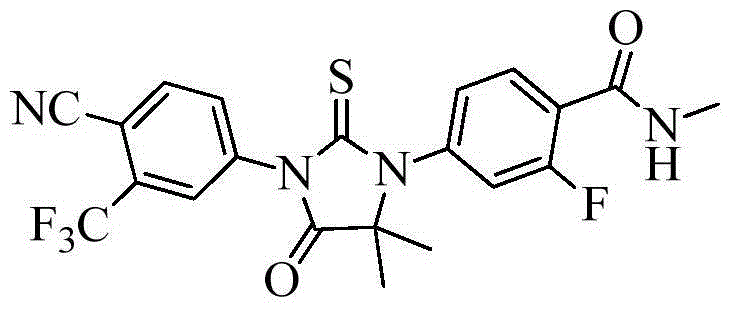
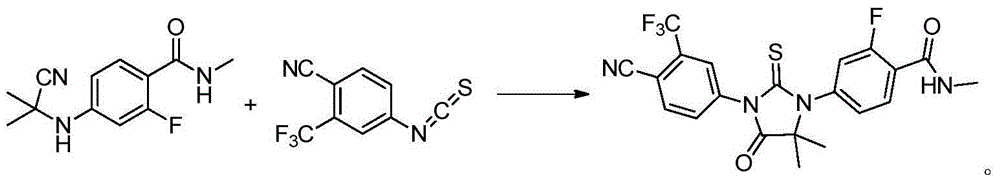
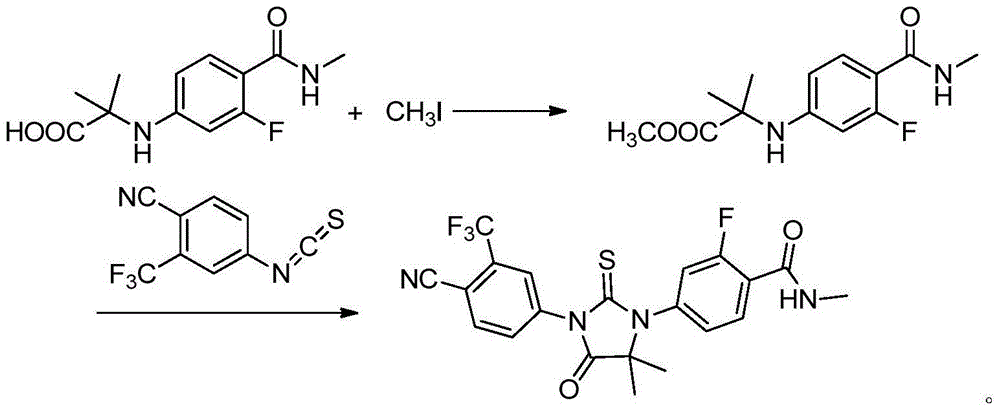
Preparation method of enzalutamide
A technology of enzalutamide and diisopropylcarbodiimide, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of unsuitability for industrial production, unsuitability for scale-up, low reaction yield, etc., to achieve suitable industrial production and reduce impurities The effect of producing and improving the purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: 4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxoimidazolidine-1 Preparation of -yl)-2-fluoro-N-methylbenzamide
[0041] 2-((3-fluoro-4-(methylcarbamoyl)phenyl)amino)-2-methylpropanoic acid (500g, 2.0mol, 1eq), 3-buten-1-ol (140g, 2.0mol, 1.0eq), dimethylaminopyridine (24g, 0.2mol), 1,3-diisopropylcarbodiimide (500g, 4.0mol) and dichloromethane (2L) were sequentially added to a 10L reaction flask, The internal temperature was controlled at 20-30°C, and the reaction was stirred for 12 hours. Add 2L of water into the reaction flask, extract, extract the aqueous phase with dichloromethane 2L×3, combine the dichloromethane layers, wash twice with 2L of saturated sodium chloride solution, dry over anhydrous sodium sulfate, filter, and concentrate to dryness under reduced pressure. Add 4L of acetone and stir, filter, add 10L of n-hexane to the solution, stir and crystallize for 2h to obtain the crude intermediate 1 (480g, 1.6mol).
[0042] Dis...
Embodiment 2
[0045] Example 2: 4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxoimidazolidine-1 Preparation of -yl)-2-fluoro-N-methylbenzamide
[0046] 2-((3-fluoro-4-(methylcarbamoyl)phenyl)amino)-2-methylpropanoic acid (500g, 2.0mol, 1eq), 2-propen-1-ol (116g, 2.0 mol, 1.0eq), dimethylaminopyridine (24g, 0.2mol), 1,3-diisopropylcarbodiimide (500g, 4.0mol) and dichloromethane (2L) were added to the 10L reaction flask in turn, and the control The internal temperature is 20-30°C, and the reaction is stirred for 12 hours. Add 2L of water into the reaction flask, extract, extract the aqueous phase with dichloromethane 2L×3, combine the dichloromethane layers, wash twice with 2L of saturated sodium chloride solution, dry over anhydrous sodium sulfate, filter, and concentrate to dryness under reduced pressure. Add 4L of acetone, stir, filter, add 10L of n-hexane to the solution, stir and crystallize for 2h, and obtain the crude intermediate 1 (441g, 1.5mol).
[0047] Disso...
Embodiment 3
[0050] Example 3: 4-(3-(4-cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxoimidazolidine-1 Preparation of -yl)-2-fluoro-N-methylbenzamide
[0051] 2-((3-fluoro-4-(methylcarbamoyl)phenyl)amino)-2-methylpropanoic acid (500g, 2.0mol, 1eq), 4-penten-1-ol (172g, 2.0mol, 1.0eq), dimethylaminopyridine (24g, 0.2mol), 1,3-diisopropylcarbodiimide (500g, 4.0mol) and dichloromethane (2L) were sequentially added to a 10L reaction flask, The internal temperature was controlled at 20-30°C, and the reaction was stirred for 12 hours. Add 2L of water into the reaction flask, extract, extract the aqueous phase with dichloromethane 2L×3, combine the dichloromethane layers, wash twice with 2L of saturated sodium chloride solution, dry over anhydrous sodium sulfate, filter, and concentrate to dryness under reduced pressure. Add 4L of acetone, stir, filter, add 10L of n-hexane to the solution, stir and crystallize for 2h, and obtain the crude product of Intermediate 1 (451g, 1.4mol).
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com