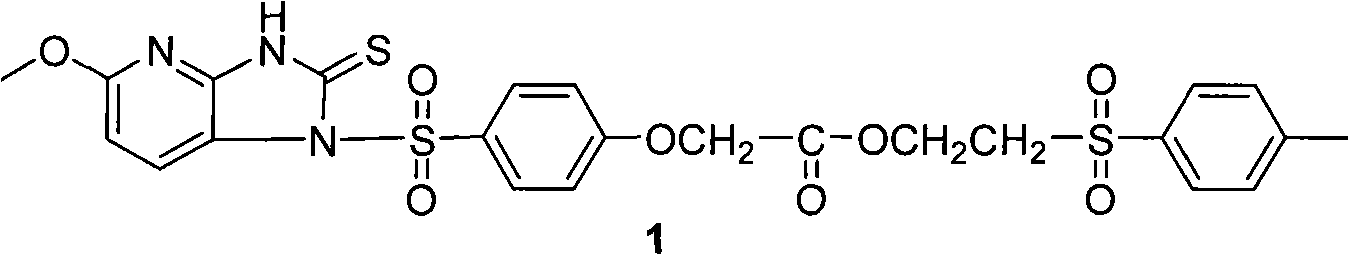
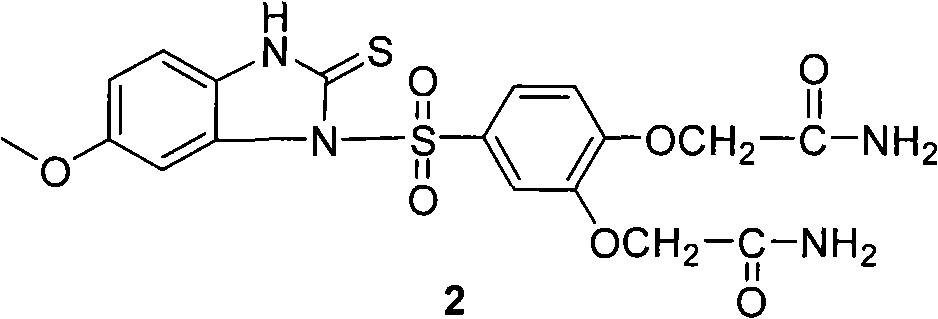
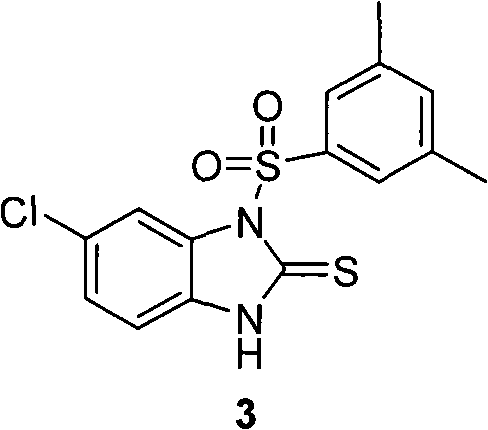
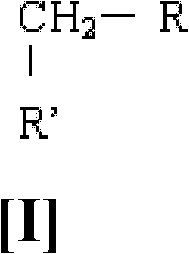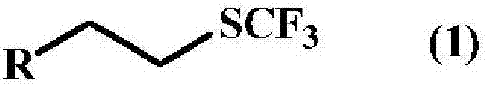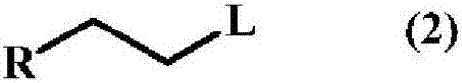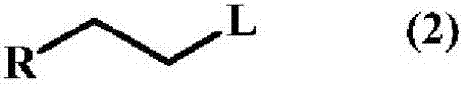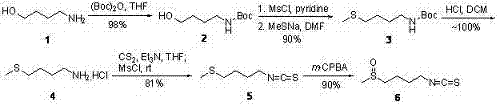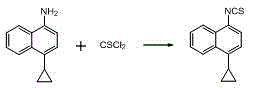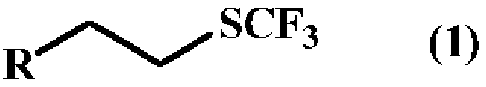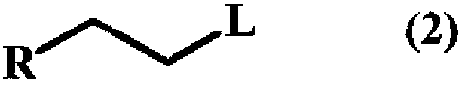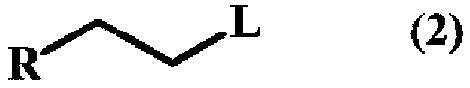Patents
Literature
30 results about "Thiophosgene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
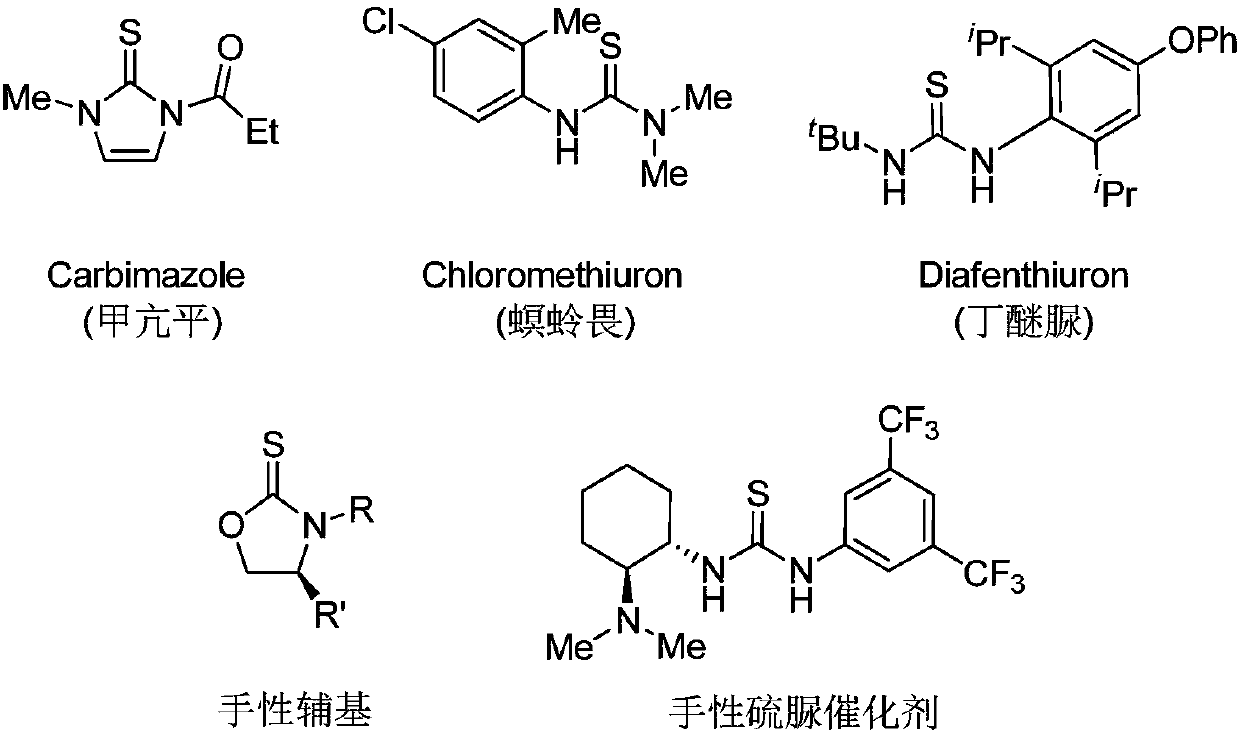
Thiophosgene is a red liquid with the formula CSCl₂. It is a molecule with trigonal planar geometry. There are two reactive C–Cl bonds that allow it to be used in diverse organic syntheses.
Preparation method of gout curative medicine Lesinurad and midbody of Lesinurad
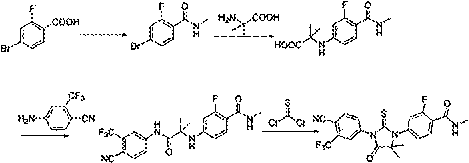
The invention provides a novel Lesinurad midbody and provides a synthesis process of Lesinurad, which is economic, efficient, safe, environment-friendly and applicable to large-scale industrial production. The invention further provides a method for preparing the conventional Lesinurad midbody and Lesinurad by using the novel Lesinurad midbody. When synthesized from the novel Lesinurad midbody provided by the invention, the Lesinurad has advantages that the price of necessary raw materials is low, the raw materials are easy to obtain, heavy metal and solvents which are harmful to the environment are not used, the midbody and a product can be easily separated and purified, the operation is easy, application of thiophosgene which is high in toxicity and not easy to operate is avoided, and the total reaction yield is approximate to or higher than that in the prior art.
Owner:SUZHOU PENGXU PHARM TECH CO LTD
Hexa alkyl guanidine salt ion liquid and preparing process
