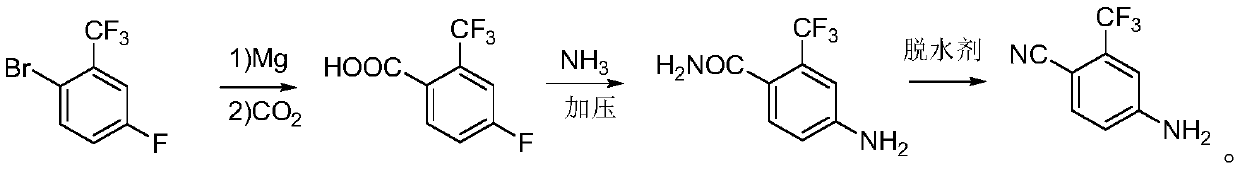
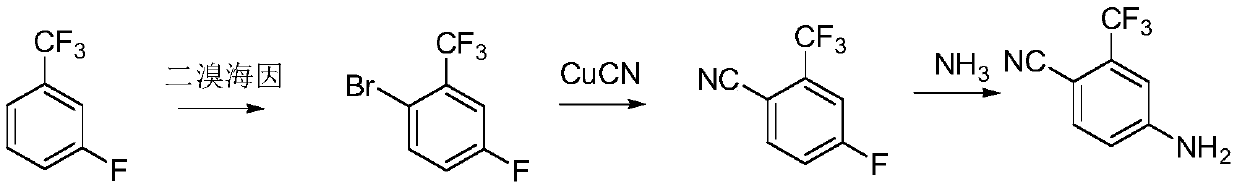
Preparation method of 4-amino-2-trifluoromethyl benzonitrile
The invention relates to the technical field of chemical engineering, in particular to a preparation method of 4-amino-2-trifluoromethyl benzonitrile, which comprises the following steps: after 2-bromo-5-fluorobenzotrifluoride is subjected to a Grignard reaction, feeding carbon dioxide, and hydrolyzing to obtain 4-fluoro-2-trifluoromethyl benzoic acid; adding the 4-fluoro-2-trifluoromethyl benzoicacid into a pressure kettle, feeding liquid ammonia, and reacting under the action of a catalyst to generate 4-amino-2-trifluoromethyl benzamide; heating and dehydrating the 4-amino-2-trifluoromethylbenzamide with a dehydrating agent to generate the 4-amino-2-trifluoromethyl benzonitrile. According to the preparation method, highly toxic cuprous cyanide is not adopted, so that the safety risk ofproduction is reduced, and three wastes do not contain cyanide ions or a large amount of copper ions, are easier to treat and have small harm to the environment.
Owner:常州沃腾化工科技有限公司
Asymmetric aromatic diamine having naphthalenone binaphthyl structure, preparation and use thereof
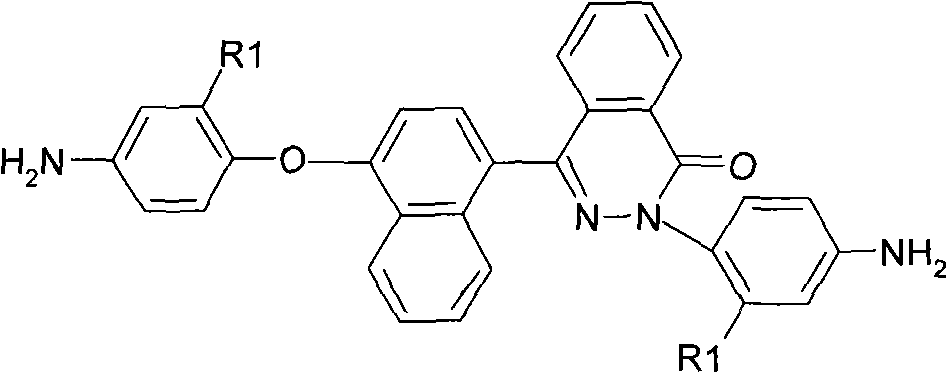
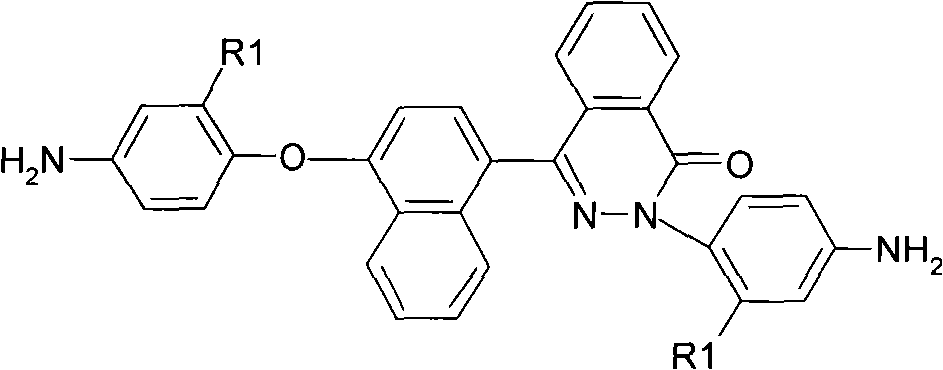
The invention relates to asymmetric aromatic diamine containing the structure of phenodiazine ketone binaphthyl and a preparation method and application thereof. The general structure is as the right formula and the preparation is as follows: 1) 4-(4-hydroxy naphthyl)-2, 3-naphthyridine-1-ketone and parachloronitrobenzene or 2-chlorine-5-nitro benzotrifluoride with molar ratio of 1:2 react under alkaline condition to obtain a binitro compound; 2) the binitro compound is reduced by palladium-charcoal and hydrazine hydrate with the existence of an organic solvent and the diamine is obtained. The application is to prepare polyimide. The asymmetric aromatic diamine containing the structure of phenodiazine ketone binaphthyl prepared by the invention has high purity and is stable under room temperature; polyimide prepared hereby has excellent solubility, high temperature resistance, mechanical property, film forming ability, optical property and other excellent comprehensive performances.
Owner:DONGHUA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com