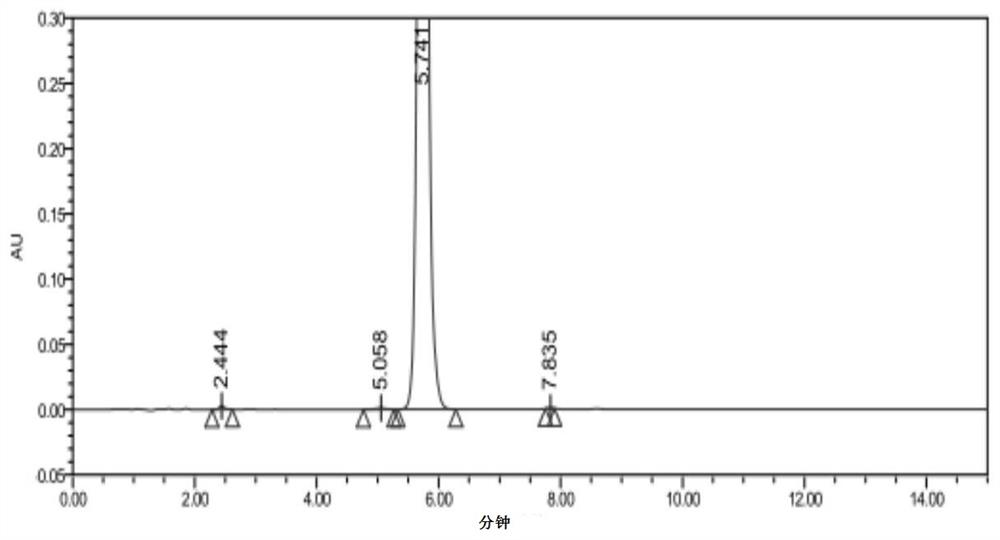
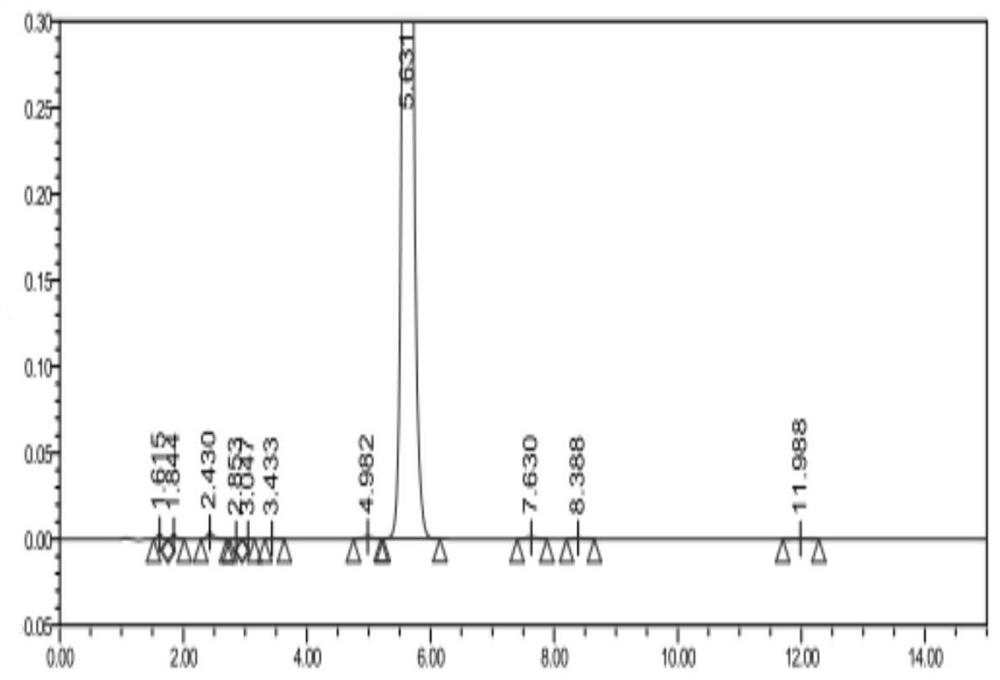
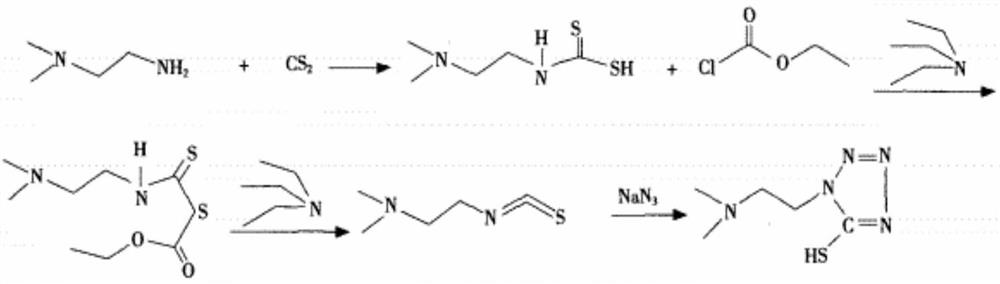
Synthesis of 1-(2-dimethylaminoethyl)-5-mercaptotetrazole
A technology of dimethylaminoethyl and mercaptotetrazole, which is applied in the field of medicine, can solve problems such as non-compliance with environmental protection situation, unfavorable industrial transformation, and large safety hazards, and achieve high industrial application value, increased yield, and improved safety. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] (1) In a 500ml three-necked flask, dissolve 40g of N,N-dimethylethylenediamine and 2g of triethylamine in 250ml of dichloromethane and stir well, then add 62g of thiophosgene dropwise at a controlled temperature of 18-20°C for 2 hours. After the addition was completed, the temperature was raised to reflux after 0.5h of heat preservation to continue the reaction for 3h, and the dichloromethane was spin-dried to obtain 63.3g of isothiocyanate;
[0051] (2) In a 500ml three-neck flask, dissolve 52.3g of azidotrimethylsilane in 300ml of acetonitrile and stir evenly, and add the isothiocyanate obtained in step (1) dropwise at a controlled temperature of 38-40°C for 1 hour. After the completion of the insulation reaction for 2h. Cool down to 20°C and add 30wt.% concentrated hydrochloric acid dropwise to adjust the acid to pH=2.5. After filtering, rinse the filter cake three times with 120ml ethanol to obtain 1-(2-dimethylaminoethyl)-5-mercaptotetrazolium hydrochloride Salt 9...
Embodiment 2
[0057] (1) In a 500ml three-necked flask, dissolve 40g of N,N-dimethylethylenediamine and 2g of DMAP in 250ml of dichloromethane and stir well, add 64.5g of thiophosgene dropwise at a temperature of 20-22°C, dropwise for 2.5h Finished; warming up to reflux after 0.6h of heat preservation to continue reaction for 4h, spin-dried dichloromethane to obtain 66.7g of isothiocyanate;
