Enzalutamide polymorphic forms and its preparation
a technology of enzalutamide and polymorphic forms, which is applied in the field of polymorphic forms of enzalutamide, can solve the problems of inability to predict the number of polymorphic forms of a given compound, and differences in pharmaceutical products in solubility, bioavailability, stability and other properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
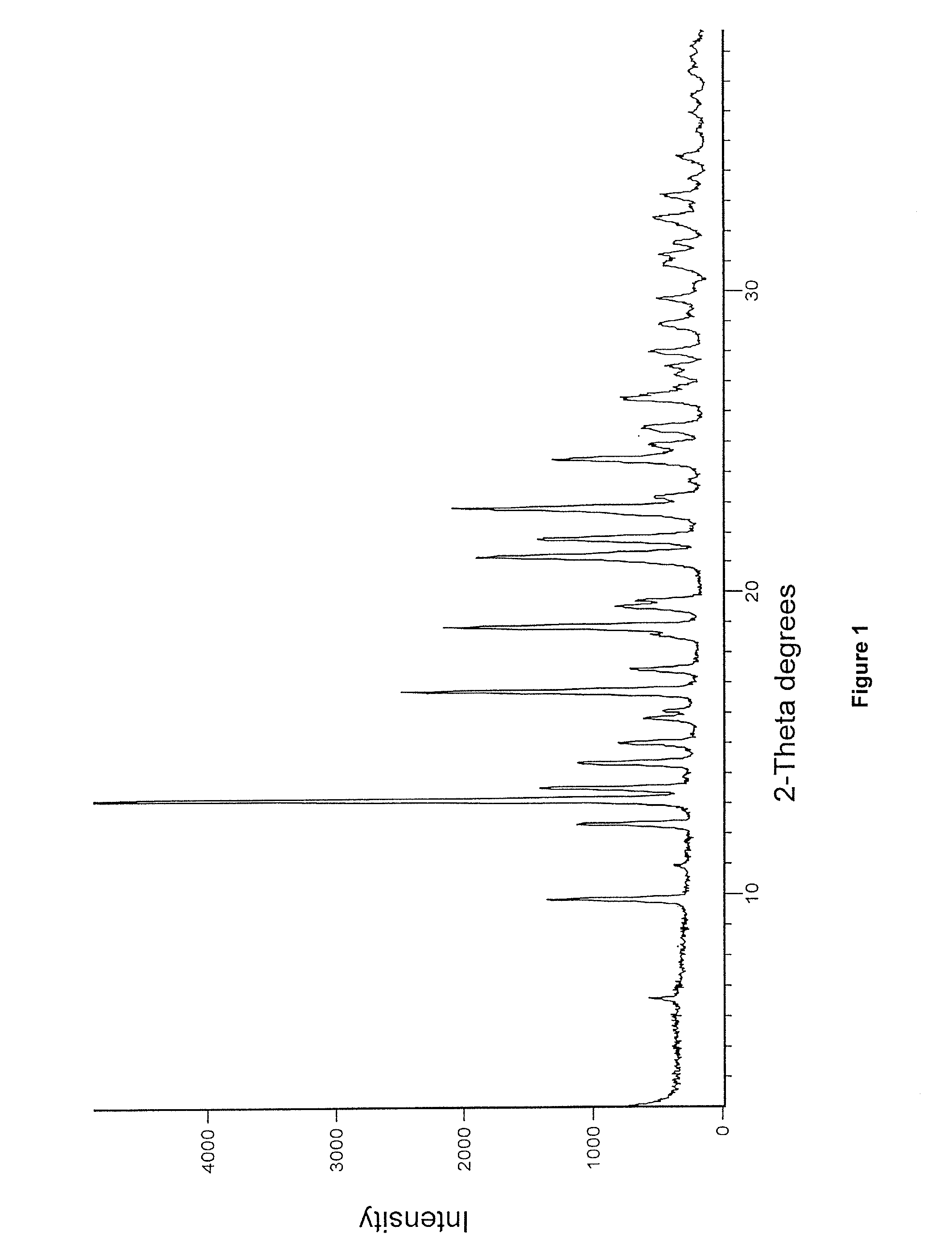
Preparation of Crystalline Enzalutamide Form R1
[0104]Methyl 2-((3-fluoro-4-(methylcarbamoyl)phenyl)amino)-2-methylpropanoate (45 g), 4-isothiocyanato-2-(trifluoromethyl)benzonitrile (95.6 g), dimethylsulphoxide (45 mL) and isopropyl acetate (90 mL) charged into round bottom flask, heated to 85° C. and maintained at 85° C. for 16 hours. Methanol (7.5 mL) was added to the reaction mixture at 65° C. and stirred at 65-68° C. for 30 minutes. Isopropyl acetate (360 mL) and water (180 mL) was added to the reaction mixture at 20° C. and stirred for 20 minutes. Layers were separated, charcoal (4.5 g) was added to the organic layer and stirred for 15 minutes. The resultant reaction mixture was passed through celite bed and filtrate solvent was evaporated under reduced pressure at below 50° C. followed by traces of isopropyl acetate was chased with isopropyl alcohol (90 mL). To the crude compound, isopropyl alcohol (450 mL) was added, heated to 75° C. and stirred at 75° C. for 20 minutes. The ...
