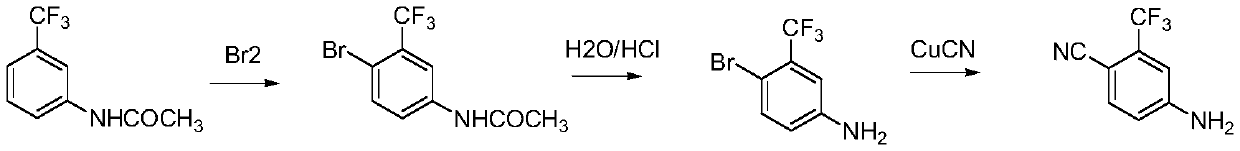
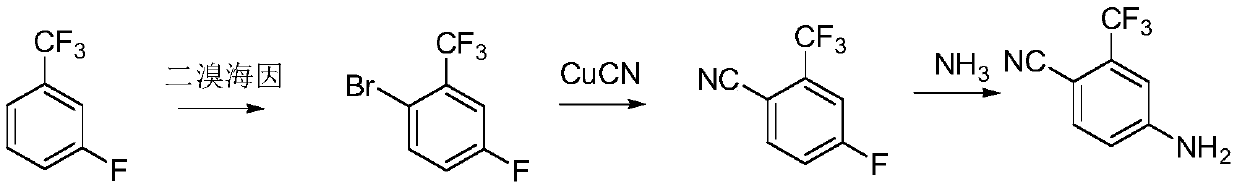
Preparation method of 4-amino-2-trifluoromethyl benzonitrile
A technology of trifluoromethyl benzonitrile and trifluoromethyl benzoic acid, which is applied in the field of preparation of 4-amino-2-trifluoromethyl benzonitrile, can solve the problem of large environmental impact, health hazards of operators, and restrictions on industrialization application and other issues, to achieve the effect of less environmental damage, reduced safety risks, and easy handling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
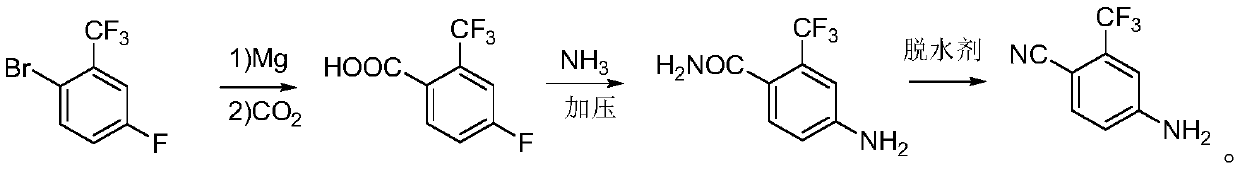
[0037] (1) Preparation of 4-fluoro-2-trifluoromethylbenzoic acid
[0038] In a 500ml four-neck round bottom flask, put 5.76g (0.24mol) of magnesium chips, 1 grain of iodine, and 50ml of tetrahydrofuran under the protection of nitrogen, and heat to about 50°C. Dissolve 48.6 grams (0.2mol) of 2-bromo-5-fluorobenzotrifluoride in 200 milliliters of tetrahydrofuran, first drop about 10 milliliters into the reaction bottle, keep stirring until the color of iodine dissipates, confirm that the reaction is initiated, and then put it in a water bath Under cooling, the remaining material was slowly added dropwise, and after the drop was completed, the mixture was incubated for 1 hour to obtain the Grignard reagent.
[0039] The Grignard reagent was cooled to 5°C with ice water, and 21 g (0.47mol) of carbon dioxide was slowly introduced, then slowly raised to 25°C and kept stirring for 2 hours, then added 88 ml of hydrochloric acid (6Mol / L), stirred for half an hour, and separated into la...
Embodiment 2
[0048] (1) Preparation of 4-fluoro-2-trifluoromethylbenzoic acid
[0049] In a 500ml four-neck round bottom flask, put 6.24g (0.26mol) of magnesium chips, 1 grain of iodine, and 50ml of 2-methyltetrahydrofuran under the protection of nitrogen, and heat to about 50°C. Dissolve 48.6 grams (0.2mol) of 2-bromo-5-fluorobenzotrifluoride in 200 milliliters of 2-methyltetrahydrofuran, first drop about 10 milliliters into the reaction flask, keep stirring until the color of iodine dissipates, and confirm the initiation After the reaction, under cooling in a water bath, the remaining material was slowly added dropwise, and after the drop was completed, the mixture was incubated for 1 hour to obtain the Grignard reagent.
[0050] The Grignard reagent was cooled to 8° C. with ice water, and 26.4 grams (0.6 mol) of carbon dioxide was slowly introduced, then slowly raised to 30° C. and kept stirring for 2 hours, then 88 milliliters of hydrochloric acid (6Mol / L) was added, stirred for half a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com