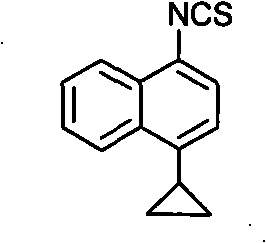
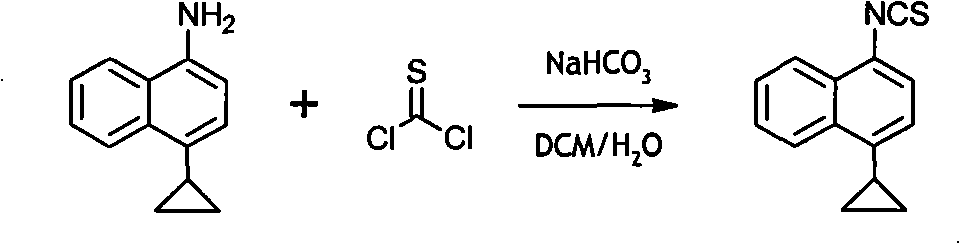
Preparation method of 4-cyclopropyl-1-naphthaline isothiocyanate and intermediate 4-cyclopropyl-1-naphthaldehyde oxime/halide
A technology of isothiocyanatonaphthalene and isothiocyanate, applied in the field of organic chemistry, can solve problems such as high labor protection costs, difficulty in large-scale production, and large environmental impact, and achieve reduced labor protection costs, improved safety, and labor protection. The effect of small environmental impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
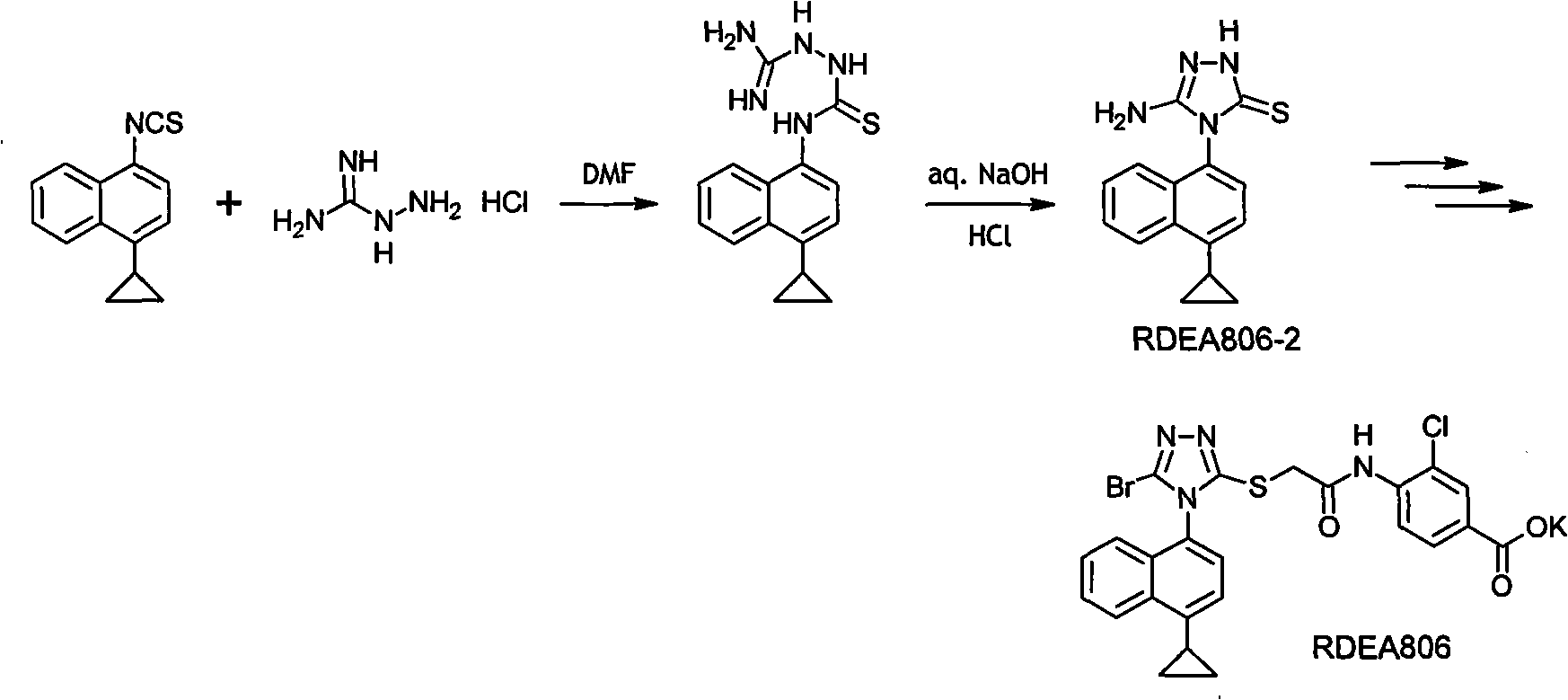
[0040] The synthetic route diagram of the present invention is as follows:
[0041]
[0042] The implementation process of synthetic route of the present invention is briefly described as follows:
[0043] 1. Obtain 4-cyclopropyl-1-naphthaldehyde oxime by reacting 4-cyclopropyl-1-naphthaldehyde with hydroxylamine or hydroxylamine derivatives. The structural formula is as follows:
[0044]
[0045] Add 9.8g (50mmol) of 4-cyclopropyl-1-naphthaldehyde, 5.2g (75mmol, 1.5eq.) of hydroxylamine hydrochloride, and 4.1g (75mmol, 1.5eq.) of anhydrous sodium acetate into 100ml of methanol, stir and reflux for reaction 8 hours, cooled to room temperature, filtered, the filtrate was dispersed in 200ml of water, filtered, rinsed with water, dried and crystallized with n-hexane to obtain 10.0 g of 4-cyclopropyl-1-naphthaldoxime white crystalline solid with a yield of 95%.
[0046] Wherein, the preparation method of the 4-cyclopropyl-1-naphthaldehyde used in step (1):
[0047] Dissol...
Embodiment 2