The present invention relates to hexa-alkyl guanidine salt ion liquid and its preparation process. The materials 1 ,3-dimethyl-2-imidazolinone and tetra-alkyl urea are produced into intermediate Wilsman salt under the action of phosphorus oxychlooride, oxalyl chloride, thiophosgene, phosgene or dichluoro sulfoxide; the intermediate is then reacted with C10 below aliphatic amine to produce penta-alkyl guanidine; and penta-alkyl guanidine is finally reacted with alkyl bromide or alkyl iodide and methyl or ethyl ester to produce hexa-alkyl guanidine salt ion liquid. The bromine salt and iodine salt may further reacted with various inorganic salt to produce hexa-alkyl guanidine salt ion liquid containing various negative ions. The present invention lays down foundation for the research and development of ionic liquid and provides systemic technology for the industrial production of hexa-alkyl guanidine salt ion liquid..
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
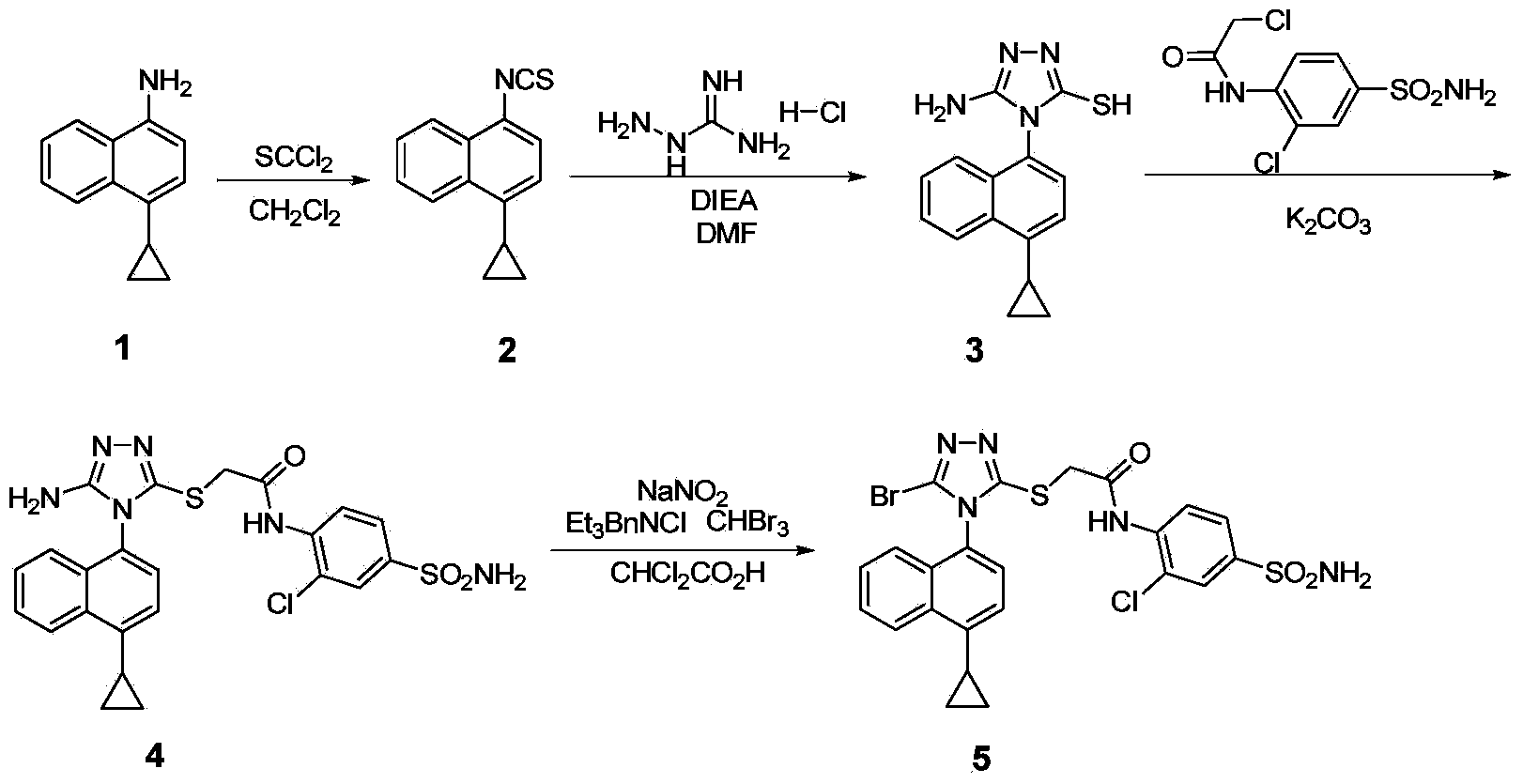
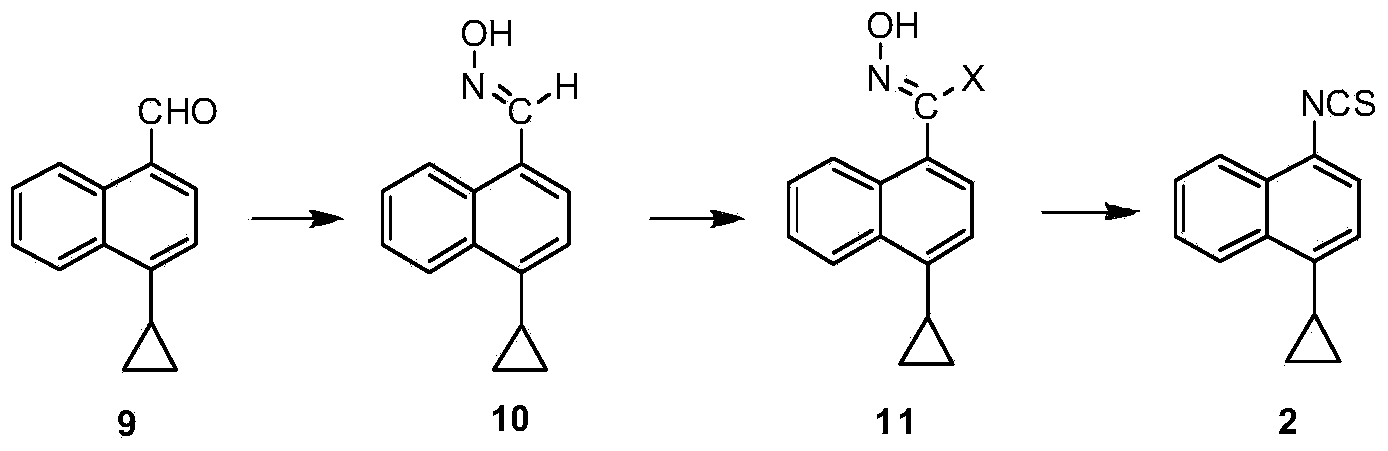
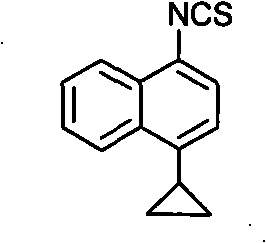
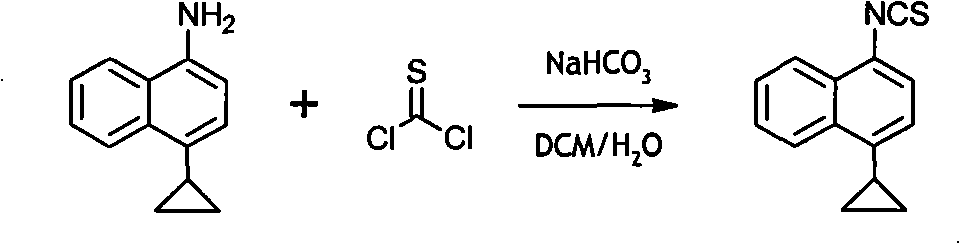
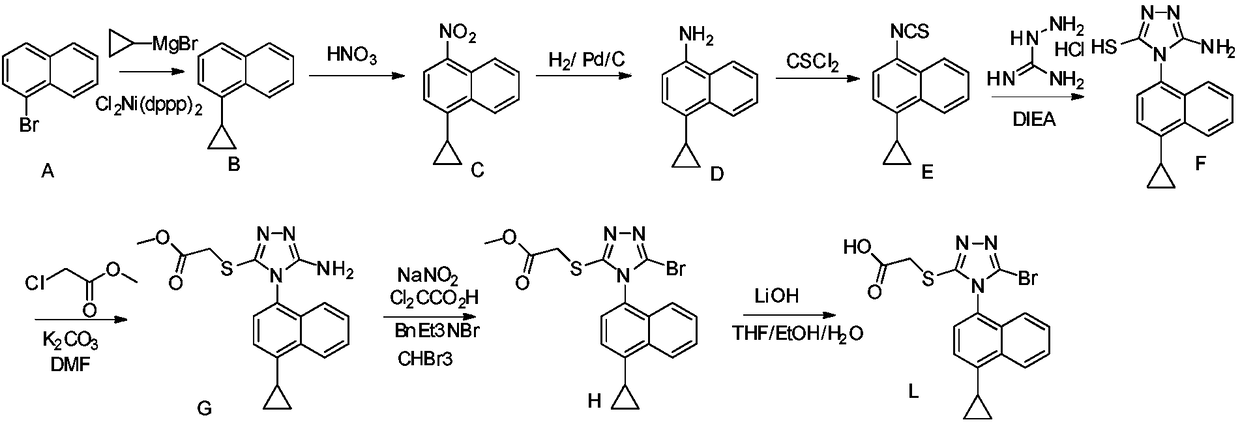
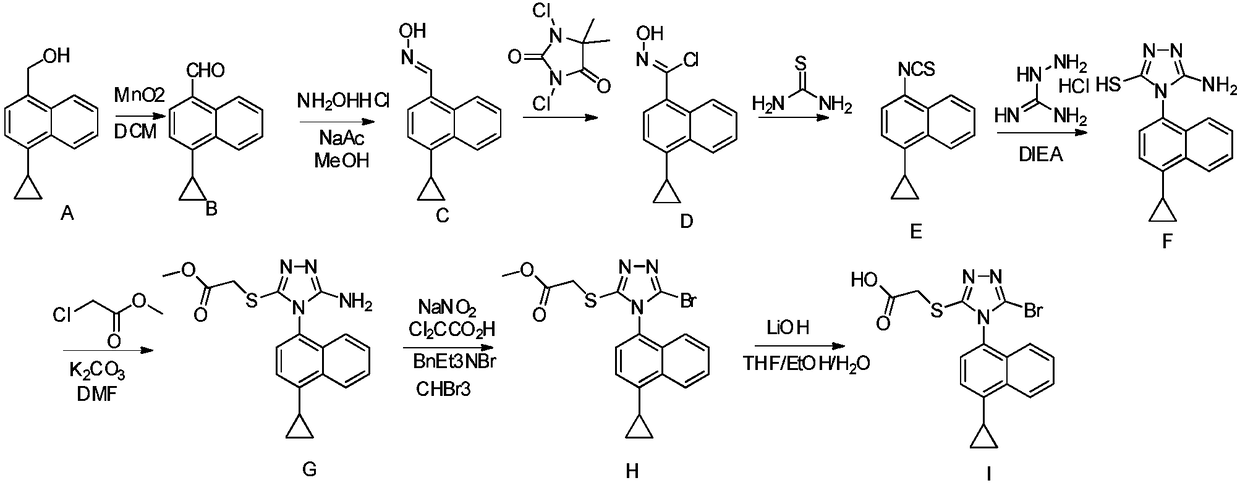
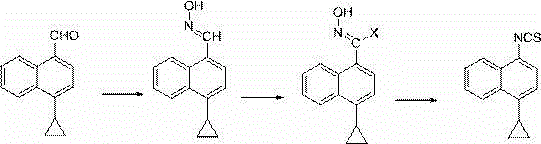
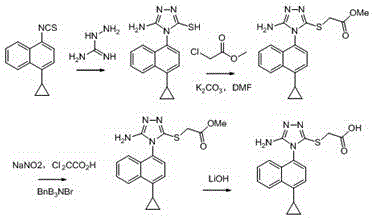
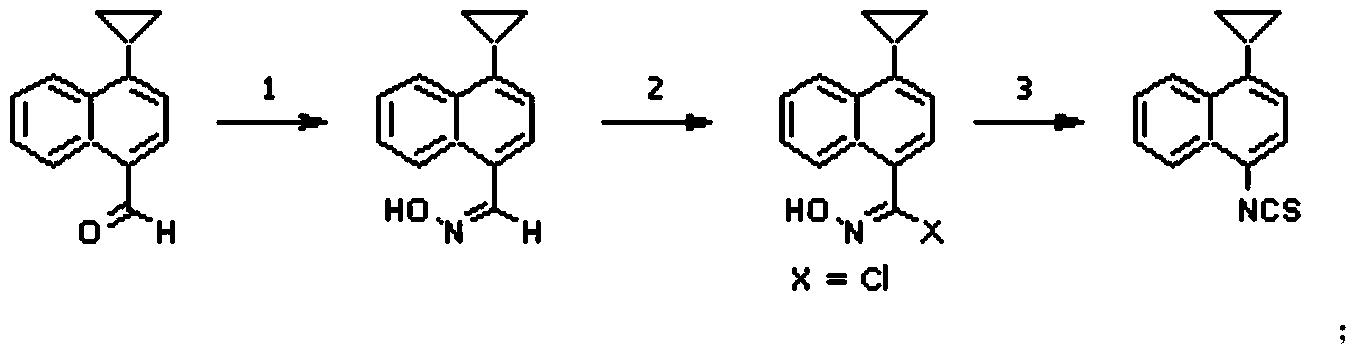
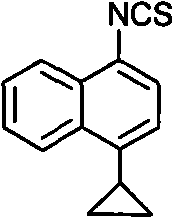
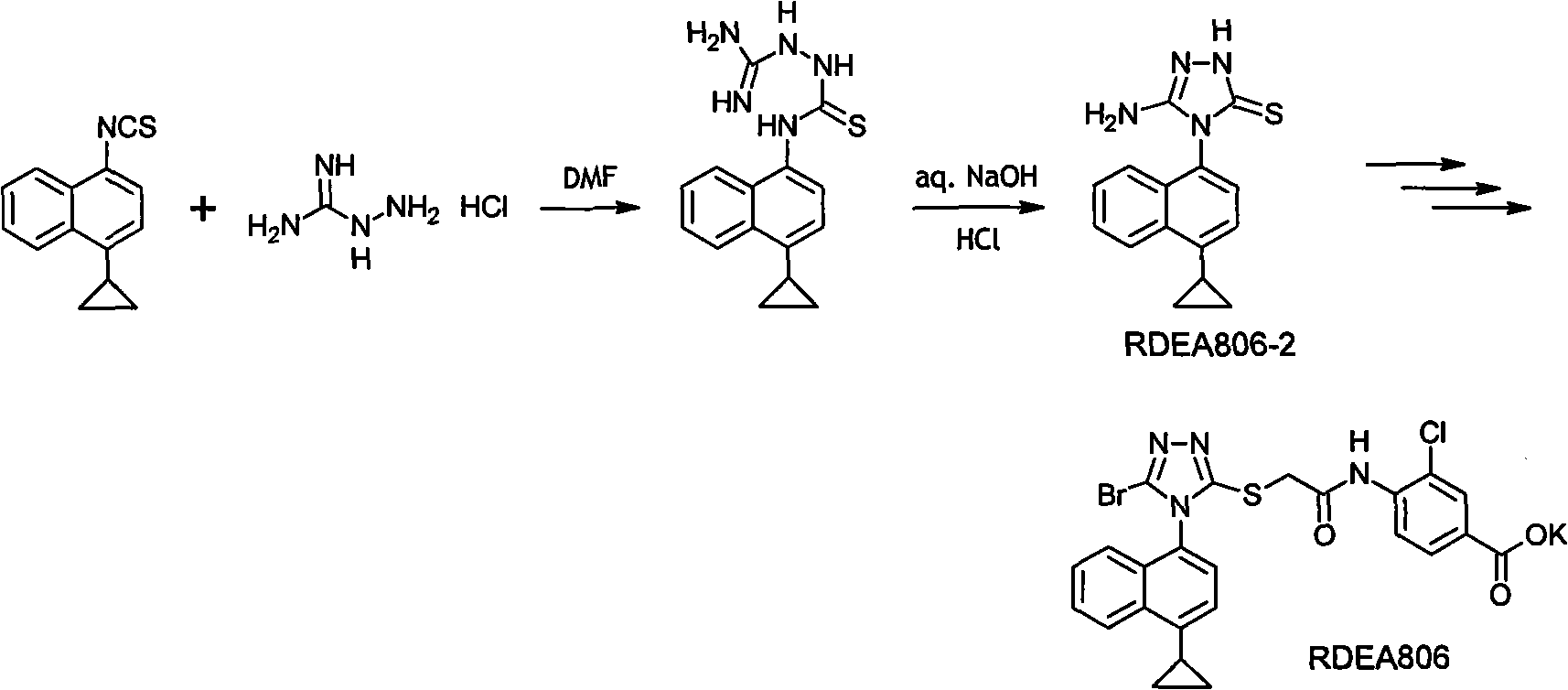
Preparation method of 4-cyclopropyl-1-naphthaline isothiocyanate and intermediate 4-cyclopropyl-1-naphthaldehyde oxime/halide
The invention provides a preparation method of 4-cyclopropyl-1-naphthaline isothiocyanate, which is characterized in that the 4-cyclopropyl-1-naphthaline isothiocyanate is synthesized by taking 4-cyclopropyl-1-naphthaldehydes as an initial raw material through three-step reaction of oximation, halogenation and vulcanization. Meanwhile, the invention discloses a preparation method of an intermediate 4-cyclopropyl-1-naphthaldehyde oxime / halide of the 4-cyclopropyl-1-naphthaldehydes. When the 4-cyclopropyl-1-naphthaline isothiocyanate is prepared with the method disclosed in the invention, thiophosgene, which has strong toxicity and bad smell, is not needed to be used. The invention has the advantages of convenience in operation, small influence to environment, good safety, good product quality and high yield. Moreover, the separation and purification of a product is easy and is simple to operate.
