3H-1,2-dithio-cyclopentene-3-thioketone compound and application thereof
A technology of dithiocyclopentene and dithiolane, applied to 3H-1,2-dithiocyclopentene-3-thione compounds, and its application in the preparation of drugs for the treatment or prevention of cerebral apoplexy In this field, it can solve the problem of less therapeutic drugs and achieve the effect of inhibiting the inflammatory response and reducing the volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
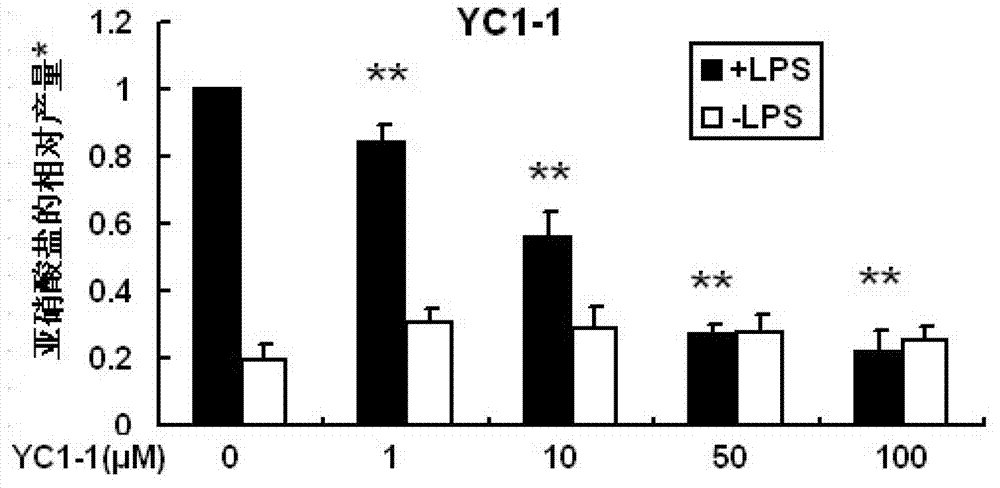
[0034] Example 1: Synthesis of 5-p-hydroxyphenyl-3H-1,2-dithiole-3-thione compound (YC1-1)
[0035] Add methyl p-hydroxybenzoyl acetate (307mg, 1.89mmol), phosphorus pentasulfide (420mg, 1.9mmol), sulfur (56mg, 0.93mmol) into 20mL of toluene, heat and reflux for 5h, the reaction is complete, filter, and the filtrate is concentrated Developed by column chromatography (petroleum ether: acetone = 10:1), 134 mg of red solid was obtained with a yield of 40%. m.p.191.3~192.1℃. 1 H NMR (400MHz, DMSO-d 6 ), 10.46(s,1H,OH),7.78(d,2H,J=8.7Hz,ArH),7.68(s,1H,=CH),6.90(d,2H,J=8.7Hz,ArH).HR-MS :Calcd.For C 9 h 6 OS 3 [M+H] + :226.9654,Found:226.9645.
Embodiment 2
[0036] Example 2: Synthesis of 5-p-fluorophenyl-3H-1,2-dithiole-3-thione (YC1-2)
[0037] Using methyl p-fluorobenzoylacetate, phosphorus pentasulfide and sulfur as raw materials, the synthetic method can be found in the synthesis of YC1-1, and the yield is 34%. m.p.108.3~110.0℃. 1 HNMR (400Hz, CDCl 3 ),δ(ppm):7.66(s,2H,ArH),7.37(s,1H,=CH),7.18(s,2H,ArH). 13 C-NMR (400MHz, CDCl 3 ), δ(ppm): 215.398, 171.482, 166.623, 163.247, 135.893, 129.124, 129.007, 117.060, 116.765.HR-MS: Calcd.For C 9 h 5 FS 3 [M+H] + :228.9610,Found:228.9610.
Embodiment 3
[0038] Example 3: Synthesis of 5-(2-thienyl)-3H-1,2-dithiole-3-thione (YC1-3)
[0039] Using methyl 2-thiopheneformylacetate, phosphorus pentasulfide and sulfur as raw materials, the synthesis method can be found in the synthesis of YC1-1, and the yield is 36%. m.p.119.2~120.9℃. 1 HNMR (400Hz, CDCl 3 ),δ(ppm):7.59(s,1H,=CH),7.54(s,1H,ArH),7.32(s,1H,ArH),7.15(s,1H,ArH). 13 C-NMR (400MHz, CDCl 3 ), δ(ppm): 214.456, 165.134, 134.629, 133.866, 131.083, 129.247, 129.065.HR-MS: Calcd.ForC 7 h 7 S 4 [M+H] + :216.9269,Found:216.9268.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com