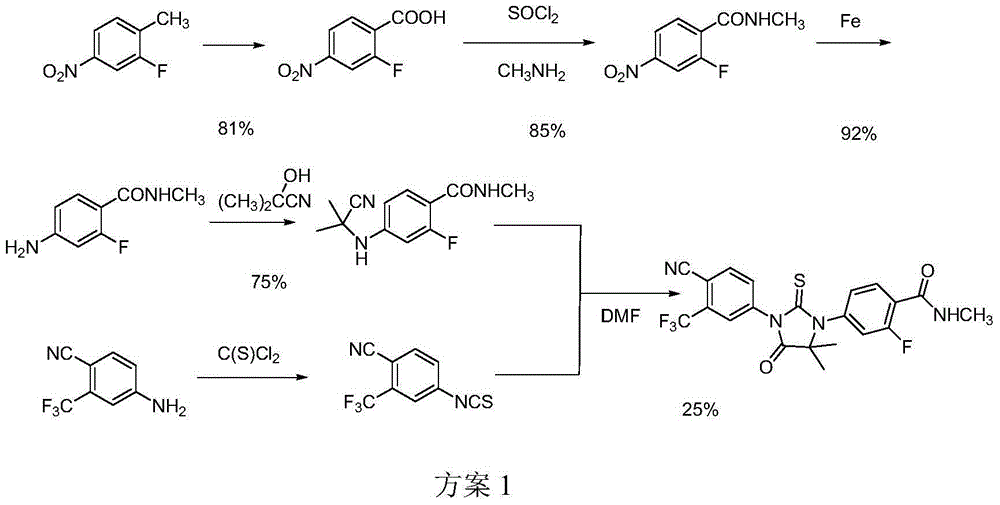
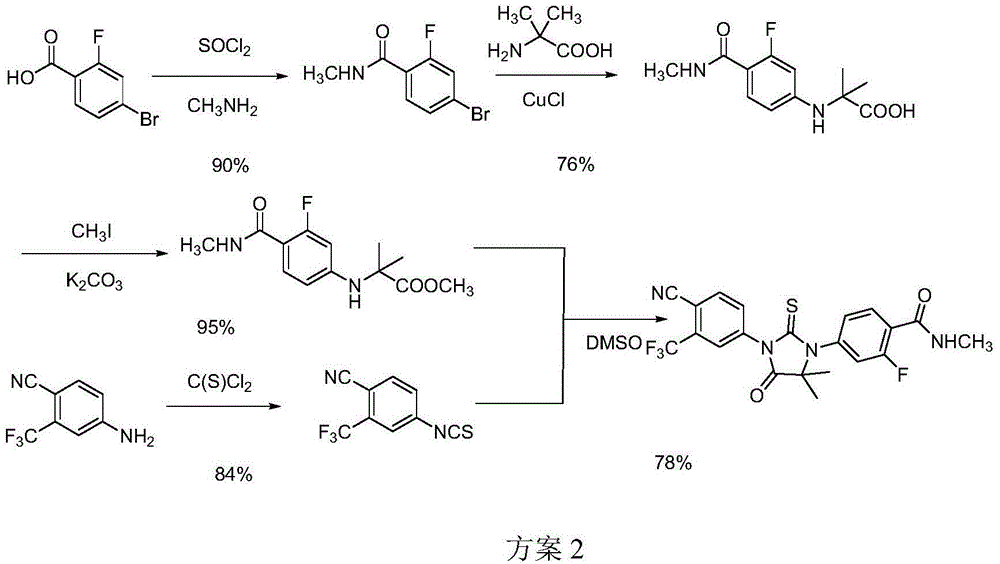
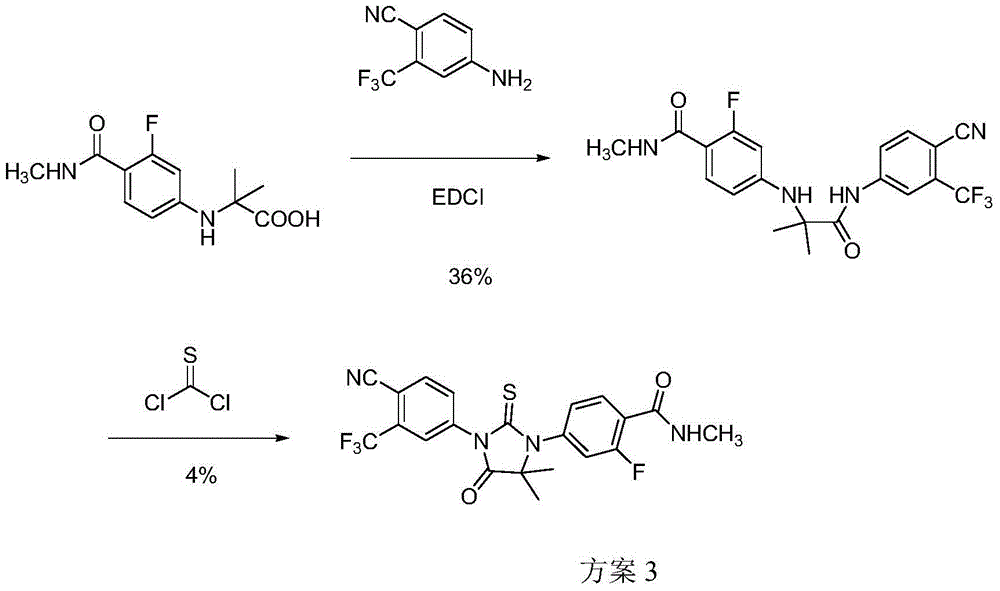
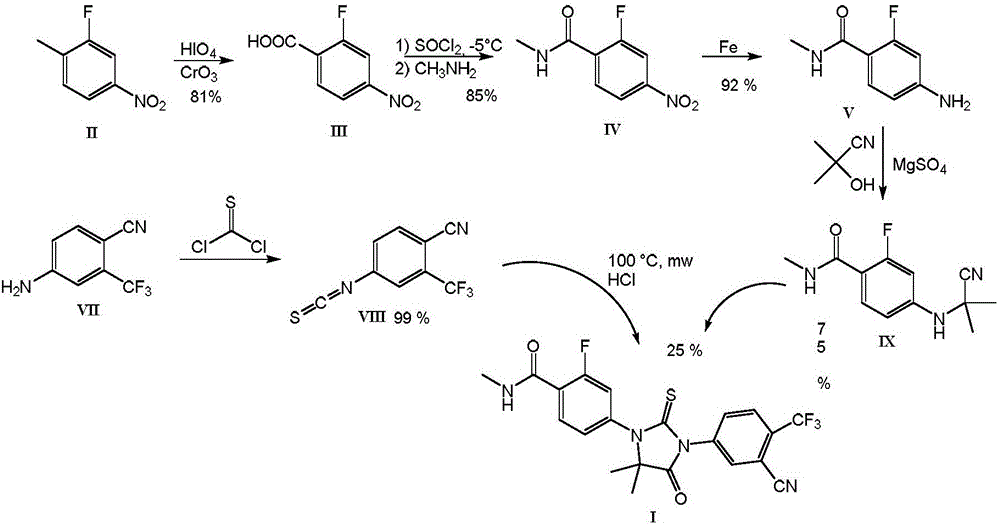
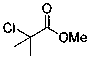
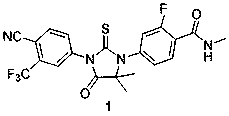
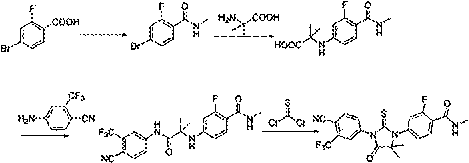
Patents
Literature
96 results about "Enzalutamide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
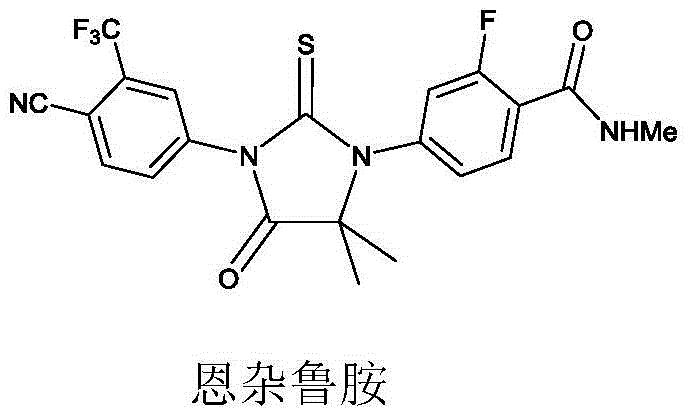
Enzalutamide is used to treat men with a certain type of prostate cancer.
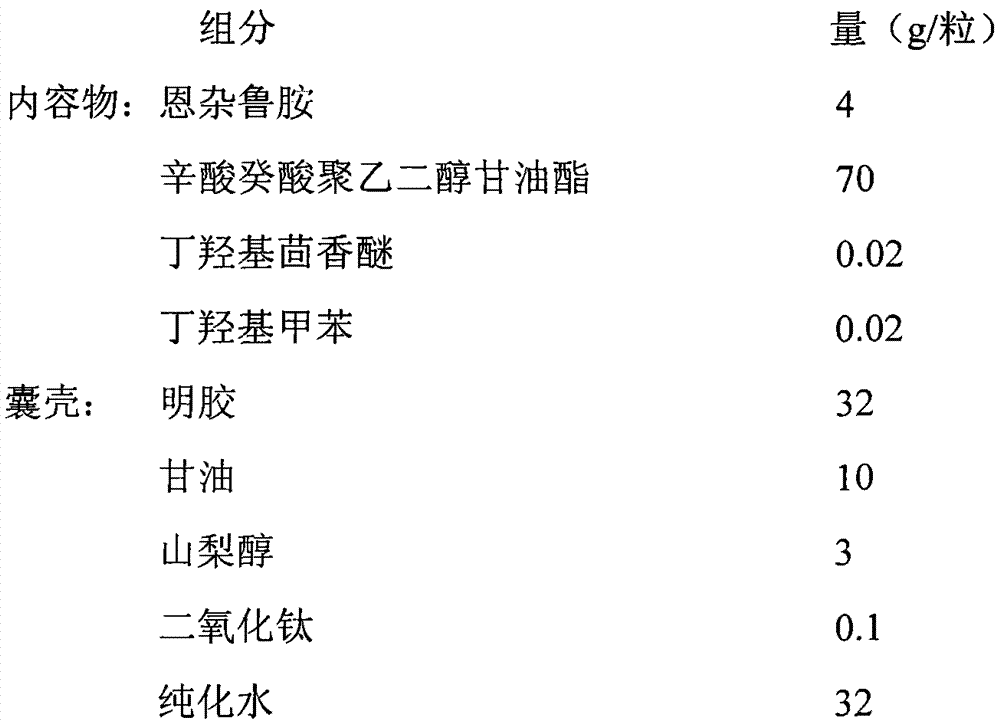
Enzalutamide soft capsule and preparation method thereof
ActiveCN104857517AHigh dissolution rateImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsPolyethylene glycolBHA - Butylated hydroxyanisole
The invention discloses an enzalutamide soft capsule and a preparation method thereof. The enzalutamide soft capsule comprises capsule content and a capsule shell, wherein the capsule content comprises enzalutamide and pharmaceutical adjuvants; the capsule shell is formed by gelatin, glycerin, sorbitol, titanium dioxide, purified water and the like. The soft capsule can be prepared with the method comprising following steps: Labrasol, butylated hydroxyanisole, butylated hydroxytoluene and enzalutamide are mixed to obtain the capsule content material of the soft capsule; the gelatin, the glycerin, the sorbitol, titanium dioxide and the purified water are mixed to obtain the capsule shell material of the soft capsule; the capsule content material and the capsule shell material of the soft capsule are pelleted on a soft capsule making machine to obtain the enzalutamide soft capsule. The enzalutamide soft capsule and the preparation method have the following advantages: the soft capsule is convenient to administrate and carry, the drug stability is good, effective components are dissolved quickly, and the bioavailability is high.
Owner:NANJING HEALTHNICE MEDICAL TECH +1
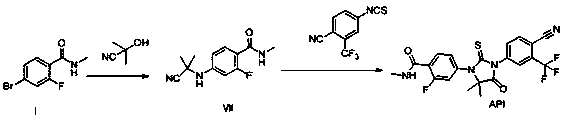
Preparation method of enzalutamide
The invention provides a preparation method of enzalutamide. The method comprises the following steps: adding a compound with formula F to a solvent; reacting with a methylamine source; and after the reaction, collecting the compound with formula G from the reaction products, so as to obtain the enzalutamide. The invention overcomes the major problems of the prior art, gets rid of the time-consuming and labor-consuming operation such as column chromatography, gets rid of the highly toxic reagent such methyl iodide, and has the advantages of mild reaction conditions, simple and easy post-treatment, increased overall yield, reaction time reduction, production cost reduction, and applicability to industrial scale-up. The reaction general formula is as below.
Owner:SHANGHAI INST OF PHARMA IND +1
A process for producing enzalutamide
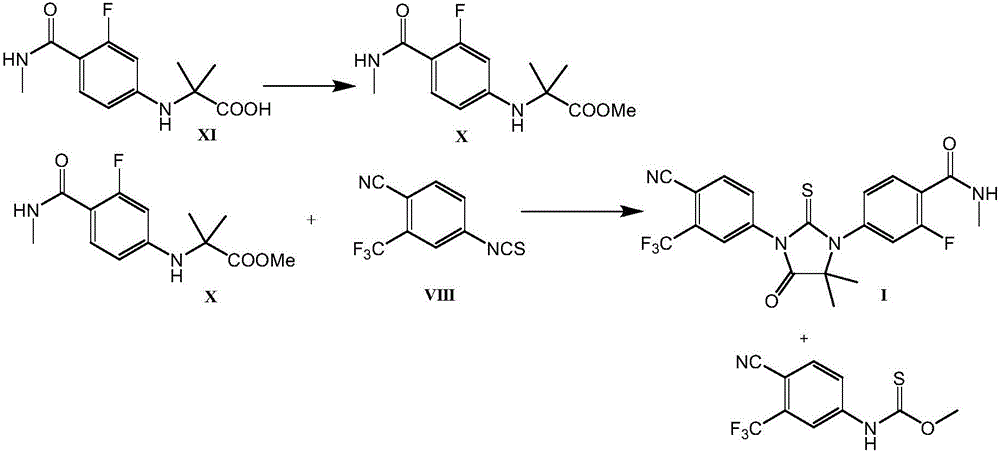
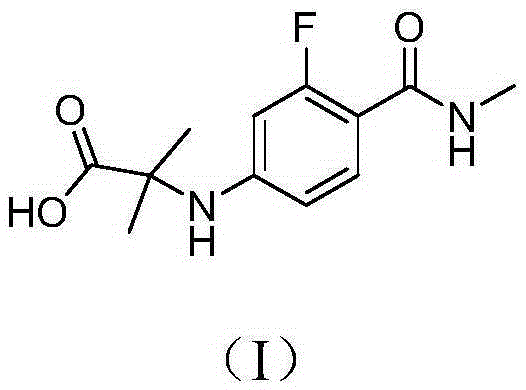
This invention provides a new process for producing enzalutamide of formula I, starting from the intermediate of formula XI, which cyclizes with the isothiocyanate of formula VIII in the presence of a base, an alcohol of the general formula R-OH as an additive, in a suitable solvent, wherein R is an alkyl with the number of carbon atoms C1C20, or a substituted alkyl with the number of carbon atoms C1C20, an aryl with the number of carbon atoms C6-C16, or a substituted aryl with the number of carbon atoms C6-C16.
Owner:ZENTIVA AS
One-pot method for synthetizing enzalutamide
The invention provides a one-pot method for synthetizing enzalutamide and belongs to the field of medicinal chemical synthesis. The method comprises the following steps: firstly, carrying out copper-catalyzed Buchwald reaction on N-methyl-4-bromo-2-fluoro-benzamide and 2-methyl alanine, adding halogenated hydrocarbon, reacting to generate ester, finally adding a key intermediate 4-isothiocyano-2-(trifluoromethyl) cyanophenyl and carrying out bucherer-Bergs reaction to generate the enzalutamide. The method is simple in operation and high in product yield and has the advantages that the intermediate products are not needed to be separated and directly reacted, so that the process flow cycle is shortened; the final product is easy to separate and purify.
Owner:SHANGHAI DINGYA PHARM CHEM CO LTD
Novel method for compounding enzalutamide
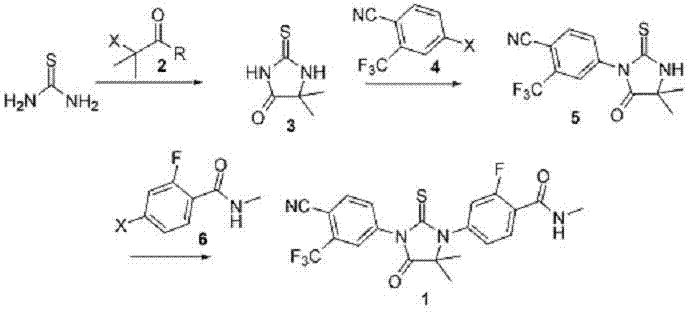
The invention discloses a novel method for compounding enzalutamide, belongs to the technical field of pharmacy synthetic technology, and particularly relates to novel technology for compounding enzalutamide. Most known methods for compounding enzalutamide relate to use of highly toxic and mephitical thiophosgene and highly toxic oxobutyronitrile, and have relatively great difficulty for industrialization. According to the invention, non-toxic and harmless thiourea and an isobutyric acid derivative are used for a reaction to obtain a key parent nucleus of 5,5-dimethyl-2-thioketone imidazole-4-ketone, and therefore, thiophosgene and oxobutyronitrile are effectively avoided. The method has a mild reaction condition, is relatively high in yield, and therefore has a great industrial prospect.
