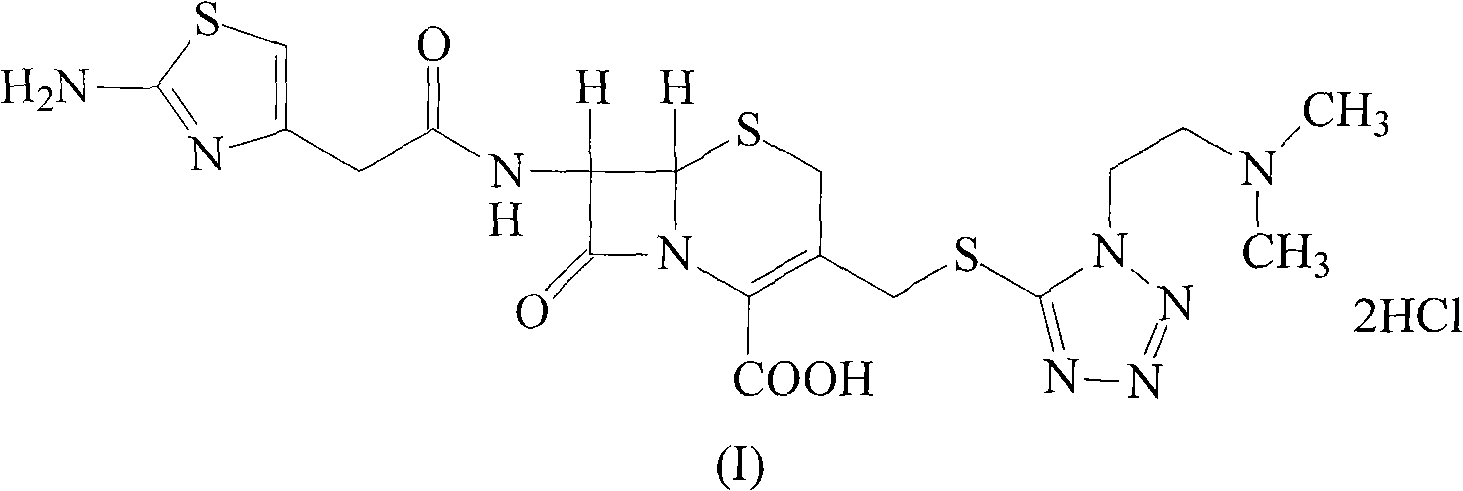
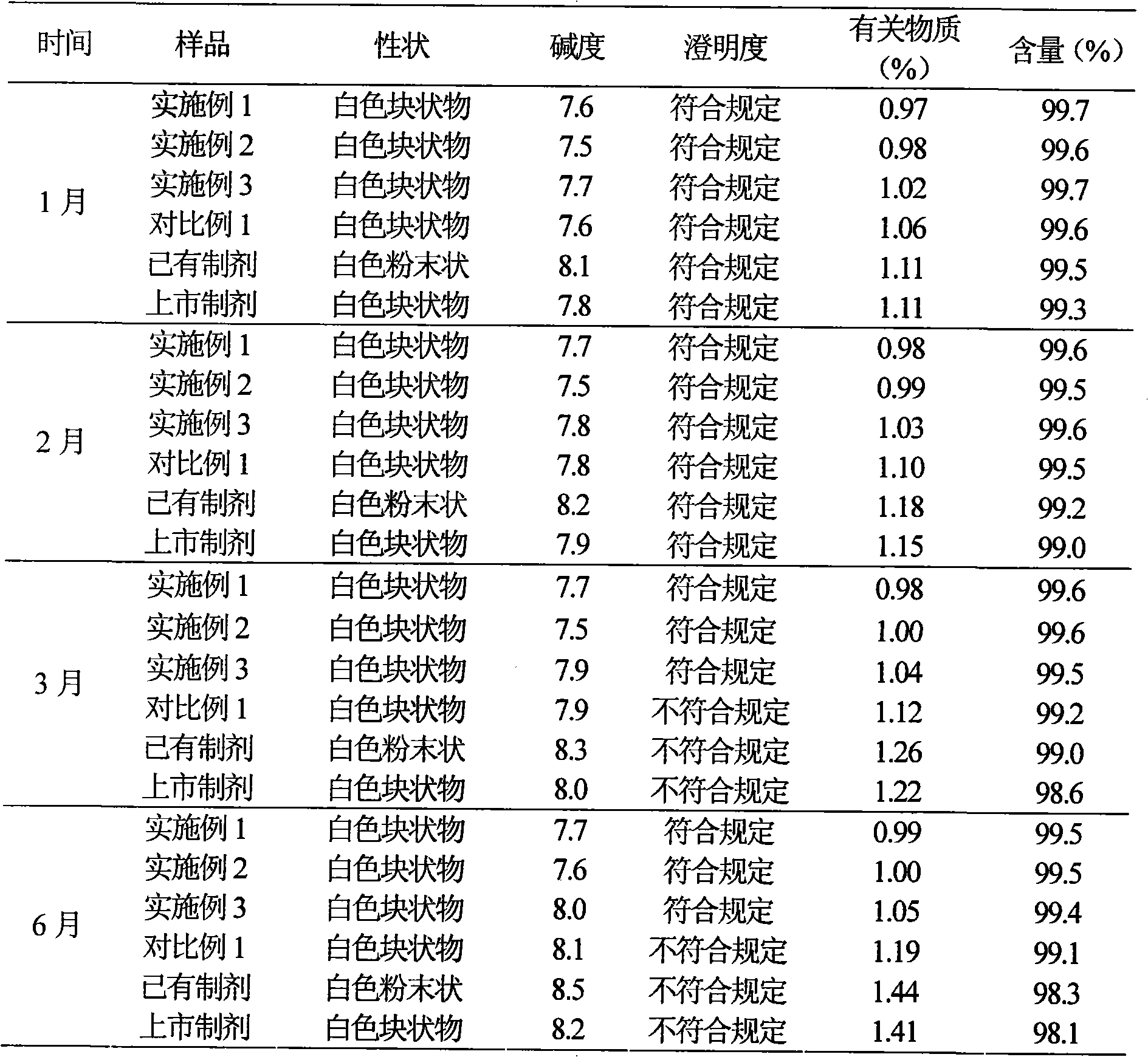
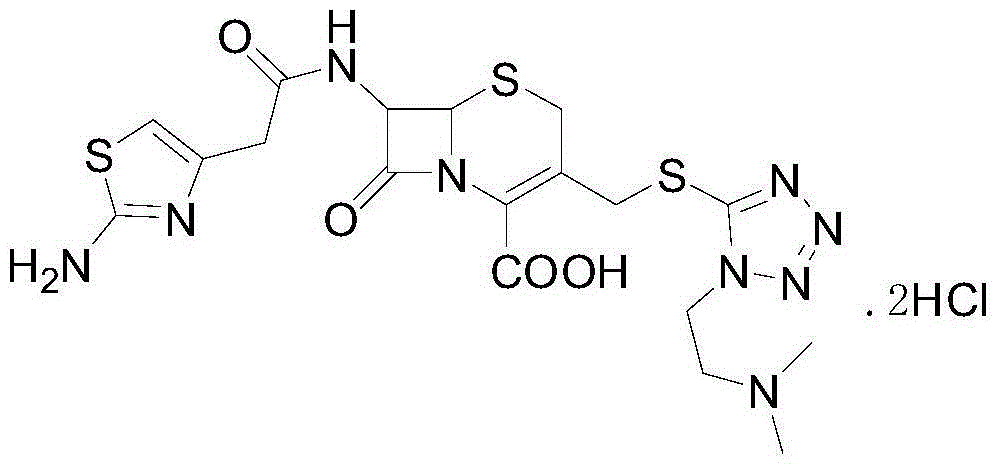
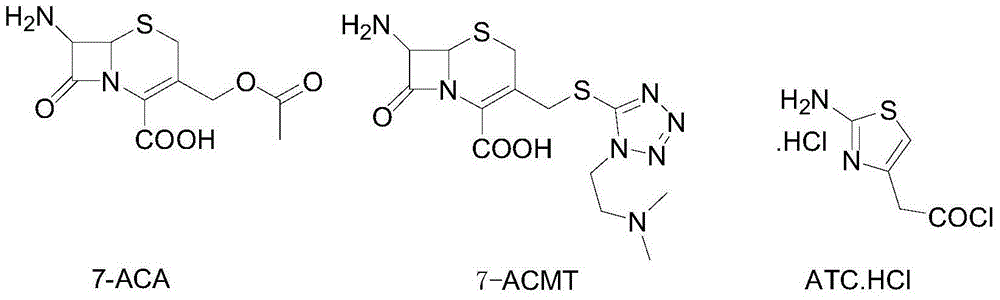
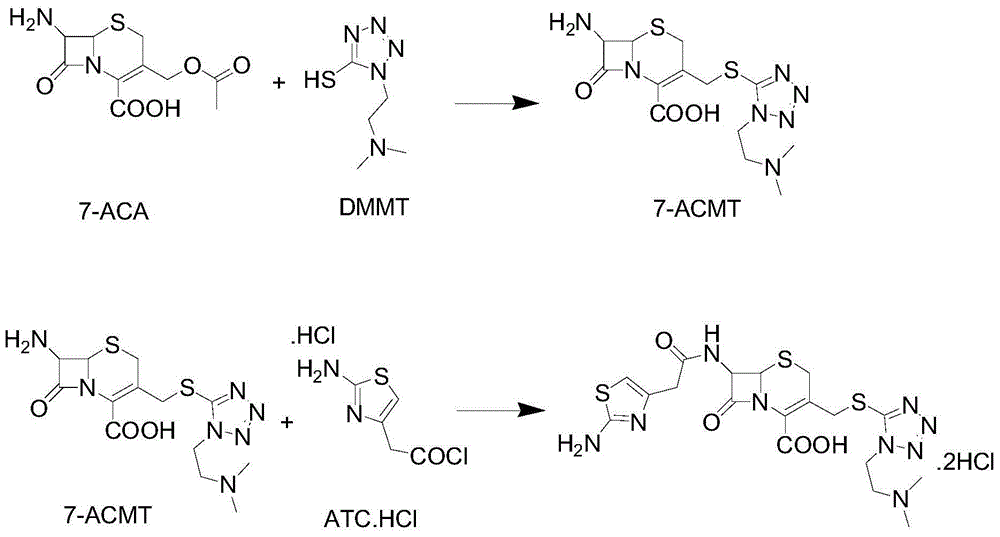
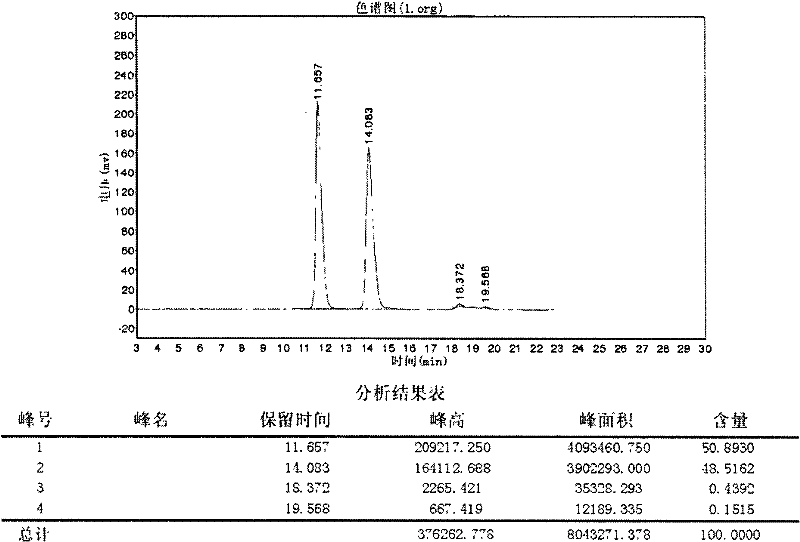
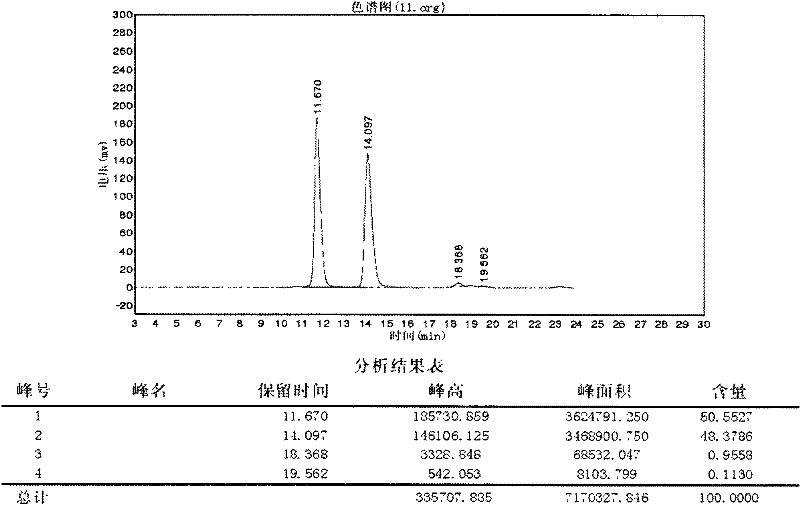
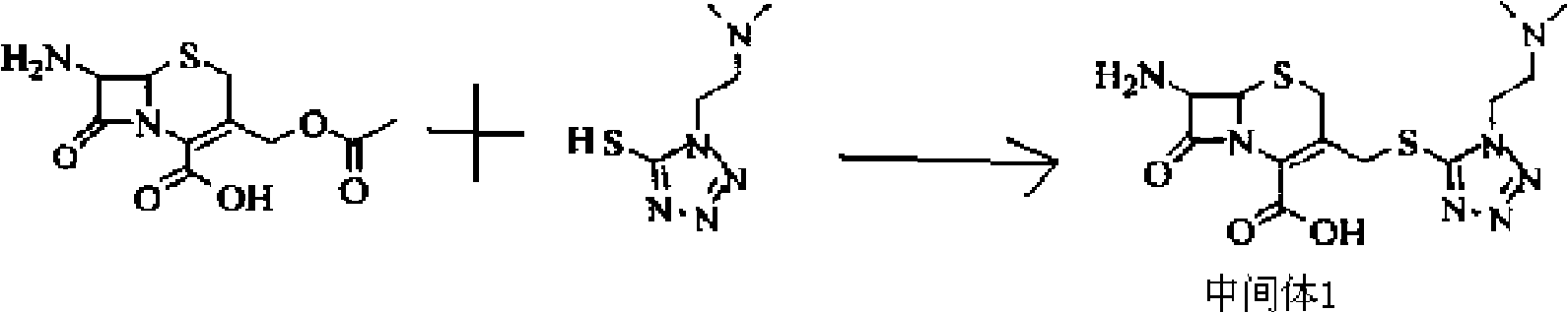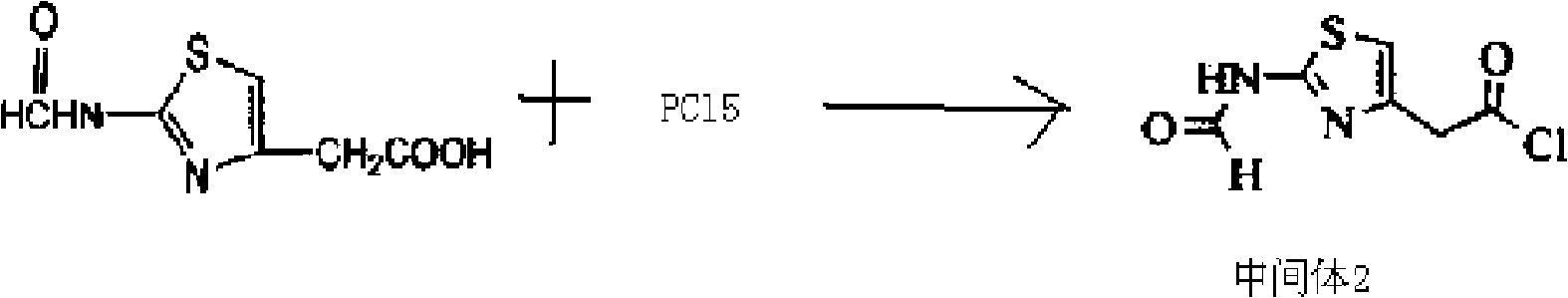Patents
Literature
82 results about "Cefotiam Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
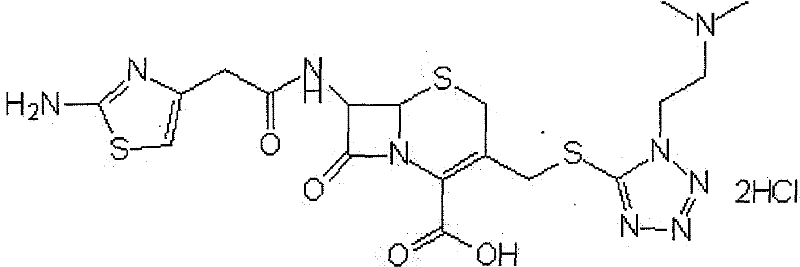
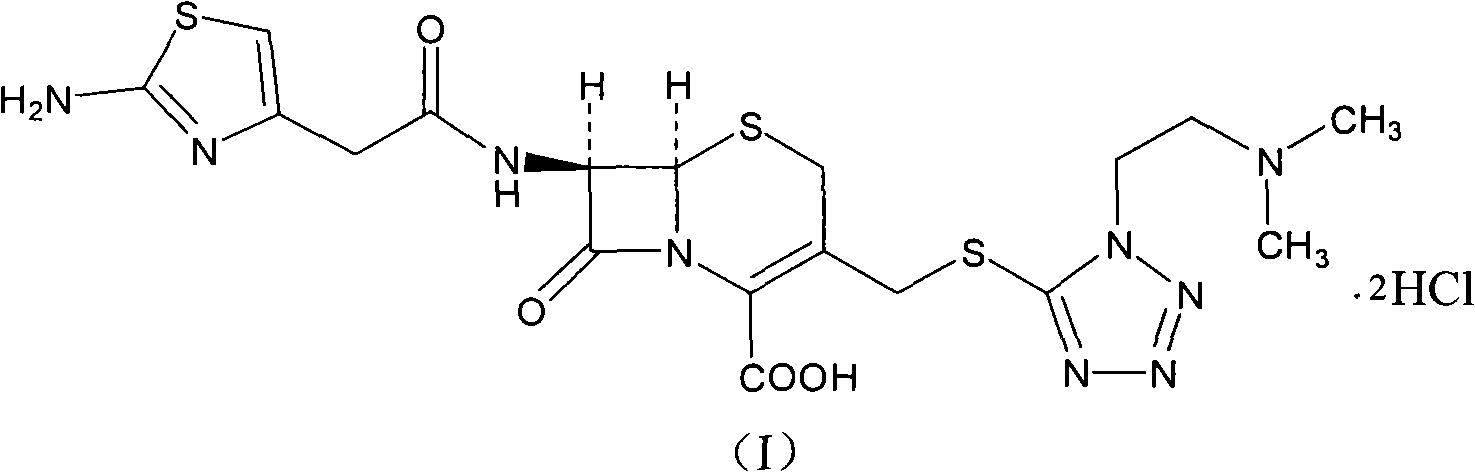
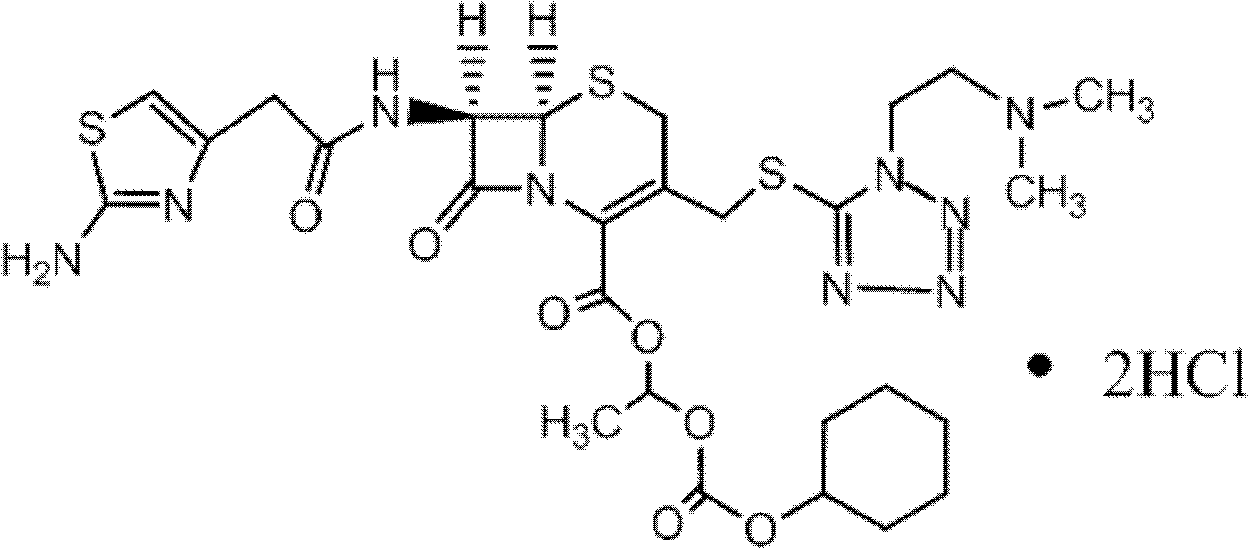
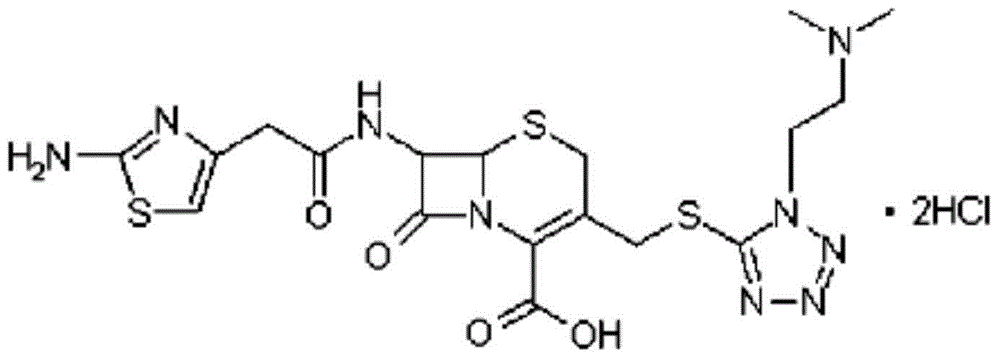
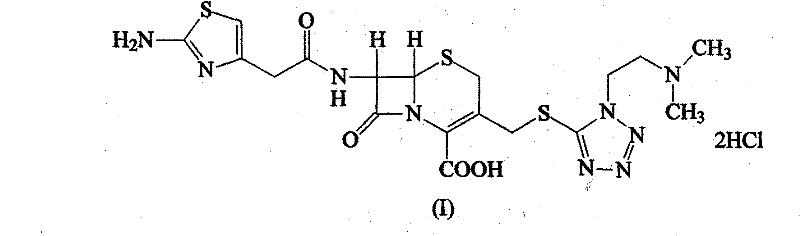
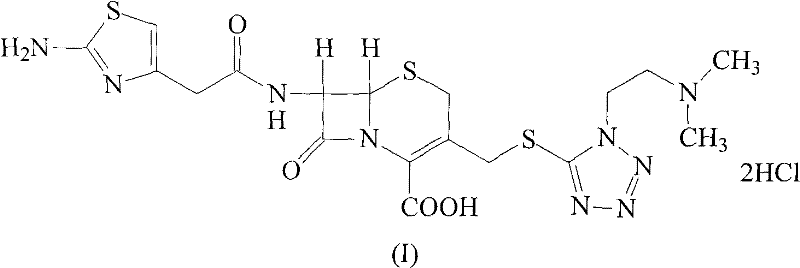
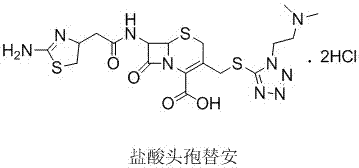
