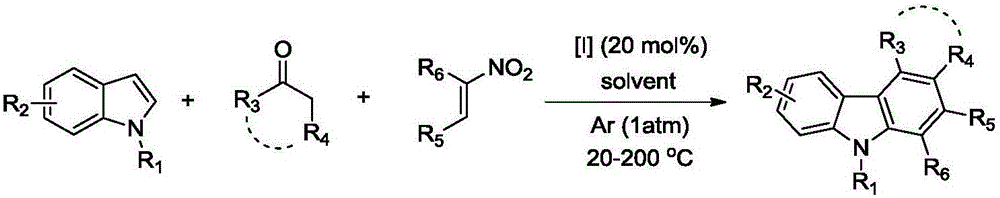
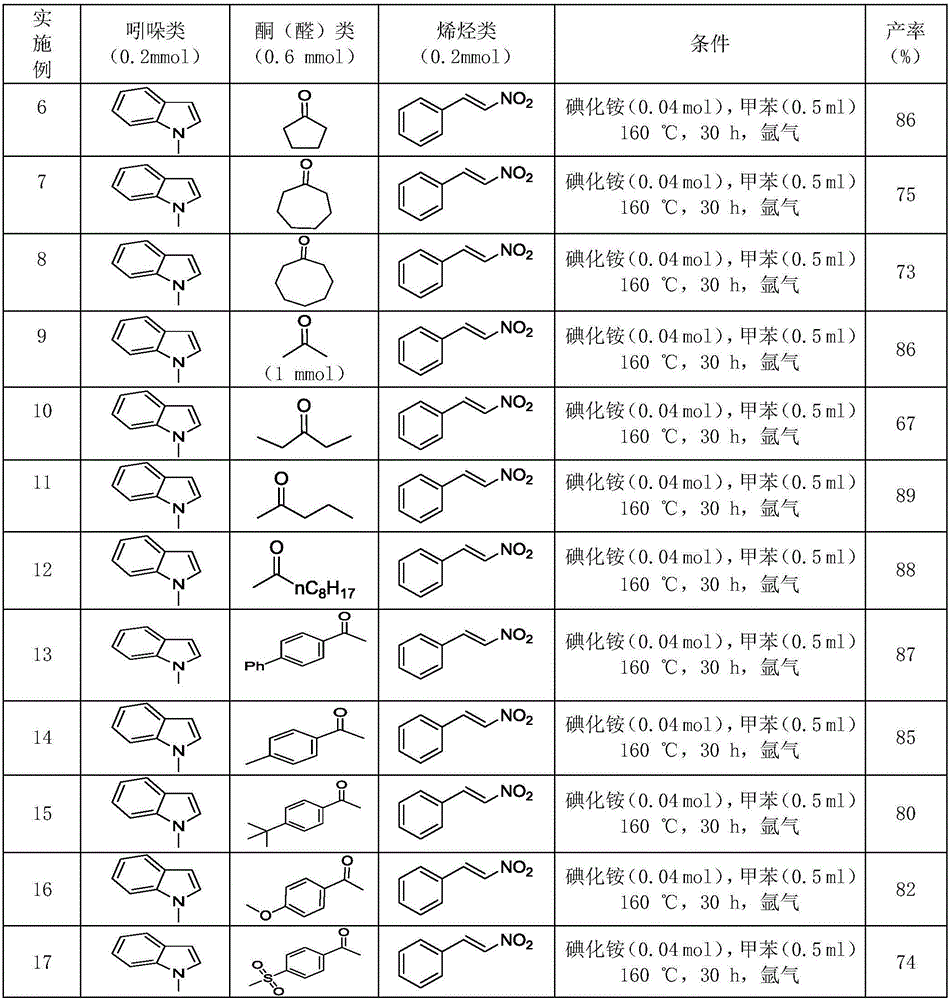
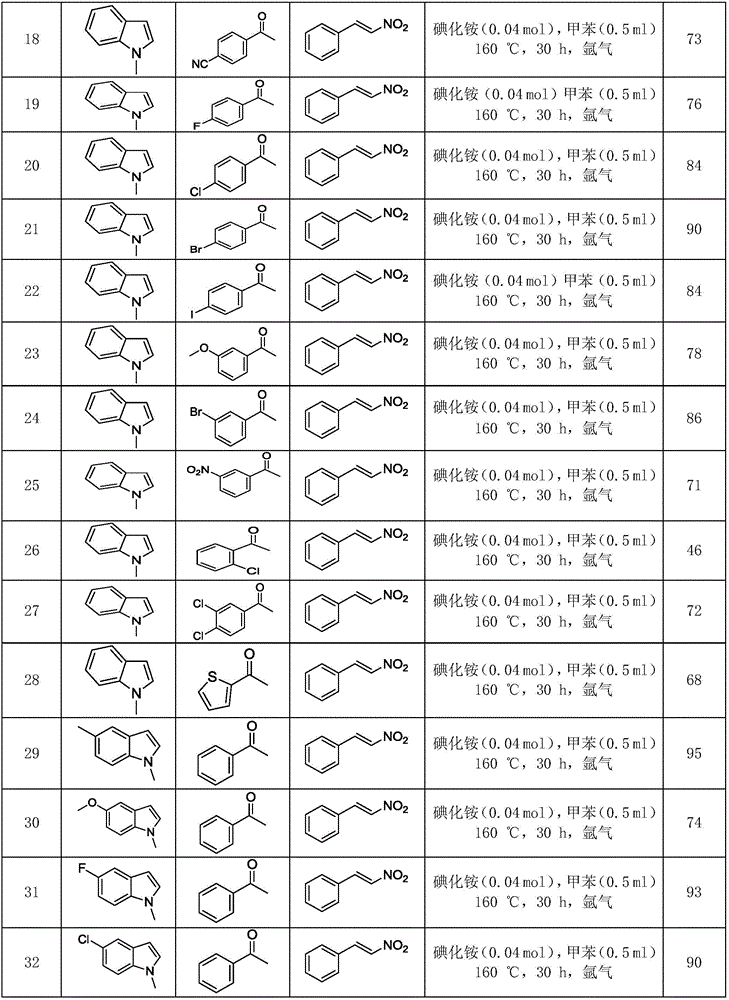
Polysubstituted carbazole, derivative and synthesis method thereof
A multi-substitution and derivative technology, applied in organic chemistry and other fields, can solve problems such as complex synthesis steps, achieve the effects of saving raw materials, excellent chemical properties, and reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: 9-methyl-2, the synthesis of 4-diphenyl-9-hydrocarbazole
[0054]
[0055] Take a reaction tube, add 0.2mmol (25.0μL) 1-methylindole, 0.2mmol (29.8mg) trans-nitrostyrene, 0.6mmol (70.4μL) acetophenone, 0.04mmol (5.8 mg) ammonium iodide, 0.5 mL of toluene, reacted at 160° C. for 30 hours, and conventionally processed to obtain 56.0 mg of pure product with a yield of 84%.
[0056] The NMR and high-resolution mass spectrometry data of embodiment 1 product are as follows:
[0057] 1 H NMR (400MHz, CDCl 3 ,ppm)δ7.76-7.74(m,2H),7.69-7.66(m,2H),7.57(d,J=1.6Hz,1H),7.55-7.44(m,6H),7.41-7.33(m, 4H),7.00-6.96(m,1H),3.87(s,3H); 13 C NMR (100MHz, CDCl 3 , ppm) δ141.8, 141.7, 141.6, 141.2, 138.7, 137.8, 129.2, 128.7, 128.4, 127.5, 127.5, 127.1, 125.5, 122.2, 122.1, 120.2, 119.4, 118.6, 108.2, 105.8 for CHR 25 h 19 N[M+H] + 334.1590,found 334.1592.
Embodiment 2
[0058] Example 2: Synthesis of 7-methyl-5-phenyl-2,3,4,7-tetrahydro-1-hydrobenzo[c]carbazole
[0059]