Owner:成都伊诺达博医药科技有限公司
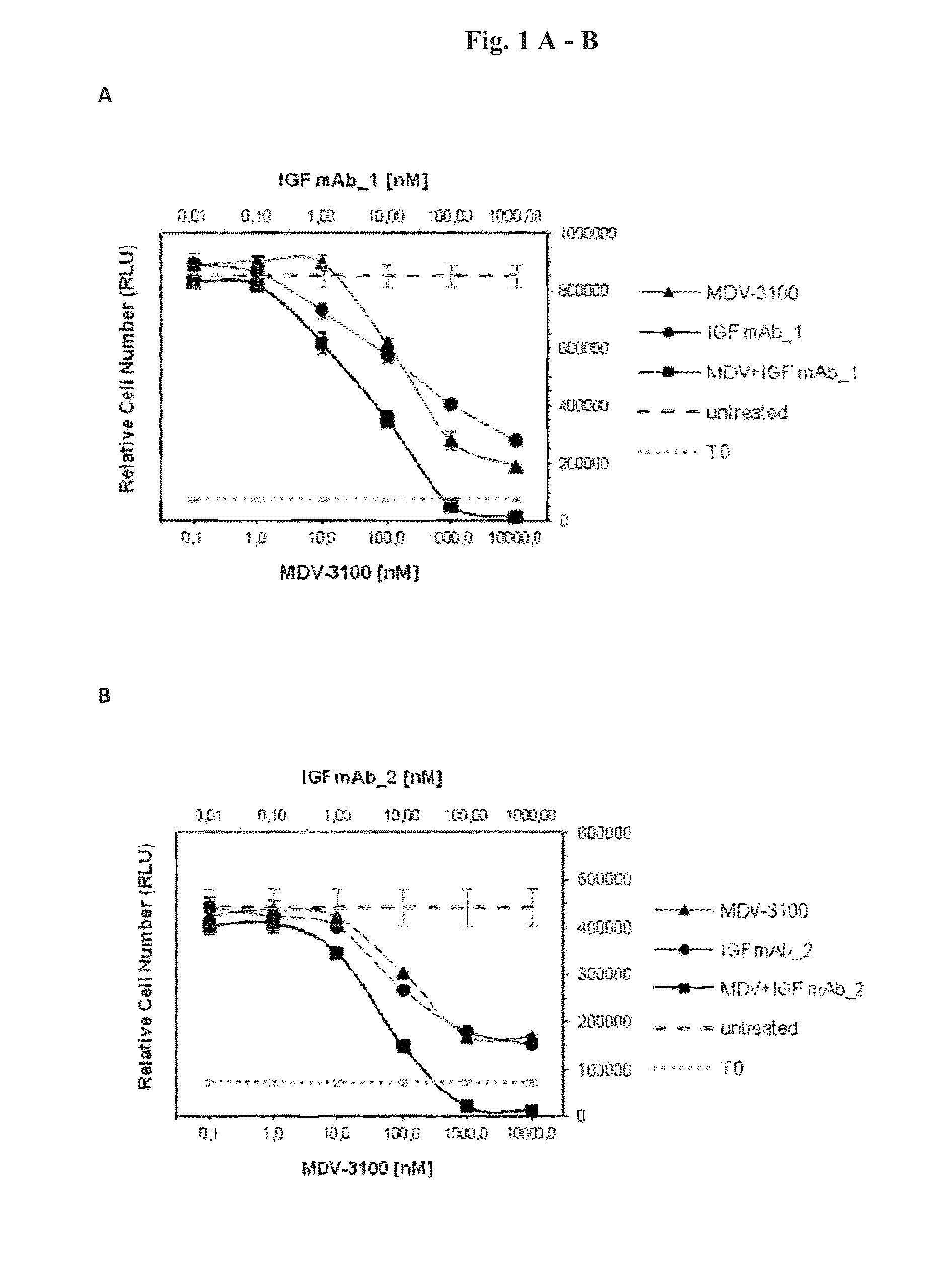
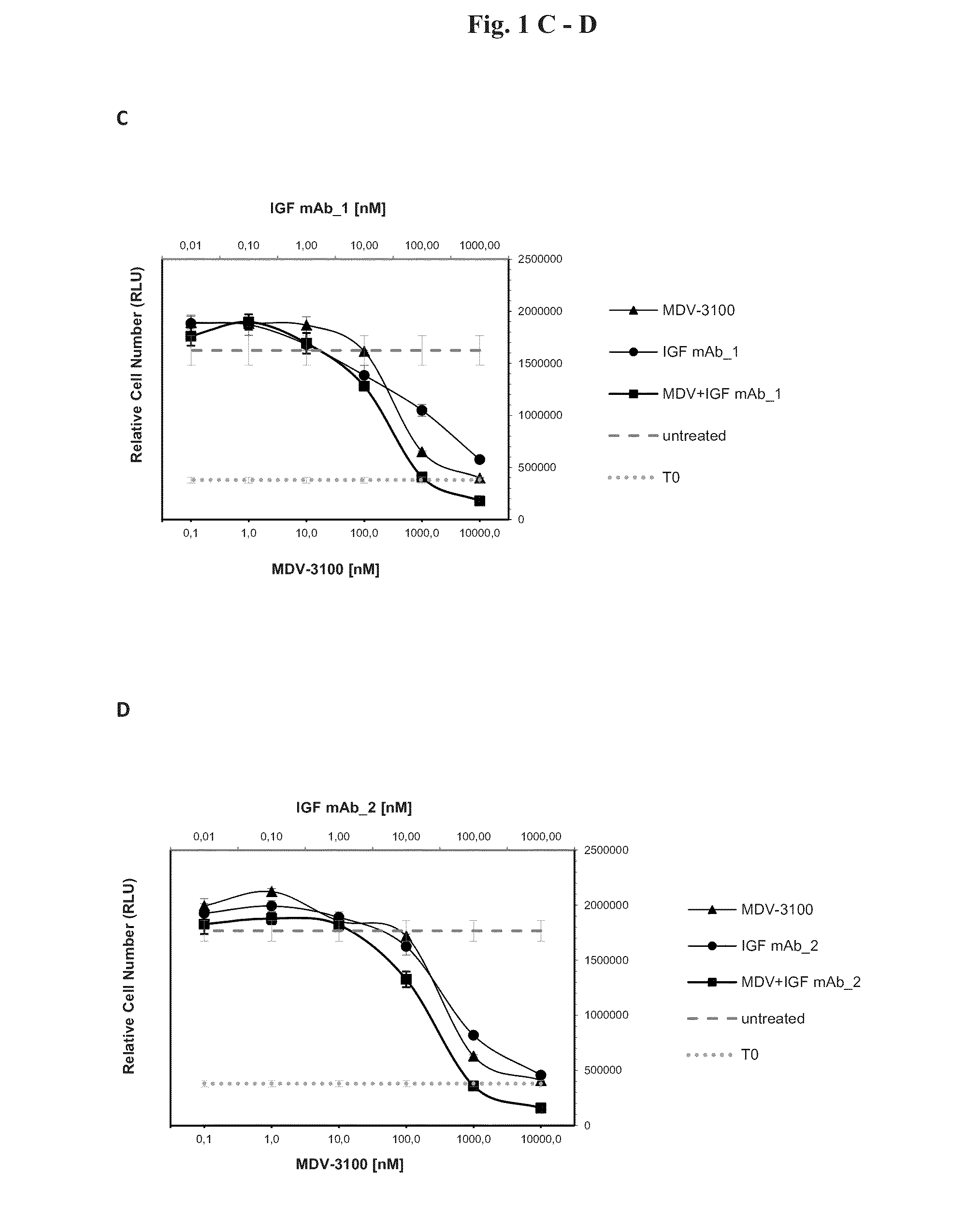
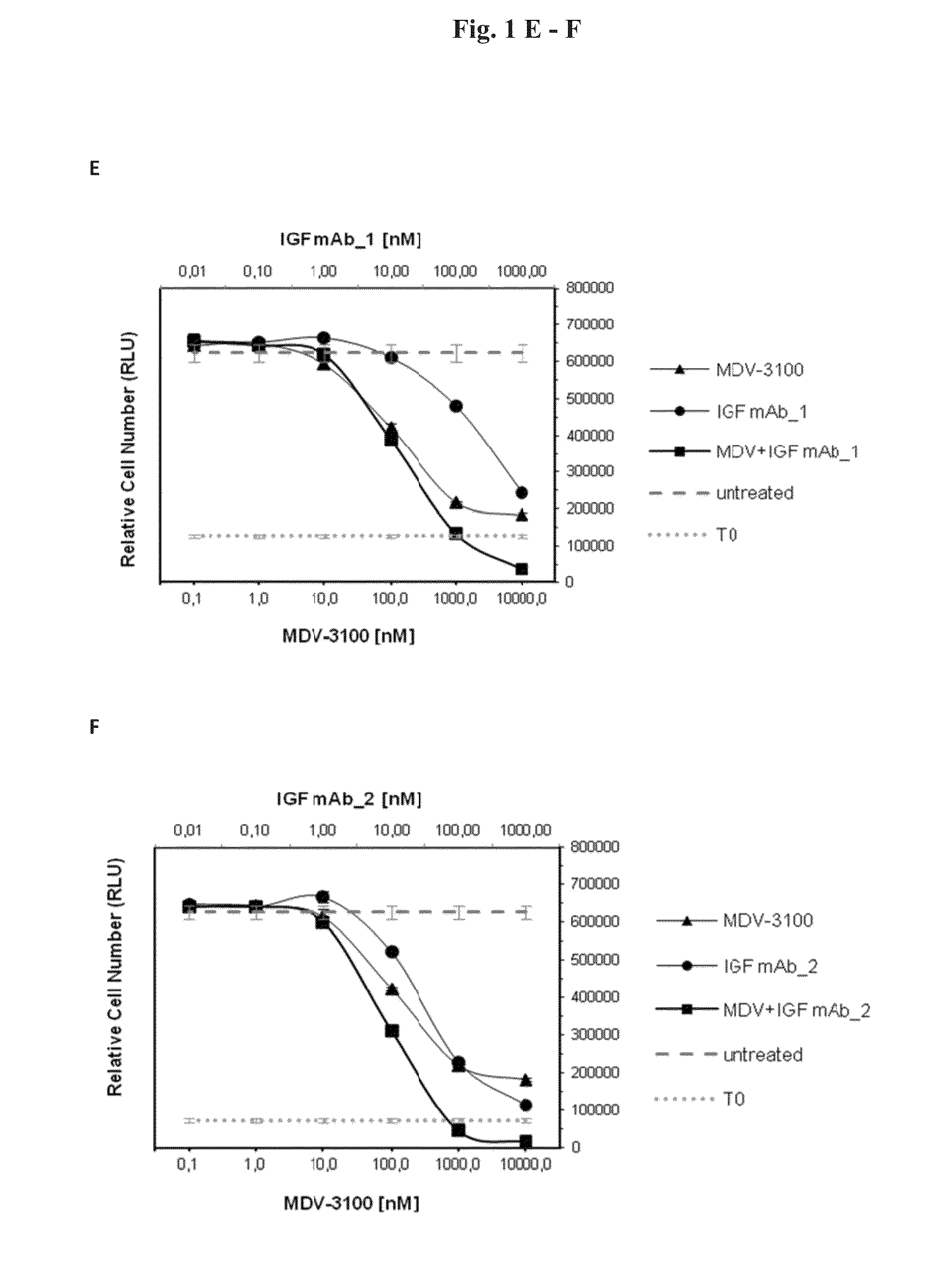
Combination therapy for neoplasia treatment
InactiveUS20140255413A1Improve the level ofOrganic active ingredientsImmunoglobulins against growth factorsInsulin-like growth factorPROSTATE NEOPLASIA
The present invention relates to an insulin-like growth factor (IGF) receptor antagonist for use in the treatment of prostate neoplasia, including benign prostatic hyperplasia (BPH), prostate cancer, and particularly CRPC, wherein the antagonist is used in combination with an androgen receptor antagonist. An embodiment of the invention is where the androgen receptor antagonist is enzalutamide.
Owner:BOEHRINGER INGELHEIM INT GMBH
Preparation process of prostatic cancer medicine Enzalutamide
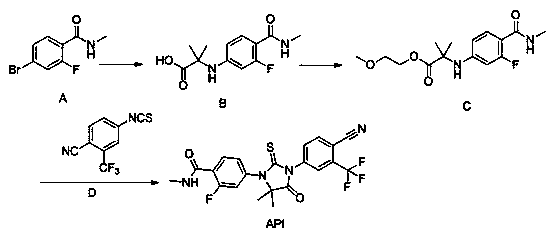
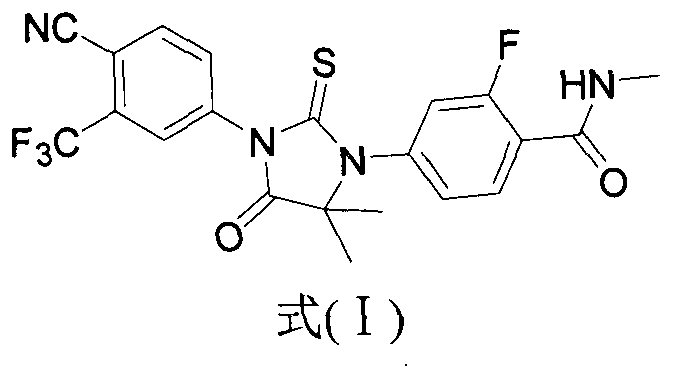
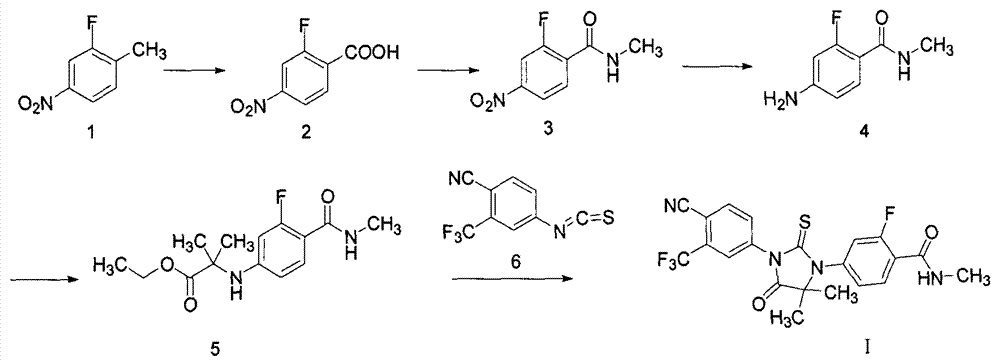
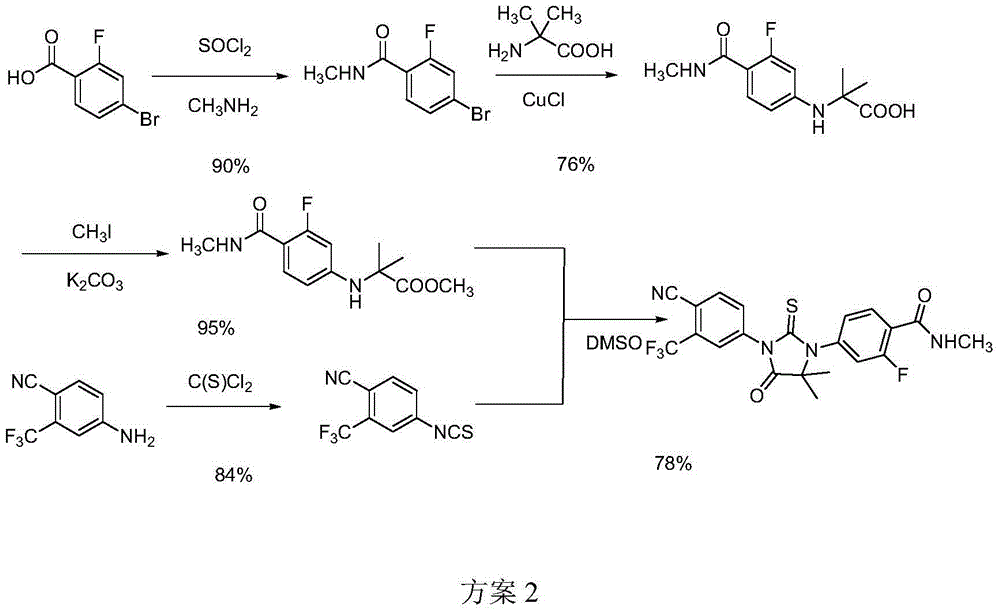
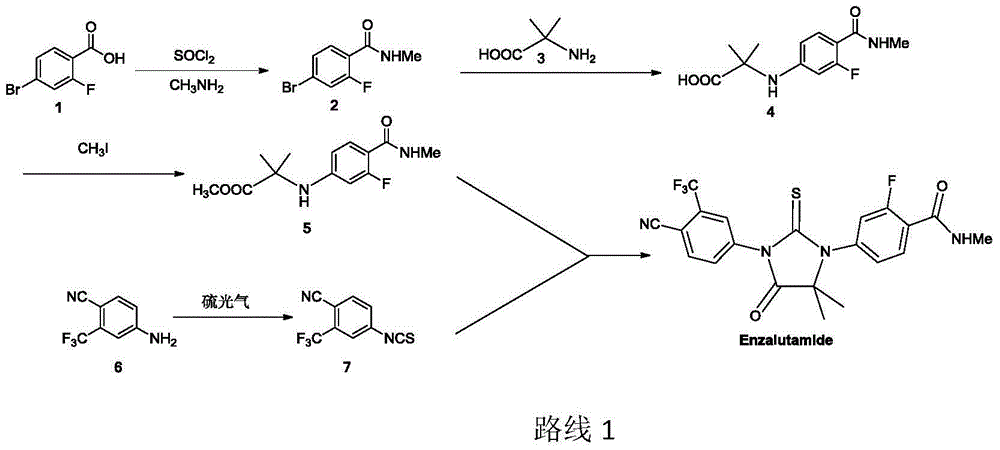
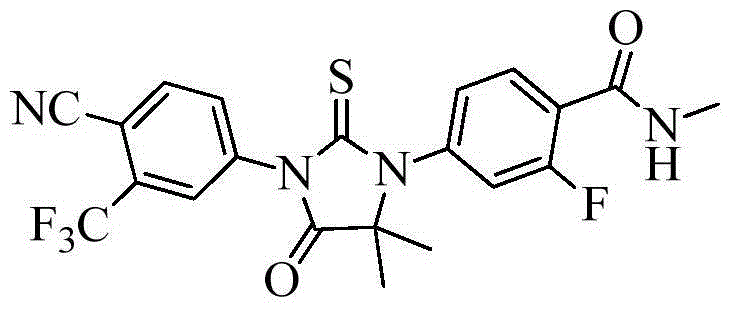
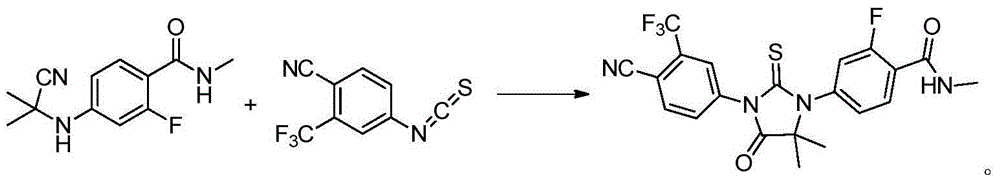
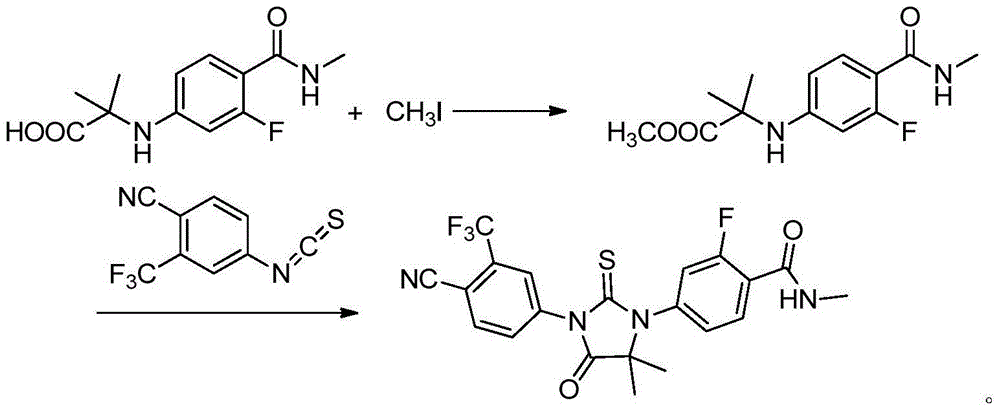
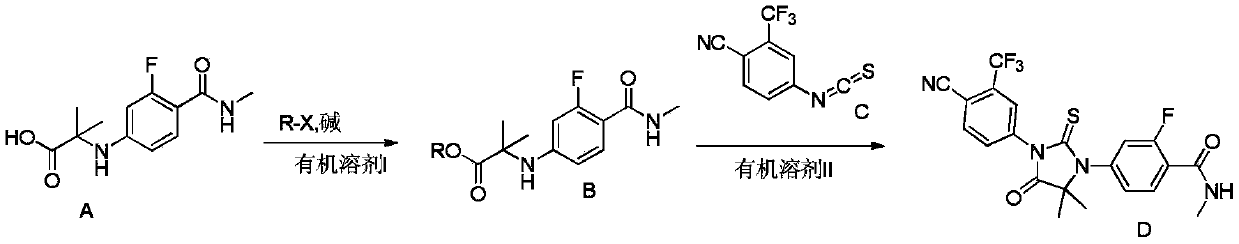
The invention provides a preparation process of a prostatic cancer medicine Enzalutamide. The preparation process includes: with N-methyl-4-bromo-2-fluoro-benzamide being a raw material, performing acatalytic nucleophilic substitution reaction with 2-amino isobutyric acid under alkaline condition to generate 2-(3-fluoro-4-(methylcarbamoyl)phenylamino)-2-methylpropionic acid; performing esterification to generate 2-methoxyethyl-2-((3-fluoro-4-(methylcarbamoyl)phenyl)amino)-2-methylpropionate; performing a ring closing reaction on the product with 2-trifluoromethyl-4-isothiocyanobenzonitrile togenerate the finish product 4-(3-(4-cyano-3-trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-sulfoimidazole-1-yl)-2-fluoro-N-methylbenzamide, namely, Enzalutamide. The method overcomes the major defectsin the prior art and avoids use of high-toxic reagent such as iodomethane, is gentle in reaction conditions and is convenient and simple in after treatment, is improved in total yield and reduced in reaction time, is reduced in preparation cost, is green and environment-friendly, and is suitable for industrial large-scale production.
