Combination therapy for neoplasia treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Inhibitory Effect of IGF and AR Signaling Blockade on Prostate Cancer Cell Proliferation
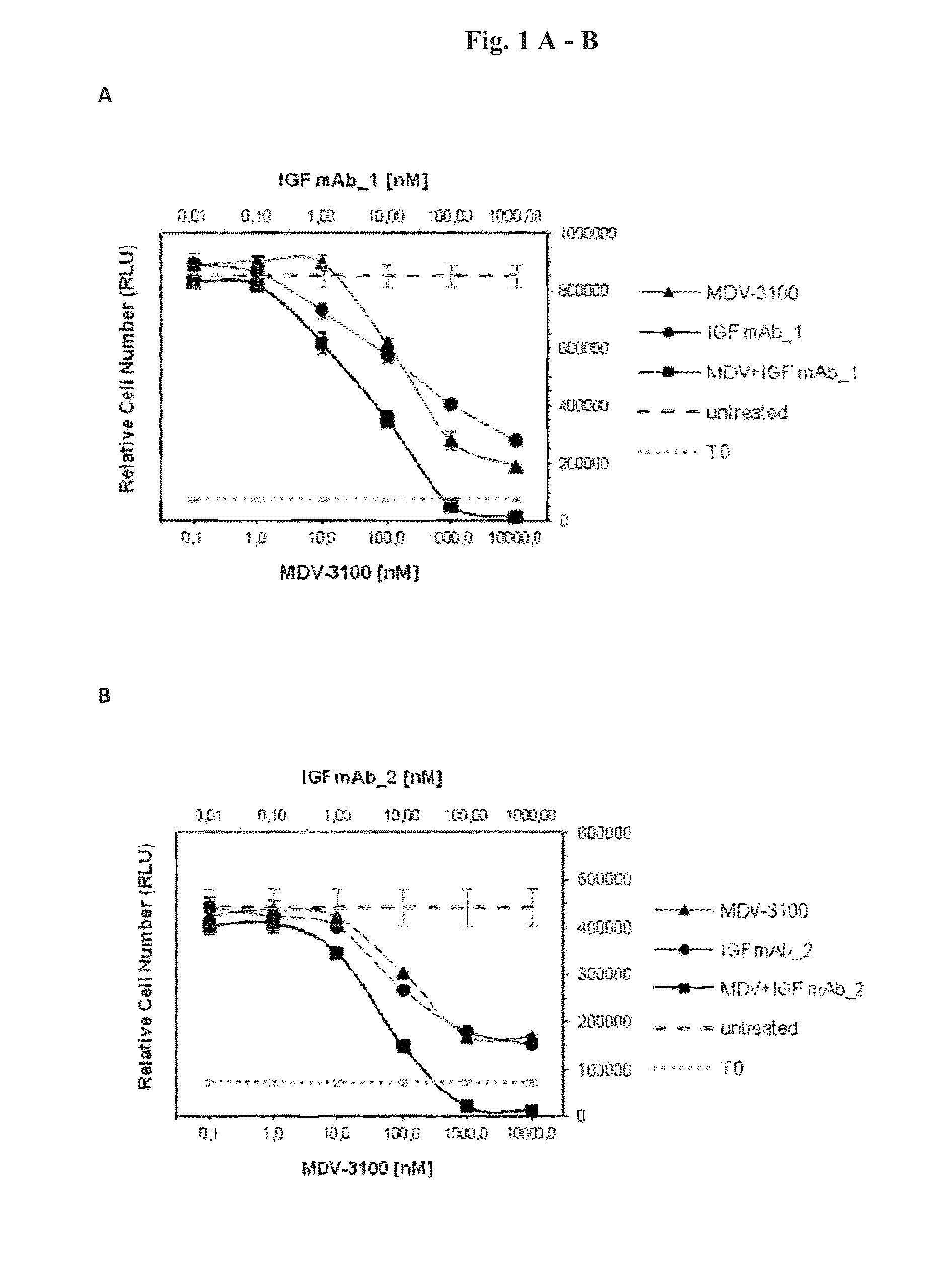
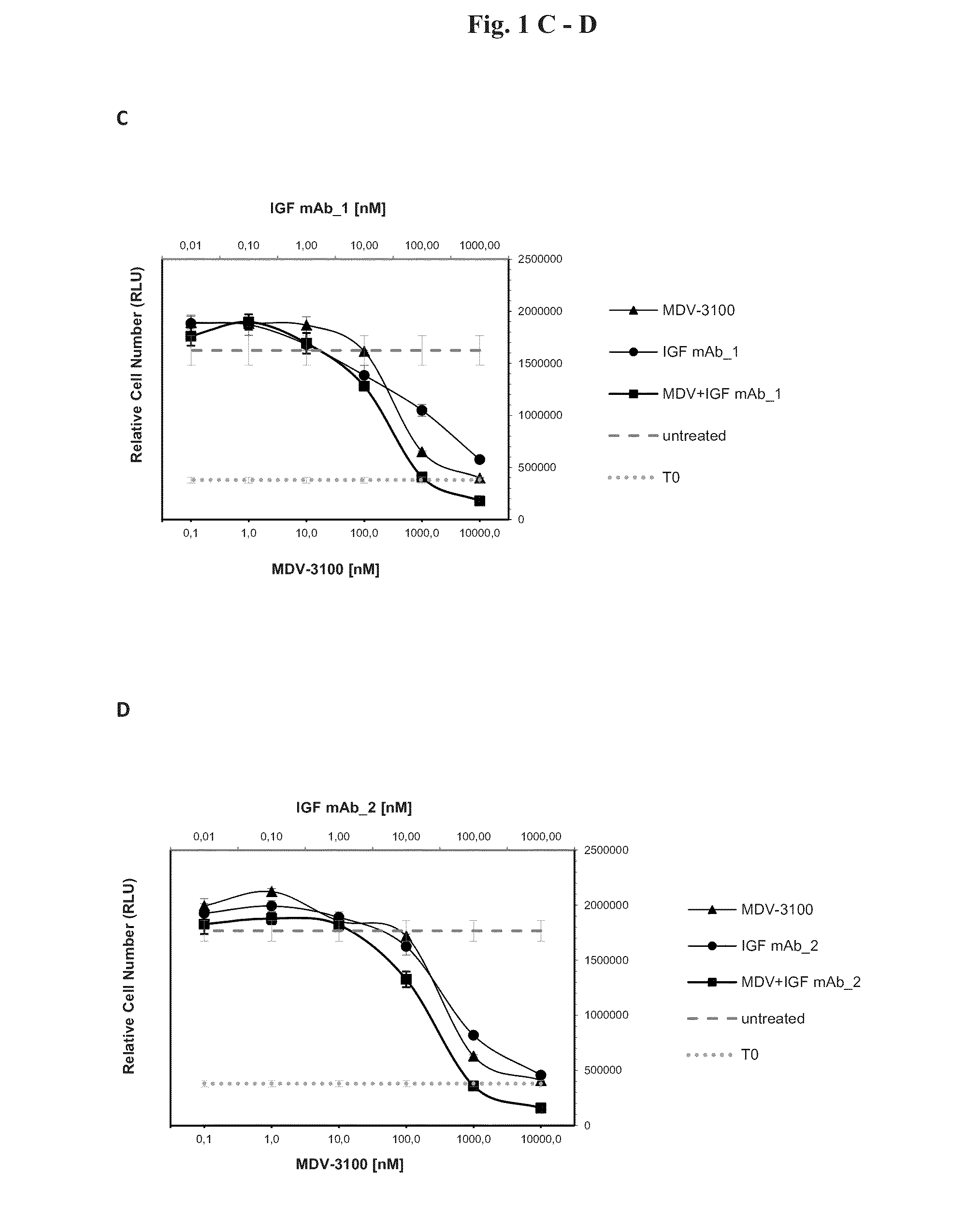
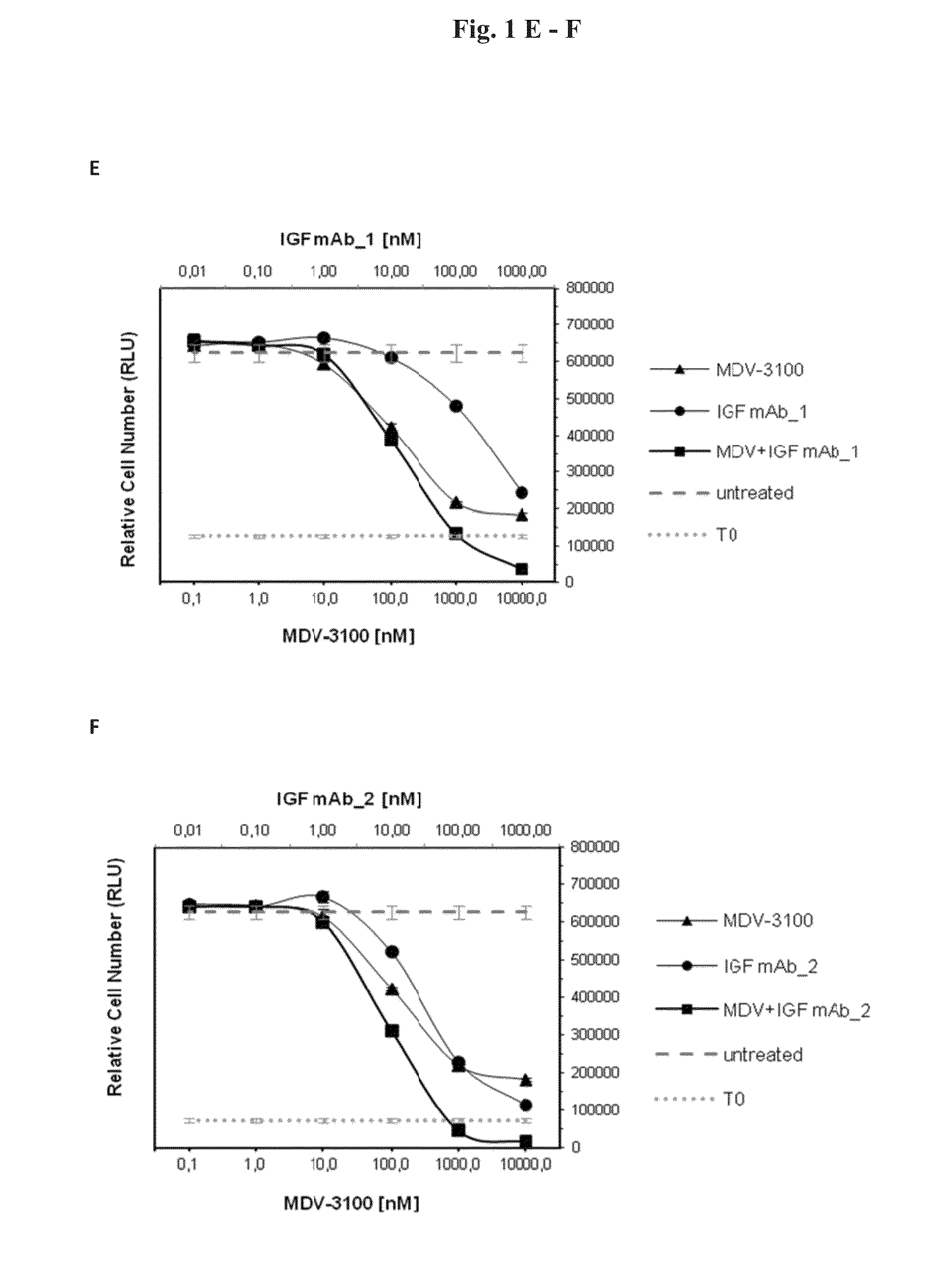
[0096]In order to test the anti-proliferative effects of the combination of AR and IGF-1 / 2 inhibition, 10 different prostate cancer cell lines (Bob, C4-2, C-4-2B, DUCaP, MDA PCa 2b, P4E6, PC-3, Shmac 4, Shmac 5, VCaP) were treated with the AR antagonist MDV-3100 and fully human monoclonal antibodies against the IGF ligands (IGF mAb—1 and IGF mAb—2), as single agents and in combination, in 2D cell proliferation assays (Table 1). Three of the tested cell lines (VCaP and DUCaP—both cell lines were derived from the same prostate cancer patient from different sites of metastasis, and MDA PCa 2b) showed single agent anti-proliferative response to both the AR and IGF signaling inhibition alone, and an enhanced effect when combined (FIG. 1).