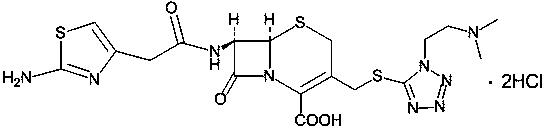
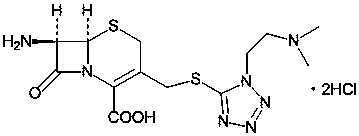
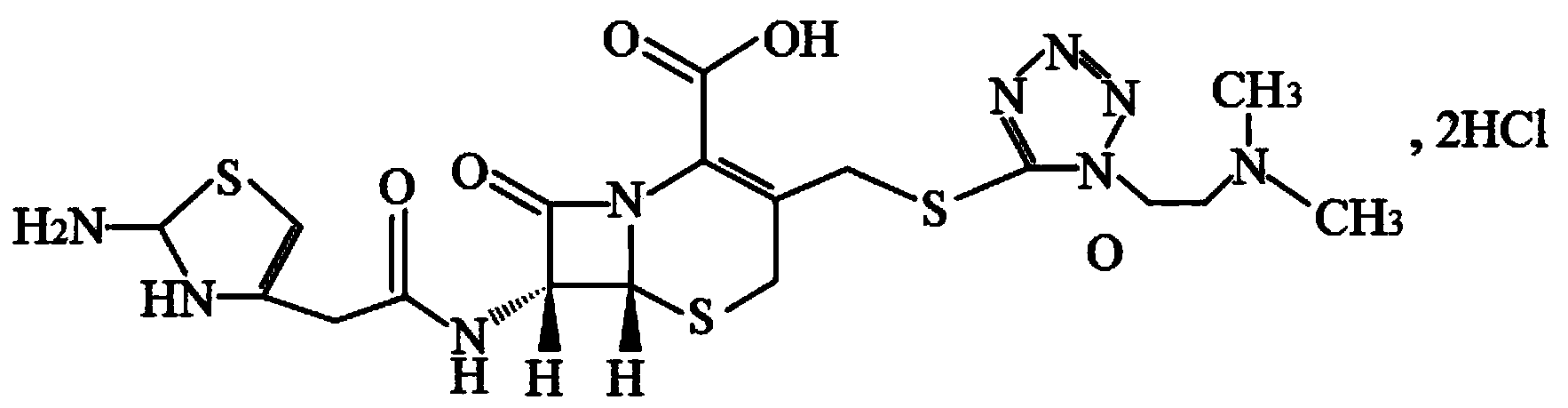
The hydrochloride salt form of cefotiam, a third-generation, semi-synthetic, beta-lactam cephalosporin antibiotic with antibacterial activity. Cefotiam binds to penicillin-binding proteins (PBPs), transpeptidases that are responsible for crosslinking of peptidoglycan. By preventing crosslinking of peptidoglycan, cell wall integrity is lost and cell wall synthesis is halted.
Cefotiam hydrochloride medicament composition sterile powder injection and preparation method thereof
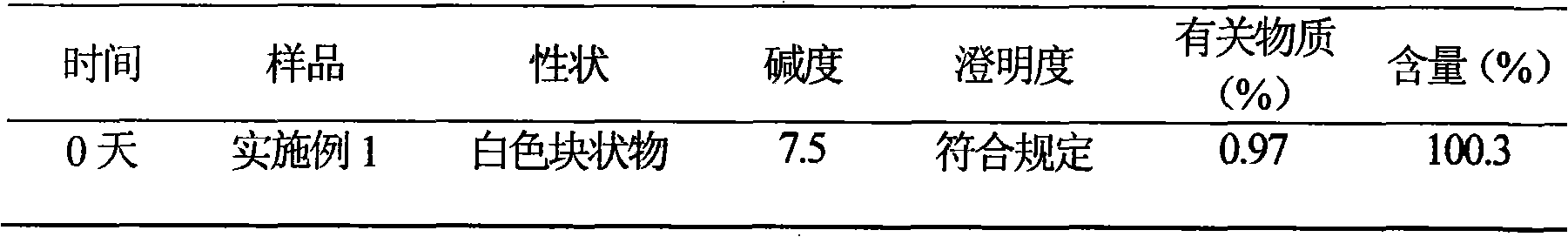
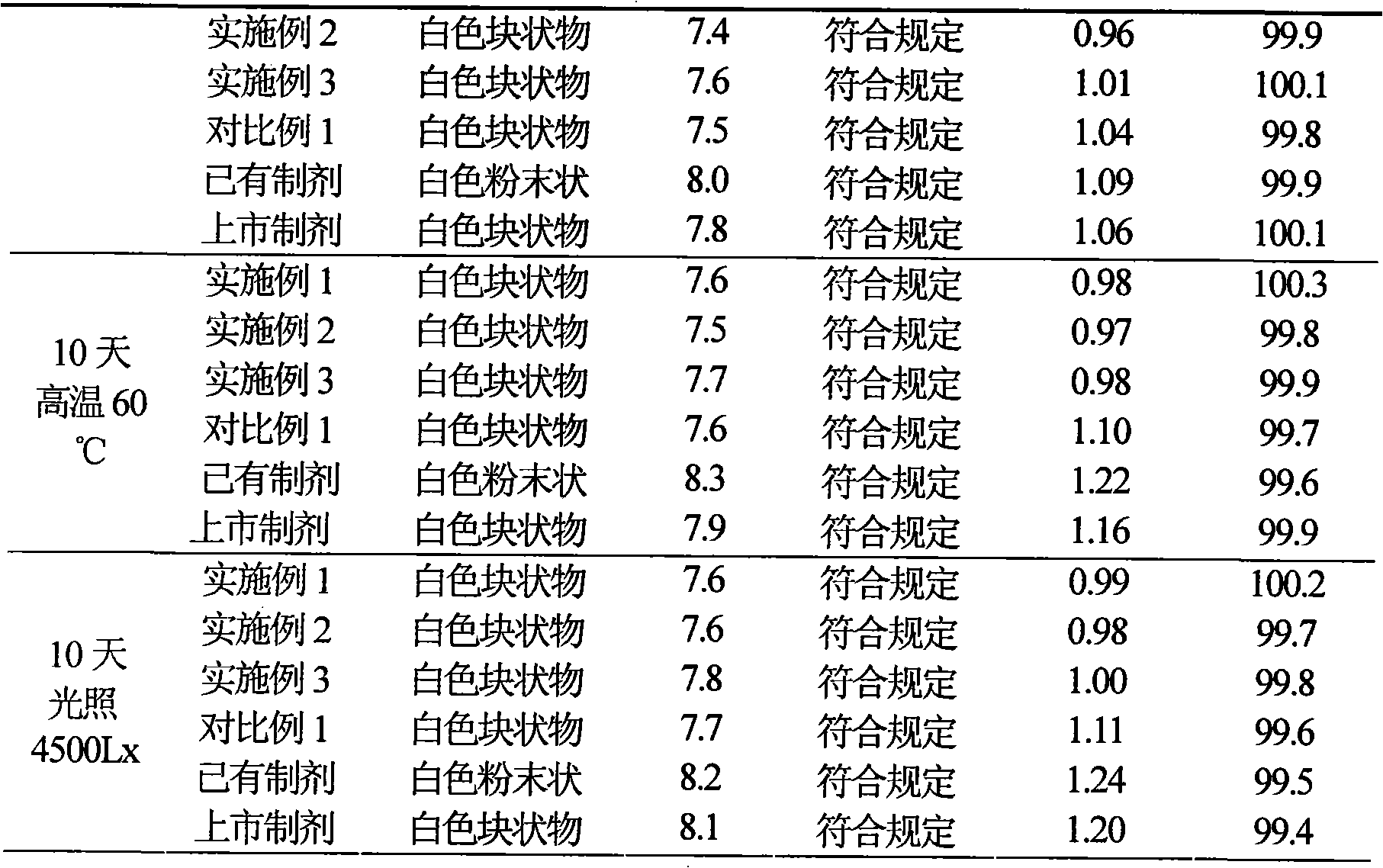
ActiveCN101584665AImprove stabilityHigh purityAntibacterial agentsOrganic active ingredientsCefotiam HydrochlorideSodium carbonate anhydrous
The present invention discloses a cefotiam hydrochloride medicament composition sterile powder injection, including 500 to 600 weight shares of cefotiam hydrochloride and 110 to 150 weight shares of anhydrous sodium carbonate. The cefotiam hydrochloride medicament composition sterile powder injection has advantages of a good stability and a high purity. The invention also discloses a method for preparing the cefotiam hydrochloride medicament composition sterile powder injection, including steps of weighing the cefotiam hydrochloride and the anhydrous sodium carbonate according to the formula amount separately and mixing them in a sterile container uniformly. The method is simple, and the cefotiam hydrochloride prepared by the above method has a good stability.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Cefotiam salt compound and pharmaceutical composition made therefrom
InactiveCN101544662APromote precipitationEasy to operateAntibacterial agentsPowder deliveryCefotiam HydrochloridePurification methods
The invention relates to a high-purity cefotiam hydrochloride compound prepared from crystal cefotiam or a crude product of crystal cefotiam salt. The invention also relates to a product obtained according to a purification method of the invention, in particular to a pure cefotiam hydrochloride and a pharmaceutical composition containing the product.