Owner:BEIJING KAILAI TIANCHENG MEDICINE TECH CO LTD
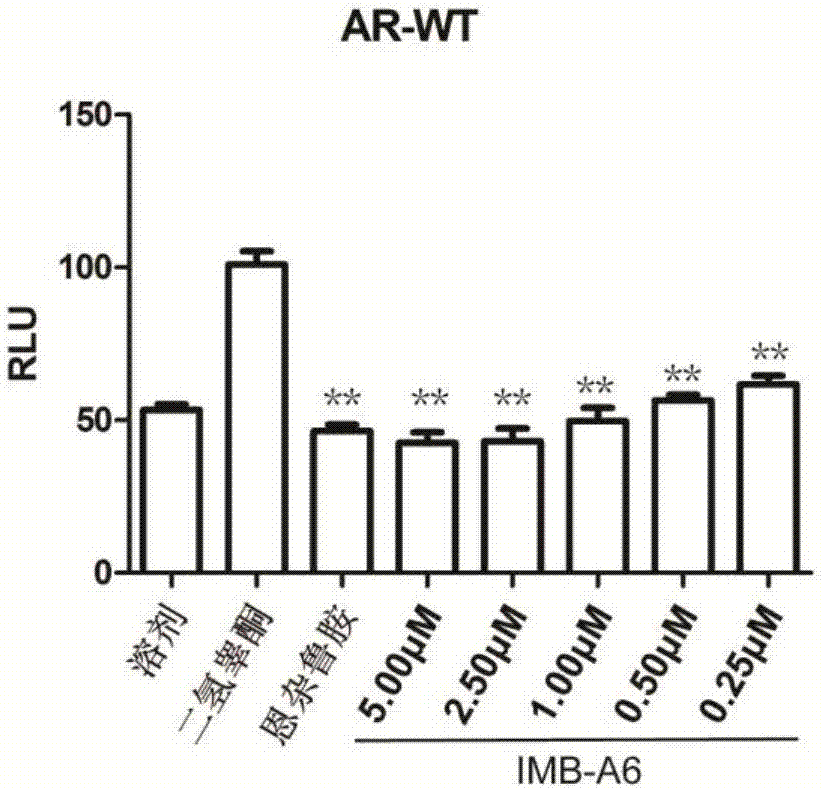
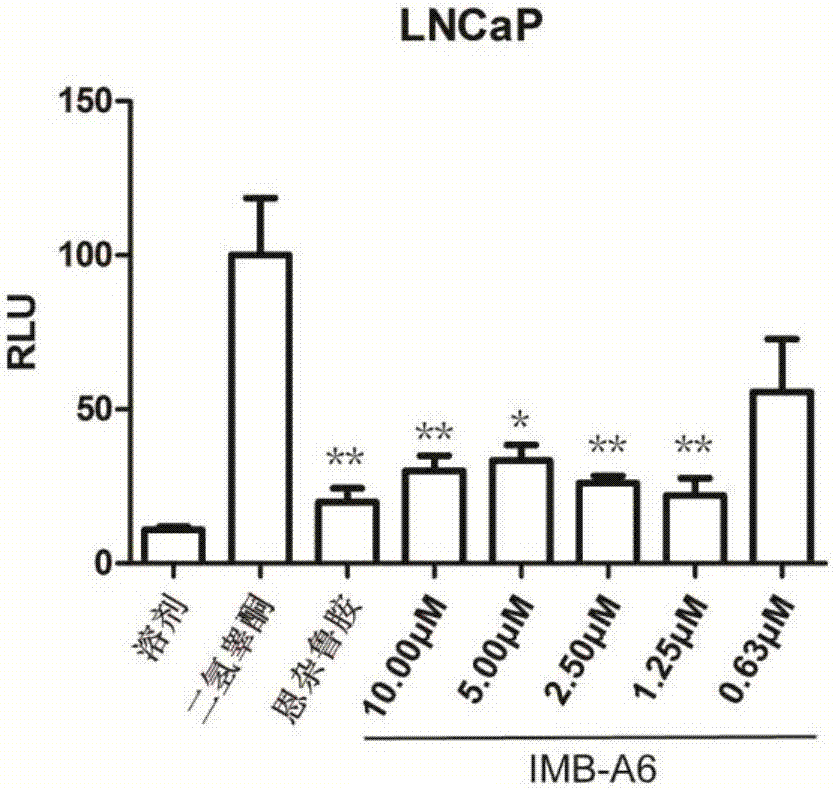
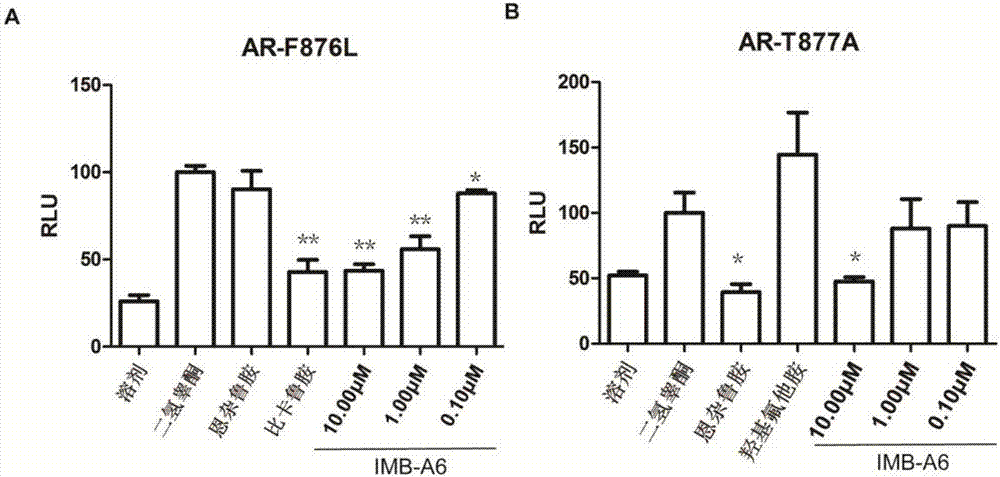
Application of IMB-A6 serving as androgen receptor antagonist
ActiveCN106902112AEnhanced inhibitory effectHigh activityUrinary disorderDermatological disorderDiseaseProstate cancer cell
The invention relates to the field of medicine primers, and particularly discloses an application of IMB-A6 serving as an androgen receptor antagonist and an application of the IMB-A6 to preparation of androgen imbalance disease treating medicines and male contraception pills. By building a reliable androgen activity screening method, the inhibiting effect of a plurality of medicine primers on androgen receptor activity is tested to finally find that the IMB-A6 is a novel androgen receptor antagonist, and activity of wild and mutagenic androgen receptors can be inhibited. Further, activity of the prostate cancer resistance of the IMB-A6 is tested to find that the IMB-A6 has good activity for AR positive prostate cancer cells such as LNCaP and has no activity for AR negative prostate cancer cells, the expression activity of the androgen receptors can be inhibited, and the IMB-A6 is similar to positive medicines such as enzalutamide.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Method for synthesizing enzalutamide
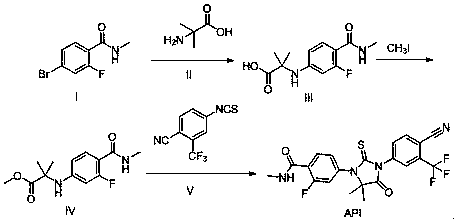
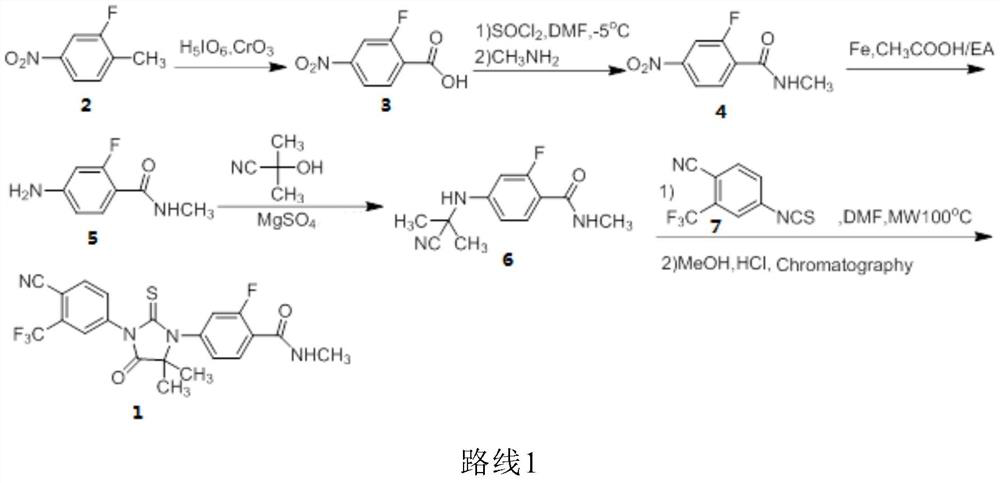
The invention relates to the field of chemical synthesis and in particular relates to synthesis of a medicine enzalutamide for treating male late castration-resistant prostate cancer. The method is characterized by comprising the following steps: by taking 2-fluoro-4-nitrotoluene as a raw material, sequentially performing oxidation, acylation and reduction, thereby obtaining N-methyl-2-fluoro-4-aminobenzamide; carrying out a nucleophilic reaction between N-methyl-2-fluoro-4-aminobenzamide and ethyl 2-bromoisobutyrate, condensing the generated 2-(3-fluoro-4-methylamino formyl phenyl) amino aniline-2-methylethyl propionate and 4-isothiocyanic-2-trifluoromethylbenzonitrile in the presence of a condensing agent, thereby obtaining the enzalutamide. The method disclosed by the invention is easy to operate, stable, reliable and mild in conditions, application of a toxic reagent is reduced, the total reaction yield is more than 20 percent, the purity of the synthesized product is high and is larger than 99.0 percent.
Owner:CHINA PHARM UNIV
Application of auranofin in preparation of medicine for treating castration-resistant prostate cancer
InactiveCN112022871AShorten the timeOrganic active ingredientsAntineoplastic agentsProstate cancerPharmaceutical drug
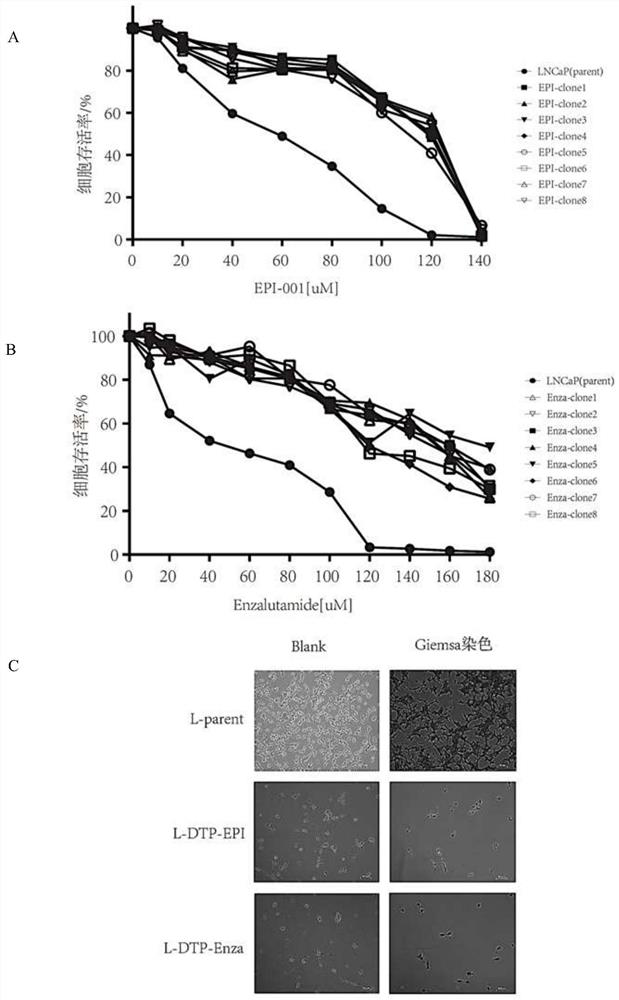
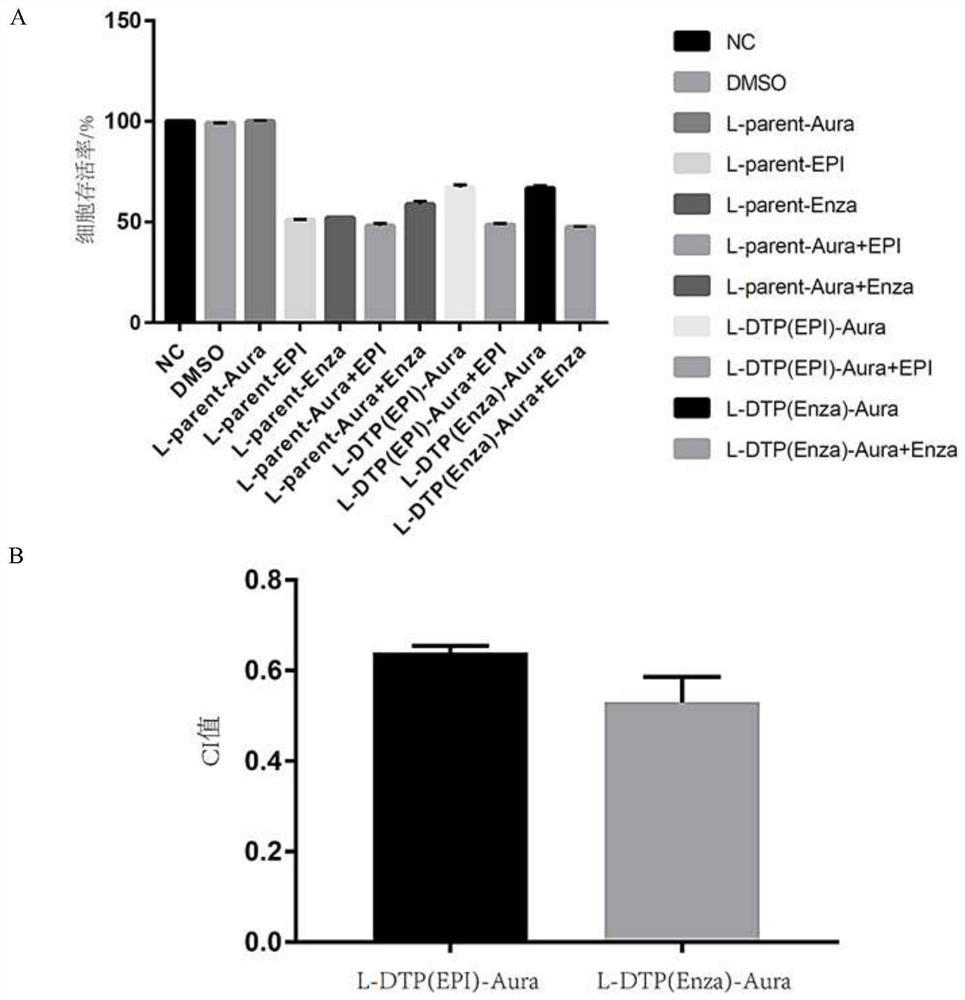
The invention discloses an application of auranofin (Aura) in preparation of a medicine for treating castration-resistant prostate cancer (CRPC), and belongs to the technical field of biological medicines. The invention provides a new strategy used for preparing the CRPC treatment medicine from the Aura and based on combined use of Enza and Aura for the first time, and multi-angle and multi-levelverification research is carried out. The Aura can be used for treating the castration-resistant prostate cancer, significantly improves the castration-resistant prostate cancer inhibition effect of the Enza, realizes new use of the old drug, can greatly shorten the time from drug discovery to clinical transformation, and has important clinical treatment significance.