Owner:ZHEJIANG HUABANG MEDICAL & CHEM
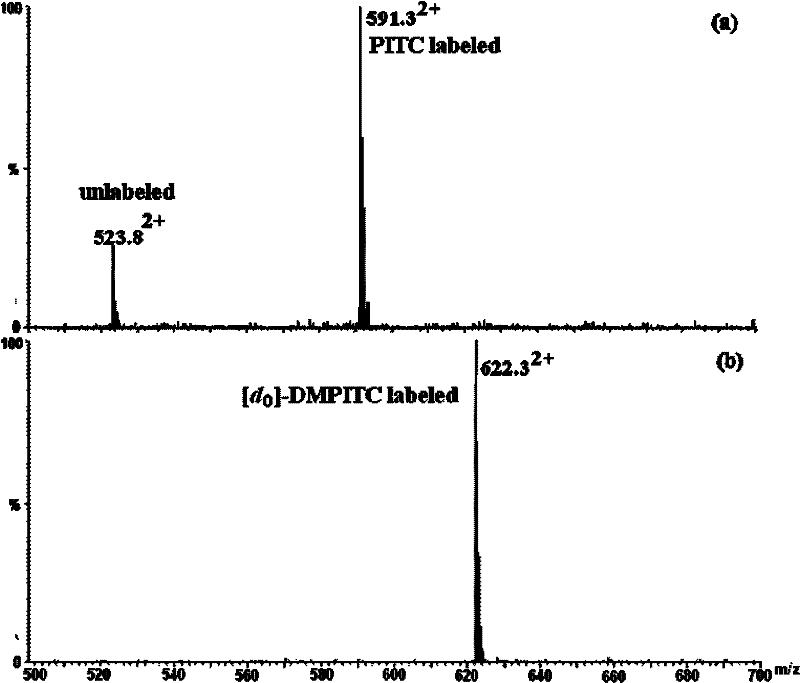
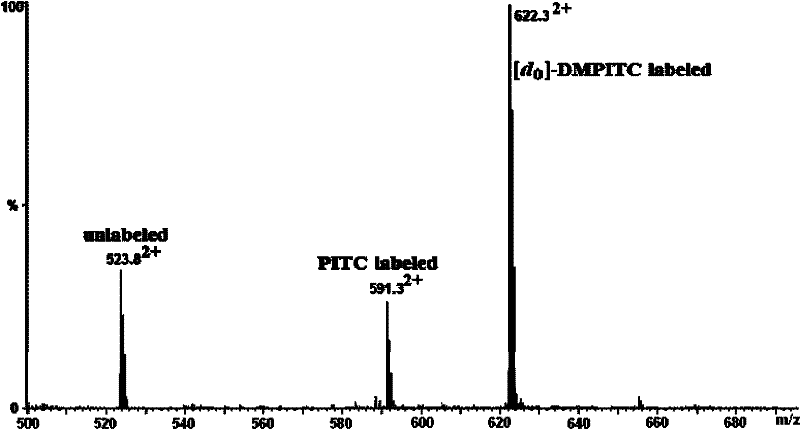
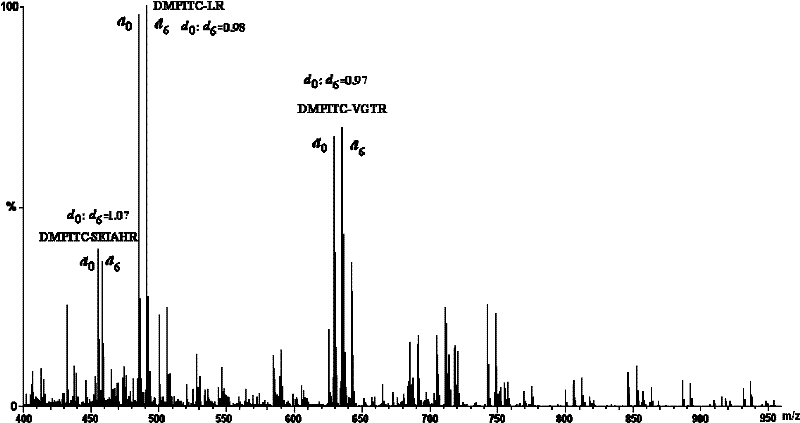
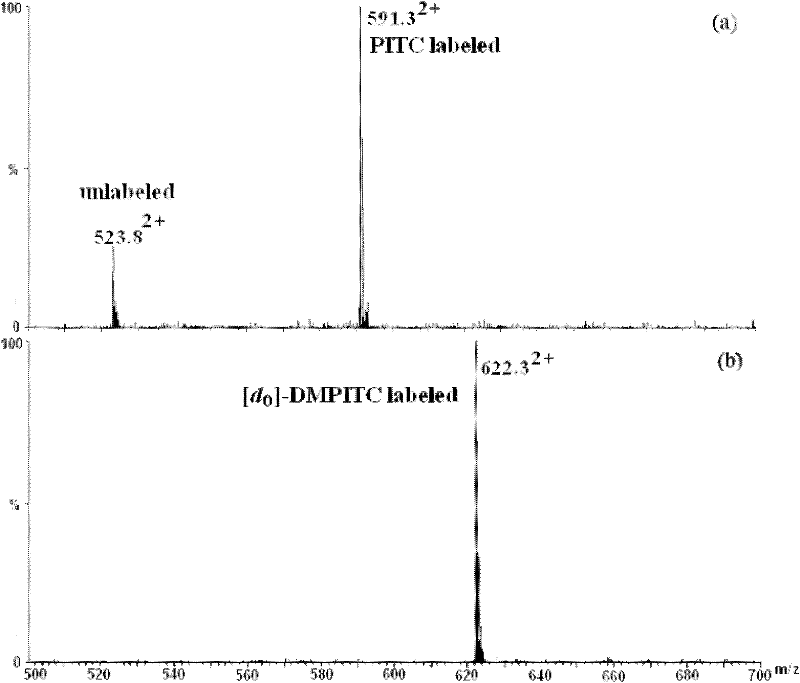
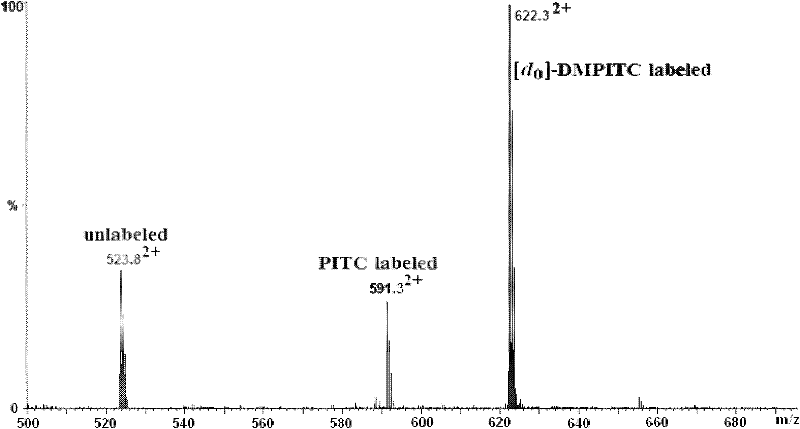
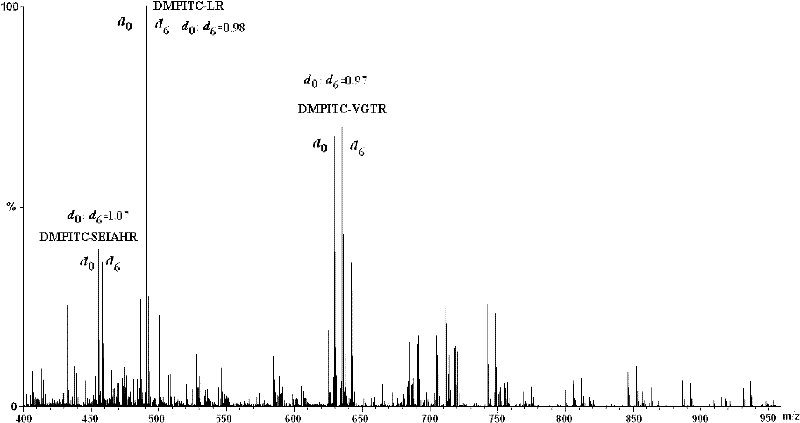
Isotope labeling reagent as well as preparation method and application thereof
ActiveCN102174025AIncrease signal strengthEasy AdditionOrganic chemistryComponent separationSodium bicarbonateSodium methoxide
The invention discloses an isotope labeling reagent which is an isothiocyanopyrimidine isotope labeling reagent with a chemical name of [d0] / [d6]-4,6-dimethoxine-2-isothiocyanate. The preparation method of the reagent comprises the following steps of: firstly, reacting 4,6-dichloro-2-amiopyrimidine with sodium methoxide / deuterosodium methoxide to obtain a [d0] / [d6]-4,6-dimethoxy-2-amiopyrimidine crude product; carrying out reflux reaction on the obtained crude product, thiophosgene and sodium bicarbonate to obtain a final crude product; and then carrying out column chromatography to obtain the isotope labeling reagent disclosed by the invention. The isotope labeling reagent disclosed by the invention can be applied to the identification analysis of polypeptide N-end residues and the quantitative analysis of protein, can speed up the labeling reaction rate and improve a signal of the labeled peptide section, is beneficial to strengthening the accuracy and the credibility of the identified peptide section and has the advantages of less molecular weight of the labelled part, relative simple structure, stable performance, simpleness and convenience for operation, and the like.
Owner:SHANGHAI INST OF ORGANIC CHEMISTRY - CHINESE ACAD OF SCI
A method for preparation of perfluoroalkyl sulfenyl chloride
A process for the preparation of perfluoroalkyl sulfenyl chloride; said process comprising i) reacting a compound of formula (I) wherein R represents an aromatic group with or without a substituent; and R' represents a halogen, with at least one fluoride compound and thiophosgene in a solvent to obtain a reaction mass; ii) pulverizing the reaction mass with the help of beads to obtain a mixture containing trifluoromethyl thiomethyl benzene derivative; iii) isolating the trifluoromethyl thiomethyl benzene derivative from the mixture; iv) distilling the trifluoromethyl thiomethyl benzene derivative to obtain a purified trifluoromethyl thiomethyl benzene derivative; v) dissolving the purified trifluoromethyl thiomethyl benzene derivative in a solvent selected from the group consisting of dichloromethane, toluene, benzene, ethylene dichloride, mono chloro benzene, carbon tctra chloride, ortho dichloro benzene and tri chloro benzene; and vi) cleaving the trifluoromethyl thiomethyl benzene derivative by selective chlorinolysis by passing chlorine gas at a temperature in the range of about -10 to about 30 DEG C to obtain perfluoroalkyl sulfenyl chloride.
Owner:克基·霍尔穆斯吉·阿加尔达
Novel method for compounding medicament of Enzalutamide for resisting prostate cancer
The invention discloses a novel method for compounding a medicament of Enzalutamide for resisting the prostate cancer, belongs to the technical field of pharmacy synthetic technique, and particularly relates to novel technique for compounding Enzalutamide. Most of known methods for compounding Enzalutamide relate to use of highly toxic and mephitical thiophosgene and highly toxic oxobutyronitrile and have great difficulty in industrialization. According to the invention, a non-toxic and harmless Lawesson's agent is adopted, and a key parent nucleus of a carbonyl group is subjected to a sulfenylation reaction, so that thiophosgene and oxobutyronitrile are effectively avoided. The reaction condition of the method is mild, and the yield is relatively high, therefore, the novel method has a great industrialization prospect.
Owner:成都伊诺达博医药科技有限公司
Method for producing trifluoromethyl thioalkyl compound
A method for producing a trifluoromethyl thioalkyl compound represented by general formula (1) (wherein R represents a C1-C10 alkyl group which may be substituted by R1, or the like; R1 represents a C1-C4 alkyl group or the like; and R2 represents a hydrogen atom, a halogen atom, a C1-C4 alkyl group or the like), which is characterized by adding thiophosgene at an addition temperature of 45 DEG C or more for an addition time of 0.25 hour or more in the presence of a compound represented by general formula (2) (wherein R is as defined in general formula (1); and L represents a halogen atom, a C1-C4 alkylsulfonyloxy group or the like) and a fluorine compound.
Owner:KUMIAI CHEM IND CO LTD
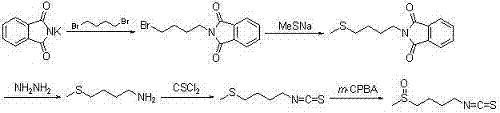
Synthetic method for sulforaphane
ActiveCN102249968BAvoid hydrazinolysisSimple and fast operationOrganic chemistryBulk chemical productionSulforaphaneSodium methanethiolate
The synthesis method of sulforaphane of the present invention belongs to the field of medicine synthesis. After the amino group in 4-amino-1-butanol is protected by the Boc group, its hydroxyl group is converted into a methylsulfonyl ester by methylsulfonyl chloride, and then reacted with sodium methylthiolate to generate 4-methylthiobutyl-1 - tert-butoxyamide. Under acidic conditions, the Boc protecting group is removed to obtain 4-methylthio-1-butylamine. The latter reacted with carbon disulfide for 1 hour in the presence of triethylamine, and then added p-toluenesulfonyl chloride for half an hour to generate 4-methylthiobutyl-1-thioisocyanate. Finally m-CPBA is oxidized to produce sulforaphane. The present invention avoids the complex phthalimide hydrazinolysis reaction in post-treatment, and does not need to use toxic thiophosgene to prepare thioisocyanate; the total yield is 64%, significantly higher than the total yield of 8% reported in the literature The whole preparation process is easy to operate, saves time, and is suitable for large-scale production.
Owner:江苏宁录科技股份有限公司
Thiourea and oxazolidine thione compounds and synthesizing method and application thereof
ActiveCN107056668AImprove universalityStrong toleranceOrganic chemistry methodsMetallocenesHalohydrocarbonPhosphorus pentasulfide
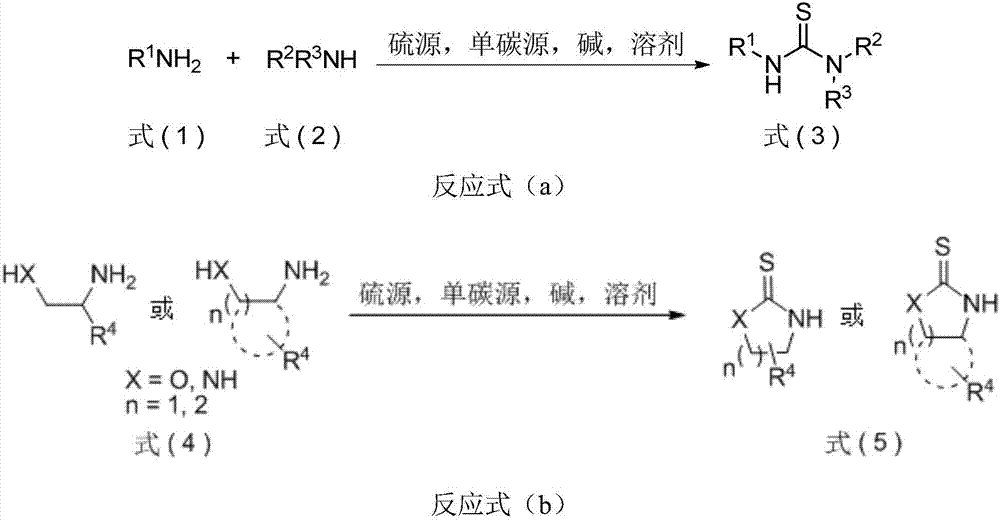
The invention discloses thiourea and oxazolidine thione compounds shown in formulas (3) and (5) and a synthesizing method and application thereof. The synthesizing method comprises the following steps of in a reaction solvent, using primary amine, secondary amine and other amine compounds as raw materials, using an inorganic vulcanizing reagent as a sulfur source, using single-carbon halohydrocarbon as a single-carbon source, and under the action of alkaline, reacting to obtain the thiourea and oxazolidine thione compounds shown in the formulas (3) and (5). The synthesizing method has the advantages that the cost of the raw materials is low, the obtaining is easy, the reaction is simple, and the tolerability of functional groups is strong; the use of main thiocarbonyl reagents, such as volatile and flammable carbon disulfide, high-toxic and strong-corrosive thiophosgene, and high-toxic and smelly-odor Lawesson reagent or phosphorus pentasulfide, is avoided; the synthesizing method is successfully applied to the gram-level synthesizing of chiral thiourea catalysts, chiral oxazolidine thione prothetic groups and commercial pesticide; the practical value is stronger, and the application prospect is wide.