Owner:HAINAN LINGKANG PHARMA CO LTD
New method for purifying cefotiam hydrochloride
The invention relates to a method for purifying cefotiam hydrochloride, which comprises the following steps of: 1) dissolving a raw material of cefotiam hydrochloride in water, treating by using an acid salt substance, reducing temperature, and filtering off a precipitate separated out so as to obtain water-containing filtrate; 2) adding a solvent immiscible with water into the aqueous solution for extraction, separating to remove an organic phase containing impurities, and obtaining aqueous solution containing the cefotiam hydrochloride; and 3) adding a poor solvent of the cefotiam hydrochloride into the aqueous solution, controlling temperature for recrystallization, centrifugally washing crystals separated out, and drying to obtain the purified cefotiam hydrochloride. The refined cefotiam hydrochloride purified by the method has the cefotiam hydrochloride content of not less than 86 percent and the polymer impurity content of less than 3 percent; and injection prepared from the purified cefotiam hydrochloride has extremely low insoluble particle content.
Owner:HAINAN LINGKANG PHARMA CO LTD
Hydrochloric acid cefotiam crystalline compound, preparation method thereof and medicine combination containing compound
InactiveCN102659818AHigh purityImprove thermal stabilityAntibacterial agentsOrganic active ingredientsCefotiam HydrochloridePowder diffraction
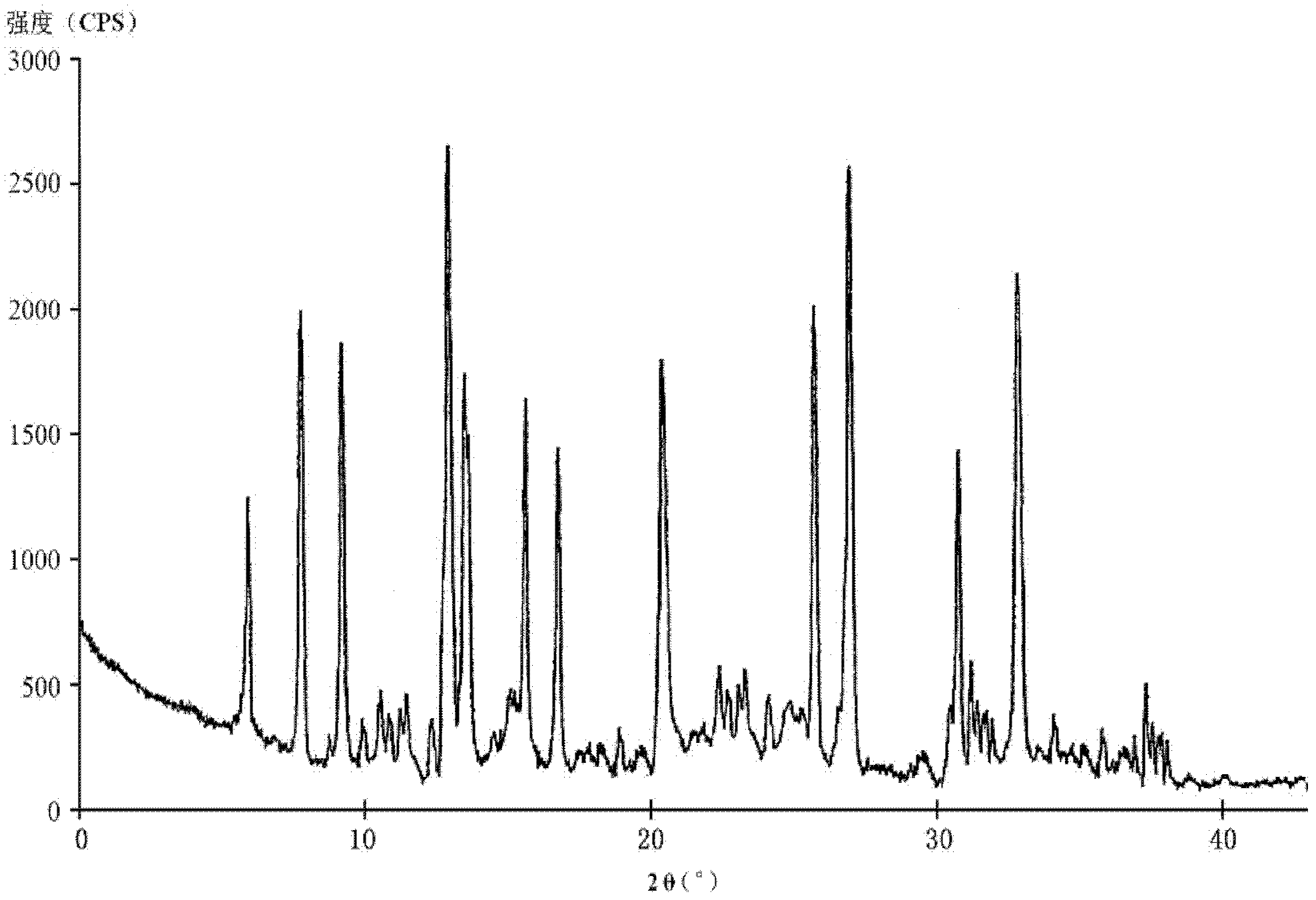
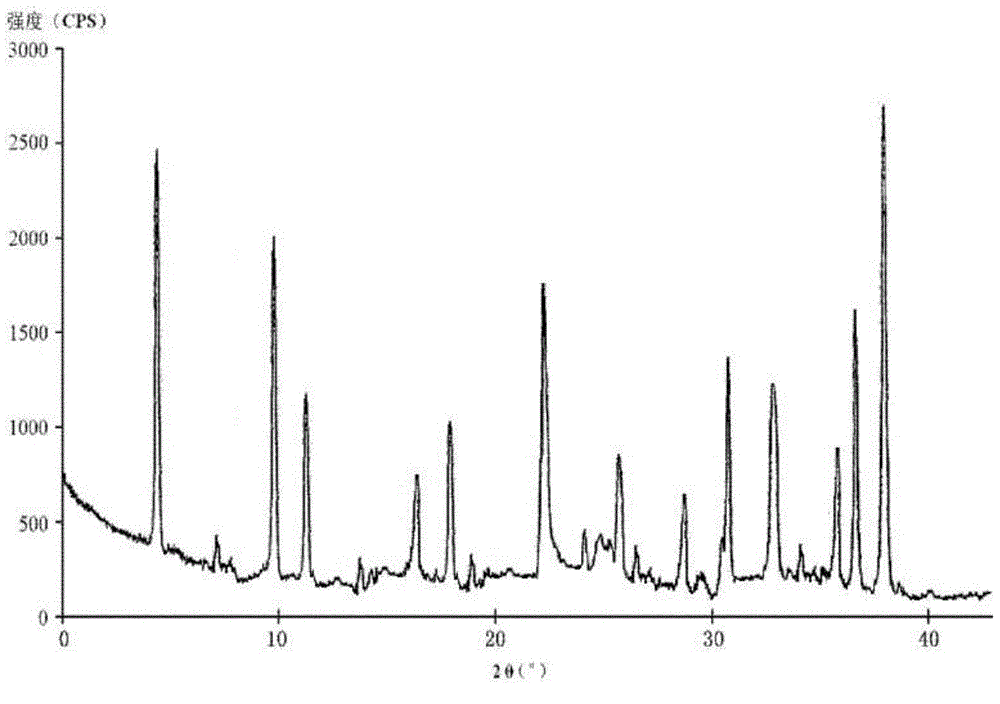
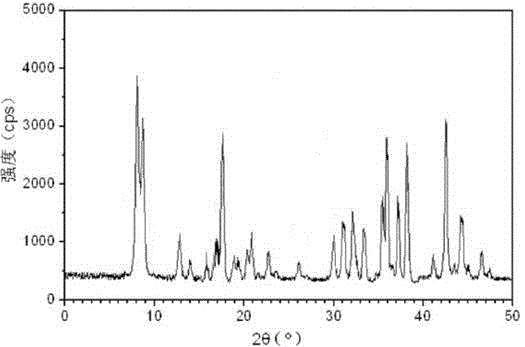
The invention relates to a hydrochloric acid cefotiam crystalline compound and a medicine combination containing the same. The crystalline compound is determined by a powder X-ray diffraction measurement method, and an X-ray powder diffraction pattern represented with a diffraction angle of 2theta+ / -0.2 degree shows characteristic diffraction peak at the positions of 5.9 degrees, 7.9 degrees, 9.3 degrees, 13.0 degrees, 13.6 degrees, 15.7 degrees, 16.8 degrees, 20.4 degrees, 25.7 degrees, 27.0 degrees, 30.9 degrees and 32.9 degrees. The crystalline compound is high in purity, has good heat stability, and can hardly absorb moisture. Simultaneously, the invention further provides a preparation method of the crystalline compound and the medicine combination containing the crystalline compound. The method is simple in process, high