Owner:JIANGNAN UNIV
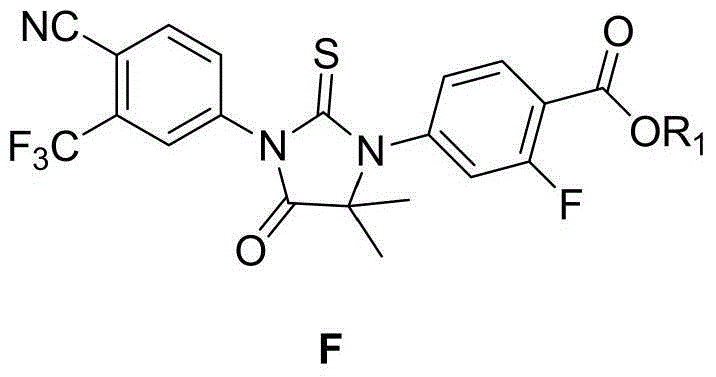
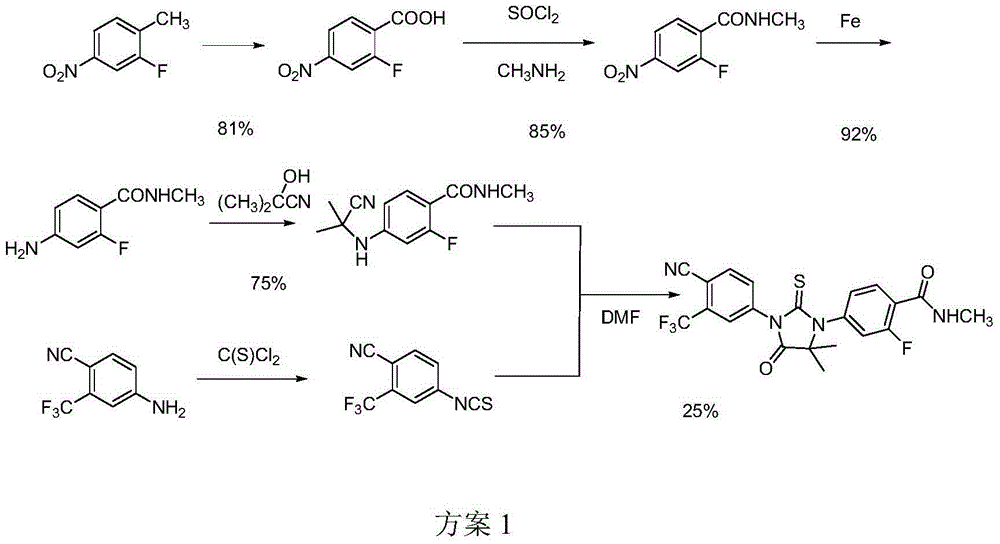
Preparation method of enzalutamide intermediate F
InactiveCN104803919ARaw materials are easy to getThe reaction is easy to operateOrganic chemistryRoom temperatureProtic solvent
The invention provides a preparation method of an enzalutamide intermediate F. The method comprises the steps of: (1) reacting a compound with a formula D and a compound with a Formula E in a solvent; (2) after the reaction, cooling to room temperature and adding a protic solvent; and (3) adding a polar solvent, and then collecting the compound with the formula F from the reaction products. The preparation process has the advantages of easily available raw materials, easy reaction operation, simple post-treatment and no usage of hazardous reagent in the preparation method, and is applicable to industrial production. The reaction general formula is as below.
Owner:SHANGHAI INST OF PHARMA IND +1
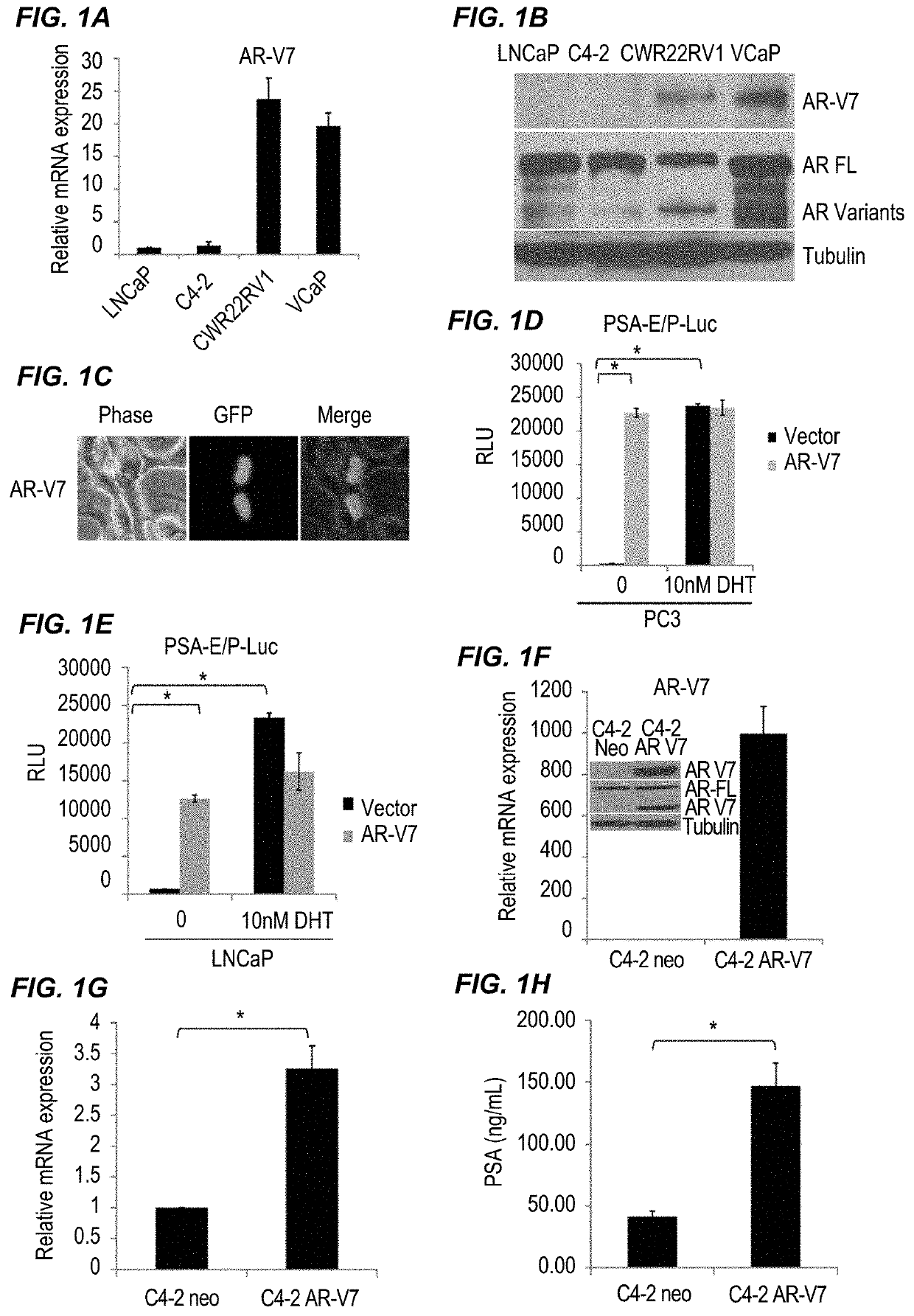
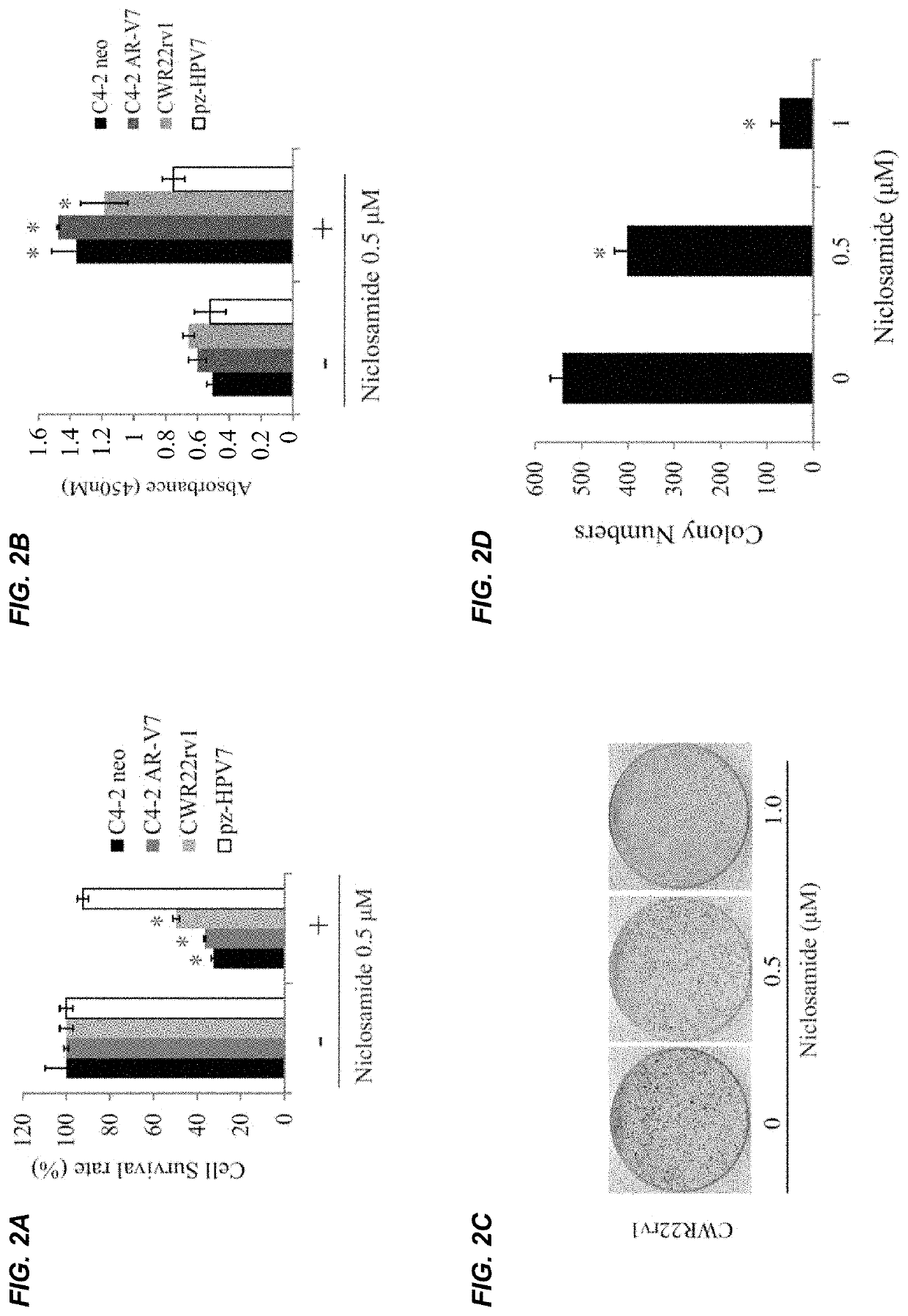
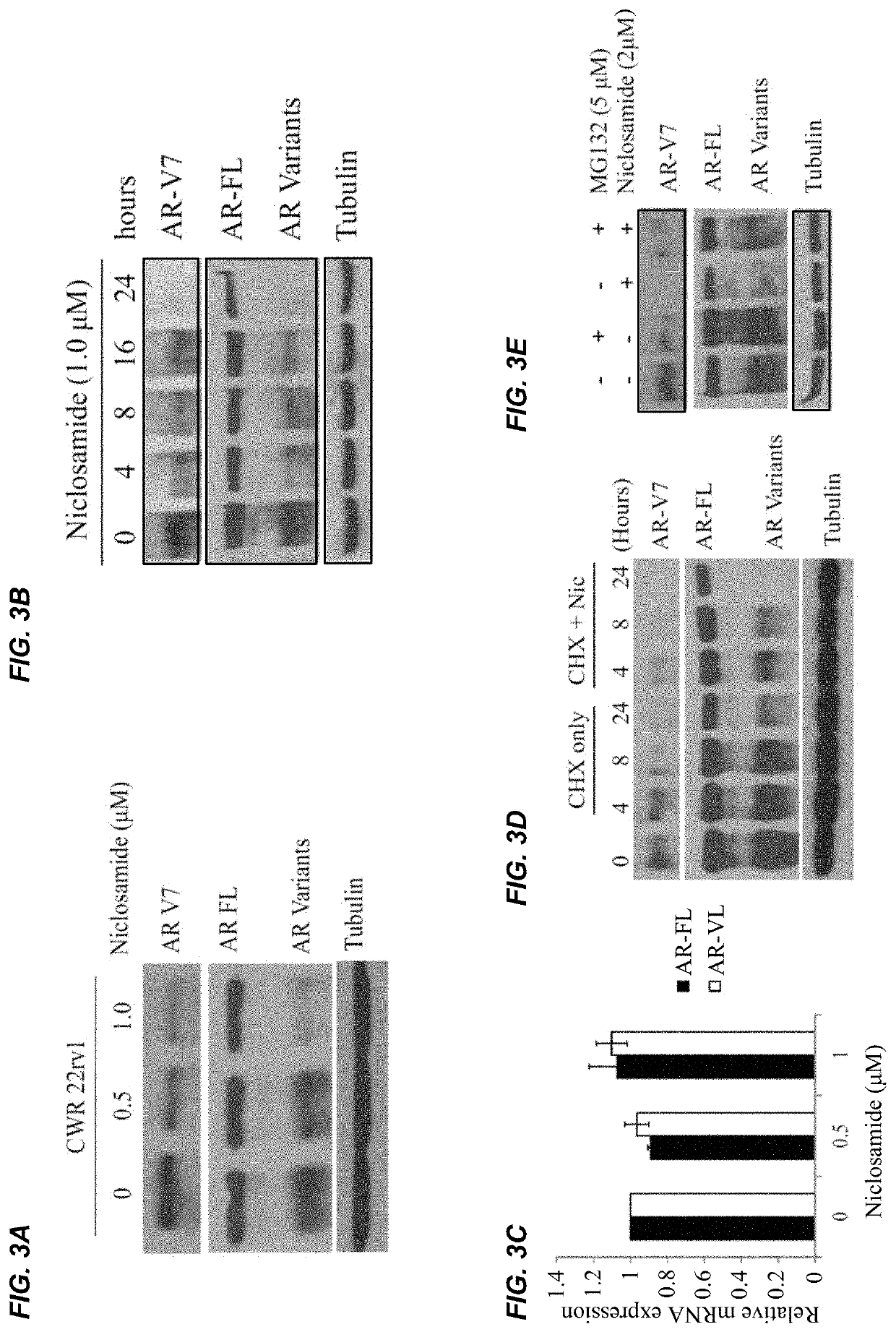
Treatment of metastatic prostate cancer
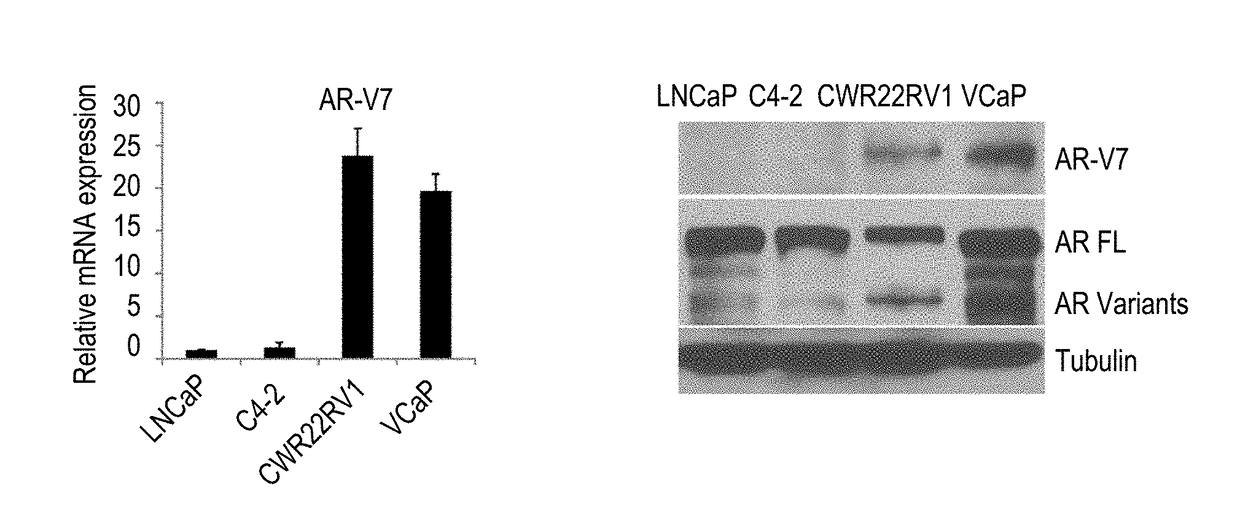
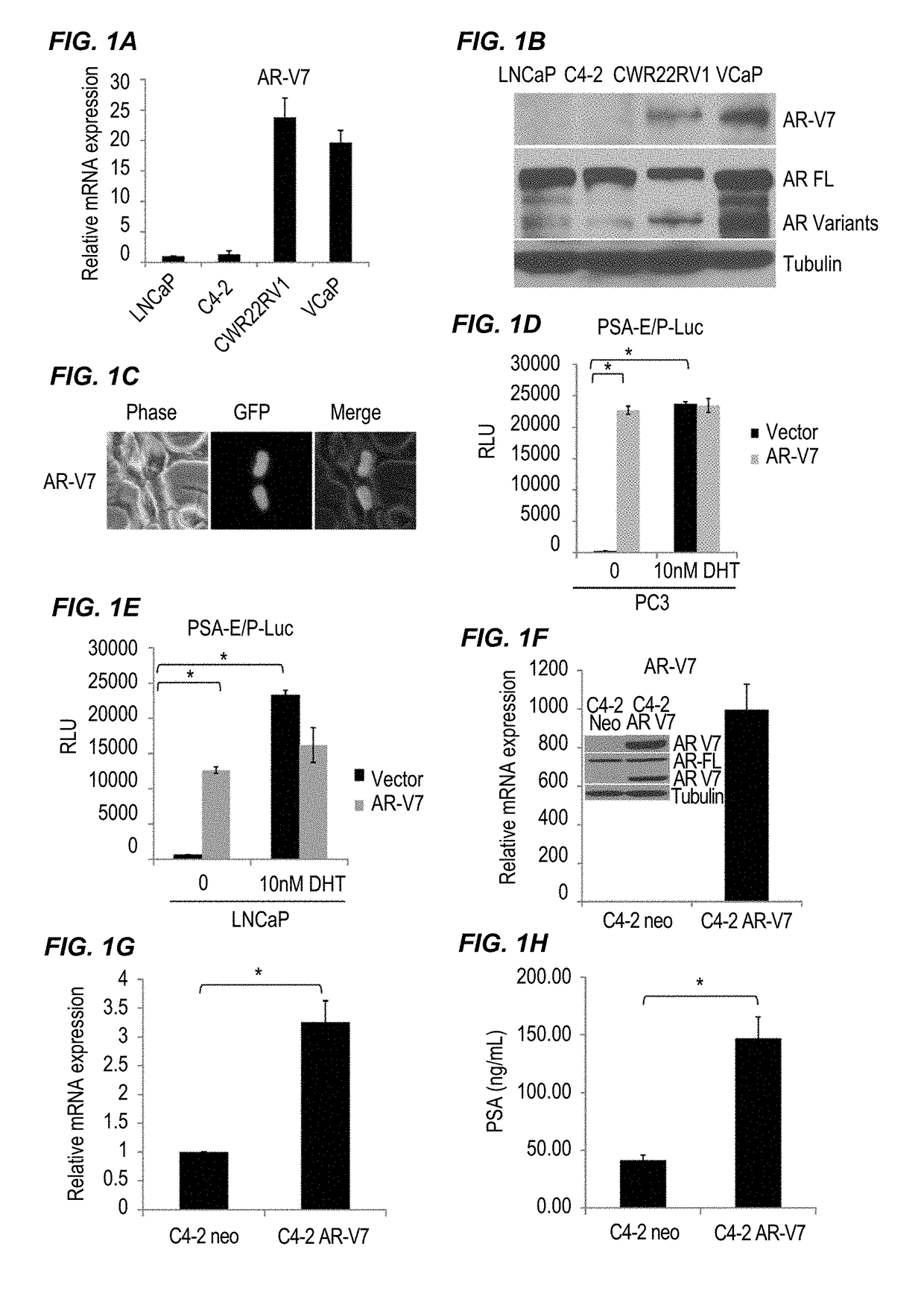
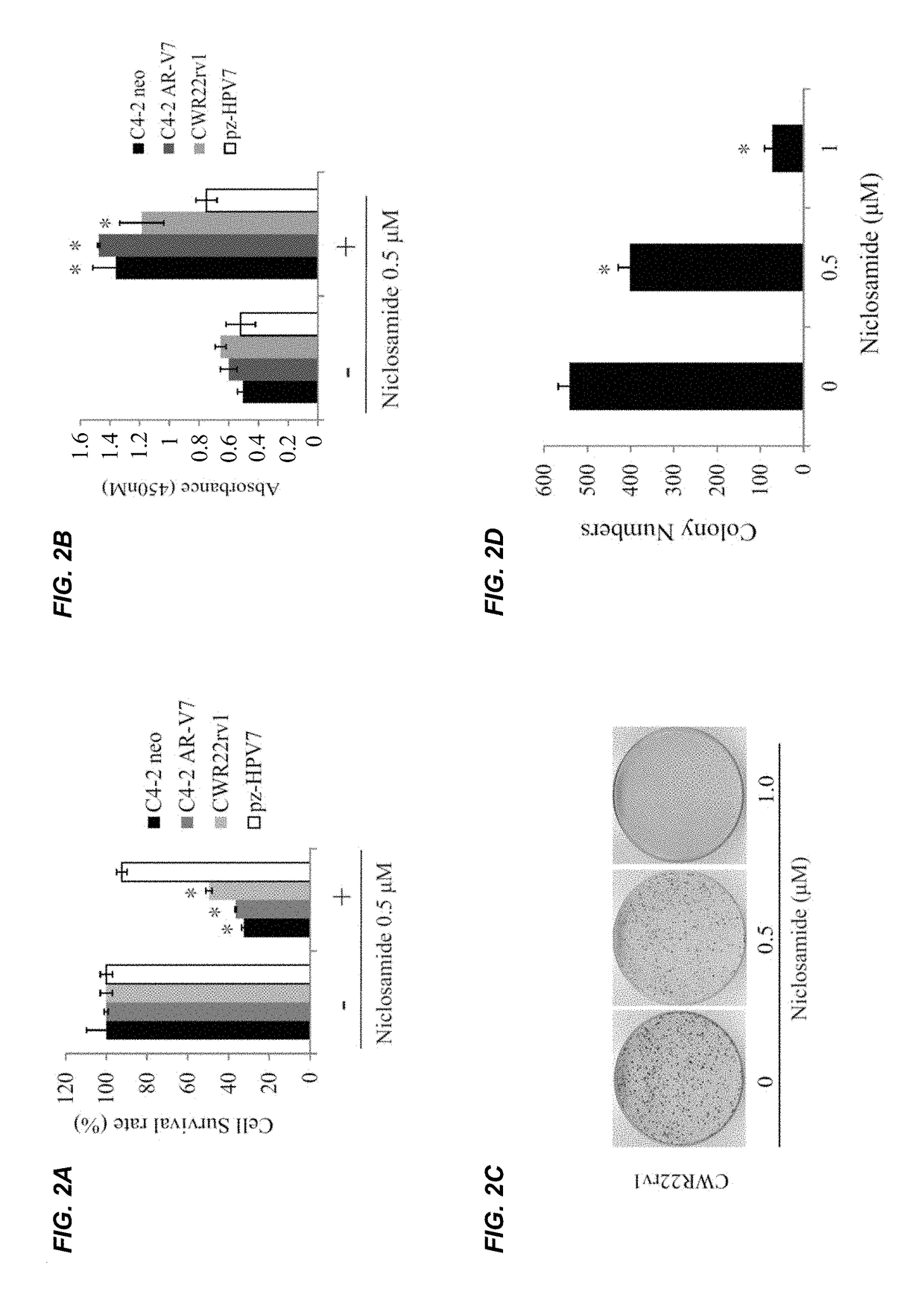
InactiveUS20170315127A1Good treatment effectPeptide/protein ingredientsMicrobiological testing/measurementNiclosamideAnti-Androgen
The present invention provides new compositions and methods for treating prostate cancer, e.g., drug-resistant prostate cancer, such as anti-androgen drug (e.g., enzalutamide) resistant and / or castration resistant prostate cancer (CRPC). These new compositions include, but are not limited to, pharmaceutical compositions that include an AR-V7 inhibitor, such as niclosamide. Alternatively, these new compositions can include, but are not limited to, pharmaceutical compositions that include an AKR1C3 inhibitor, such as indomethacin. These new methods include, but are not limited to, methods of administering an AR-V7 inhibitor, such as niclosamide, and / or an AKR1C3 inhibitor, such as indomethacin, to treat patients having prostate cancer. The present invention also provides methods of inhibiting androgen receptor variant expression, e.g. AR-V7, and methods of killing cells expressing AR-V7. The present invention further provides methods of inhibiting AKR1C3 expression or activity, and methods of killing cells that express AKR1C3.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Novel method for compounding medicament of Enzalutamide for resisting prostate cancer