Owner:EAST CHINA NORMAL UNIV
New preparation method of anti-gout medicine Lesinurad and its key intermediate
ActiveCN108947919AAvoid damageImprove conversion rateOrganic chemistryBulk chemical productionLesinuradTriflic acid
The invention provides a new preparation method of an anti-gout medicine Lesinurad and its key intermediate. By using the process provided by the invention, a compound IV can be directly converted into a product III without separation, the reaction yield is greatly increased, and the operation step can be simplified. In addition, the synthesis of the novel intermediate provided by the invention does not require the high-toxicity thiophosgene and carbon disulfide, which greatly improves the safety and environmental protection of the process. The novel synthesis preparation process of Lesinuradis highly efficient, economical, safe, environmentally friendly and suitable for industrial production. In the specification, wherein R is cyclopropane, halogen, trifluoro mesylate, mesylate, and p-toluenesulfonate, and preferably R is cyclopropane; R3 represents COCH3, or R3 represents benzyl or CH2R4, wherein R4 represents an ester group, CN, CH2OH or a phenyl group substituted with one or moreselected from C1-C6 alkyl group and halogen; and X is a halogen.
Owner:SHANGHAI AOBO PHARMTECH INC LTD +1
Preparation method of gout treatment drug Lesinurad and Lesinurad intermediate
Provided are a novel Lesinurad intermediate, providing a synthetic process more economical, more efficient, safer, more environmentally friendly, and suitable for large-scale industrial production for the preparation of Lesinurad. Also provided is a method for preparing the known Lesinurad intermediate and Lesinurad via the provided novel Lesinurad intermediate. Using the novel Lesinurad intermediate of the present invention to synthesize Lesinurad has the following advantages of using cheap and easily available required raw materials, avoiding the use of heavy metals and solvents harmful to the environment, easily separating and purifying the intermediate and the product with a simple operation, avoiding the use of the thiophosgene having high toxicity and difficulty of operation, and enabling the total yield of the reaction to reach a level equal to or higher than the prior art.
Owner:SUZHOU PENGXU PHARM TECH CO LTD
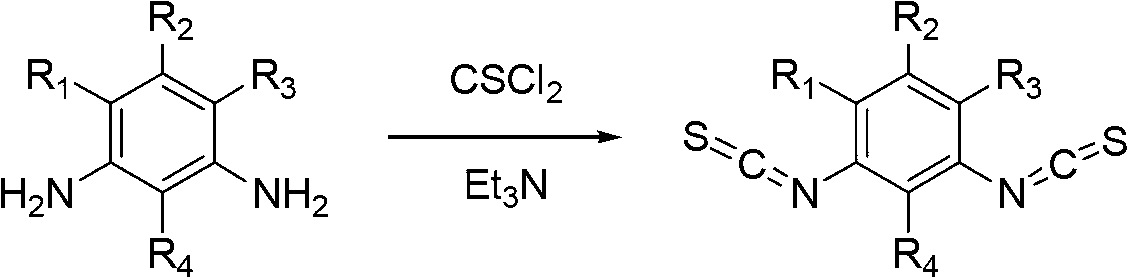
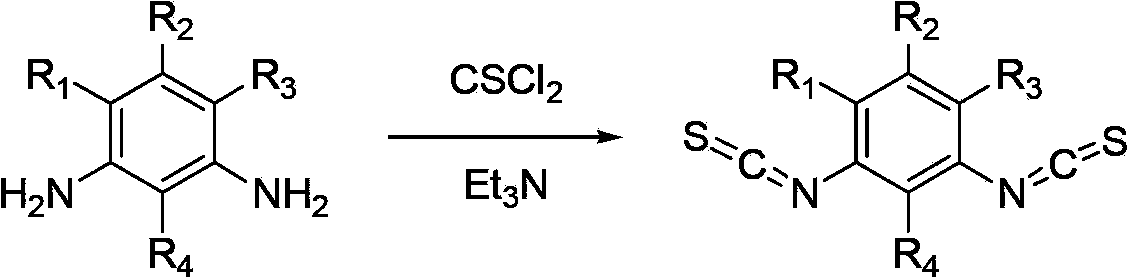
Method for synthesizing substituted m-phthalic isothiocyanate by one-pot method, and synthesized substituted m-phthalic isothiocyanate compound
InactiveCN102702056AWide range of responsesSimple post-processingOrganic chemistrySulfurCombinatorial chemistry
The invention relates to the field of compound synthesis, in particular to a method for synthesizing substituted m-phthalic isothiocyanate by a one-pot method, and a synthesized substituted m-phthalic isothiocyanate compound. According to the method, m-phenylenediamine, m-phenylenediamine derivatives and thiophosgene are used as raw materials, triethylamine is used as acid-binding agents, and the one-pot method is used for synthesizing the substituted m-phthalic isothiocyanate. Compared with the prior art, the method provided by the invention utilizes m-phenylenediamine and m-phenylenediamine derivatives and has the advantages that 1) the multi-step reaction is changed into the one-step reaction; 2) the post treatment is simple, and the operation is easy; 3) the reaction condition is mild; 4) the yield is high; 5) the cost is reduced; 6) the micro reaction is changed into the massive reaction, and the method is suitable for industrial production; and 7) the application is wide, and the method is applicable to reaction of various substituted amine.
Owner:BEIJING NORMAL UNIVERSITY
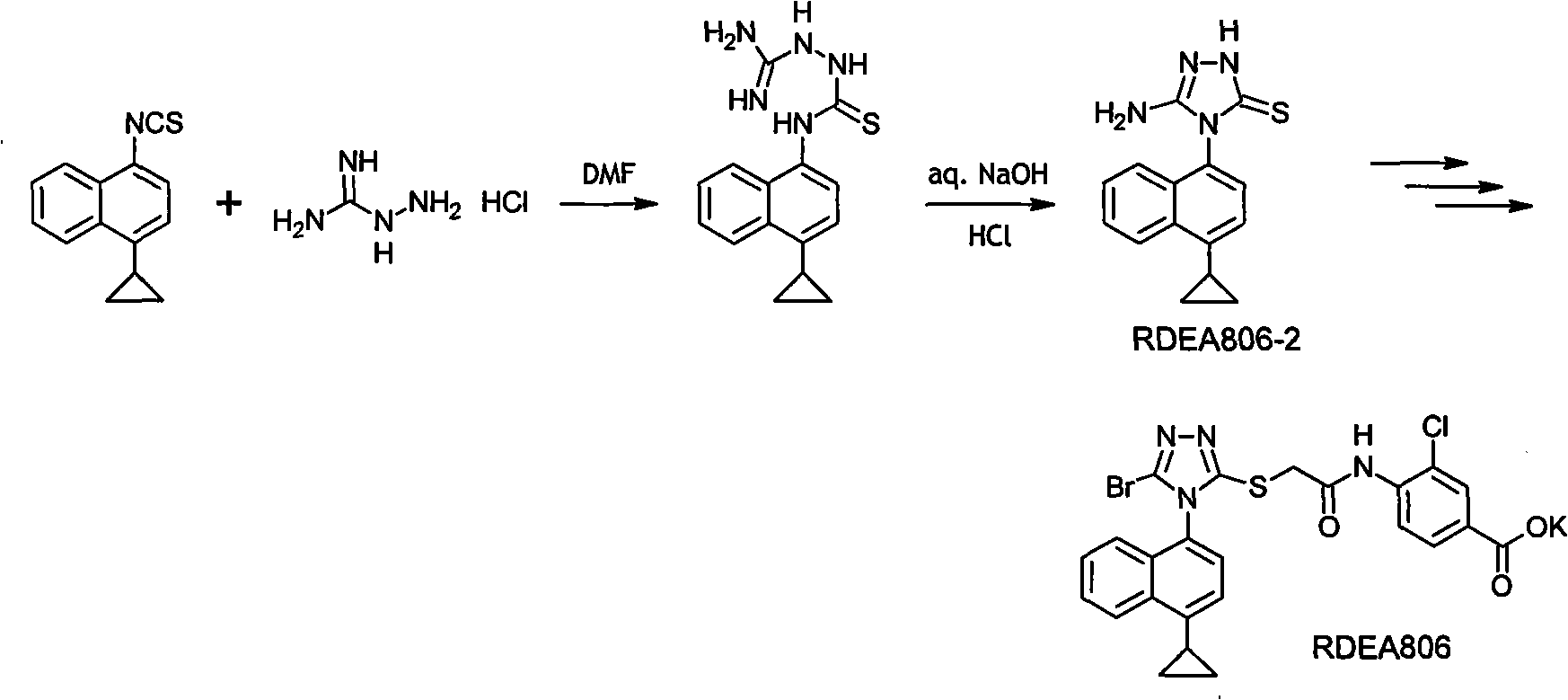
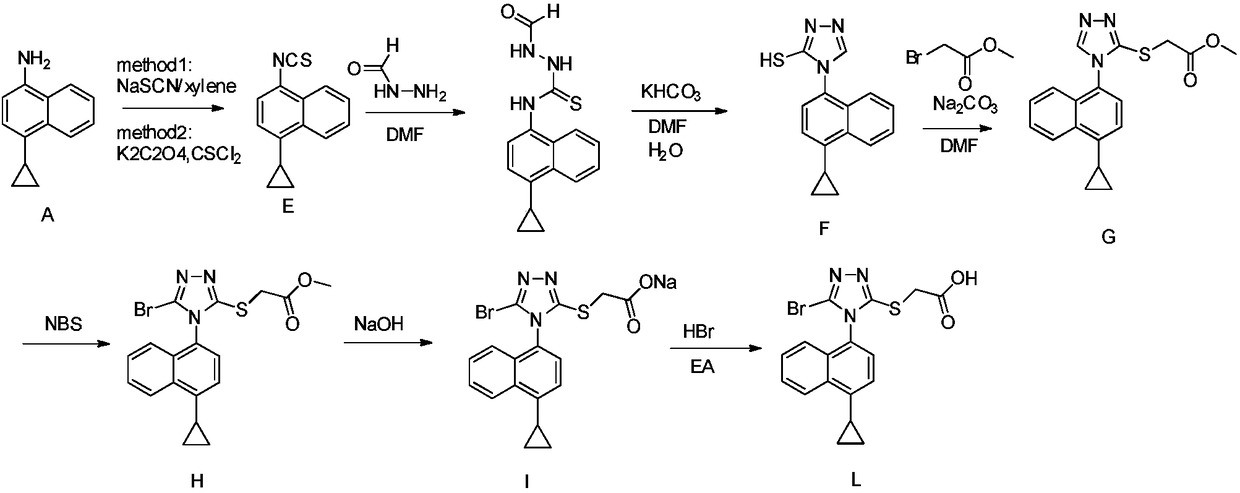
A method for synthesizing 4-(4-cyclopropylnaphthalen-1-yl)-1H-1,2,4-triazole-5(4H)-thione
The present invention discloses a method for synthesizing 4-(4-cyclopropylnaphthalen-1-yl)-1H-1,2,4-triazole-5(4H)-thione. The method includes the following steps: using 4-cyclopropyl-1-naphthylamine (formula A) as a starting reactant, reacting the formula A with carbon disulfide to generate 4-cyclopropyl-1-naphthylamino dithiocarbamate (formula B) under a organic alkaline condition, reacting the formula B with bis (trichloromethyl) carbonate (BTC) or acylating reagents like ethyl chloroformate and methyl chloroformate, etc. to generate 4-cyclopropyl-1-naphthylamino dithiocarbamate chloro-carbonic acid anhydride, conducting decomposition reaction to the resulting product without separation and purification to produce 4-cyclopropyl-1-naphthyl isothiocyanate. The method uses carbon disulfide instead of thiophosgene with greater toxicity, and provides simple process and stable reaction. Furthermore, raw materials are readily available, and industrialization is easy to be realized with a total recovery of more than 65%.