example 2
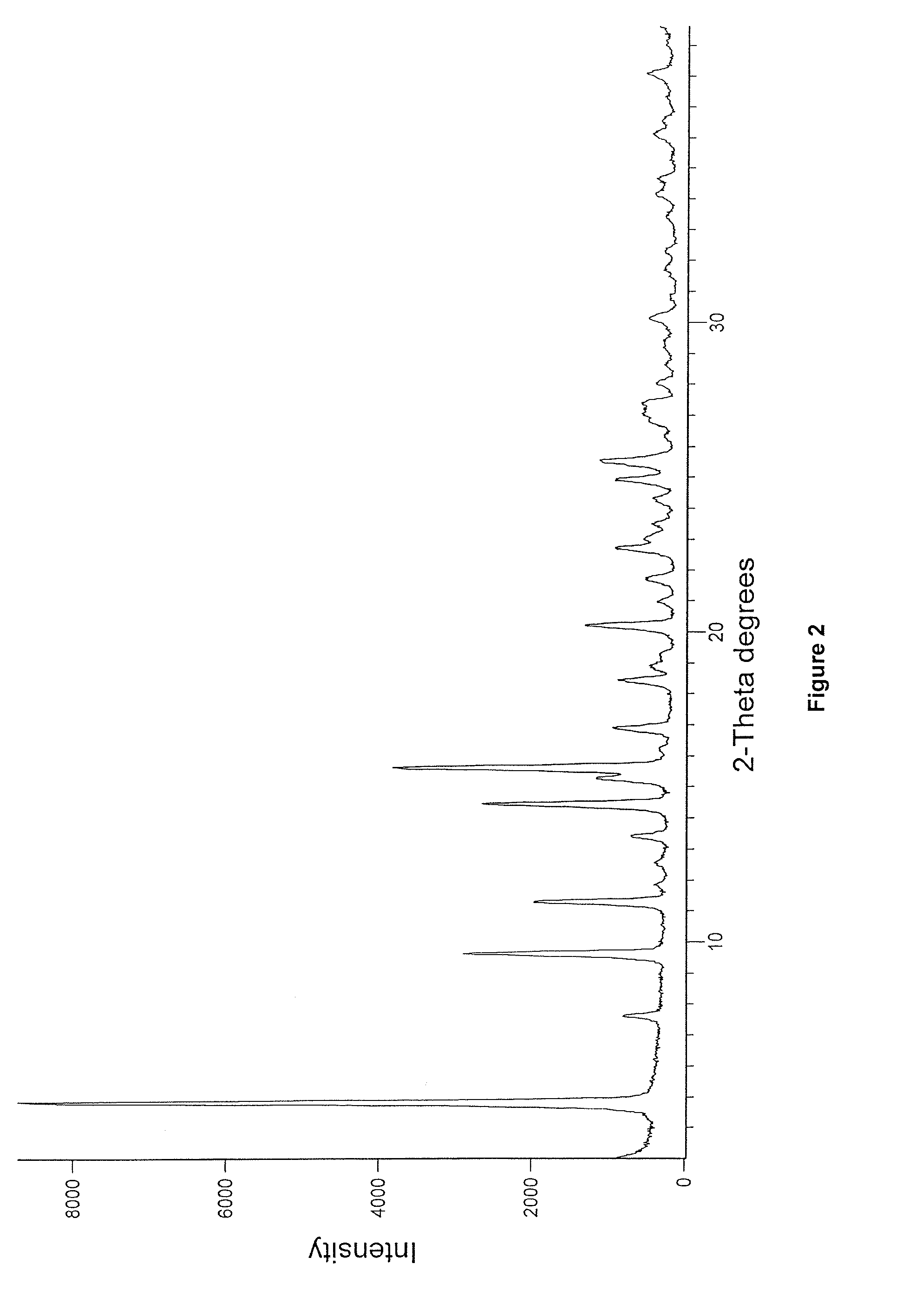
Preparation of Crystalline Enzalutamide Form R1
[0106]Methyl 2-((3-fluoro-4-(methylcarbamoyl)phenyl)amino)-2-methylpropanoate (200 g), 4-isothiocyanato-2-(trifluoromethyl)benzonitrile (425 g), dimethylsulphoxide (200 mL) and isopropyl acetate (400 mL) charged into round bottom flask, heated to 85° C. and maintained at 83° C. for 14 hours. Methanol (30 mL) was added to the reaction mixture at 65° C. and stirred at 65-67° C. for 30 minutes. Isopropyl acetate (1400 mL), water (600 mL) and isopropyl alcohol (400 mL) was added to the reaction mixture at 20° C. and stirred for 15 minutes. Layers were separated and organic layer was dried over sodium sulphate. Charcoal (20 g) was added to the organic layer and stirred for 25 minutes. The resultant reaction mixture was passed through celite bed and filtrate solvent was evaporated under reduced pressure at below 50° C. To the crude compound, isopropyl alcohol (1600 mL) was added, heated to 75° C. and stirred at 75° C. for 20 minutes. The resu...