The invention discloses a novel method for compounding a medicament of Enzalutamide for resisting the prostate cancer, belongs to the technical field of pharmacy synthetic technique, and particularly relates to novel technique for compounding Enzalutamide. Most of known methods for compounding Enzalutamide relate to use of highly toxic and mephitical thiophosgene and highly toxic oxobutyronitrile and have great difficulty in industrialization. According to the invention, a non-toxic and harmless Lawesson's agent is adopted, and a key parent nucleus of a carbonyl group is subjected to a sulfenylation reaction, so that thiophosgene and oxobutyronitrile are effectively avoided. The reaction condition of the method is mild, and the yield is relatively high, therefore, the novel method has a great industrialization prospect.
Owner:成都伊诺达博医药科技有限公司
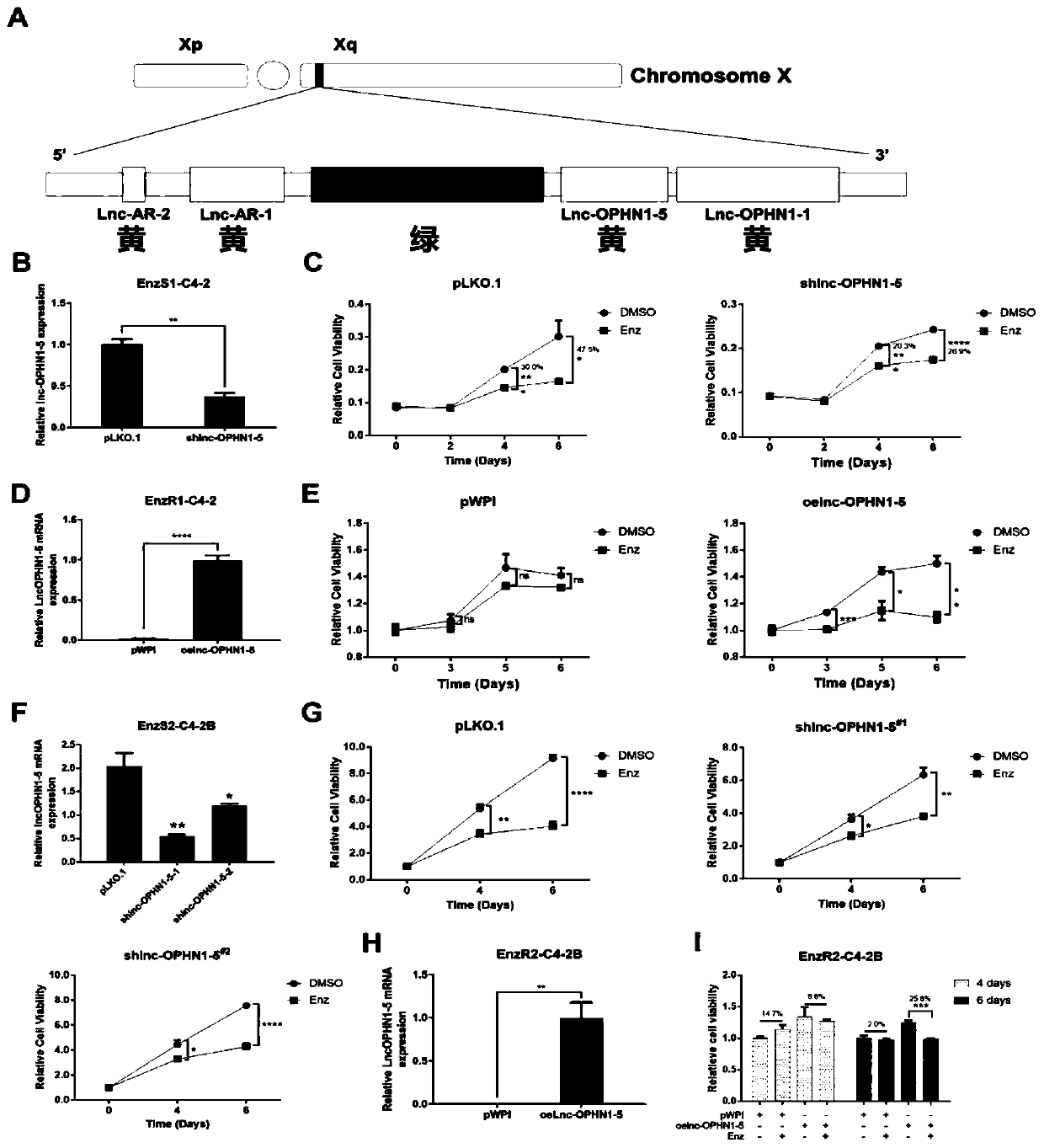
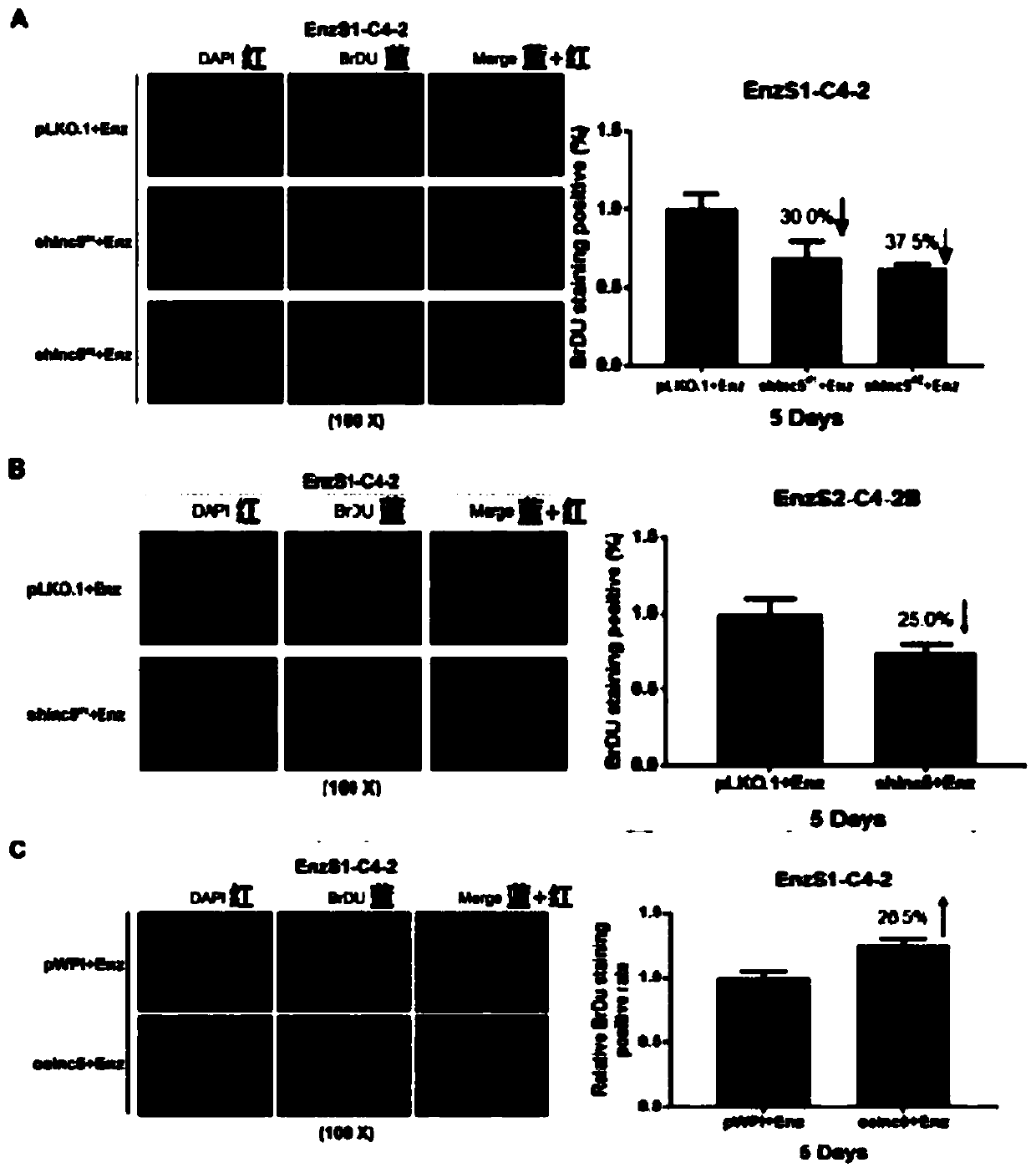
LncRNA related to enzalutamide treatment sensitivity and application of lncRNA to prostate cancer treatment by enzalutamide
InactiveCN110302197AExtend the life cycleOrganic active ingredientsGenetic material ingredientsGene technologyOncology
The invention provides lncRNA related to enzalutamide treatment sensitivity and application of the lncRNA to prostate cancer treatment by enzalutamide, and relates to the field of biological gene technologies. The lncRNA related to the enzalutamide treatment sensitivity is lnc-OPHN1-5, and the lnc-OPHN1-5 can increase the sensitivity of prostate cancer (PCa) to the enzalutamide (Enz) by reducing the translation of ARmRNA and the synthesis of AR protein. The lncRNA related to the enzalutamide treatment sensitivity overcomes the disadvantages of the prior art, and provides a new medical idea andstrategy for the treatment of castrated resistant prostate cancer (CRPC) and through the application of the lnc-OPHN1-5 in the prostate cancer treatment by the enzalutamide to improve the survival cycle of patients.