Owner:ANHUI WANBANG MEDICAL TECH
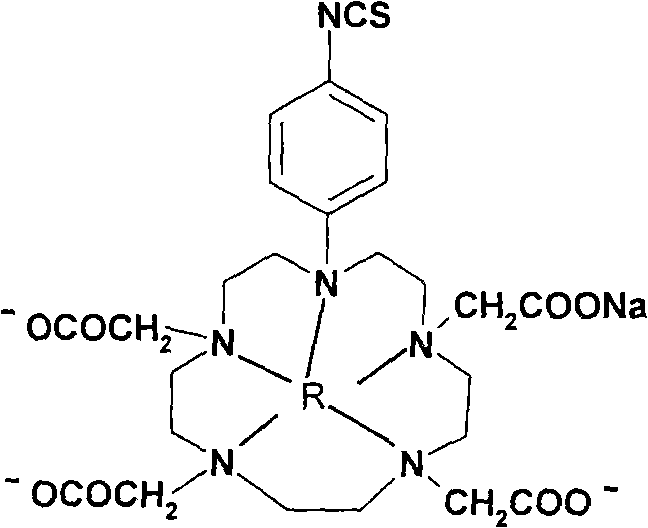
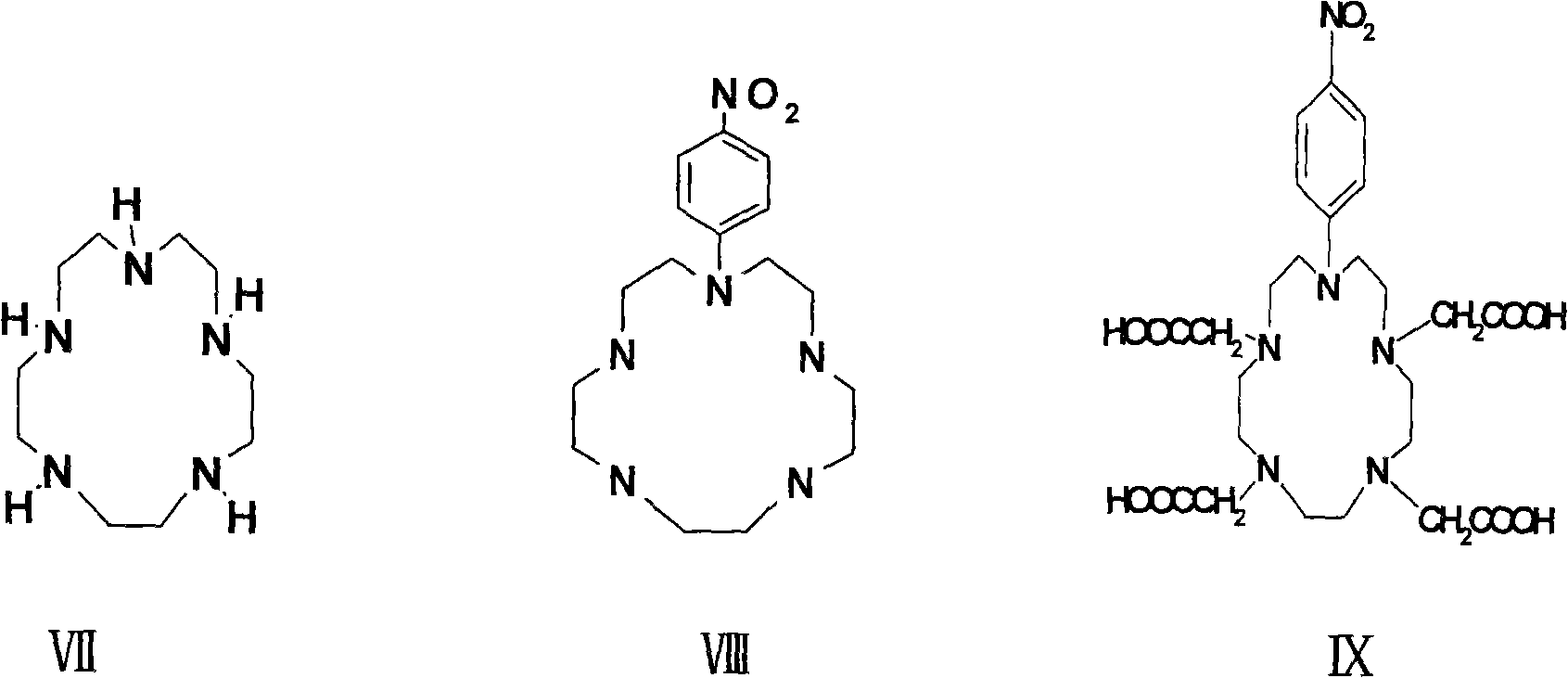
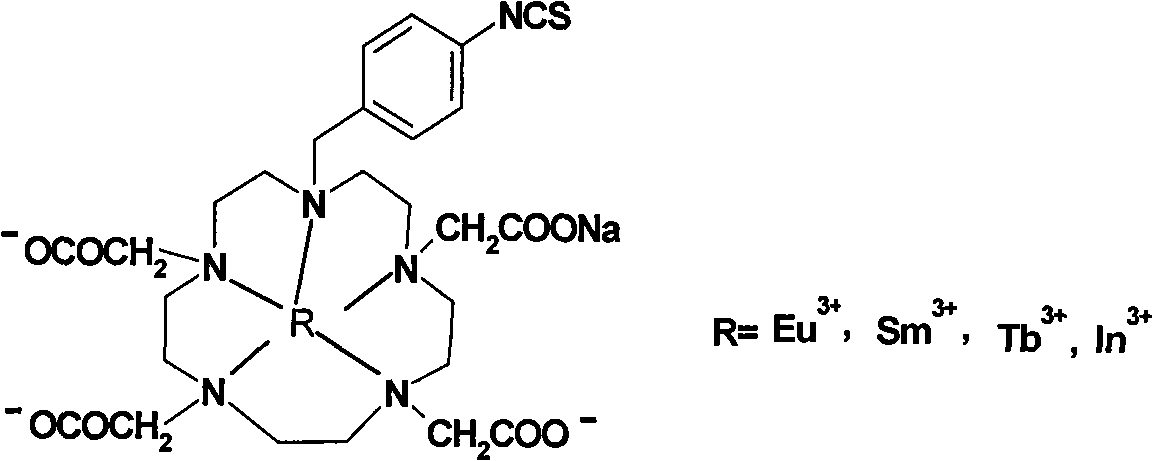
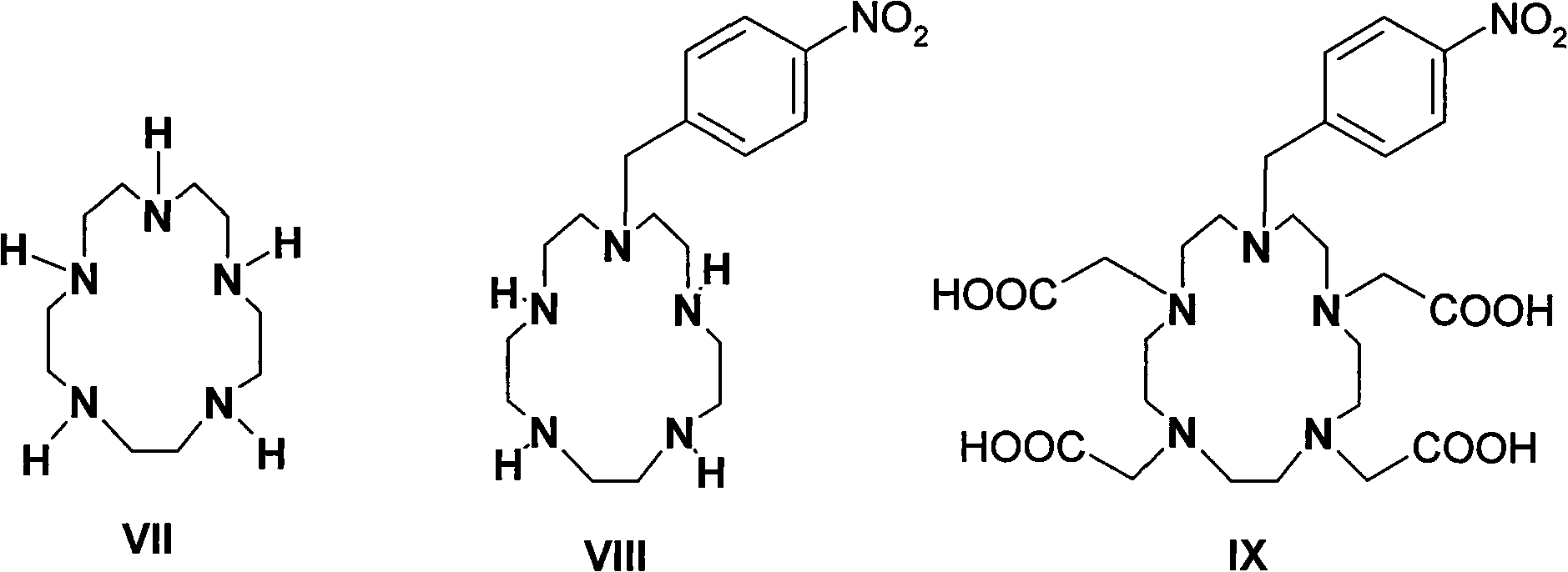
P-isosulfhydryl phenyl five-membered nitrogen heterocyclic ring tetraacethyl chelate and preparation thereof
The invention relates to a chelate of isothiocyanate phenyl pentaazacyclo tetraacetic acid and a method for preparing the same. The method comprises the following steps of condensation, hydrolysis, and substitution, the product of which undergoes condensation with bromoacetate, and chelating with a lanthanide to produce p-nitrobenzene pentaazacyclo tetraacetic acid which undergoes deoxidization with 5 percent Pd / C and finally the reaction with thiophosgene to obtain the target compound I. The chelate of isothiocyanate phenyl pentaazacyclo tetraacetic acid is an unknown compound, and the formed rigid aperture compound is chelated with the lanthanide including europium, indium, scythe, and terbium, and the structure of the chelate is determined through element analysis, analysis with infrared and ultraviolet, magnetic resonance imaging, and mass spectrum, the prepared chelate is mainly used for time-resolved fluoroimmunoassay.
Owner:INST OF RADIATION MEDICINE CHINESE ACADEMY OF MEDICAL SCI
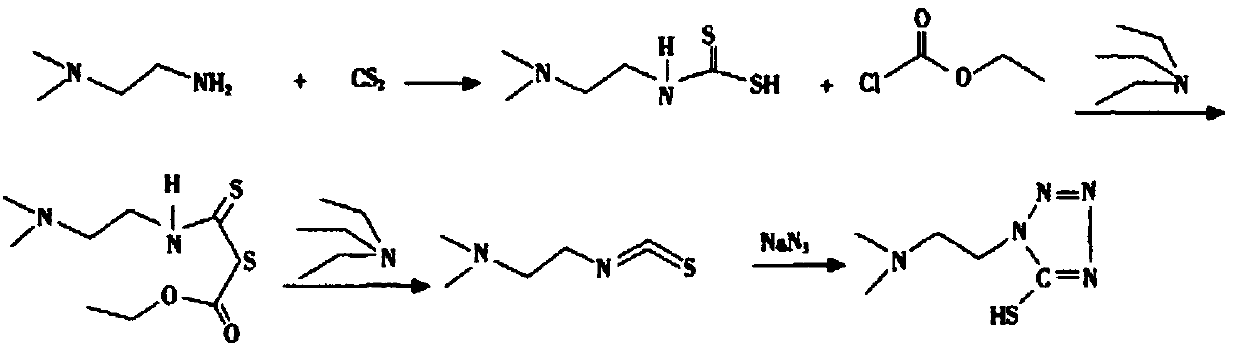
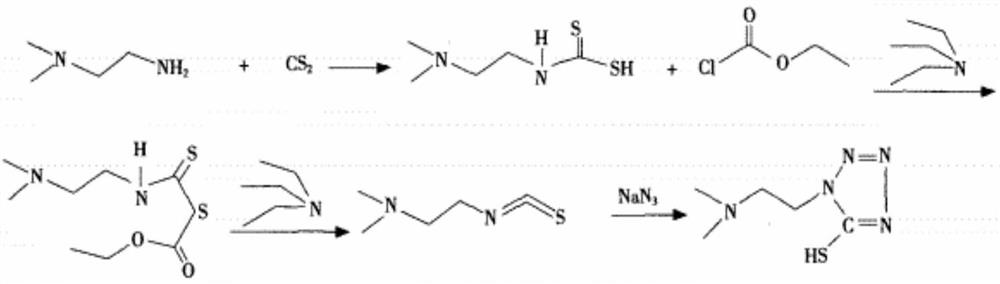
Synthesis method of 1-(2-dimethylaminoethyl)-5-mercaptotetrazole
ActiveCN110950816AThe synthesis process is simpleHigh yieldOrganic chemistryAzidotrimethylsilaneTetrazole
The invention belongs to the technical field of medicines, and particularly relates to a synthesis method of 1-(2-dimethylaminoethyl)-5-mercaptotetrazole. The method comprises the following steps: reacting N, N-dimethylethylenediamine with thiophosgene to obtain isothiocyanate, reacting isothiocyanate with azidotrimethylsilane to obtain 1-(2-dimethylaminoethyl)-5-mercaptotetrazole hydrochloride, dissolving 1-(2-dimethylaminoethyl)-5-mercaptotetrazole hydrochloride in water, and carrying out decolorizing and crystallizing to obtain 1-(2-dimethylaminoethyl)-5-mercaptotetrazole. According to themethod, the isothiocyanate is synthesized from the N, N-dimethylethylenediamine and the thiophosgene through a one-step method, so that the synthesis process is simplified and the yield is greatly increased; non-toxic azidotrimethylsilane is used for replacing virulent sodium azide in the cyclization process, so that the safety of the process is improved, and the method is more suitable for industrial amplification.