[0060] Take a reaction tube, add 0.2mmol (25.0μL) 1-methylindole, 0.2mmol (29.8mg) trans-nitrostyrene, 0.6mmol (62.0μL) cyclohexanone, 0.04mmol (5.8 mg) ammonium iodide, 0.5 mL of toluene, reacted at 160° C. for 30 hours, and conventionally processed to obtain 55.4 mg of pure product with a yield of 89%.
[0061] The NMR and high-resolution mass spectrometry data of embodiment 2 product are as follows:
[0062] 1 H NMR (400MHz, CDCl 3 ,ppm)δ8.21(d,J=8.0Hz,1H),7.48-7.34(m,7H),7.23-7.34(m,1H),7.15(s,1H),3.81(s,3H),3.46 (t, J=6.4Hz, 2H), 2.72(t, J=6.2Hz, 2H), 2.05-1.93(m, 2H), 1.84-1.78(m, 2H); 13 C NMR (100MHz, CDCl 3 ,ppm)δ143.1,141.2,140.4,138.9,132.7,129.5,127.9,126.6,125.4,124.7,123.2,123.0,120.1,118.6,108.0,107.4,28.9,28.9,28.6,23.5,23.0 for C cald; 23 h 21 N[M+H] + 312.1747,found 312.1746.
Embodiment 3
[0063] Example 3: Synthesis of 1,9-dimethyl-2,4-phenyl-9-hydrocarbazole
[0064]
[0065] Take a reaction tube, add 0.2mmol (25.0μL) 1-methylindole, 0.2mmol (36.2mg) 1-phenyl-2 nitropropene, 0.6mmol (70.4μL) acetophenone, 0.04 mmol (5.8 mg) of ammonium iodide, 0.5 mL of toluene, reacted at 160° C. for 30 hours, and conventionally processed to obtain 53.5 mg of pure product with a yield of 77%.
[0066] The NMR and high-resolution mass spectrometry data of embodiment 3 product are as follows:
[0067] 1 H NMR (400MHz, CDCl 3 ,ppm)δ7.62-7.60(m,2H),7.51-7.43(m,7H),7.40-7.33(m,4H),7.03(s,1H),6.97-6.93(m,1H),4.18( s,3H),2.77(s,3H); 13 C NMR (100MHz, CDCl 3 , ppm) δ142.8, 142.4, 141.2, 141.1, 140.8, 134.9, 130.0, 129.3, 128.3, 128.0, 127.3, 126.7, 125.4, 123.7, 122.5, 122.2, 120.3, 118.7, 116.9, 108.6 forC 26 h 21 N[M+H] + 348.1747, found 348.1731.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com