Owner:THE FIRST AFFILIATED HOSPITAL OF ANHUI MEDICAL UNIV
Enzalutamide intermediate preparation method
InactiveCN105330560ALow priceWide variety of sourcesOrganic compound preparationCarboxylic acid amides preparationQuinolineN-methylbenzamide
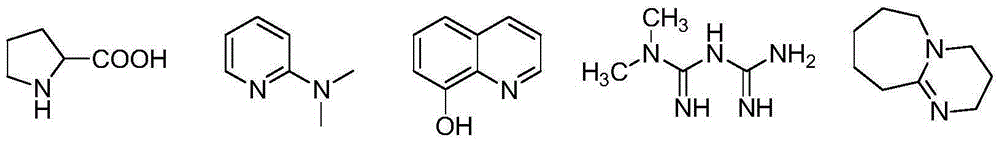
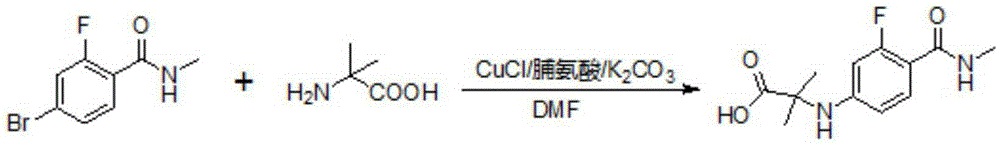
The present invention discloses an enzalutamide intermediate preparation method, wherein 2-bromo-4-fluoro-N-methylbenzamide and 2-amino-isobutyric acid are subjected to a substitution reaction under catalysis of cuprous halide, assisted catalysis of a nitrogen-containing ligand and the effect of an acid-binding agent, and the nitrogen-containing ligand is proline, o-phenanthroline, 8-hydroxy quinoline, metformin or 1,8-diazabicycloundec-7-ene. According to the present invention, the method has characteristics of safety, environmental protection, simple operation, low cost, high product yield, and great production use value.
Owner:FERGUSON WUHAN BIOTECH
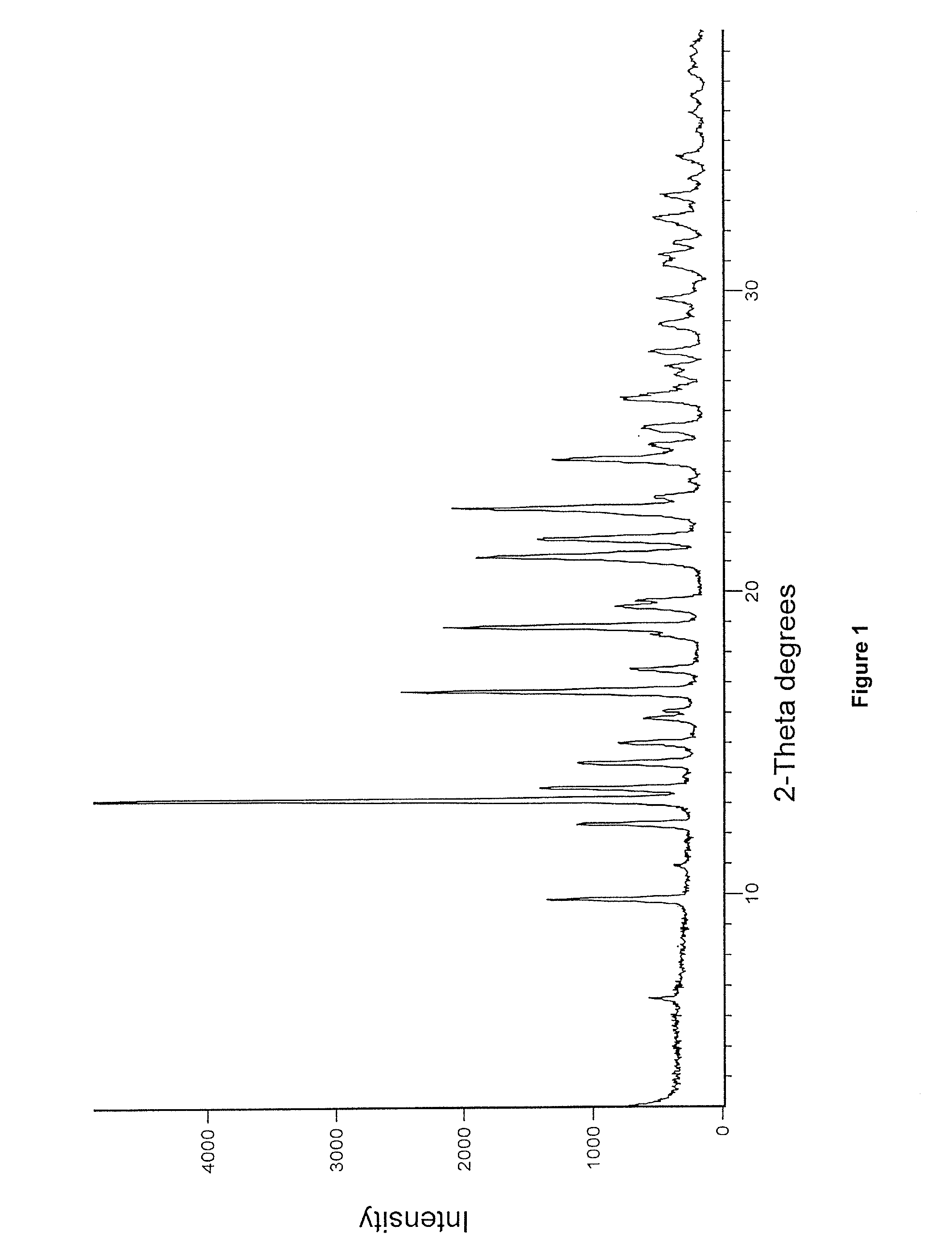
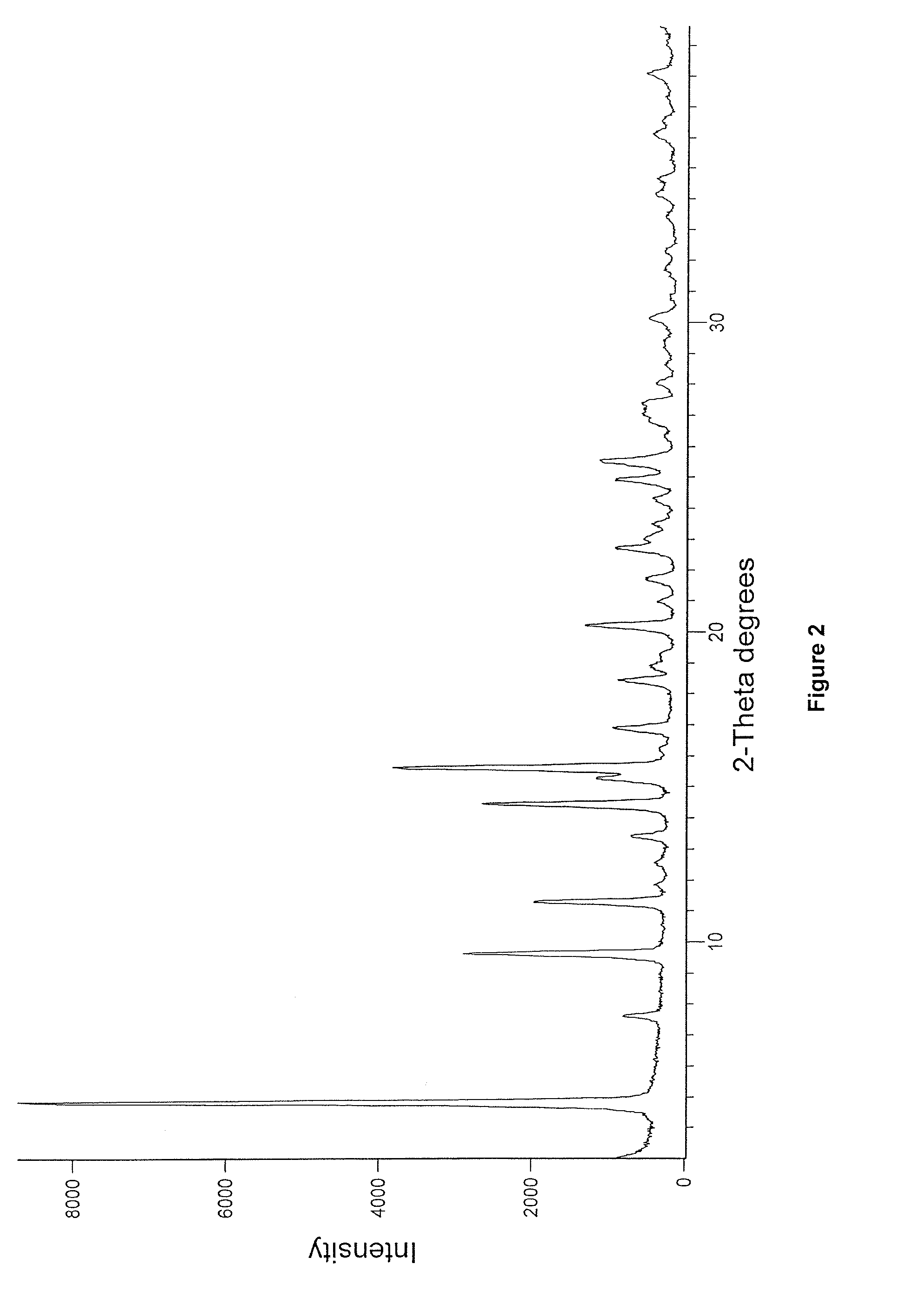
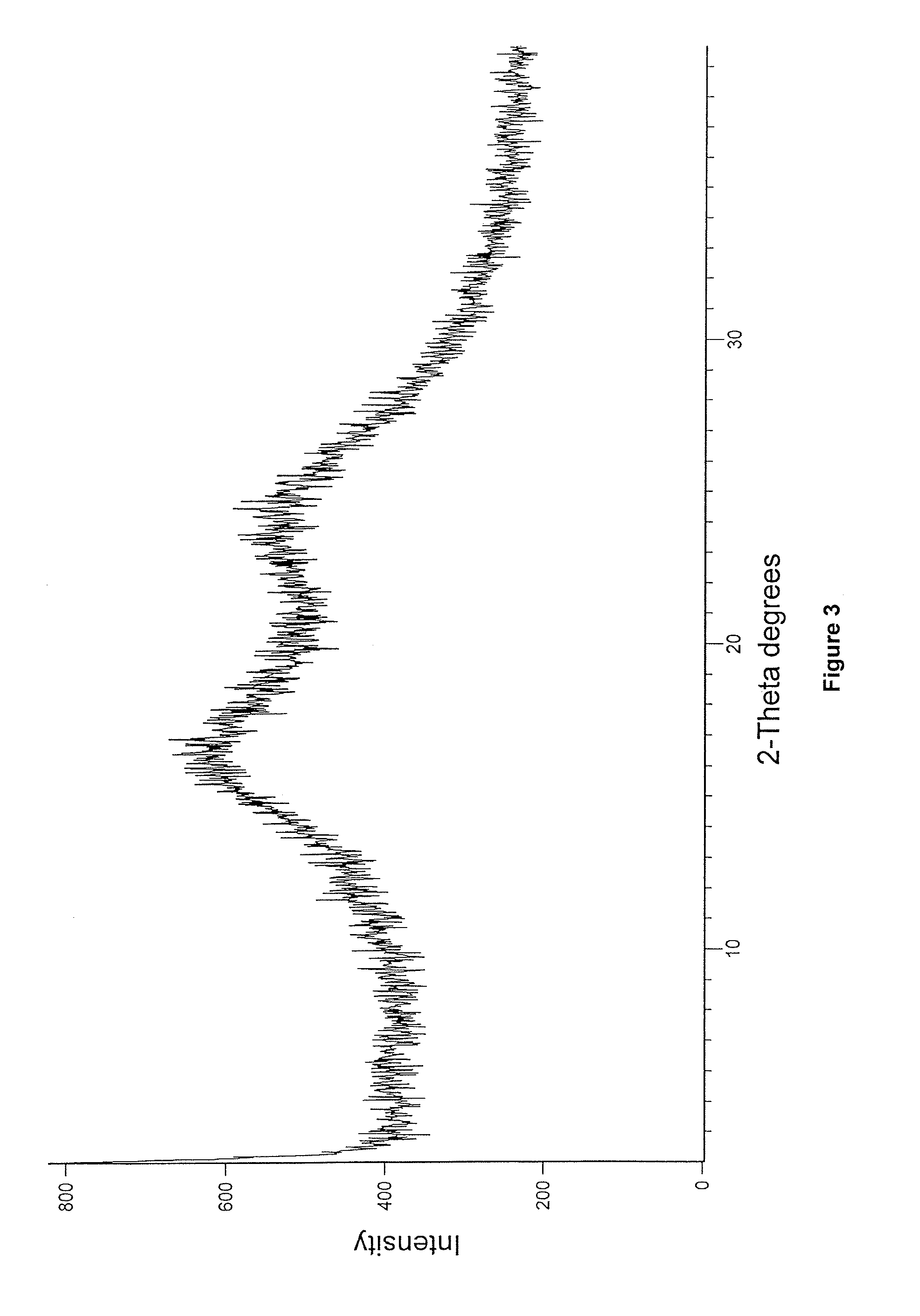
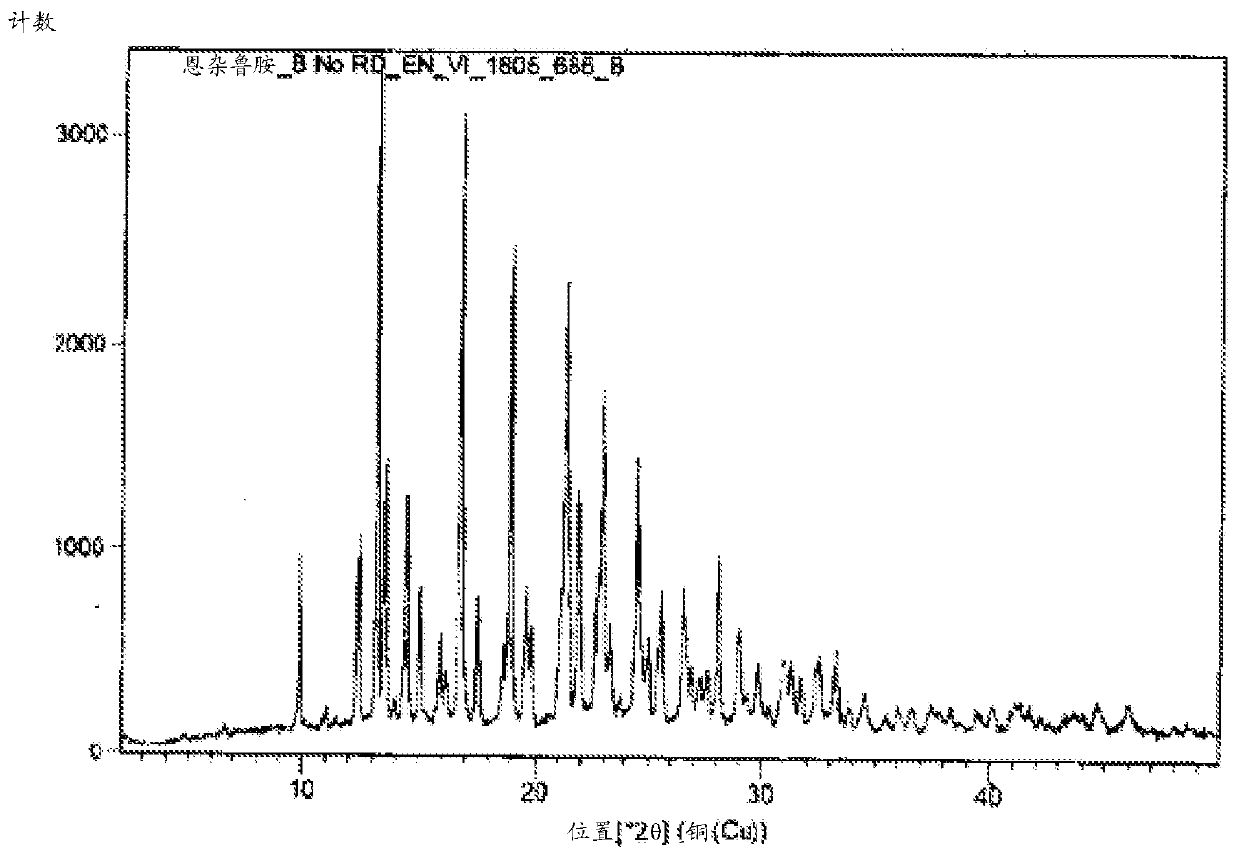
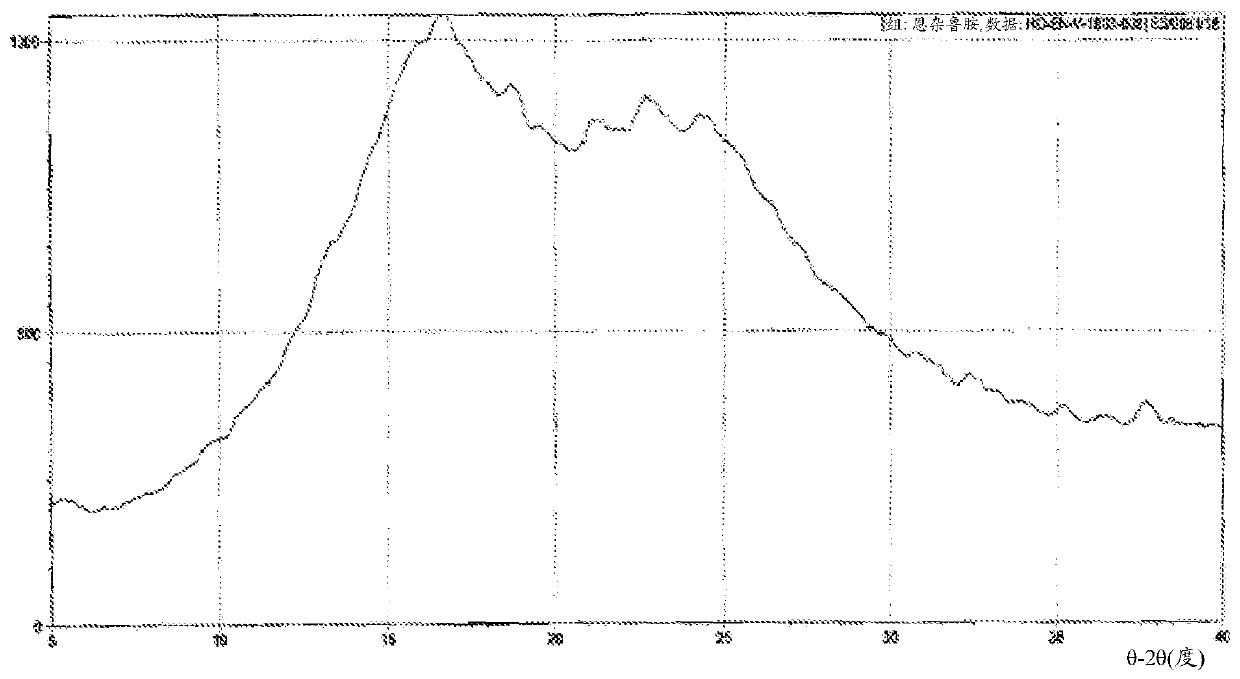
Enzalutamide polymorphic forms and its preparation
The present application relates to crystalline and amorphous forms of Enzalutamide. The present application further relates to amorphous solid dispersions of Enzalutamide with pharmaceutically acceptable carriers. The present application also relates to a process for the preparation of Form R1 of Enzalutamide.