Owner:SHANDONG JINCHENG PHARMACCUTICAL CHEM CO LTD
Isotope labeling reagent as well as preparation method and application thereof
ActiveCN102174025BReduce complexityAccurate identificationOrganic chemistryComponent separationSodium methoxideSodium bicarbonate
The invention discloses an isotope labeling reagent which is an isothiocyanopyrimidine isotope labeling reagent with a chemical name of [d0] / [d6]-4,6-dimethoxine-2-isothiocyanate. The preparation method of the reagent comprises the following steps of: firstly, reacting 4,6-dichloro-2-amiopyrimidine with sodium methoxide / deuterosodium methoxide to obtain a [d0] / [d6]-4,6-dimethoxy-2-amiopyrimidine crude product; carrying out reflux reaction on the obtained crude product, thiophosgene and sodium bicarbonate to obtain a final crude product; and then carrying out column chromatography to obtain the isotope labeling reagent disclosed by the invention. The isotope labeling reagent disclosed by the invention can be applied to the identification analysis of polypeptide N-end residues and the quantitative analysis of protein, can speed up the labeling reaction rate and improve a signal of the labeled peptide section, is beneficial to strengthening the accuracy and the credibility of the identified peptide section and has the advantages of less molecular weight of the labelled part, relative simple structure, stable performance, simpleness and convenience for operation, and the like.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
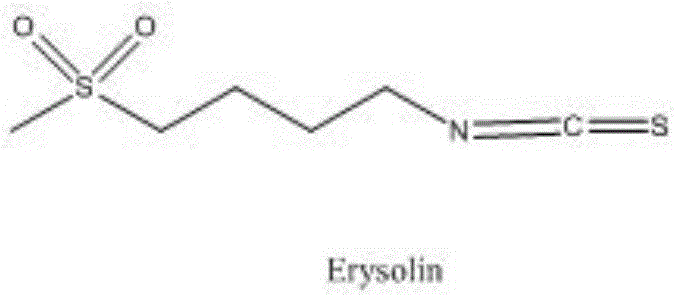
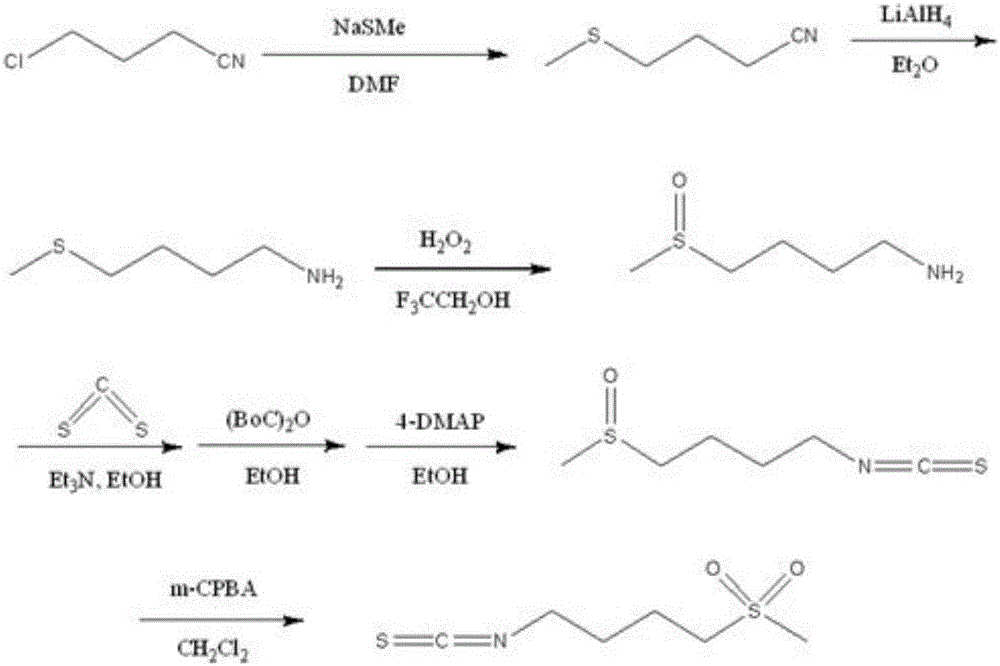
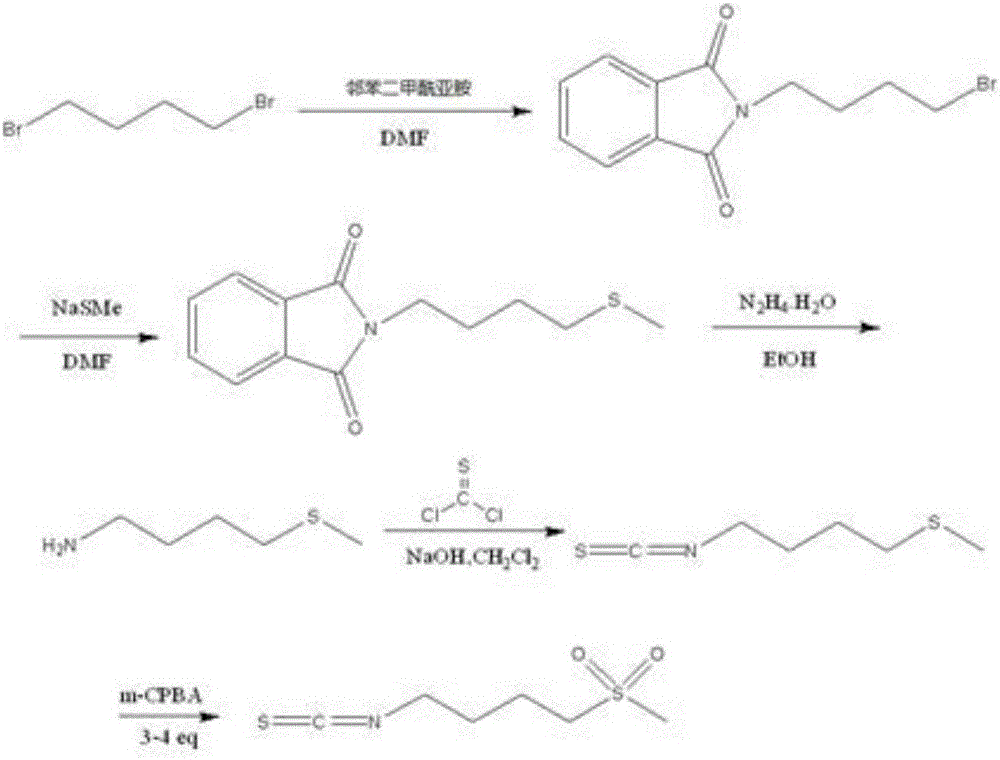
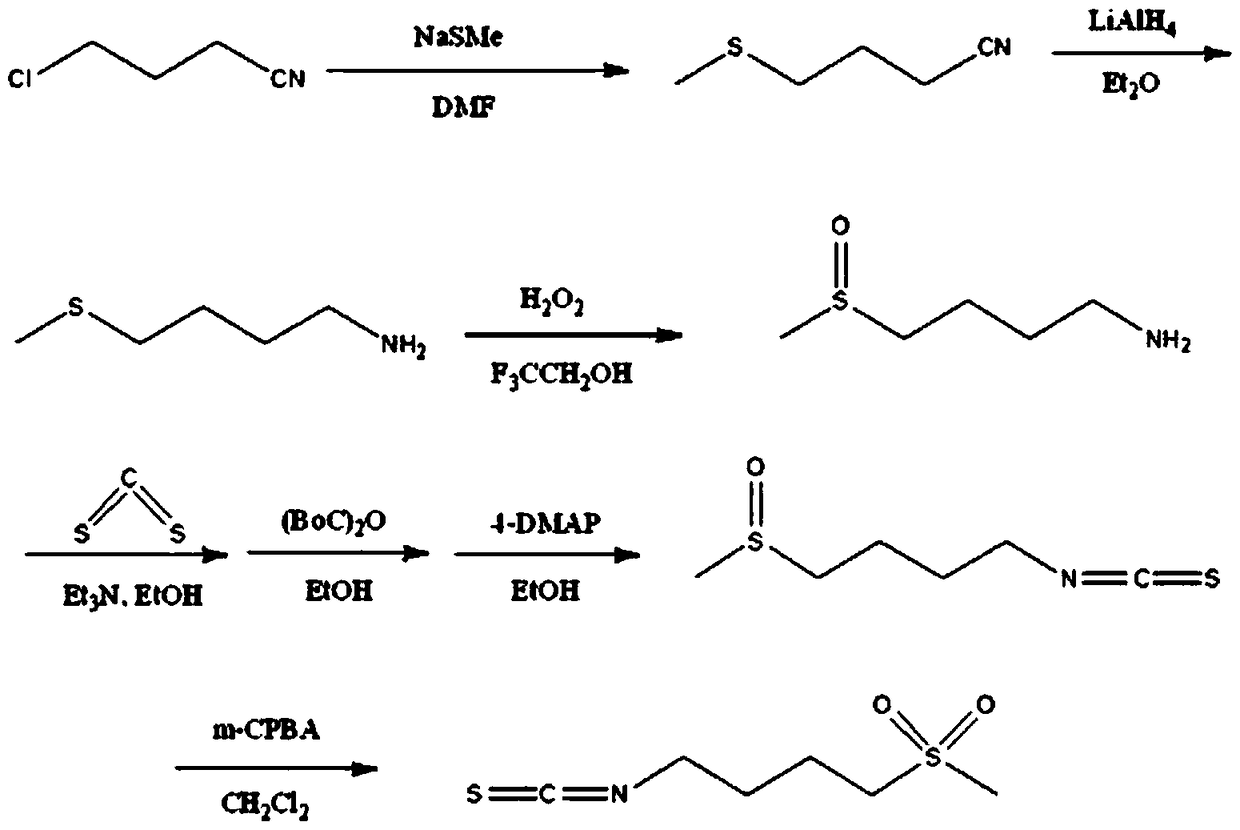
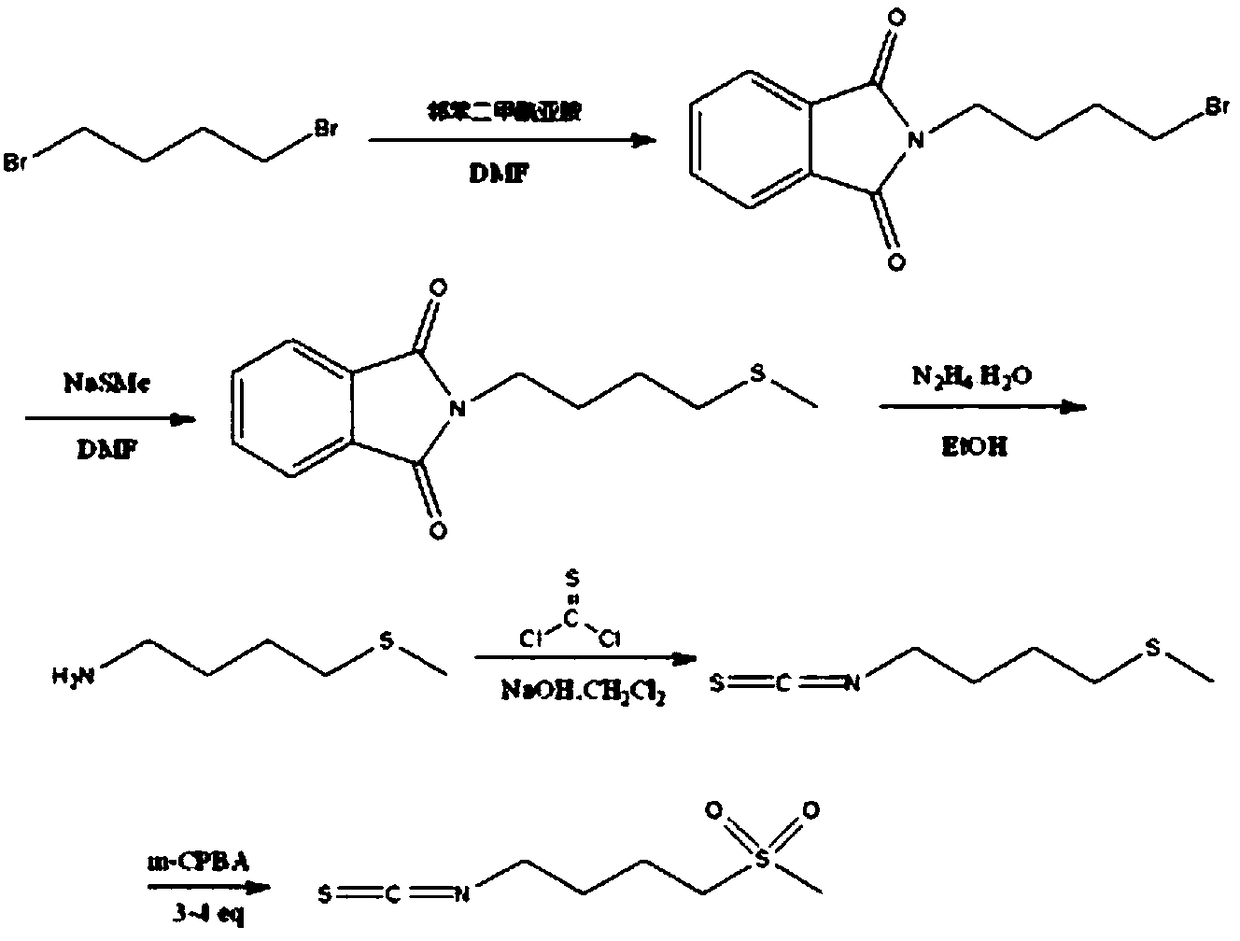
Synthesis method of 4-methanesulfonylbutyl isothiocyanate
ActiveCN106496086AThe synthesis steps are simpleReduce yieldOrganic chemistryChemical synthesisSynthesis methods
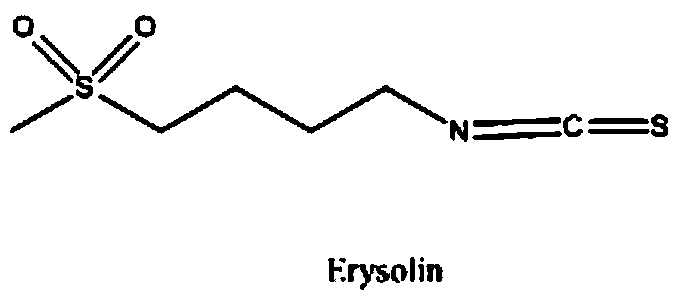
The invention relates to a chemical synthesis method of 4-methanesulfonylbutyl isothiocyanate (Erysolin). The method comprises that 1-bromo-4-chlorobutane and hydrous sodium methyl mercaptide as starting raw materials undergo a reaction to produce chlorothioether, the chlorothioether and phthalimide potassium salt undergo a reaction to produce a tertiary amine conjugate, the tertiary amine conjugate undergoes a hydrazinolysis reaction to produce thioether primary amine, the thioether primary amine forms isothiocyanate under action of thiophosgene, and the isothiocyanate is oxidized to form Erysolin. The chemical synthesis method is simple in operation, uses a small amount of dangerous reagents, gives consideration to a cost and a yield and is suitable for Erysolin industrial production.
Owner:SHENYANG PHARMA UNIVERSITY
Para-thiocyano benzyl five-nitrogen heterocyclic ring tetraacethyl chelate and preparation method thereof
The invention relates to a para-thiocyano benzyl five-nitrogen heterocyclic ring tetraacethyl chelate and a preparation method thereof. The preparation method comprises the following steps of: condensing, hydrolyzing, substituting, and condensing the product with bromoacetic acid; then, chelating with lanthanides to obtain para-nitro benzyl five-nitrogen heterocyclic ring tetraacethyl, and reducing with 5% Pd / C; and finally, reacting with thiophosgene to obtain a target compound I. The para-thiocyano benzyl five-nitrogen heterocyclic ring tetraacethyl chelate is an unknown compound, the formed rigid cryptate is chelated with the lanthanides of europium, indium, samarium, terbium and the like, and the structure is determined by elementary analysis, infrared spectra, ultraviolet spectra, nuclear magnetism and mass spectra. The prepared chelate is mainly used for time-resolved fluoroimmunoassay.