[0059] Add 9.8g (50mmol) of 4-cyclopropyl-1-naphthalene formaldehyde, 8.2g (50mmol, 2.0eq.) of hydroxylamine sulfate, and 4.1g (75mmol, 1.5eq.) of anhydrous sodium acetate into 100ml of methanol, stir and reflux to react 8 hours, cooled to room temperature, filtered, the filtrate was dispersed in 200ml of water, filtered, rinsed with water, dried and crystallized with n-hexane to obtain 9.6 g of 4-cyclopropyl-1-naphthaldoxime as a white crystalline solid with a yield of 91%.
[0060] Dissolve 9.5g (45mmol) of the obtained intermediate 4-cyclopropyl-1-naphthaldoxime in 100ml of acetonitrile, and add 3.5g (15mmol, 1.0eq.) of trichloroisocyanuric acid in batches. After the addition was complete, the stirring reaction was continued for 2.0 hours. The reaction solution was dispersed into 500ml of water, a large amount of white solid crystals were precipitated, filtered, rinsed with water, sucked dry, and dried in vacuum to obtain 9.1g of 4-cyclopropyl-1-naphthaldoxime chloride with...
Embodiment 3
[0063] Add 9.8g (50mmol) of 4-cyclopropyl-1-naphthaldehyde, 40ml (60mmol, 1.2eq.) of 1.5M hydroxylamine methanol solution, add 100ml methanol, stir and reflux for 1.5 hours, cool to room temperature, and disperse the reaction solution to 200ml of water, filtered, washed with water, dried and crystallized with n-hexane to obtain 10.0 g of white crystalline solid of 4-cyclopropyl-1-naphthalene formaldehyde oxime, yield 95%.
[0064] Dissolve 9.5 g (45 mmol) of the resulting intermediate 4-cyclopropyl-1-naphthalene formaldehyde oxime in 100 ml of acetonitrile, and slowly add 8.9 g of N-bromosuccinimide (50 mmol, 1.0 eq.) in 100 ml of MeCN solution. After the dropwise addition was completed, the mixture was kept warm and stirred for 2.0 hours. The reaction solution was dispersed into 800ml of water, a large amount of white solid crystals were precipitated, filtered, rinsed with water, sucked dry, and dried in vacuum to obtain 12.6g of 4-cyclopropyl-1-naphthaldoxime bromide with a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com