Owner:DR REDDYS LAB LTD
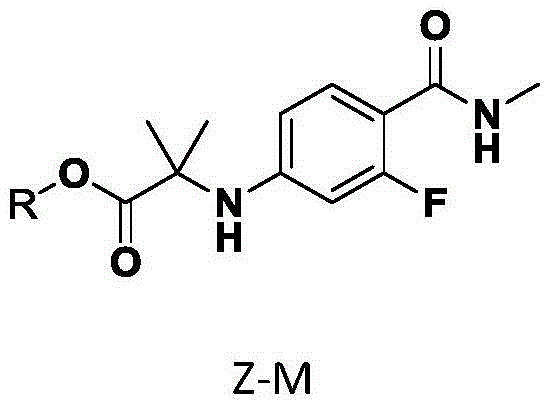
New compound for synthesizing Enzalutamide
ActiveCN105367441AReduce usageImprove conversion rateOrganic compound preparationCarboxylic acid amides preparationMethyl propionateEdman degradation
The present invention provides a compound Z-M for synthesizing enzalutamide, and a preparation method thereof, wherein R is selected from allyl, propargyl, benzyl, glycidyl or other groups containing alpha and beta unsaturated groups. According to the present invention, with the new series of the compound Z-M, the use of the toxic reagent iodomethane can be avoided during the key intermediate 2-(3-fluoro-4-(methylcarbamoyl)phenylamino)2-methyl methyl propionate preparation, and the conditions of the enzalutamide synthesis Edman degradation reaction of the new intermediate Z-M and an isothiocyanate compound 7 are superior to the reaction conditions of the methyl ester intermediate in the original literature report. The structure of the compound Z-M is defined in the specification.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +2
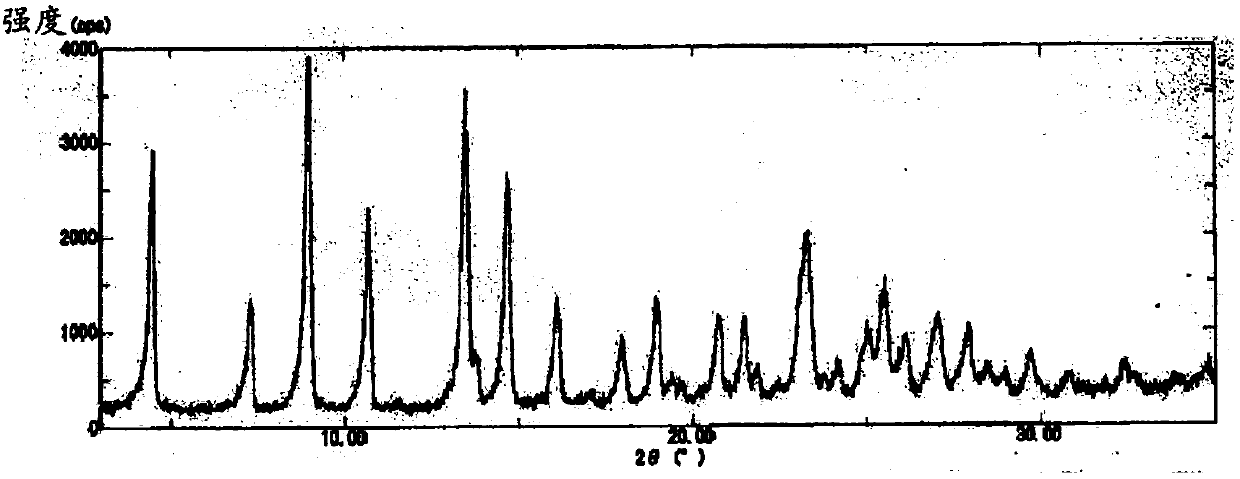
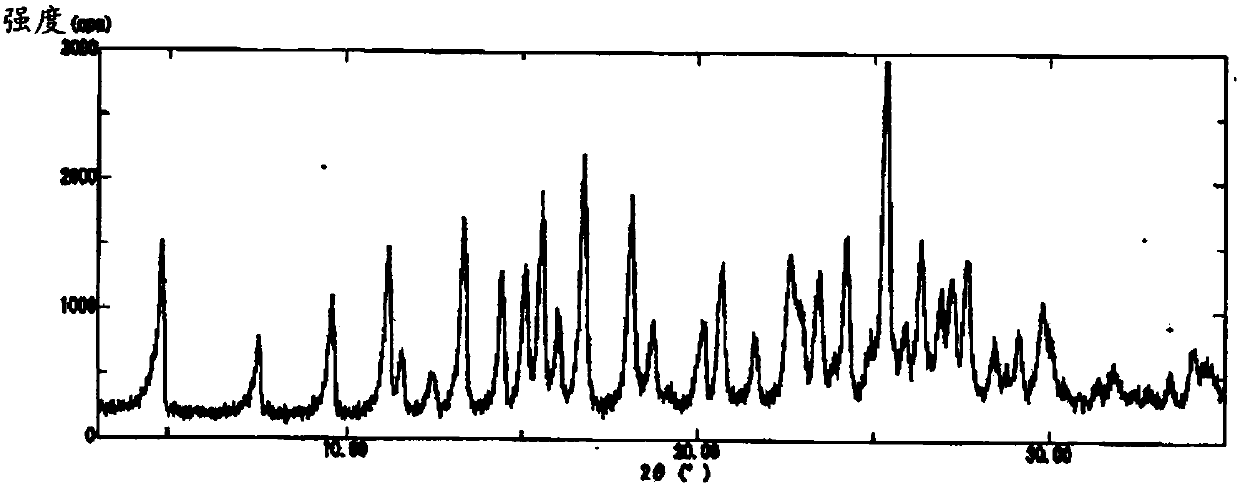
Process for producing enzalutamide crystal form
ActiveCN107635969AReduce crystallizationReduced crystalline formOrganic active ingredientsFrom normal temperature solutionsCrystallographySolvation
The purpose of the present invention is to provide a new process for producing an enzalutamide crystal form which is reduced in the contents of 2-propanol and B-form crystals, the process including obtaining wet crystals of enzalutamide in a crystallization step during the production of the enzalutamide crystal form and subsequently subjecting the enzalutamide to solvation. The present invention relates to a process for producing an enzalutamide crystal form, characterized by including a crystallization step in which wet crystals of enzalutamide are obtained and a step for drying the wet crystals and by including, after the crystallization step, a cleaning step in which a solvent mixture of a good solvent and a poor solvent is used.
Owner:ASTELLAS PHARMA INC
A kind of enzalutamide soft capsule and preparation method thereof
ActiveCN104857517BHigh dissolution rateImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsOctanoic AcidsGlycerol
Owner:NANJING HEALTHNICE MEDICAL TECH +1
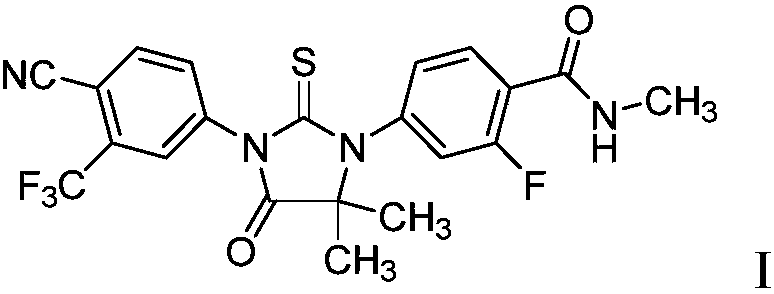
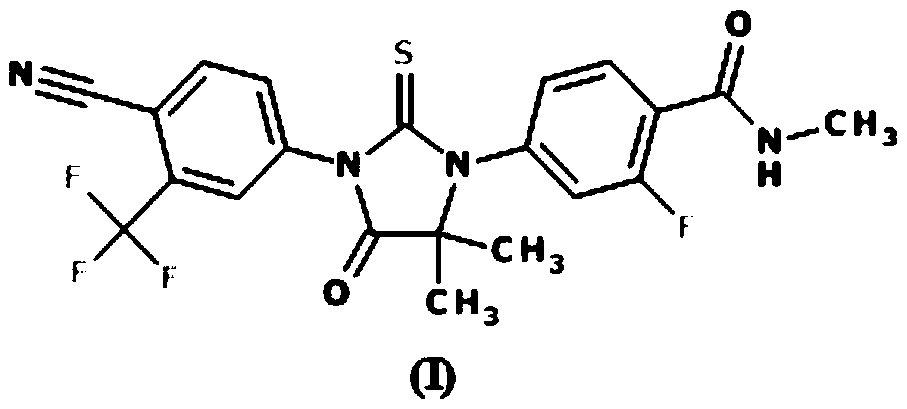
A novel process for preparing enzalutamide
ActiveCN107690427AOrganic active ingredientsOrganic chemistryBiochemical engineeringProcess engineering
The present invention provides a process for the efficient preparation of enzalutamide of the following formula I:
Owner:SCINOPHARM TAIWAN LTD
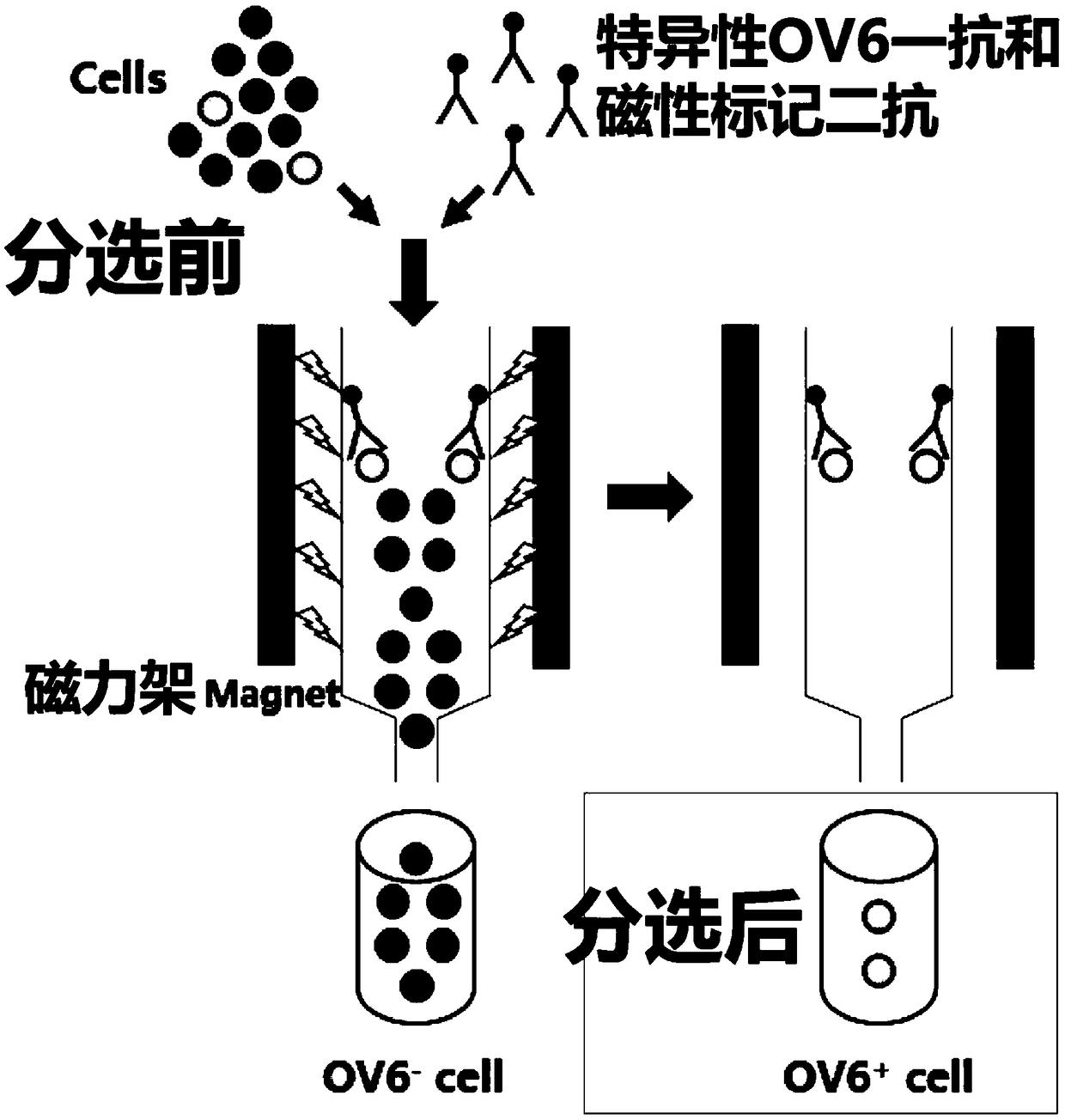
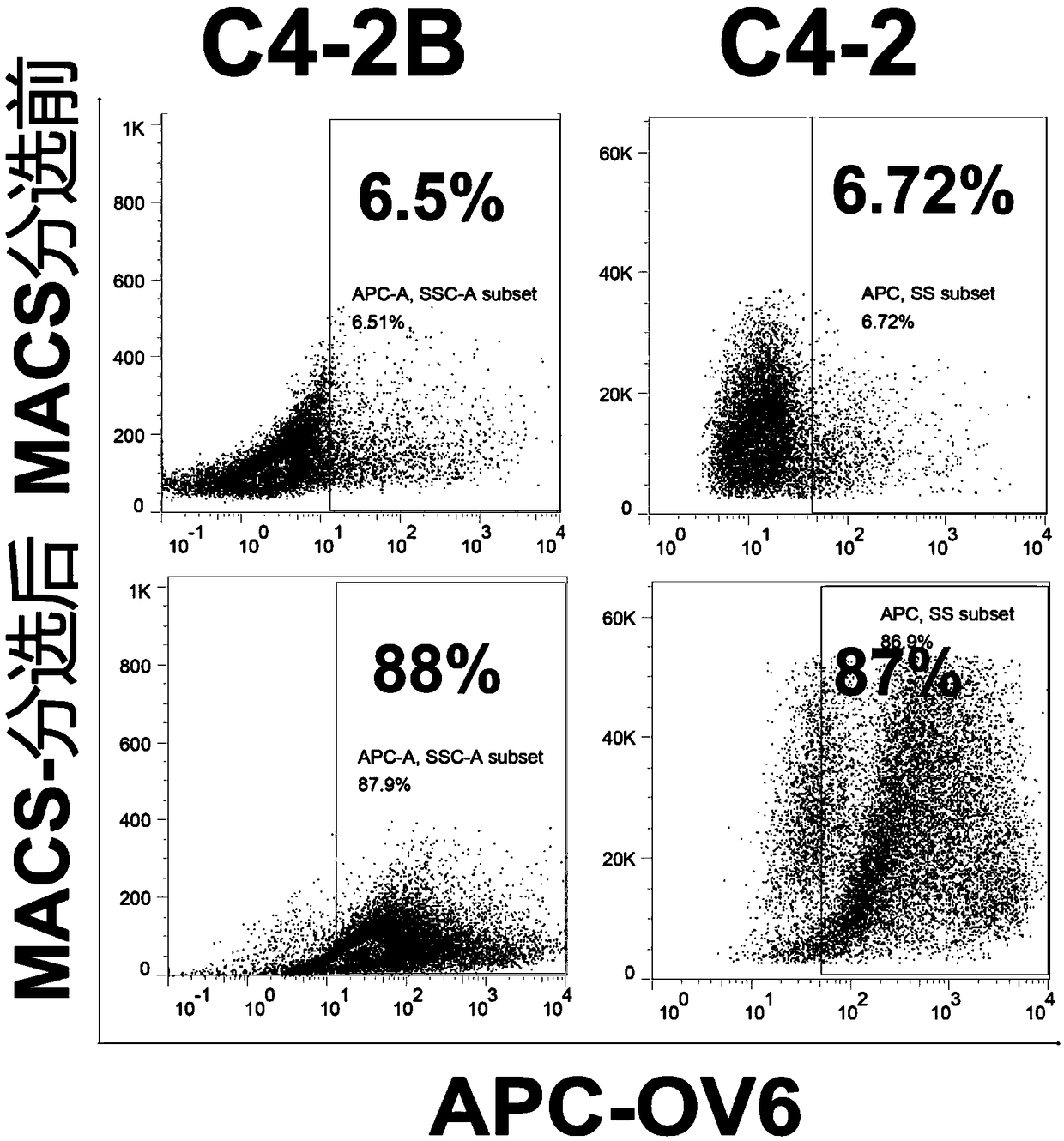
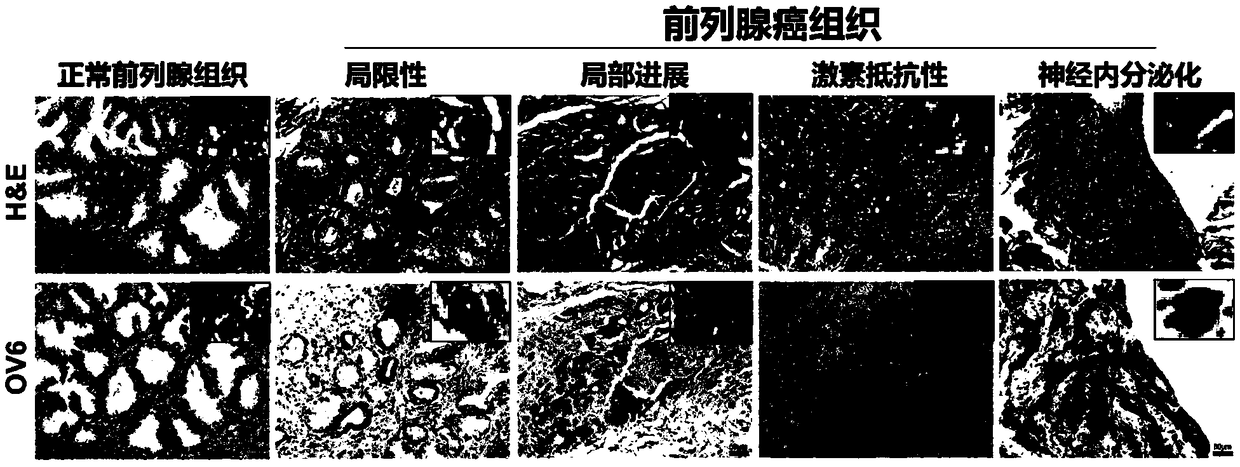
Prostatic cancer stem cell marker, application of antibody OV6 in preparation of prostatic cancer stem cell marking material and marking method
InactiveCN108727495AHigh ball rateHigh tumor formation rateImmunoglobulins against animals/humansTumor/cancer cellsProstate cancer cellFiltration
The invention relates to the field of medical technique research and discloses a prostatic cancer stem cell marker, application of an antibody OV6 in the preparation of a prostatic cancer stem cell marking material and a marking method. The marking method comprises the following steps: marking a prostate cancer cell line or a prostate tissue cell by virtue of a mouse-source OV6 antibody; incubating the prostate cancer cell line or the prostate tissue cell together with rat anti-mouse IgG and magnetic beads; putting the prostate cancer cell line or the prostate tissue cell on an MACS MS column,and carrying out filtration separation, so as to obtain an OV6 positive prostatic cancer cell; and finally detecting the marking efficiency of the OV6 positive prostatic cancer cell by virtue of a flow cytometry. Furthermore, enzalutamide is utilized for realizing drug stimulation so as to enhance the marking effect before the marking of the mouse-source OV6 antibody. According to the marking method, the prostatic cancer stem cell can be remarkably marked, and the OV6 positive cell has high expression dryness-related gene and high granulation rate and tumor formation rate. The marking methodhas relatively high specificity and sensitivity and important clinical values.
Owner:SHANGHAI CHANGHAI HOSPITAL
Treatment of metastatic prostate cancer
ActiveUS20200041516A1Peptide/protein ingredientsMicrobiological testing/measurementOncologyPharmacology
The present invention provides new compositions and methods for treating prostate cancer, e.g., drug-resistant prostate cancer, such as anti-androgen drug (e.g., enzalutamide) resistant and / or castration resistant prostate cancer (CRPC). These new compositions include, but are not limited to, pharmaceutical compositions that include an AR-V7 inhibitor, such as niclosamide. Alternatively, these new compositions can include, but are not limited to, pharmaceutical compositions that include an AKR1C3 inhibitor, such as indomethacin. These new methods include, but are not limited to, methods of administering an AR-V7 inhibitor, such as niclosamide, and / or an AKR1C3 inhibitor, such as indomethacin, to treat patients having prostate cancer. The present invention also provides methods of inhibiting androgen receptor variant expression, e.g. AR-V7, and methods of killing cells expressing AR-V7. The present invention further provides methods of inhibiting AKR1C3 expression or activity, and methods of killing cells that express AKR1C3.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Indazole hydrazide compound and application thereof