[0058] (2) In a 500ml three-necked flask, dissolve 54.2g of azidotrimethylsilane in 300ml of acetonitrile and stir evenly, and add the isothiocyanate obtained in step (1) dropwise at a controlled temperature of 35-40°C for 1.2 hours. After the addition was completed, the reaction was incubated for 2.5 hours. Cool down to 21°C and add 30wt.% concentrated hydrochloric acid dropwise to adjust the acidity to pH=2.3. After filtering, rinse the filter cake three times with 150ml methanol to obtain 1-(2-dimethylaminoethyl)-5-mercaptotetrazolium hydrochloric acid Salt 91.8g;
[0059] (3) Add 1-(2-dimethylami...
Embodiment 3
[0061] (1) In a 500ml three-necked flask, dissolve 40g of N,N-dimethylethylenediamine and 2g of pyridine in 250ml of dichloromethane and stir well, then add 63.3g of thiophosgene dropwise at a controlled temperature of 20-22°C for 2.5 hours After the addition was completed, the temperature was raised to reflux to continue the reaction for 4 hours after being kept warm for 1 hour, and the dichloromethane was spin-dried to obtain 63.7 g of isothiocyanate;
[0062] (2) In a 500ml three-necked flask, dissolve 53.7g of azidotrimethylsilane in 300ml of acetonitrile and stir evenly, and add the isothiocyanate obtained in step (1) dropwise at a controlled temperature of 40-42°C for 1.5 hours. After the addition was completed, the reaction was incubated for 3 hours. Cool down to 22°C and add 30wt.% concentrated hydrochloric acid dropwise to adjust the acidity to pH=2.3. After filtering, rinse the filter cake three times with 150ml isopropanol to obtain 1-(2-dimethylaminoethyl)-5-mercap...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com