Owner:INST OF RADIATION MEDICINE CHINESE ACADEMY OF MEDICAL SCI
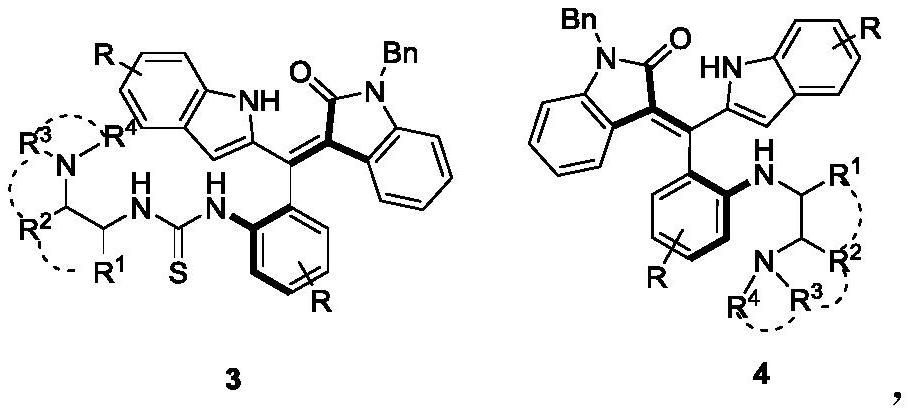
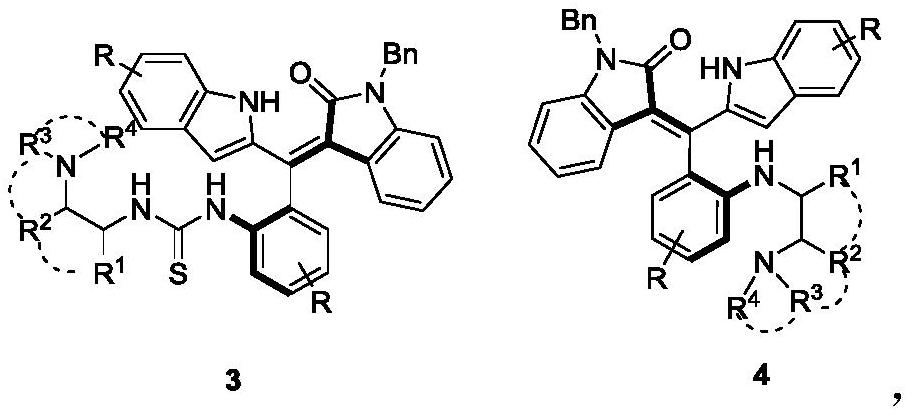
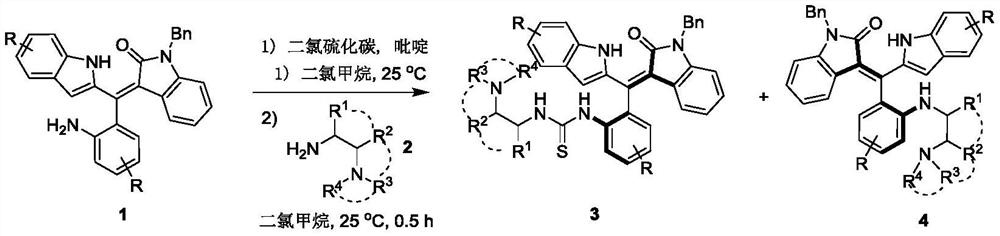
Axial chiral styrene tertiary amine thiourea catalyst as well as preparation method and application thereof
PendingCN112264091AStereoselectivity is well controlledGood catalyticOrganic chemistry methodsOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystThiourea
The invention discloses an axial chiral styrene tertiary amine thiourea catalyst as well as a preparation method and application thereof. The chemical structures of the axial chiral styrene tertiary amine thiourea catalyst are shown as formulas 3 and 4. The preparation method comprises the following steps: (1) putting a compound shown as a formula 1 and carbon dichloride sulfide serving as raw materials into dichloromethane, adding pyridine, stirring for reaction at 25 DEG C, and performing TLC tracking reaction until the reaction is finished; (2) concentrating the product obtained in the step(1), adding dichloromethane and a compound shown in a formula 2, stirring for reaction at 25 DEG C, and performing TLC tracking reaction until the reaction is finished; and (3) concentrating and purifying the product obtained in the step (2) to obtain the compound shown in the formula 3 and the formula 4. The catalyst disclosed by the invention is relatively good in stereoselectivity control andrelatively good in catalytic effect. The preparation method disclosed by the invention is simple in steps, the cost is reduced, and two catalysts with different configurations can be obtained in one step; and the method is mild in reaction condition, and the enantioselectivity is improved.
Owner:XUZHOU NORMAL UNIVERSITY
Method for preparing sulfonyl ring thiourea from mono-sulfonyl diamine in aqueous phase
InactiveCN101880211ASimple manufacturing processReduce usageFunctional group formation/introductionOrganic solventThiourea
The invention discloses a method for preparing sulfonyl ring thiourea from mono-sulfonyl diamine in an aqueous phase, and belongs to a preparative technique of sulfonyl ring thiourea compounds. The method comprises the following processes of: adding mono-sulfonyl diamine, carbon disulfide, and one of sodium hydroxide, potassium hydroxide, sodium carbonate and potassium carbonate into water according to the molar ratio of the mono-sulfonyl diamine to the carbon disulfide to alkali or the molar ratio of the mono-sulfonyl diamine to the carbon disulfide to carbonate, performing reaction with stirring to obtain a compound, and filtering and washing the compound to obtain the sulfonyl ring thiourea. The method has the advantages of overcoming numerous defects in the prior art and avoiding thiophosgene or thiocarbonyl diimidazole; and the reaction takes the water as a medium, so the method also has the advantages of no need of organic solvent and heating, simpleness, convenience, readily available raw materials, and environmental friendliness.
Owner:TIANJIN UNIV
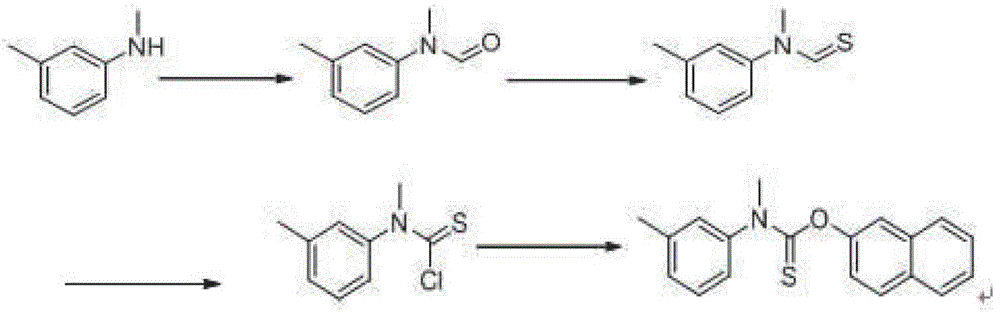
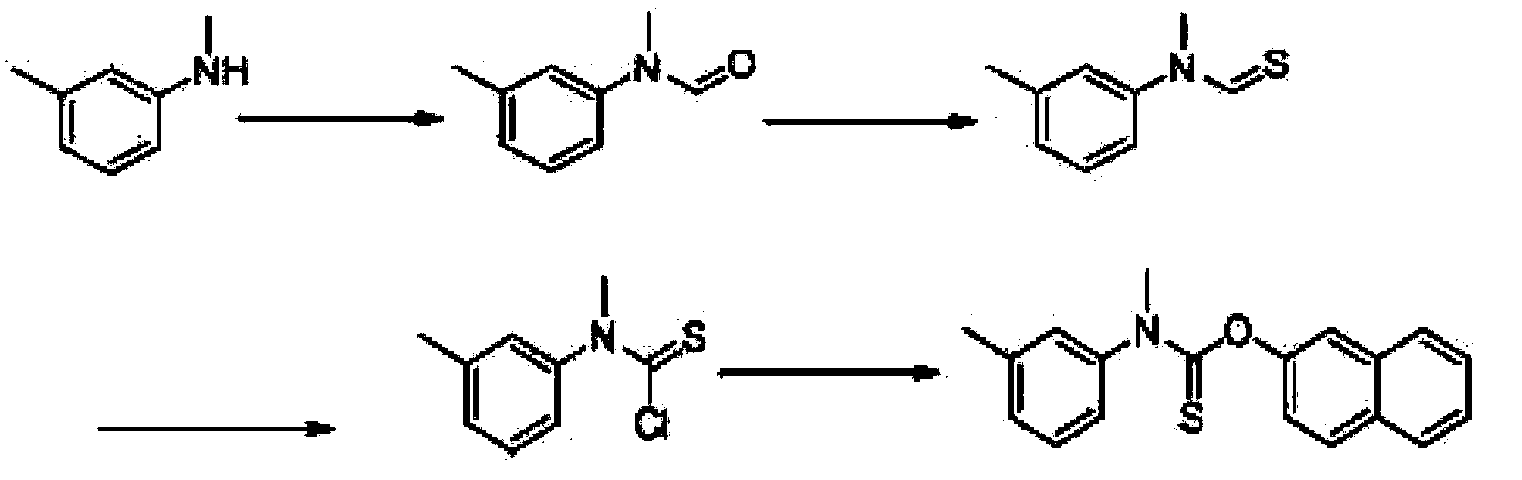
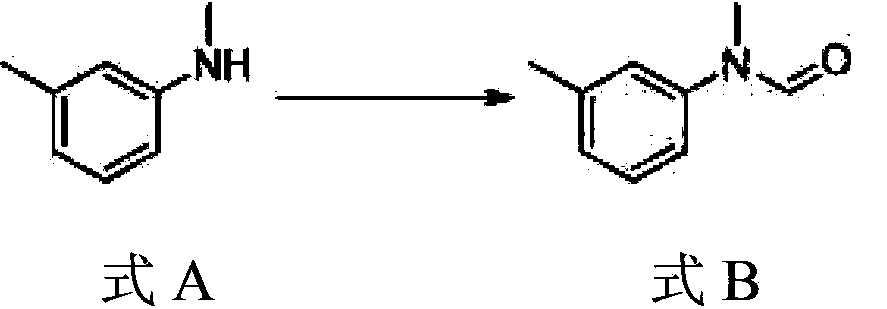
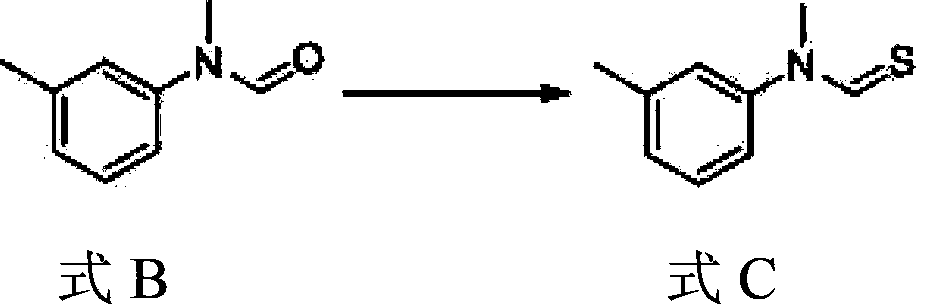
A kind of preparation method of N-methyl-n-(3-methylphenyl) thiocarbamate-2-naphthyl ester
The invention discloses a method for preparing N-methyl-N-(3-methyl phenyl) thiocarbamate-2-naphthyl. By adopting the method, usage of harmful and toxic thiophosgene or chlorine can be avoided, and physical health of staff can be protected. The method is suitable for large-scale industrial production and application, and good enterprise benefits and social benefits can be obtained.
Owner:四川摩尔生物制药有限公司
Synthesis method of anti-prostate cancer drug enzalutamide
The invention discloses a novel method for compounding a medicament of Enzalutamide for resisting the prostate cancer, belongs to the technical field of pharmacy synthetic technique, and particularly relates to novel technique for compounding Enzalutamide. Most of known methods for compounding Enzalutamide relate to use of highly toxic and mephitical thiophosgene and highly toxic oxobutyronitrile and have great difficulty in industrialization. According to the invention, a non-toxic and harmless Lawesson's agent is adopted, and a key parent nucleus of a carbonyl group is subjected to a sulfenylation reaction, so that thiophosgene and oxobutyronitrile are effectively avoided. The reaction condition of the method is mild, and the yield is relatively high, therefore, the novel method has a great industrialization prospect.
Owner:成都伊诺达博医药科技有限公司
Method for preparing N-methyl-N-(3-methyl phenyl) thiocarbamate-2-naphthyl
The invention discloses a method for preparing N-methyl-N-(3-methyl phenyl) thiocarbamate-2-naphthyl. By adopting the method, usage of harmful and toxic thiophosgene or chlorine can be avoided, and physical health of staff can be protected. The method is suitable for large-scale industrial production and application, and good enterprise benefits and social benefits can be obtained.
Owner:四川摩尔生物制药有限公司
Thiourea and oxazolidinethione compounds and their synthesis method and application
ActiveCN107056668BImprove universalityStrong toleranceOrganic chemistry methodsMetallocenesPhosphorus pentasulfideHalohydrocarbon
The invention discloses thiourea and oxazolidine thione compounds shown in formulas (3) and (5) and a synthesizing method and application thereof. The synthesizing method comprises the following steps of in a reaction solvent, using primary amine, secondary amine and other amine compounds as raw materials, using an inorganic vulcanizing reagent as a sulfur source, using single-carbon halohydrocarbon as a single-carbon source, and under the action of alkaline, reacting to obtain the thiourea and oxazolidine thione compounds shown in the formulas (3) and (5). The synthesizing method has the advantages that the cost of the raw materials is low, the obtaining is easy, the reaction is simple, and the tolerability of functional groups is strong; the use of main thiocarbonyl reagents, such as volatile and flammable carbon disulfide, high-toxic and strong-corrosive thiophosgene, and high-toxic and smelly-odor Lawesson reagent or phosphorus pentasulfide, is avoided; the synthesizing method is successfully applied to the gram-level synthesizing of chiral thiourea catalysts, chiral oxazolidine thione prothetic groups and commercial pesticide; the practical value is stronger, and the application prospect is wide.