The invention provides an indazole hydrazide compound as shown in a formula (I), wherein R is selected from substituted alkyl, substituted alkenyl or substituted phenyl; substituent groups in the substituted alkyl group and the substituted alkenyl group comprise phenyl and / or substituted phenyl; and R' is selected from H or alkyl. Compared with the prior art, the indole hydrazide compound provided by the invention can be used as an integrin avbeta3 receptor antagonist, has obvious anti-prostatic cancer activity, and has a significant inhibition effect on enzalutamide drug-resistant cell lines.
Owner:PEKING UNIV FIRST HOSPITAL
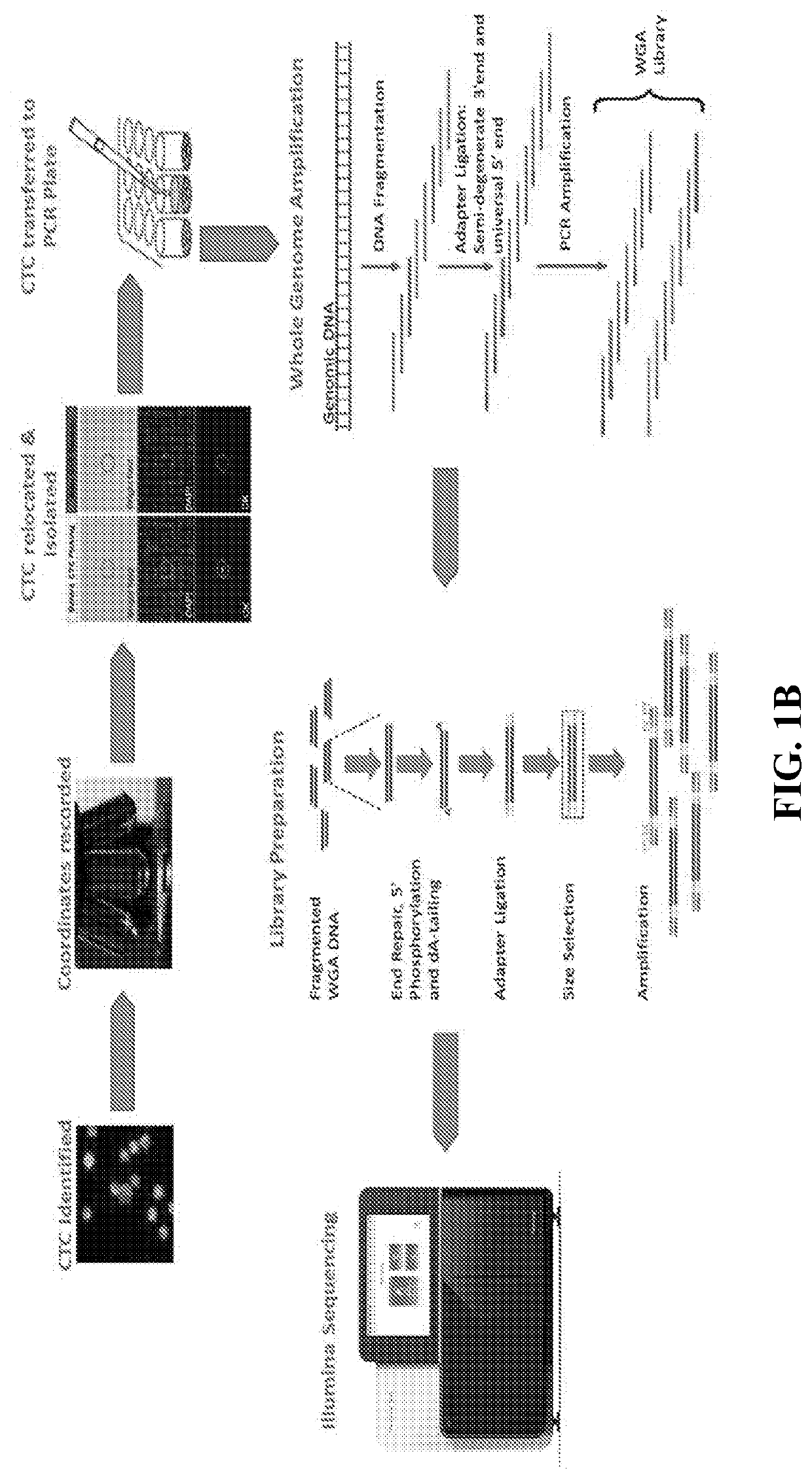
Methods of determining therapies based on single cell characterization of circulating tumor cells (CTCS) in metastatic disease
The disclosure provides a method of identifying a cell type, or the presence or absence of a cell type, associated with response to abiraterone or enzalutamide in a cancer patient, comprising (a) performing a direct analysis comprising immunofluorescent staining and morphological characterization of nucleated cells in a blood sample obtained from the patient to identify and enumerate circulating tumor cells (CTC); (b) isolating the CTCs from the sample; (c) individually characterizing parameters to generate a profile for each of the CTCs, and (d) identifying a biomarker CTC, wherein presence or absence of the biomarker CTC indicates a responsiveness in the patient to abiraterone or enzalutamide.
Owner:EPIC SCIENCES
Process for preparation of enzalutamide using novel intermediate
PendingCN111386257AOrganic compound preparationCarboxylic acid amides preparationCombinatorial chemistryPhenyl group
Process for preparation of Enzalutamide using novel intermediate. Provided herein is a process for the preparation of a novel [4-cyano-3- (trifluoromethyl)phenyl]carbamodithioic acid and its use in preparation of Enzalutamide being cost effective with higher yield, higher HPLC purity with reduced impurities.
Owner:AARTI IND LTD
Preparation method of enzalutamide
InactiveCN105461634AReduce generationImprove reaction efficiencyOrganic chemistryStereochemistryEnzalutamide
The invention provides a preparation method of enzalutamide. The method comprises the following steps: condensing an initial raw material 2-((3-fluoro-4-(methylcarbamoyl)phenyl)amino)-2-methylpropioric acid and enol to obtain an intermediate I, and reacting the intermediate I with The method has the advantages of simple operation, suitableness for industrial production, high yield and high purity.
Owner:JIANGSU HANSOH PHARMA CO LTD
A kind of preparation method of enzalutamide
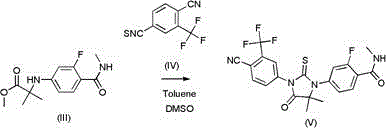
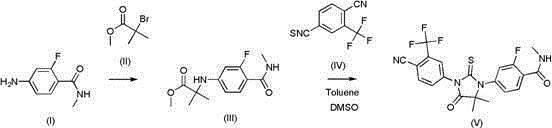
The invention relates to a method for preparing enzalutamide shown in a formula (V). The method comprises the following steps: (a) carrying out substitution reaction on 4-ammonia-2-fluorine-N-methyl formamide shown in a formula (I) and 2-bromo acid methyl ester shown in a formula (II) under the action of an acid-binding agent, so as to obtain 2-(3-fluorine-4-(methyl amino formamide) phenyl amine)-2-isobutyric acid-methyl ester shown in a formula (III); and (b) carrying out cyclization on the 2-(3-fluorine-4-(methyl amino formamide) phenyl amine)-2-isobutyric acid-methyl ester and 4-isothiocyano-2-(trifluoromethyl) benzonitrile shown in a formula (IV) in a mixed solvent of methylbenzene and dimethylsulfoxide (DMSO), so as to obtain the enzalutamide. The invention provides a preparation process of the enzalutamide, which is different from that in the prior art, safe and environment-friendly, simple and convenient to operate, and high in yield, and has great practical production value.
Owner:HANGZHOU XINBOSI BIOMEDICAL CO LTD
Method for synthesizing enzalutamide
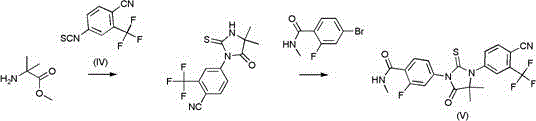
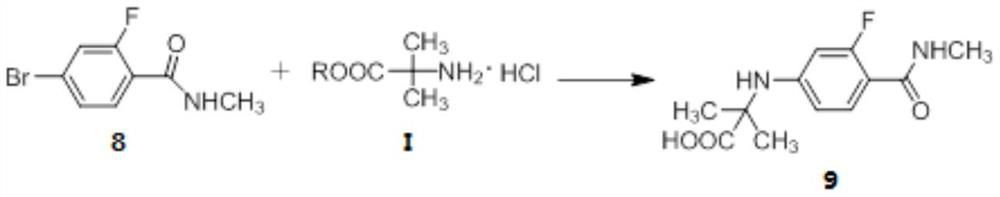
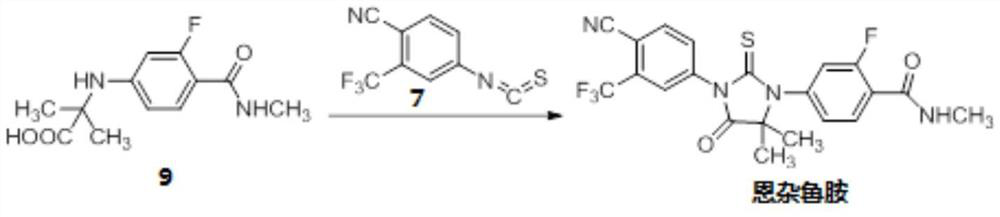
The invention provides a method for synthesizing enzalutamide. Specifically, the preparation method comprises the following steps: (1) in a first solvent, in the presence of an inorganic base, a catalyst and a ligand, enabling a compound 8 to react with hydrochloride of a compound shown as a formula I, thereby obtaining a compound 9; wherein the first solvent is composed of an organic solvent 1 and water; and (2) reacting the compound 9 and the compound 7 in a second solvent in the presence of an organic base to obtain enzalutamide. The preparation method has the advantages of short synthesisroute, high yield, mild reaction conditions, simple operation and post-treatment, high product purity, and suitableness for industrial production.
Owner:ANLITE SHANGHAI PHARMA TECH CO LTD +2
A preparation method for enzalutamide
PendingCN110396063AReduce usageAvoid operabilityOrganic compound preparationCarboxylic acid amides preparationOrganic solventSynthesis methods
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Aromatic amine protein degradation chimeric compound targeting AR and BET and application
ActiveCN111944012AOrganic active ingredientsDipeptide ingredientsProstate cancer cellCancer research
The invention relates to an aromatic amine protein degradation chimeric compound targeting AR and BET and an application thereof, and particularly provides a compound as shown in a formula I in the description. Experimental results show that the compound can degrade AR and BRD4 in a targeted manner and reduce protein expression of the AR and the BRD4 at the same time. The compound can inhibit proliferation of various prostate cancer cells, can inhibit proliferation of a prostate cancer cell line LNCaP / AR with multiple expression of an androgen receptor AR, and also shows a good inhibition effect on prostate cancer cell lines 22RV1 resistant to prostate cancer drugs (enzalutamide) on the market. The compound also shows good metabolic stability, and has good application prospects in preparation of androgen receptor and / or BET protein degradation targeting chimeras and drugs for treating androgen receptor and BET regulated related diseases.
Owner:HINOVA PHARM INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com