Method for synthesizing enzalutamide
A technology of enzalutamide and compounds, applied in the field of synthesizing enzalutamide, can solve problems such as corrosion, damage, and inhibition of the central nervous system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
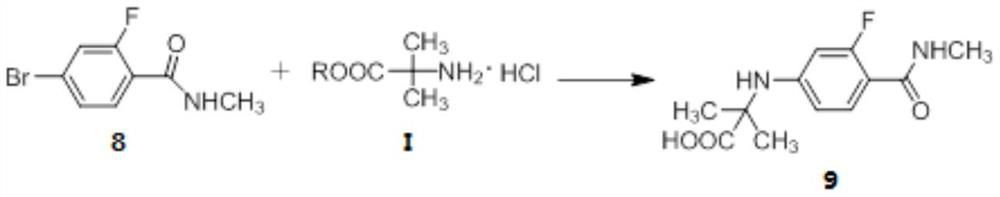
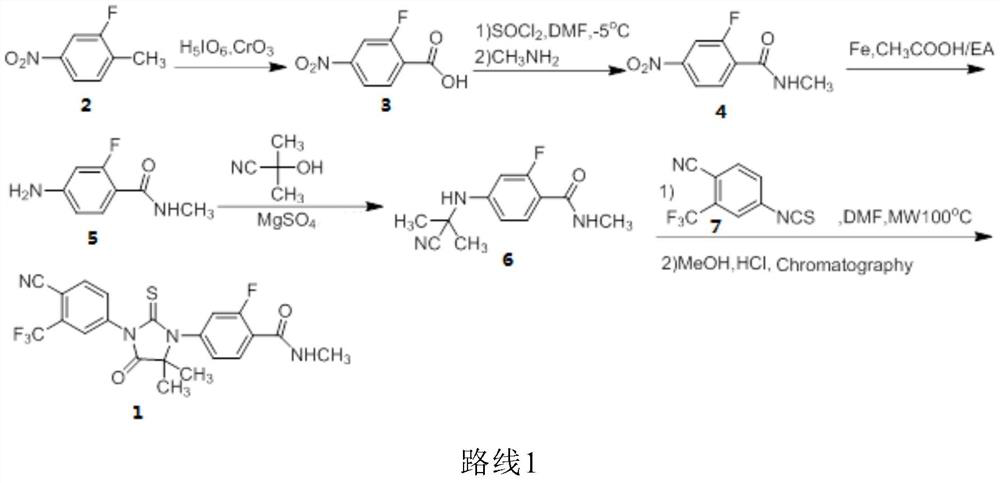
[0135] Embodiment 1: Preparation of 2-fluoro-4-bromobenzamide (compound 8)
[0136] Add 4-bromo-2-fluorobenzoic acid (300.0g, 1.4mol), isopropyl acetate (IPAc) 2L, thionyl chloride (228.0g, 1.9mol), DMF (30.0g, 0.4mol) into the reaction flask , slowly raised the temperature to 55°C, and kept the reaction for 3 hours. Cool down to 10-20°C, slowly add a mixture of methylamine aqueous solution (987.0 g, 0.79 mol) and 600 mL of isopropyl acetate dropwise, and control the temperature at 0-10°C. After the dropwise addition, the temperature was raised to 35° C., and stirring was continued for 12 hours. After the reaction was completed, 600 mL of pure water was added and stirred for 10 minutes. The layers were separated, and the aqueous layer was extracted twice with 600 ml of isopropyl acetate. The combined organic layers were dried and concentrated to dryness. Add 1.5 L of n-heptane to the residue to make a slurry, filter, and vacuum-dry at 40° C. for 6 hours to obtain 300.3 g o...
Embodiment 2
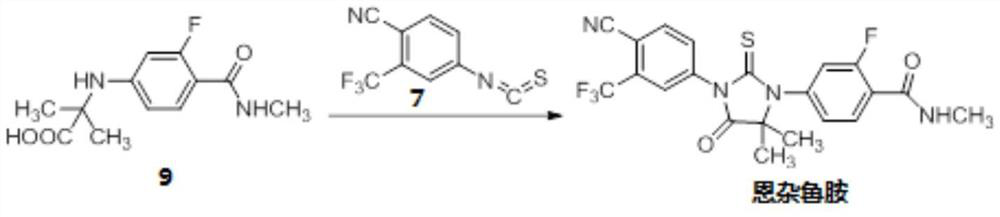
[0137] Example 2: Preparation of N-[3-fluoro-4-[(methylamino)carbonyl]phenyl]-2-methylalanine (compound 9):
[0138] Compound 8 (100.0 g, 0.43 mol) prepared in Example 1, methyl aminoisobutyrate hydrochloride (R is -CH 3 ) (99.5g, 0.65mol), K 2 CO 3 (180.0 g, 1.3 mol), CuCl (8.5 g, 85.4 mmol), DMF (600 mL) and water (60 mL), heated to 30°C. Add 2-acetylcyclohexanone (12.0 g, 85.6 mmol), heat to 105° C., and continue stirring for 12 hours. After the reaction, cool down to room temperature, add water (1.2 L) and IPAc (600 mL), and stir for 10 minutes. The separated aqueous layer was extracted twice with IPAc (600 mL). The combined organic layers were acidified with concentrated hydrochloric acid to pH 2-3, and solids were precipitated. It was further cooled to 0-5°C, filtered, and the filter cake was washed with water (300 mL). Add 700mL of absolute ethanol to the wet product, raise the temperature to reflux, and make the solution clear. Cool down to 5-10°C, filter, and v...
Embodiment 3
[0139] Example 3: Preparation of N-[3-fluoro-4-[(methylamino)carbonyl]phenyl]-2-methylalanine (compound 9):
[0140] Compound 8 (5.0 g, 21.6 mmol) prepared in Example 1, benzyl aminoisobutyrate hydrochloride (R is ) (8.9g, 38.8mmol), K 2 CO 3(10.3 g, 74.6 mmol), CuI (0.5 g, 2.6 mmol), DMSO (50 mL) and water (5 mL), heated to 40°C. Add 2-acetylcyclohexanone (0.5 g, 3.6 mmol), heat to 110° C., and continue stirring for 10 hours. After the reaction, cool down to room temperature, add water (60 mL) and IPAc (40 mL), and stir for 10 minutes. The separated aqueous layer was extracted with IPAc (40 mL). The combined organic layers were acidified with concentrated hydrochloric acid to pH 2-3, and solids were precipitated. It was further cooled to 0-5°C, filtered, and the filter cake was washed with water (20 mL). Add 40mL of absolute ethanol to the wet product, raise the temperature to reflux, and make the solution clear. Cool down to 0-5°C, filter, and dry under vacuum at 40°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com