Treatment of metastatic prostate cancer
a prostate cancer and metastatic technology, applied in the field of treatment of metastatic prostate cancer, can solve the problems of ineffective crpc, drug resistance to enzalutamide and abiraterone, and inability to cure crpc, so as to enhance the therapeutic effect of a compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ivity in Prostate Cancer Cells, Enzalutamide Resistance, and Compositions and Methods for Treatment of Prostate Cancer Cells
[0261]a. Introduction
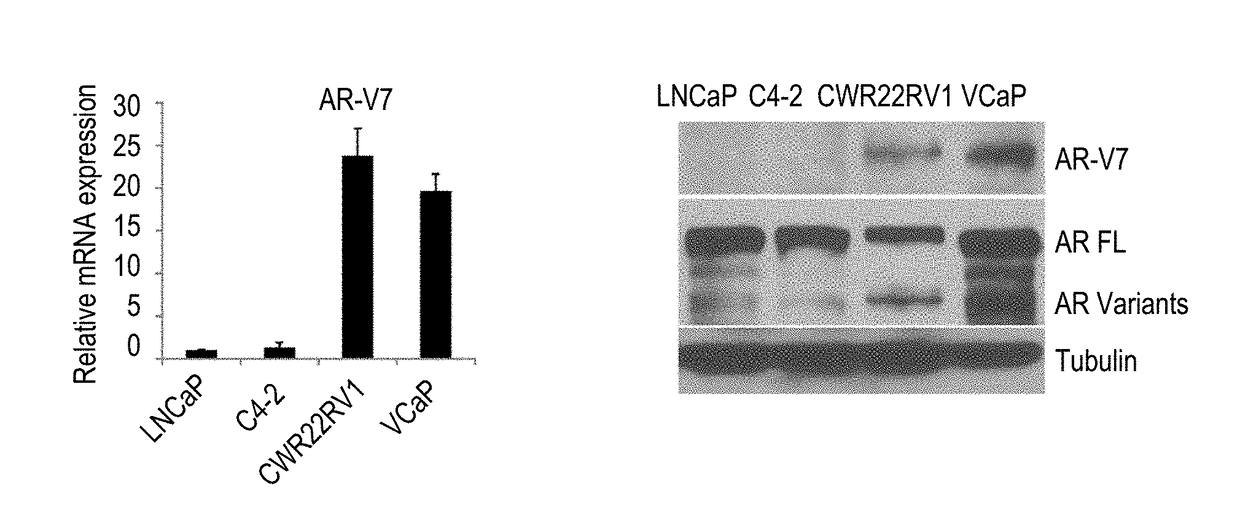
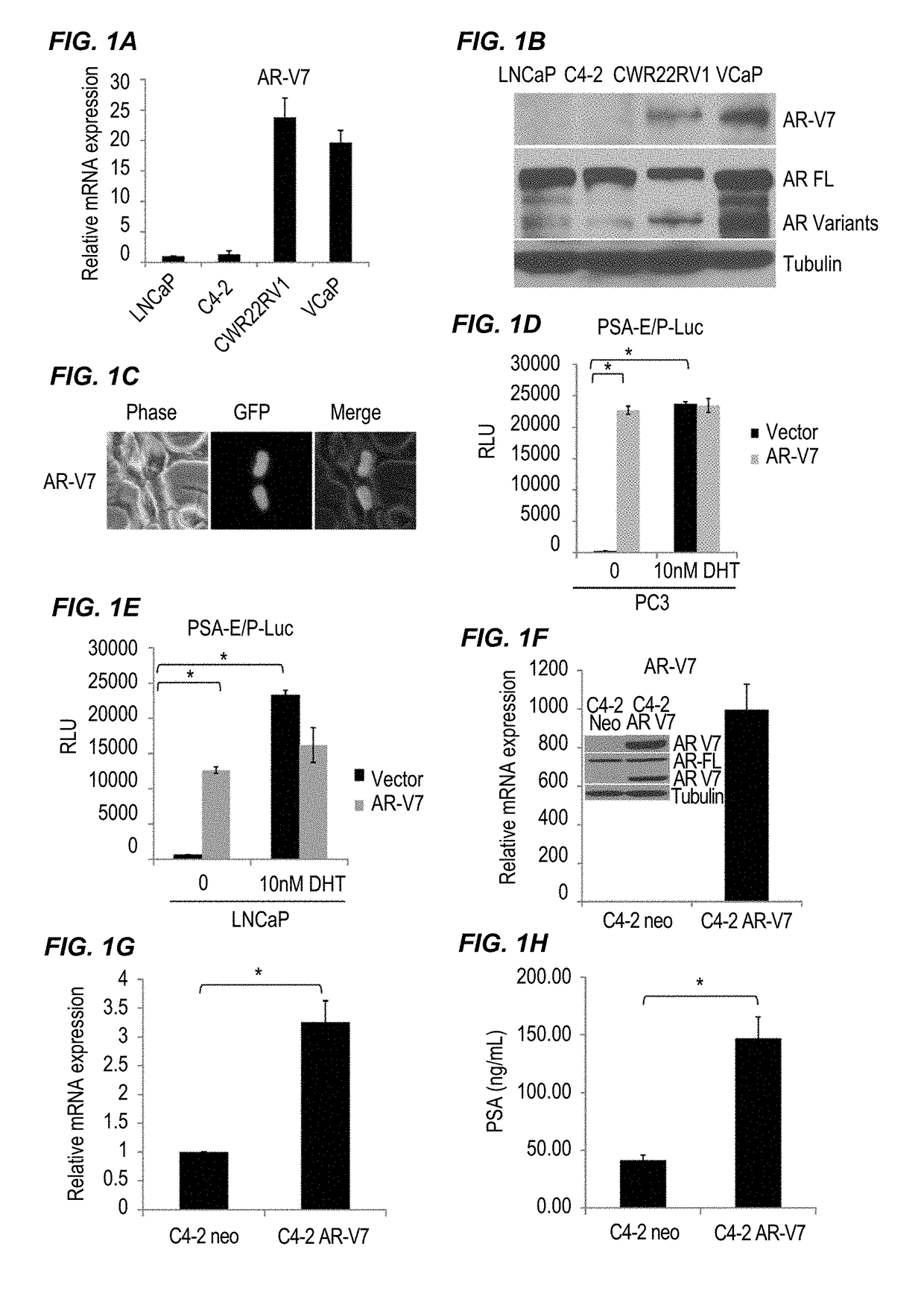
[0262]The deletion of LBD results in constitutive activation of the AR in prostate cancer cells. AR-V7 mRNA expression in different cell lines was detected as shown in FIG. 1A. CWR22rv1 and VCaP cells expressed significantly higher AR-V7 than LNCaP and C4-2 cells; the expression level of AR-V7 in CWR22rv1 cells was 25 times higher than in LNCaP and C4-2 cells, whereas VCaP cells exhibited a 15 fold increase in comparison. The results were also confirmed by Western blot, as shown in FIG. 1B, in which CWR22rv1 and VCaP cells expressed higher protein expression levels of AR variants, especially AR-V7, than