Preparation process of prostatic cancer medicine Enzalutamide
A preparation process and technology of enzalutamide, applied in the field of drug synthesis, can solve problems such as being unsuitable for industrial production, and achieve the effects of reduced preparation cost, convenient and simple post-processing, and reduced reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
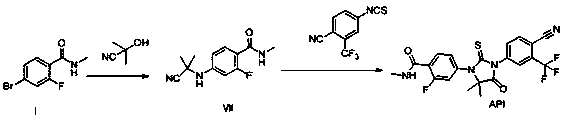
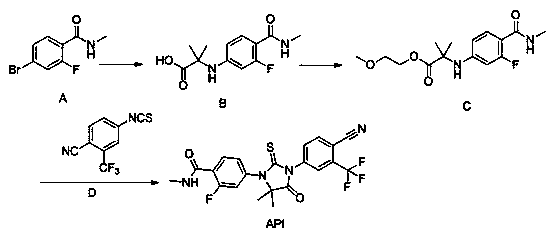
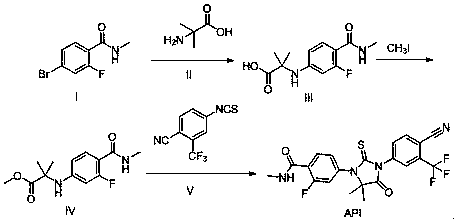
[0036] Example 1: Synthesis of 2-(3-fluoro-4-(methylcarbamoyl)phenylamino)-2-methylpropionic acid
[0037]
[0038] N-methyl-4-bromo-2-fluoro-benzamide (A) (600g, 2.6mol, 1.0eq), 2-amino-2-methylpropionic acid (400g, 3.9mol, 1.5eq), Potassium carbonate (893g, 6.5mol, 2.5eq), CuCl (51g, 0.52mol, 0.2eq), was added to DMF (3600ml), stirred well, and 2-acetylcyclohexanone (73g, 0.52mol, 0.2eq ), react at 110°C for 16 hours under inert gas. After the reaction was complete, the reaction liquid was cooled to room temperature, purified water and ethyl acetate were added to extract twice, and the organic layers were combined. The organic layer was adjusted to acidic pH with 1N hydrochloric acid solution, stirred and crystallized at 0-5°C, filtered, the filter cake was washed with purified water, and air-dried at 60°C to obtain 583g of a yellow solid, with a yield of 88.7%.
Embodiment 2
[0039] Example 2: Synthesis of 2-methoxyethyl 2-((3-fluoro-4-(methylcarbamoyl)phenyl)amino)-2-methylpropionate
[0040]
[0041]2-(3-fluoro-4-(methylcarbamoyl)phenylamino)-2-methylpropionic acid (200g, 0.79mol), ethylene glycol monomethyl ether (124mL, 0.16mol, 2.0eq) , 1-hydroxybenzotriazole (HOBt, 127g, 0.95mmol, 1.2eq), 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDCHCl, 181g, 0.95mol , 1.2eq) into 1L of dichloromethane, under the protection of an inert gas, react overnight at room temperature. Purified water (3 L) was added to the reaction solution, stirred and separated, the organic layer was washed once with water and brine, dried over sodium sulfate, concentrated under reduced pressure to remove the organic solvent, and dried in vacuo overnight to obtain 225 g of off-white solid with a yield of 91.3%. ESI-MS m / z: 313.16[M+H] + , 1 H-NMR (DMSO-d 6 ) (ppm): 7.97(d, 1H, J=10.0Hz, -Ph),6.61(s, 1H, -NH), 6.57(dd, 1H, J 1 =5.0Hz,J 2 =10.0Hz, -Ph), 6.4...
Embodiment 3
[0042] Example 3: Synthesis of 2-methoxyethyl 2-((3-fluoro-4-(methylcarbamoyl)phenyl)amino)-2-methylpropionate
[0043]
[0044] 2-(3-fluoro-4-(methylcarbamoyl)phenylamino)-2-methylpropionic acid (200g, 0.79mol), 1-bromo-2-methoxyethane (82mL, 0.87 mol, 1.1eq), 1,8-diazacyclo[5,4,0]undecene-7 (238g, 1.57mol, 2.0eq), dissolved in DMF (1.5L), under inert gas protection, React overnight at 30°C. Purified water (3 L) was added to the reaction solution, stirred for 2 hours, filtered, and the filter cake was air-dried at 50°C to obtain 228 g of off-white solid, with a yield of 92.6%. ESI-MS m / z: 313.16[M+H] + , 1 H-NMR (DMSO-d 6 ) (ppm): 7.97(d, 1H, J=10.0Hz, -Ph), 6.61(s, 1H, -NH), 6.57(dd,1H, J 1 =5.0Hz,J 2 =10.0Hz, -Ph), 6.43(dd, 1H, J 1 =5.0Hz,J 2 =10.0Hz, -Ph), 4.46(s,1H, -NH), 4.21(t, 2H, J=5.0Hz, -CH 2 ), 3.69(t, 2H, J=5.0Hz, -CH 2 ), 3.37(s, 3H,-CH 3 ), 2.76(s, 3H, -CH 3 ), 1.56(s, 6H, -CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com