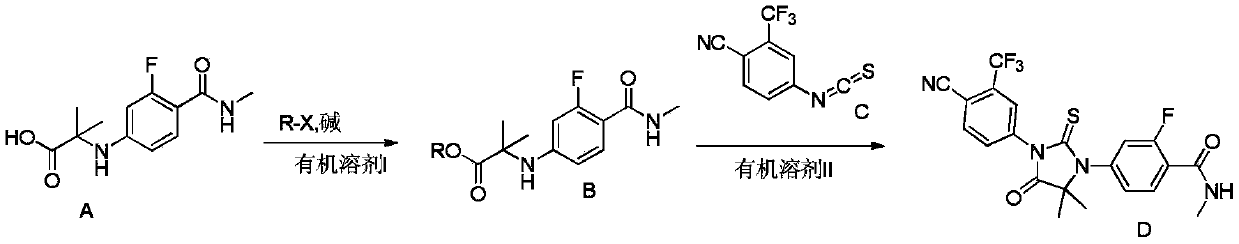
A preparation method for enzalutamide
A technology of enzalutamide and dimethylacetamide, which is applied in the field of drug synthesis, can solve problems such as unsatisfactory demulsification effect, waste water and waste liquid, and a large amount of organic solvents, so as to avoid serious situations of extraction operation and emulsification, The effect of increasing the reaction temperature and simplifying the post-treatment process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
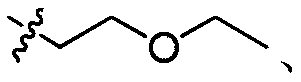
[0035] Take compound A 30.0g, K 2 CO 3 19.6g and 162mL of N,N-dimethylformamide were placed in a reaction flask, stirred, added 25.3g of 2-bromoethyl ethyl ether, heated to 60°C, and reacted for 2h. Pour the reaction solution into 270mL of water, stir for 0.5h, precipitate out solid, filter, and beat the obtained filter cake with 540mL of water for 1h, filter, beat the filter cake with 240mL of n-heptane for 1h, filter, and dry at 60°C to obtain the product B1 43.5 g, yield 93%, HPLC purity: 99.0%.
[0036] MS m / z 327[M+H]+; 1H NMR (400MHz, DMSO-d6) δ7.64(t, J=5.0Hz, 1H), 7.47(t, J=8.7Hz, 1H), 6.82(s ,1H),6.32(dd,J=8.7,2.3Hz,1H),6.15(dd,J=14.6,2.2Hz,1H),4.32–4.03(m,2H),3.56–3.43(m,2H), 3.34(q, J=7.0Hz, 2H), 2.74(d, J=4.5Hz, 3H), 1.48(s, 6H), 1.02(t, J=7.0Hz, 3H).
[0037] Take 32.6g of compound B1 and 47.9g of compound C, add 40mL of isopropyl acetate and 20mL of dimethyl sulfoxide, and react at 80-85°C for 20h. Concentrate under reduced pressure to remove is...
Embodiment 2
[0040]
[0041]Mix 30g of compound A with 22.2g of p-methoxybenzyl chloride, 19.56g of potassium carbonate, 162mL of N,N-dimethylformamide, and 0.3mL of water, and stir at 50°C for 4h. After the reaction is complete, the reaction solution is Added into 270mL of purified water, stirred for 0.5h, precipitated solid, filtered, slurried with 540mL of water for 1h, filtered, and the filter cake was slurried with 240mL of n-heptane for 1h, filtered, and dried at 60°C to obtain 42.6g of product B2, yield 97% , 98.5% purity.
[0042] MS m / z 375.5[M+H]+; 1H NMR (400MHz, CDCl3) δ7.82(ddd, J=11.1,5.6,2.1Hz,1H), 7.24–7.02(m,2H),6.88–6.73( m, 2H), 6.59(dd, J=14.0, 5.0Hz, 1H), 6.31(dq, J=8.7, 2.1Hz, 1H), 6.06(dt, J=15.2, 1.9Hz, 1H), 5.06(d , J=1.3Hz, 2H), 4.78(s, 1H), 3.92–3.61(m, 3H), 2.98(s, 3H), 1.77–1.41(m, 6H).
[0043] 10 g of compound B2 was mixed with 13.6 g of compound C, dimethyl sulfoxide and isopropyl acetate, and stirred overnight at a temperature of 80-85° C. After the TL...
Embodiment 3
[0047] Mix 30g of compound A with 22.2g of p-methoxybenzyl chloride, 11.20g of pyridine, 162mL of N,N-dimethylformamide, and 0.3mL of water, stir at 50°C for 4h, and add the reaction solution to into 270mL of purified water, stirred for 0.5h, precipitated solid, filtered, slurried with 540mL of water for 1h, filtered, and the filter cake was slurried with 240mL of n-heptane for 1h, filtered, and dried at 60°C to obtain 38.7g of product B2, with a yield of 88%. HPLC purity: 98.5%.
[0048] The mass spectrum and NMR data of product B2 are basically the same as in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com