example 2
Inhibitory Effect of IGF Signaling and Androgen Synthesis Blockade on Prostate Cancer Cell Proliferation
[0097]As a second approach to test the combination potential of androgen and IGF signaling inhibition, 8 different prostate cancer cell lines (22Rv1, BM 1604, DU-145, DUCaP, LNCaP, MDA PCa 2b, PC-3, VCaP) were treated with abiraterone acetate, which selectively inhibits CYP17A and thus de novo synthesis of androgens, alone and in combination with IGF-ligand neutralizing monoclonal antibodies (IGF mAb—1 and IGF mAb—2). The results from these assays also identified VCaP, MDA PCa 2b, and DUCaP cells to be the only cell lines which are responsive to both single agent and combination treatments. Treatment with abiraterone acetate, however, implies autocrine androgen production by the tumor cells for abiraterone acetate to show anti-proliferative effects. This might limit the number of cells sensitive to abiraterone acetate treatment. Results of 2D and 3D proliferation assays for VCaP a...
example 3
The Presence of Androgen Receptor and IGF-1R as Well as Expression of PTEN and Wt PIK3CA Characterizes Prostate Cancer Cells Sensitive to the Combination of Androgen and IGF Signaling Inhibitors
[0098]FIG. 3 shows signaling protein expression in the VCaP, MDA PCa 2b, and DUCaP cell lines, which are sensitive to AR and IGF signaling inhibition, in comparison to the insensitive cell line PC-3. Cells were treated with MDV-3100 and IGF mAb—1 as single agents, or in combination, for 24 hours and protein lysates were compared to untreated controls for protein expression of IGF-1R, AR, PTEN and AKT, and for phosphorylation of AKT-Ser473. Responsive cell lines expressed wt AR, IGF-1R, and PTEN. These characteristics were not present in PC-3 or the other tested cell lines which did not show an anti-proliferative response to either one of the single agent treatments or the combination of both agents (Table 1).
[0099]These results indicate that in the presence of androgen receptor, IGF-1R, and e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com