Owner:EAST CHINA NORMAL UNIV
The synthetic method of 4-methanesulfonylbutyl isothiocyanate
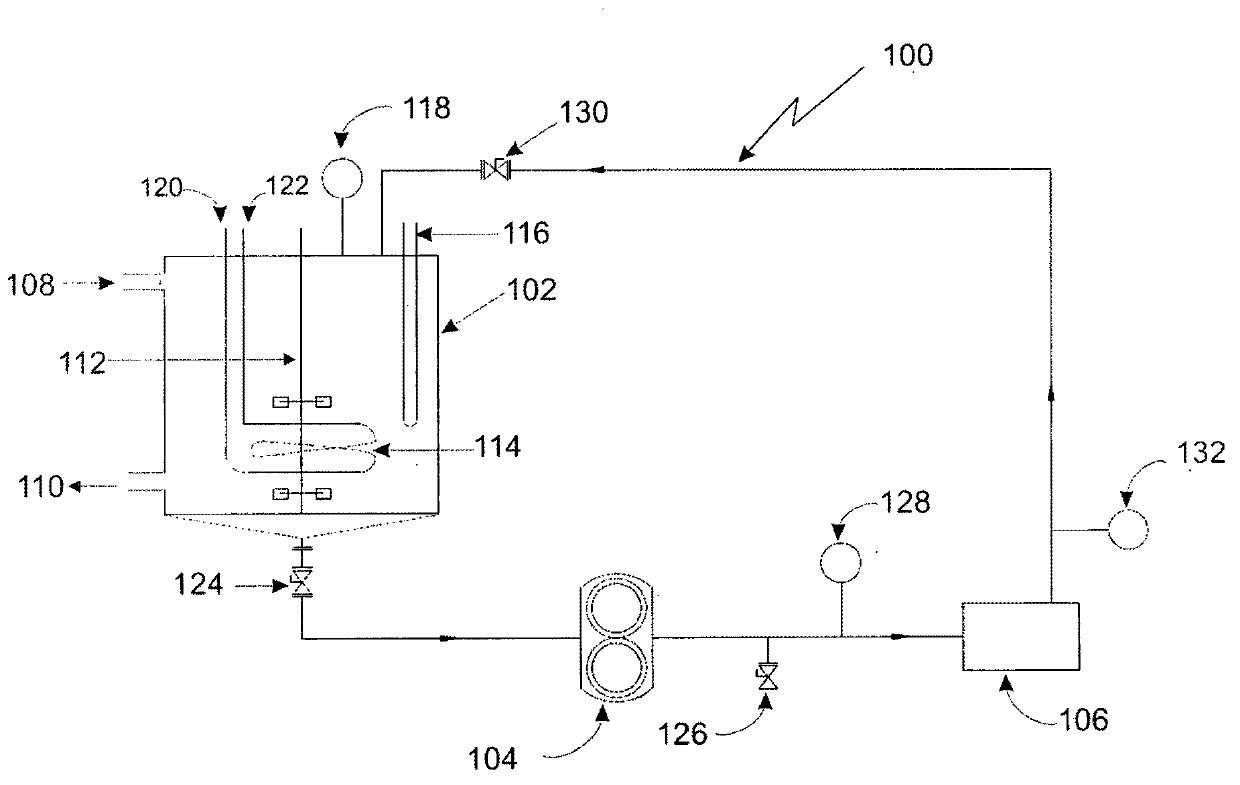
ActiveCN106496086BThe synthesis steps are simpleReduce yieldOrganic chemistryChemical synthesisSulfur
The invention relates to a chemical synthesis method of 4-methanesulfonylbutyl isothiocyanate (Erysolin). The method comprises that 1-bromo-4-chlorobutane and hydrous sodium methyl mercaptide as starting raw materials undergo a reaction to produce chlorothioether, the chlorothioether and phthalimide potassium salt undergo a reaction to produce a tertiary amine conjugate, the tertiary amine conjugate undergoes a hydrazinolysis reaction to produce thioether primary amine, the thioether primary amine forms isothiocyanate under action of thiophosgene, and the isothiocyanate is oxidized to form Erysolin. The chemical synthesis method is simple in operation, uses a small amount of dangerous reagents, gives consideration to a cost and a yield and is suitable for Erysolin industrial production.
Owner:SHENYANG PHARMA UNIVERSITY
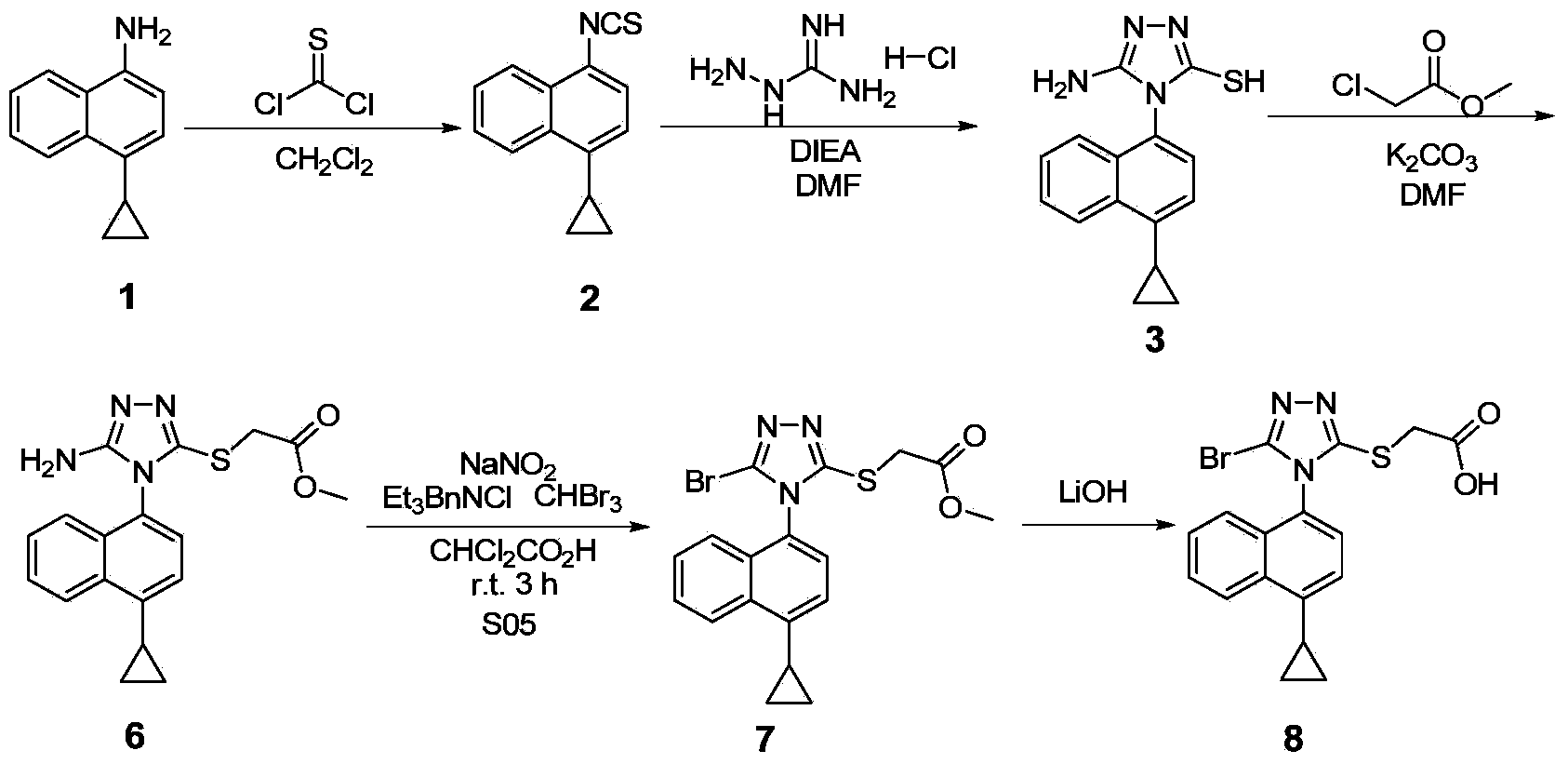
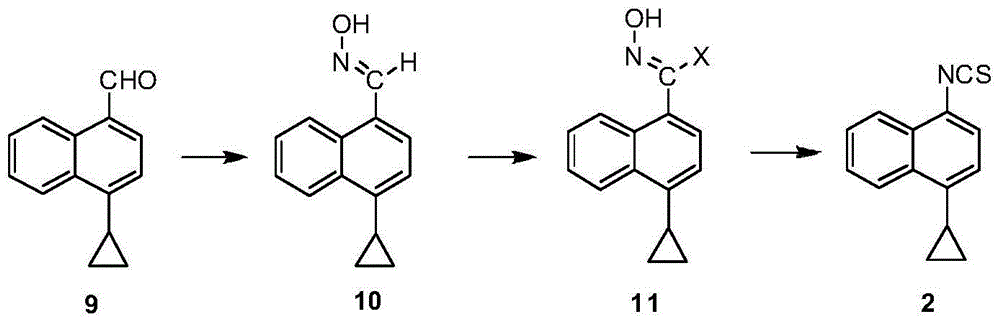
A kind of preparation method of 4-cyclopropyl-1-isothiocyanatonaphthalene and intermediate 4-cyclopropyl-1-naphthalene formaldehyde oxime/halide
The invention provides a preparation method of 4-cyclopropyl-1-naphthaline isothiocyanate, which is characterized in that the 4-cyclopropyl-1-naphthaline isothiocyanate is synthesized by taking 4-cyclopropyl-1-naphthaldehydes as an initial raw material through three-step reaction of oximation, halogenation and vulcanization. Meanwhile, the invention discloses a preparation method of an intermediate 4-cyclopropyl-1-naphthaldehyde oxime / halide of the 4-cyclopropyl-1-naphthaldehydes. When the 4-cyclopropyl-1-naphthaline isothiocyanate is prepared with the method disclosed in the invention, thiophosgene, which has strong toxicity and bad smell, is not needed to be used. The invention has the advantages of convenience in operation, small influence to environment, good safety, good product quality and high yield. Moreover, the separation and purification of a product is easy and is simple to operate.
Owner:ZHEJIANG HUABANG MEDICAL & CHEM
Method for producing trifluoromethylthioalkyl compound
A method for producing a trifluoromethylthioalkyl compound represented by the general formula (1), characterized in that, in the presence of a compound represented by the following general formula (2) and a fluorine compound, adding temperature and add thiophosgene with an addition time of 0.25 hours or more. In the general formula (1), R represents a C1-C10 alkyl group substituted or unsubstituted by R1, R1 represents a C1-C4 alkyl group, etc., R2 represents a hydrogen atom, a halogen atom, a C1-C4 alkyl group, etc. In the general formula (2), R is as defined in the above-mentioned general formula (1), and L represents a halogen atom, a C1-C4 alkylsulfonyloxy group, or the like.
Owner:KUMIAI CHEM IND CO LTD
Method for synthesizing substituted m-phthalic isothiocyanate by one-pot method, and synthesized substituted m-phthalic isothiocyanate compound
InactiveCN102702056BSimple post-processingEasy to operateOrganic chemistrySulfurCombinatorial chemistry
The invention relates to the field of compound synthesis, in particular to a method for synthesizing substituted m-phthalic isothiocyanate by a one-pot method, and a synthesized substituted m-phthalic isothiocyanate compound. According to the method, m-phenylenediamine, m-phenylenediamine derivatives and thiophosgene are used as raw materials, triethylamine is used as acid-binding agents, and the one-pot method is used for synthesizing the substituted m-phthalic isothiocyanate. Compared with the prior art, the method provided by the invention utilizes m-phenylenediamine and m-phenylenediamine derivatives and has the advantages that 1) the multi-step reaction is changed into the one-step reaction; 2) the post treatment is simple, and the operation is easy; 3) the reaction condition is mild; 4) the yield is high; 5) the cost is reduced; 6) the micro reaction is changed into the massive reaction, and the method is suitable for industrial production; and 7) the application is wide, and the method is applicable to reaction of various substituted amine.
Owner:BEIJING NORMAL UNIVERSITY
Synthesis of 1-(2-dimethylaminoethyl)-5-mercaptotetrazole
ActiveCN110950816BThe synthesis process is simpleHigh yieldOrganic chemistryAzidotrimethylsilaneTetrazole
The invention belongs to the technical field of medicine, and in particular relates to a synthesis method of 1-(2-dimethylaminoethyl)-5-mercaptotetrazole. N,N‑Dimethylethylenediamine reacts with thiophosgene to give isothiocyanate, and reacts isothiocyanate with azidotrimethylsilane to give 1‑(2‑dimethylaminoethyl)‑ 5‑mercaptotetrazolium hydrochloride, 1‑(2‑dimethylaminoethyl)‑5‑mercaptotetrazolium hydrochloride is dissolved in water, decolorized and crystallized to obtain 1‑(2‑dimethylaminoethyl )‑5‑mercaptotetrazole. The present invention uses N,N-dimethylethylenediamine and thiophosgene to synthesize isothiocyanate in one step, which simplifies the synthesis process and greatly improves the yield; the cyclization process uses non-toxic azidotrimethylsilane It replaces the highly toxic sodium azide, improves the safety of the process, and is more suitable for industrial scale-up.
Owner:SHANDONG JINCHENG PHARMACCUTICAL CHEM CO LTD
Method for preparation of perfluoroalkyl sulfenyl chloride
ActiveUS9617207B2Simple and safe and convenientEasy to operateSamplingOrganic compound preparationSulfurFluoride
The present disclosure provides a process for the preparation of perfluoroalkyl sulfenyl chloride by reacting a compound of formula [I] with at least one fluoride compound and thiophosgene.
Owner:GHARDA KEKI HORMUSJI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com