Novel method for compounding enzalutamide
A technology of enzalutamide and a new method, which is applied in the field of synthesizing enzalutamide, can solve problems such as restricting the scale-up production of enzalutamide, and achieve good industrial scale-up prospects, cheap raw materials, and mild operating conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
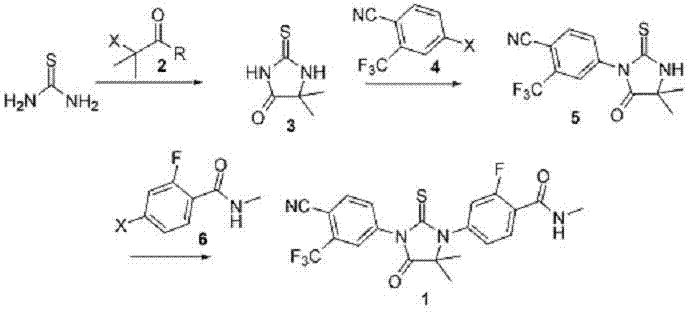
[0044] Preparation of 5,5-dimethyl-2-thione imidazol-4-one
[0045]150ml of DMF was added to a 250ml three-neck flask, and 13.6g of 2-methyl-2-chloro-propionic acid methyl ester, 7.6g of thiourea and 10.1g of triethylamine were added in sequence under stirring. Heat to 80-90°C, react for 7h, and monitor the completion of the reaction by TLC. Cool the reaction solution to room temperature, add the reaction solution to 600ml of water, stir to precipitate solid, stir for 30min, and filter. The filter cake was stirred and washed twice with water. After the filter cake was air-dried at 50-55°C to constant weight, it was recrystallized from ethanol to obtain 13.1 g of 5,5-dimethyl-2-thioneimidazol-4-one, with a yield of 91.2%.
Embodiment 2
[0047] Preparation of 5,5-dimethyl-3-(3-trifluoromethyl-4-fluorophenyl)-2-thioneimidazol-4-one
[0048] Add 100ml of DMF into a 250ml three-neck flask, add 13.1g of 5,5-dimethyl-2-thioneimidazol-4-one in sequence under stirring, cool to 0-5°C in an ice-water bath, and add 6g of sodium hydride in batches . After the addition, the ice-water bath continued to stir for 30 min. Dissolve 22.75g of 2-trifluoromethyl-4-bromobenzocyanide in 60ml of DMF, and slowly add it dropwise to the reaction solution, controlling the internal temperature to 0-5°C. After the addition was complete, return to room temperature and continue to stir for 4 h. TLC monitored the completion of the reaction. The reaction solution was added to 500ml of water, and the crude product was obtained by filtration. After drying, it was purified by column chromatography to obtain 18.5 g of 5,5-dimethyl-3-(3-trifluoromethyl-4-fluorophenyl)-2-thioneimidazol-4-one with a yield of 65.1%.
Embodiment 3
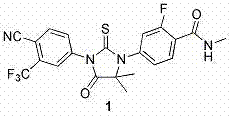
[0050] Preparation of Enzalutamide
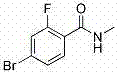
[0051] Add 100ml DMF into a 250ml three-necked flask, and add 5,5-dimethyl-3-(3-trifluoromethyl-4-fluorophenyl)-2-thioneimidazol-4-one 18.5 g, cooled to 0-5°C in an ice-water bath, and added 3.9 g of sodium hydride in batches. After the addition, the ice-water bath continued to stir for 30 min. Dissolve 13.68g of 2-fluoro-4-bromobenzamide in 30ml of DMF, and slowly add it dropwise to the reaction solution, controlling the internal temperature to 0-5°C. After the addition was complete, return to room temperature and continue to stir for 5 h. TLC monitored the completion of the reaction. The reaction solution was added to 500ml of water, and the crude product was obtained by filtration. After drying, column chromatography purified to obtain 23.9 g of enzalutamide, with a yield of 87.3%.
[0052]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com