LNCaP and C4-2 cells. AR-V7 has been shown constitutively active in prostate cancer cells. To confirm these results, EGFP-AR-V7 was transiently transfected into C4-2 cells, and 48 hours later, as shown in FIG. 1C, AR-V7 was only expressed in...
example 2
de Effects on Enzalutamide-Resistant Cells
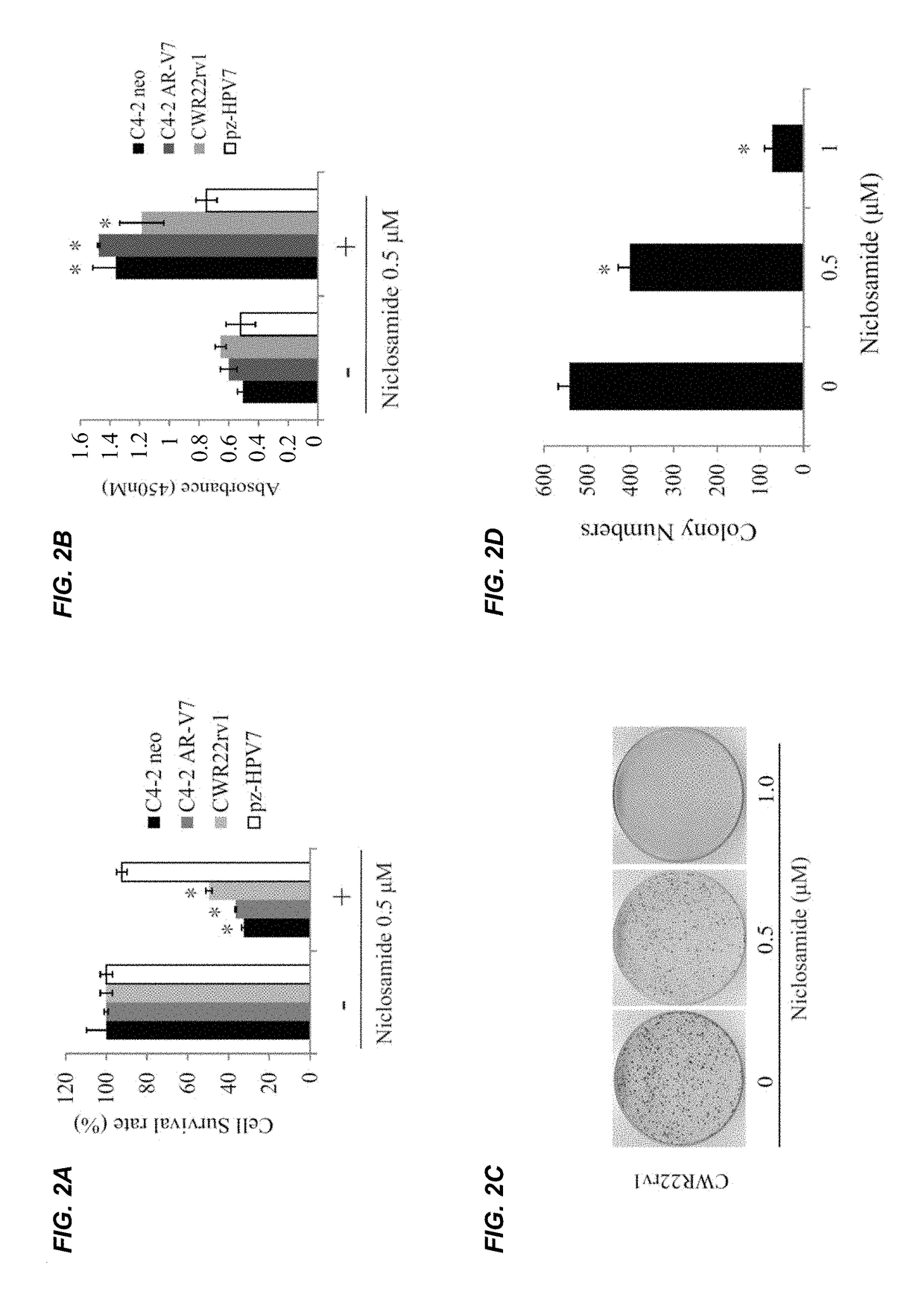
[0343]CWR22Rv1 cells (4 million) were mixed with matrigel (1:1) and injected subcutaneously into the flanks of male SCID mice, tumor-bearing mice (tumor volume around 50-75 mm3) were treated 5 days per week as follows: Control: (5% PGE8000 in H2O p.o B.I.D.), niclosamide (200 mg / kg p.o B.I.D.). Tumors were measured using calipers twice a week and tumor volumes were calculated using length×width2 / 2. Tumor tissues were harvested after 3 weeks of treatment. The results are depicted in FIGS. 40A-40D. As shown in FIGS. 40A-40C, niclosamide significantly inhibited Rv1 xenograft tumor growth when administered orally. As shown in FIG. 40D, the dosage of niclosamide was well-tolerated as illustrated by maintenance of body weight in comparison to control mice that did not receive niclosamide.
[0344]The effects of niclosamide in combination with bicalutamide were examined in vitro and in vivo. As shown in FIGS. 41A and 41B, niclosamide significantly enh...
example 3
tivation and Intracrine Androgens Confer Resistance to Enzalutamide
a. Introduction
[0346]Targeting androgen signaling via androgen deprivation therapy has been the mainstay of clinical interventions in prostate cancer (PCa). While initially effective, the majority of men experience only transient benefit and relapse with castrate-resistant prostate cancer (CRPC), which is currently incurable. Enzalutamide, a second-generation antiandrogen, was recently approved for the treatment of castration resistant prostate cancer (CRPC) in patients. Despite these advances that provide temporary respite, resistance to enzalutamide occurs frequently. Several potential mechanisms of resistance have been revealed such as AR variants expression (Antonarakis et al., 2014; Li et al., 2013; Liu et al., 2014a), IL6-STAT3-AR axis activation (Liu et al., 2014b), AR F876L mutation (Joseph et al., 2013; Korpal et al., 2013) and glucocorticoid receptor (GR) overexpression (Arora et al., 2013; Isikbay et al., ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Therapeutic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com