Application of auranofin in preparation of medicine for treating castration-resistant prostate cancer
A castration-resistant, prostate cancer technology, applied in the field of biomedicine, can solve the problems of insufficient clinical treatment of tumors and insignificant anti-tumor effects, and achieve the effect of shortening the time, time cost and economic cost of clinical transformation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The drug sensitivity test of embodiment 1 cell to EPI, Enza
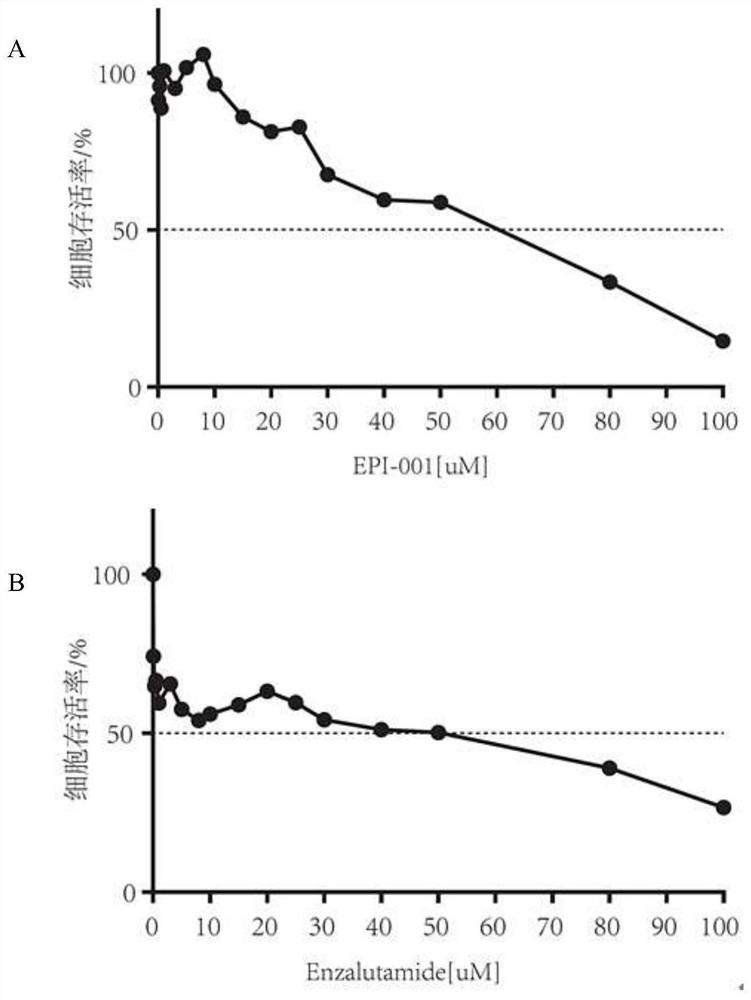
[0038] To detect the drug sensitivity of human prostate cancer cells LNCaP to EPI and Enza.
[0039] 1. Experimental method:
[0040] After the LNCaP cells were plated on a 96-well plate and adhered to the wall overnight, a series of EPI and Enza from high to low concentrations were prepared, and a control group was set up, added to the wells, and three replicate wells were set up for each concentration. After incubation in the cell incubator for 48 hours, CCK8 was added, and after 4 hours, the cell OD value at 450nm wavelength was detected by a full-wavelength multifunctional microplate reader, and the IC50 (half maximal inhibitory concentration) value of EPI and Enza acting on LNCaP cells was calculated.
[0041] 2. The result is as follows figure 1 as shown, figure 1 Among them, graph A is the dose-response curve of LNCaP cells treated with different concentrations of EPI; graph B is the dose-response c...
Embodiment 2
[0043] Example 2 Establishment of prostate cancer LNCaP-drug-tolerant persisters (L-DTP) cell lines resistant to EPI and Enza
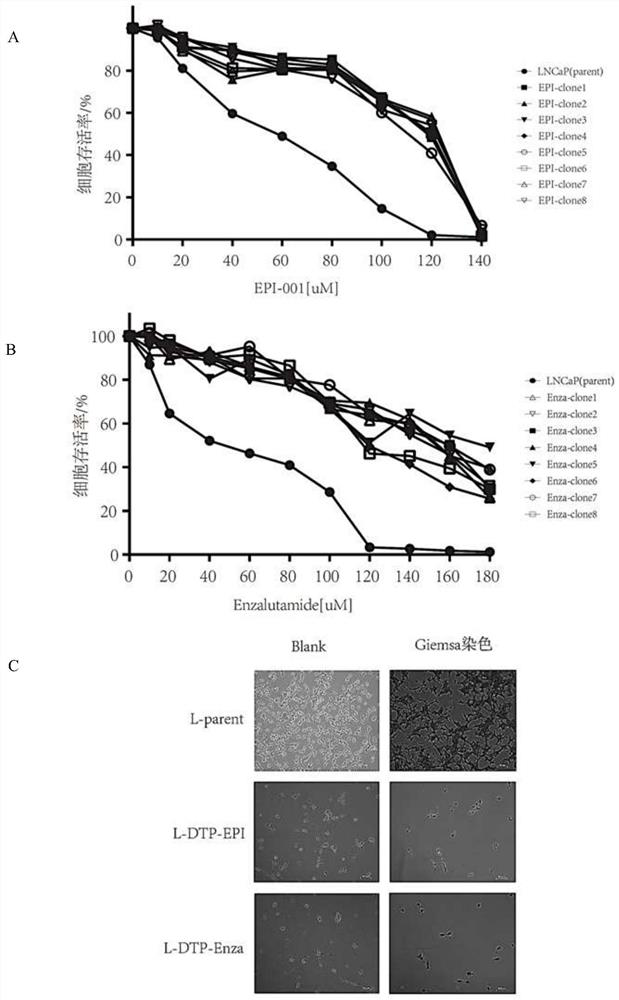
[0044] Establish prostate cancer LNCaP-drug-tolerant persisters (L-DTP) cell lines L-DTP-EPI and L-DTP-Enza that are resistant to EPI and Enza. At this time, LNCaP cells can recover drug resistance to EPI and Enza, thereby Mimic CRPC at the cellular level.