example 3
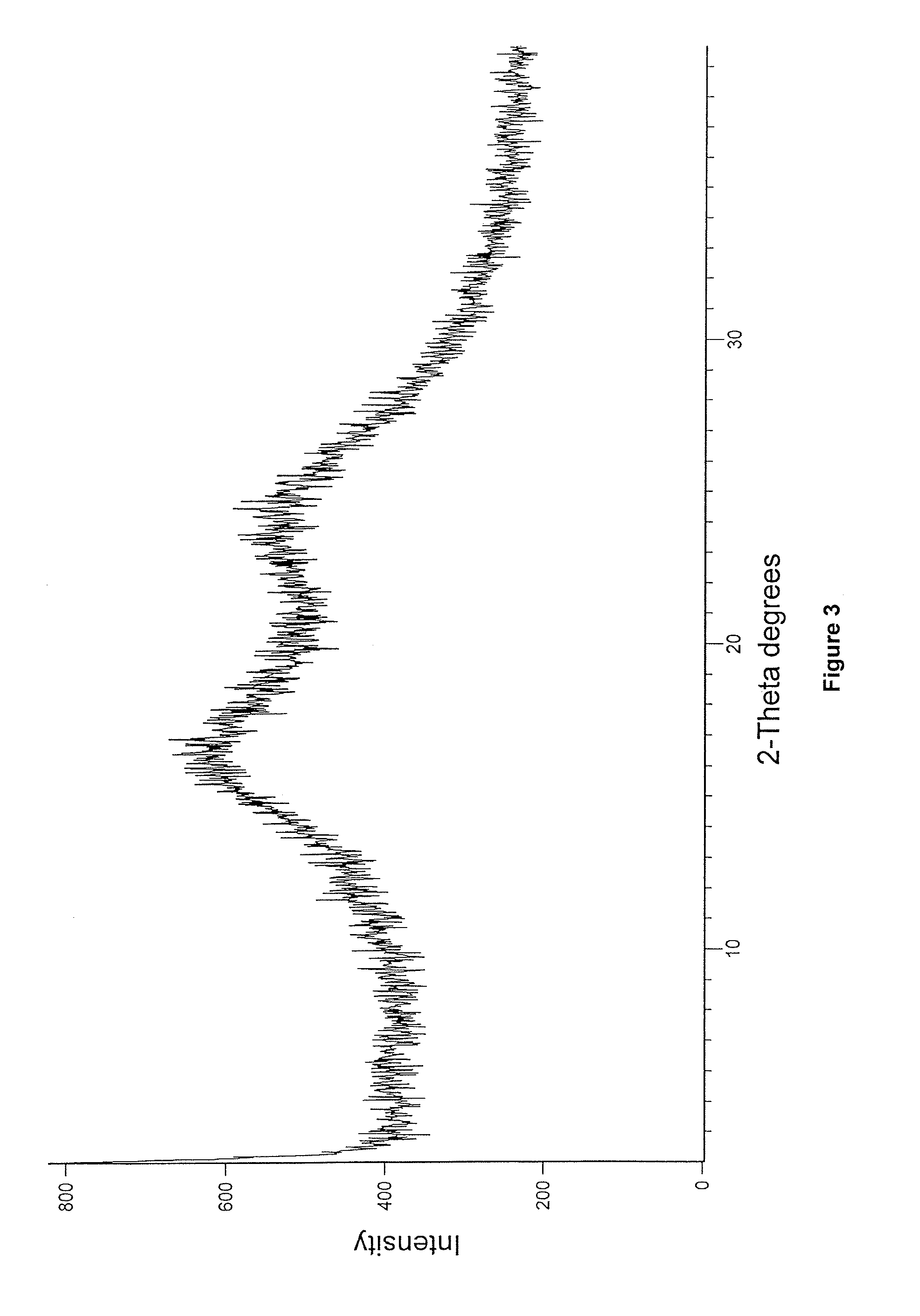
Preparation of Crystalline Enzalutamide Form R1
[0107]Enzalutamide (100 mg) and acetic acid (1 mL) was charged into a round bottom flask at 26° C. and stirred for 5 minutes. Water (10 mL) was added to the resultant reaction mass at 26° C. and stirred at 26° C. for 15 minutes. Separated solid was collected by filtration and dried at 50° C. under reduced pressure for 1 hour to afford the title compound.
[0108]Yield: 68 mg
PUM
| Property | Measurement | Unit |
|---|---|---|
| Angle | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com