[0045] 1. Experimental method:
[0046] In LNCaP cells in a 10cm dish, add EPI (60uM) and Enza (50uM) to treat the cells for 9 days after adhering to the wall overnight, and replace the fresh medium added with drugs every 3 days, and then take 8 of these two drug-resistant cells After the clones were planted in a 96-well plate and adhered to the wall, a series of EPI and Enza from high to low concentrations were prepared, and a control group was set up, added to the wells, and three replicate wells were set for each concentration. After incubation in the cell incubator for 48 hours, CCK8 was add...
Embodiment 3
[0049] Example 3 EPI, Enza respectively combined with Aura drug.
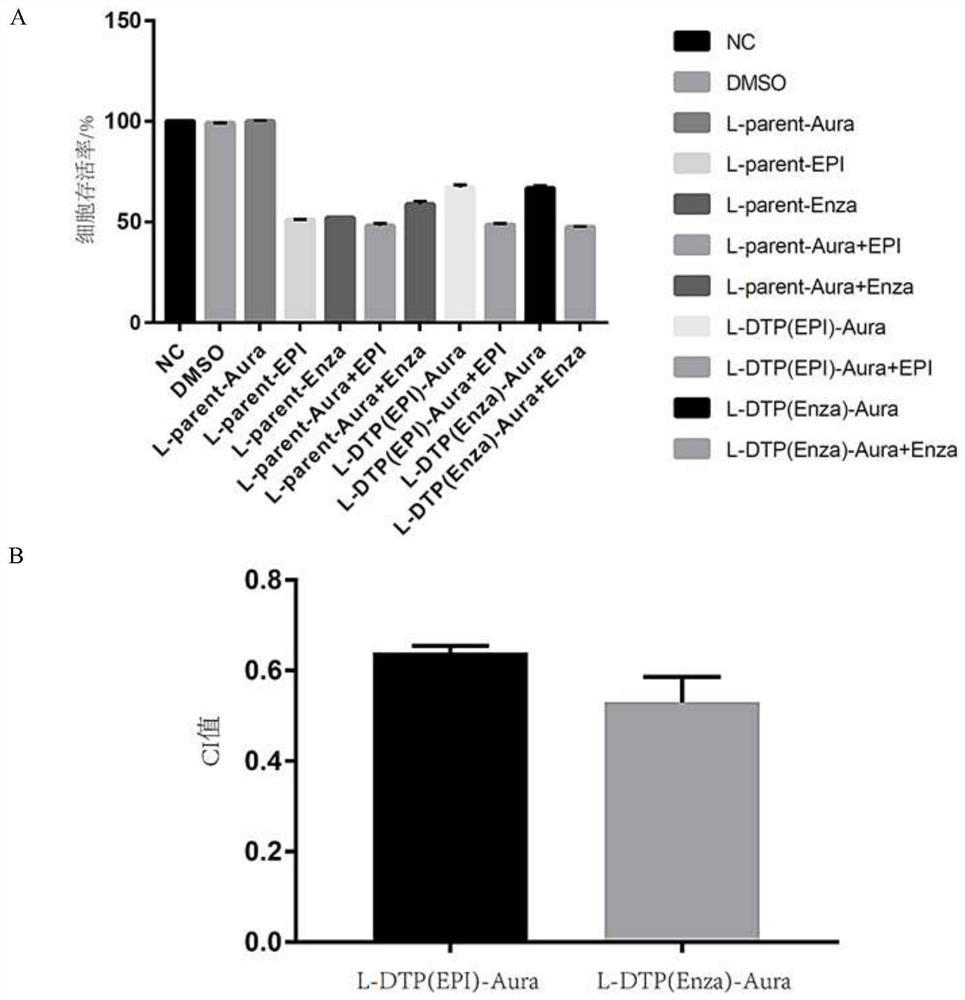
[0050] Further use of CCK8 in L-parent and L-DTP cells were used alone or in combination to illustrate the anti-tumor effect of Aura drugs in L-parent and L-DTP cells in vitro.
[0051] 1. Experimental method
[0052] Normal prostate cancer cells L-parent and drug-resistant cells L-DTP (including L-DTP-EPI and L-DTP-Enza) were planted in 96-well plates, and a series of Aura drugs from high to low were prepared In order to find the highest concentration of Aura without growth inhibition in L-parent cells, set 3 duplicate wells for each concentration, after incubation in the cell culture incubator for 48h, add CCK8, and use the full-wavelength multifunctional enzyme after 4h The standard instrument detects the cell OD value with a wavelength of 450nm, and calculates the survival rate of Aura drug alone in L-parent cells. Then use this concentration to measure the survival rate in L-parent cells with [L-parent-c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com