in yield, strong in repeatability and suitable for industrial production. The medicine combination containing the crystalline compound is good in stability, thereby improving medication safety and effectiveness and reducing occurrence rate of adverse reactions.
Owner:HAINAN HERUI PHARMA
High-purified cefotiam hydrochloride compound
InactiveCN101787037AHigh purityImprove product qualityOrganic chemistryCefotiam HydrochlorideOrganic chemistry
The invention provides a cefotiam hydrochloride compound, which is highly purified and finally obtained by the way of achieving the refine and purification purposes through a specifically designed method of acid-base conversion and macroporous absorption resin adsorption, thus optimizing the quality of preparation products and the safety of clinical medication.
Owner:HAINAN LINGKANG PHARMA CO LTD
Purification method of cefotiam hydrochloride and aseptic powder injection of cefotiam hydrochloride
ActiveCN102746324AHigh yieldLow organic residueAntibacterial agentsOrganic active ingredientsPurification methodsNitrogen gas
The invention relates to the technical field of medicaments, and particularly relates to a purification method of cefotiam hydrochloride and an aseptic powder injection of cefotiam hydrochloride. The method comprises the steps of: dissolving cefotiam sodium hydrochloride crude product with water, adding activated carbon for injection to remove pyrogen, then adding acetone, recrystallizing, washing obtained crystal with ethyl ether or methyl tert-butyl ether, filtering, carrying out suction filtering water-saturated nitrogen gas, drying under reduced-pressure to obtain cefotiam aseptic powder which is low in organic residue, pyrogen-free and high in purity.
Owner:HAINAN JINXING PHARMA
Novel preparation technology of cefotiam hexetil hydrochloride
InactiveCN103288853ASimple manufacturing methodSuitable for industrialized mass productionOrganic chemistryOrganic solventCefotiam Hydrochloride
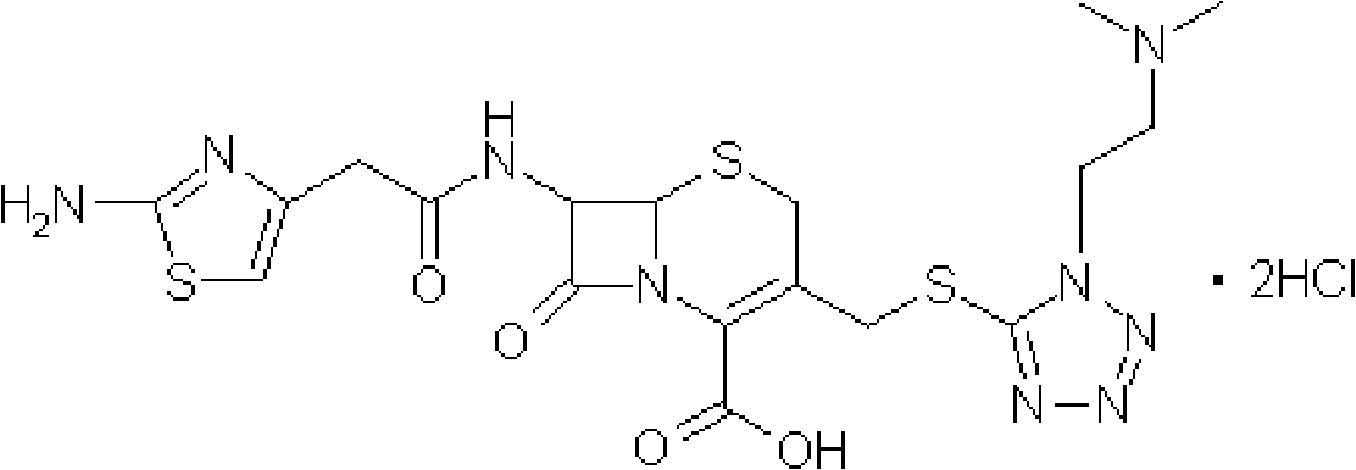
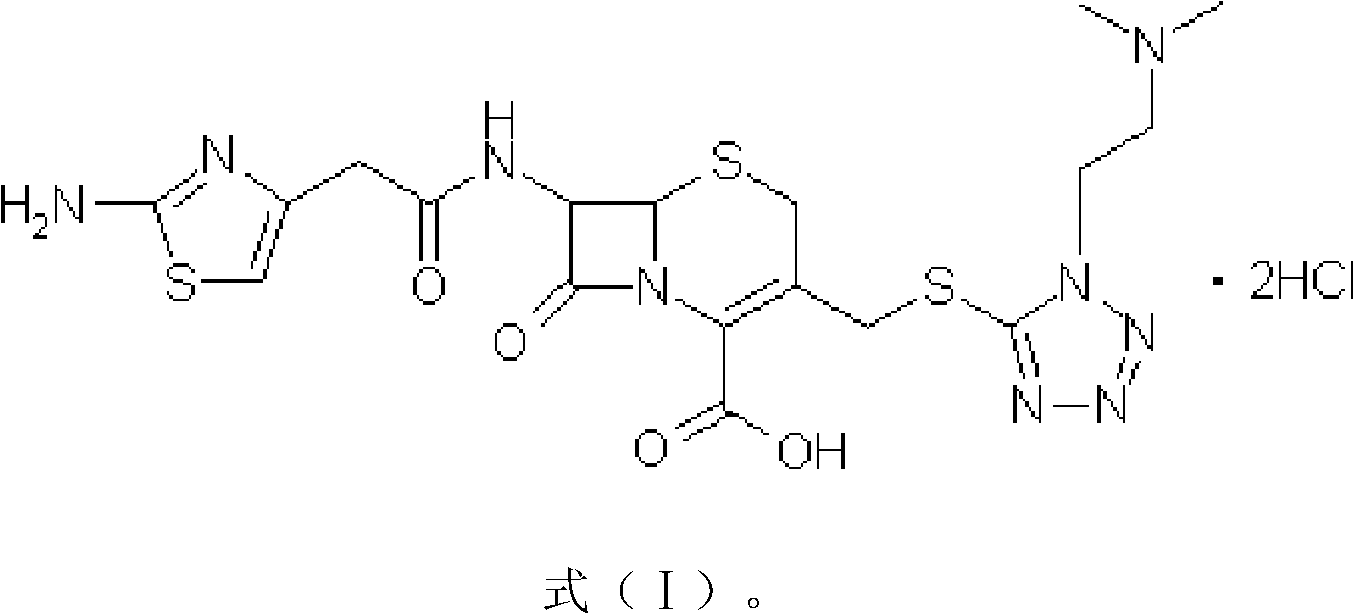
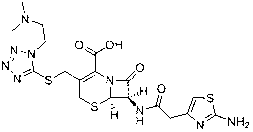
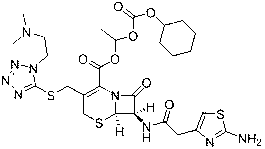
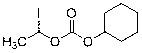
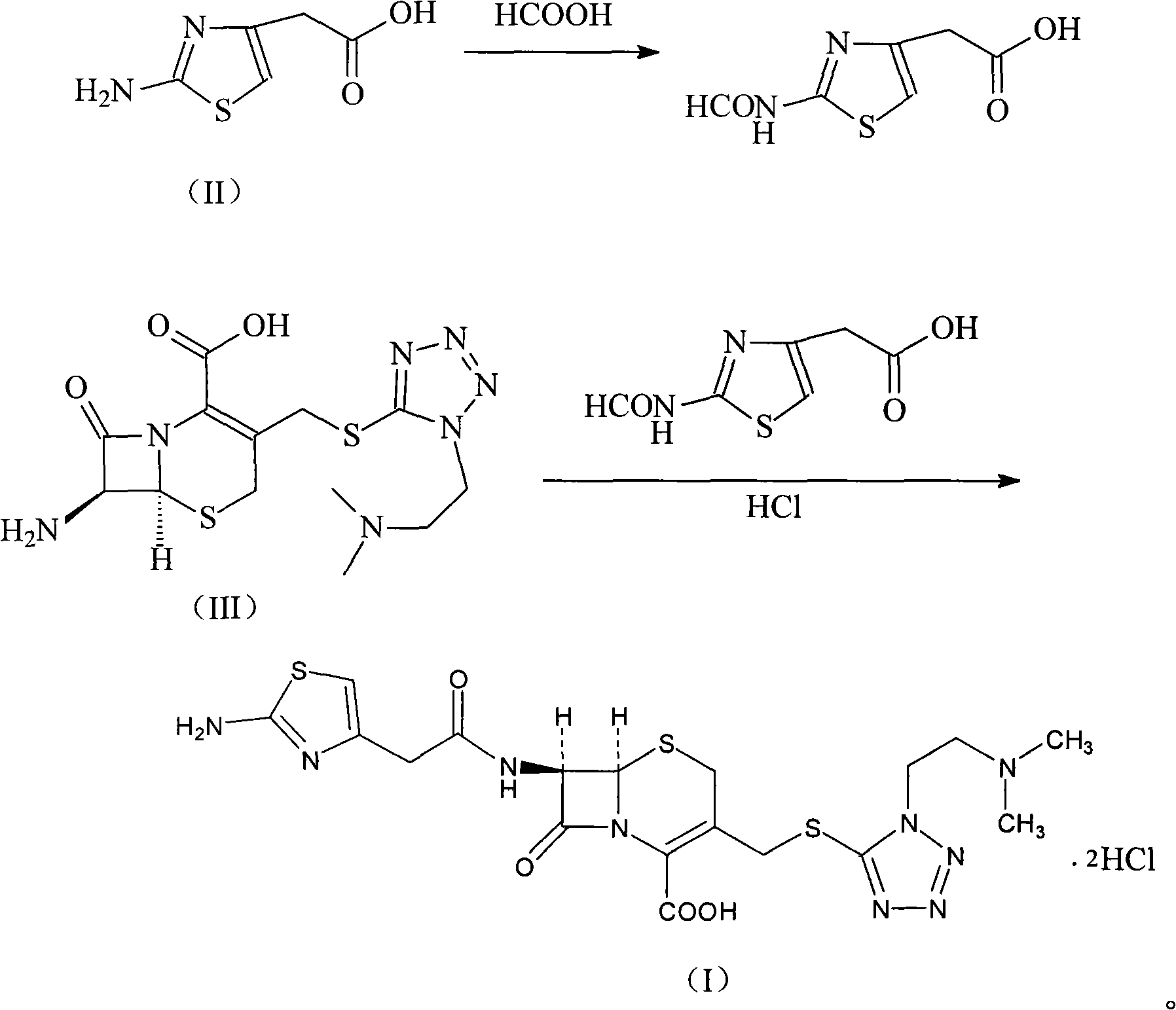
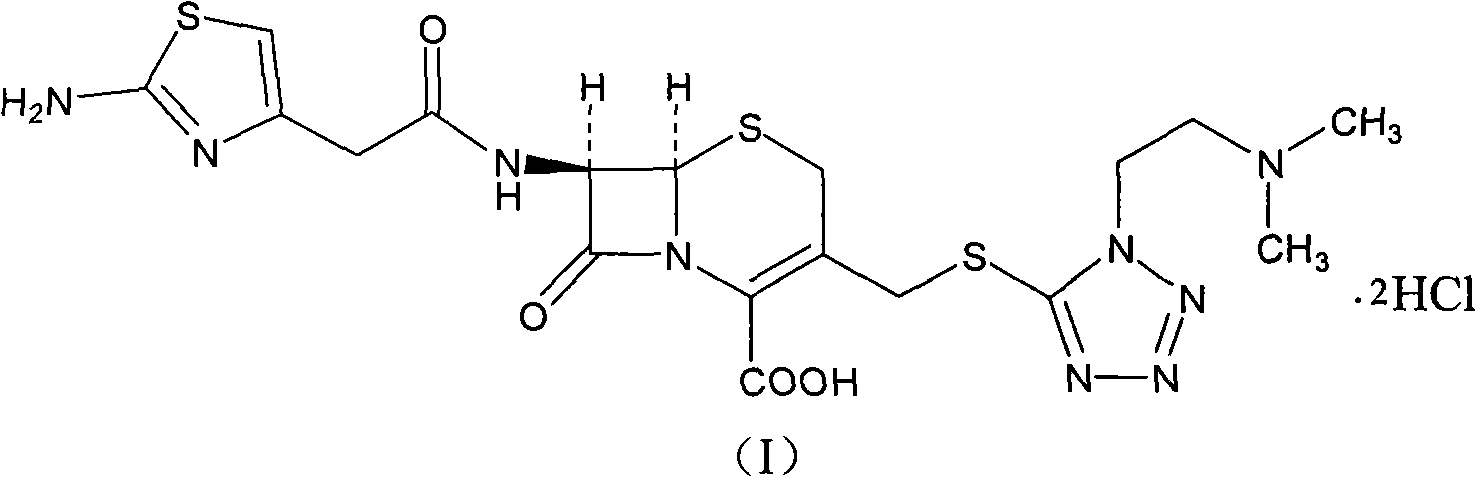
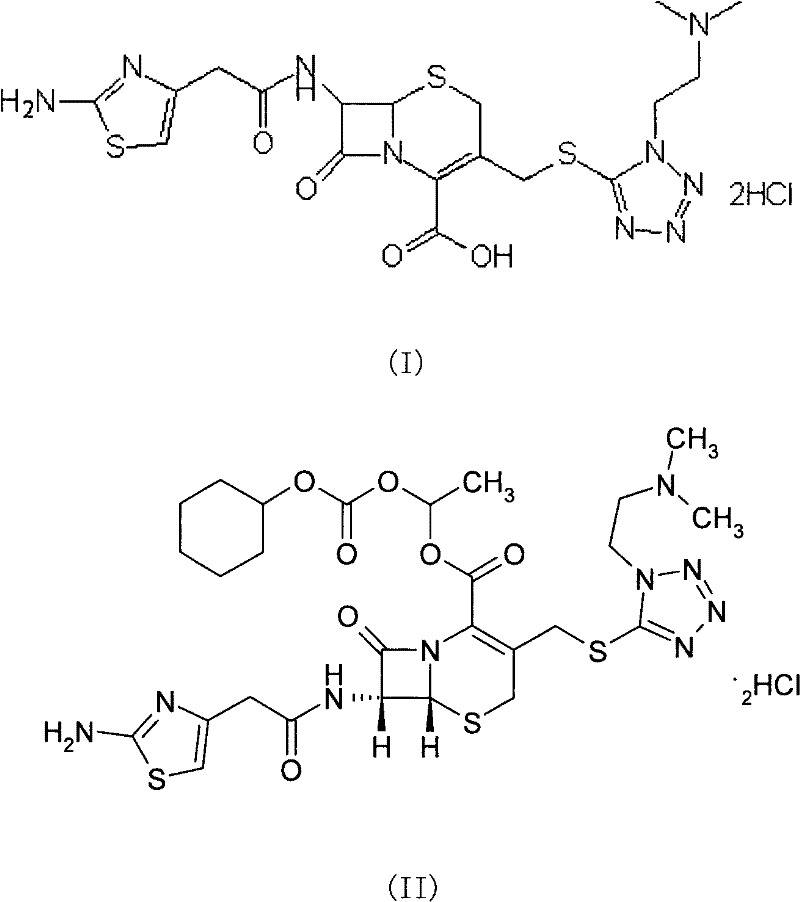
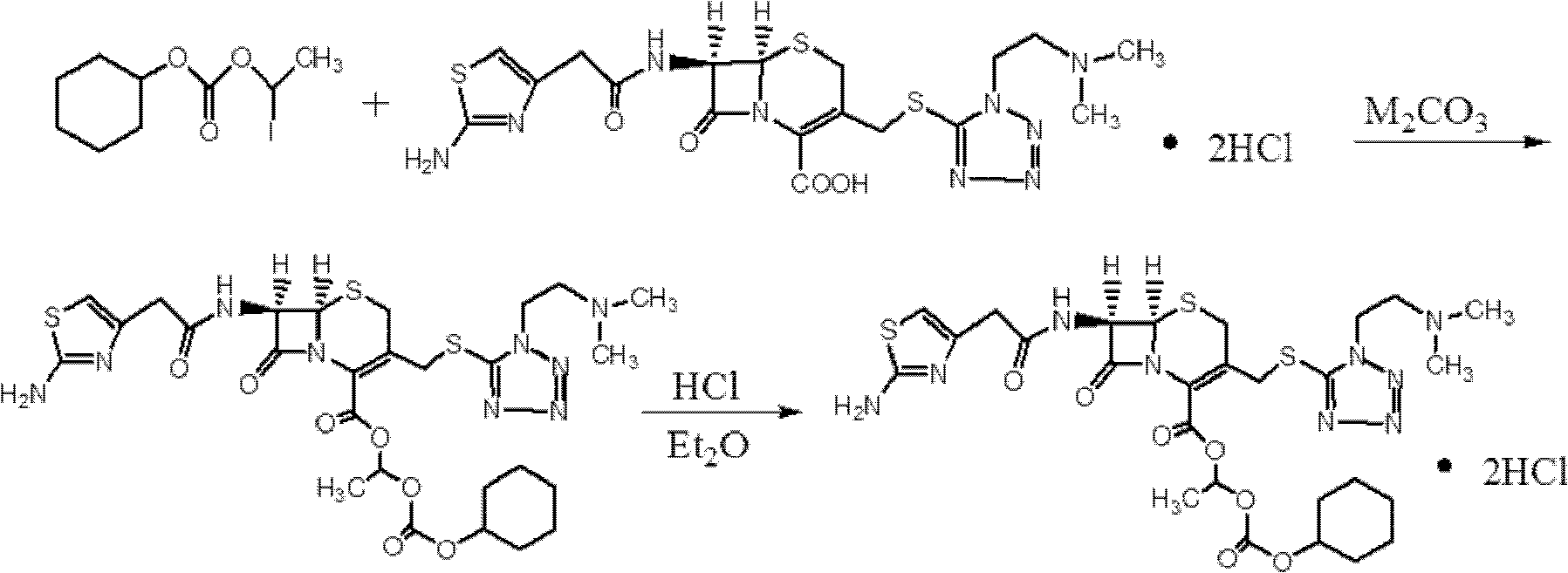
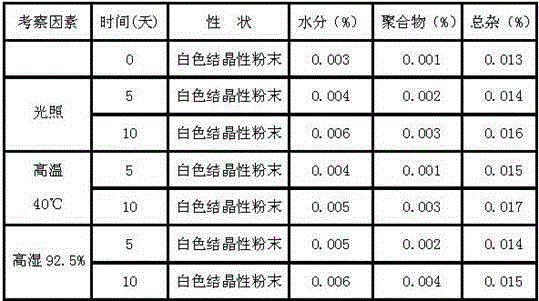
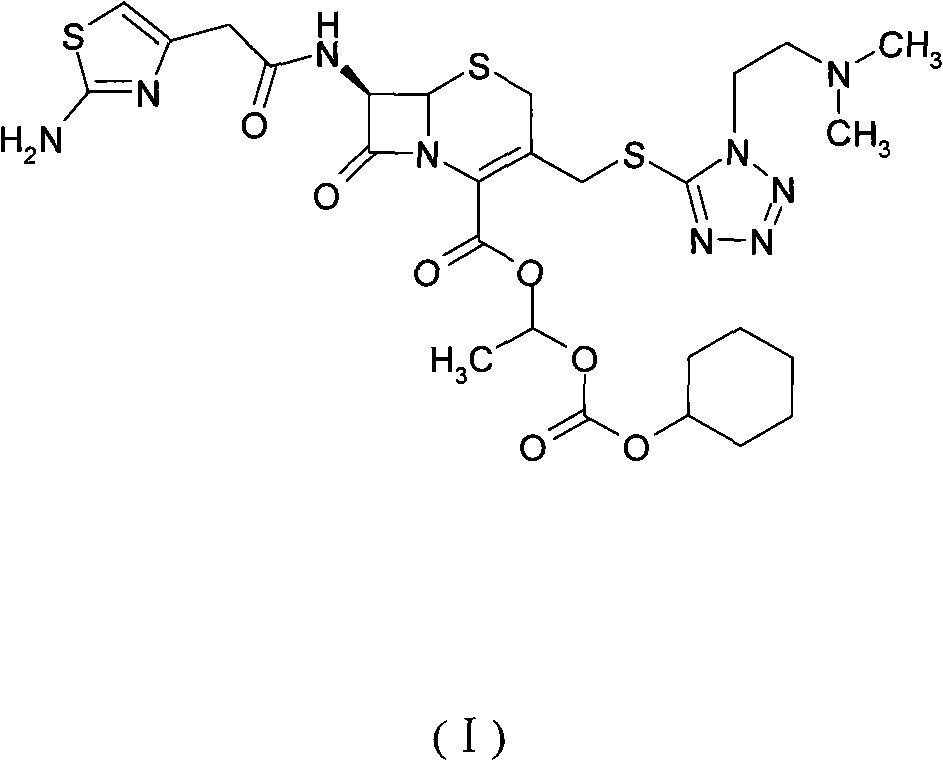
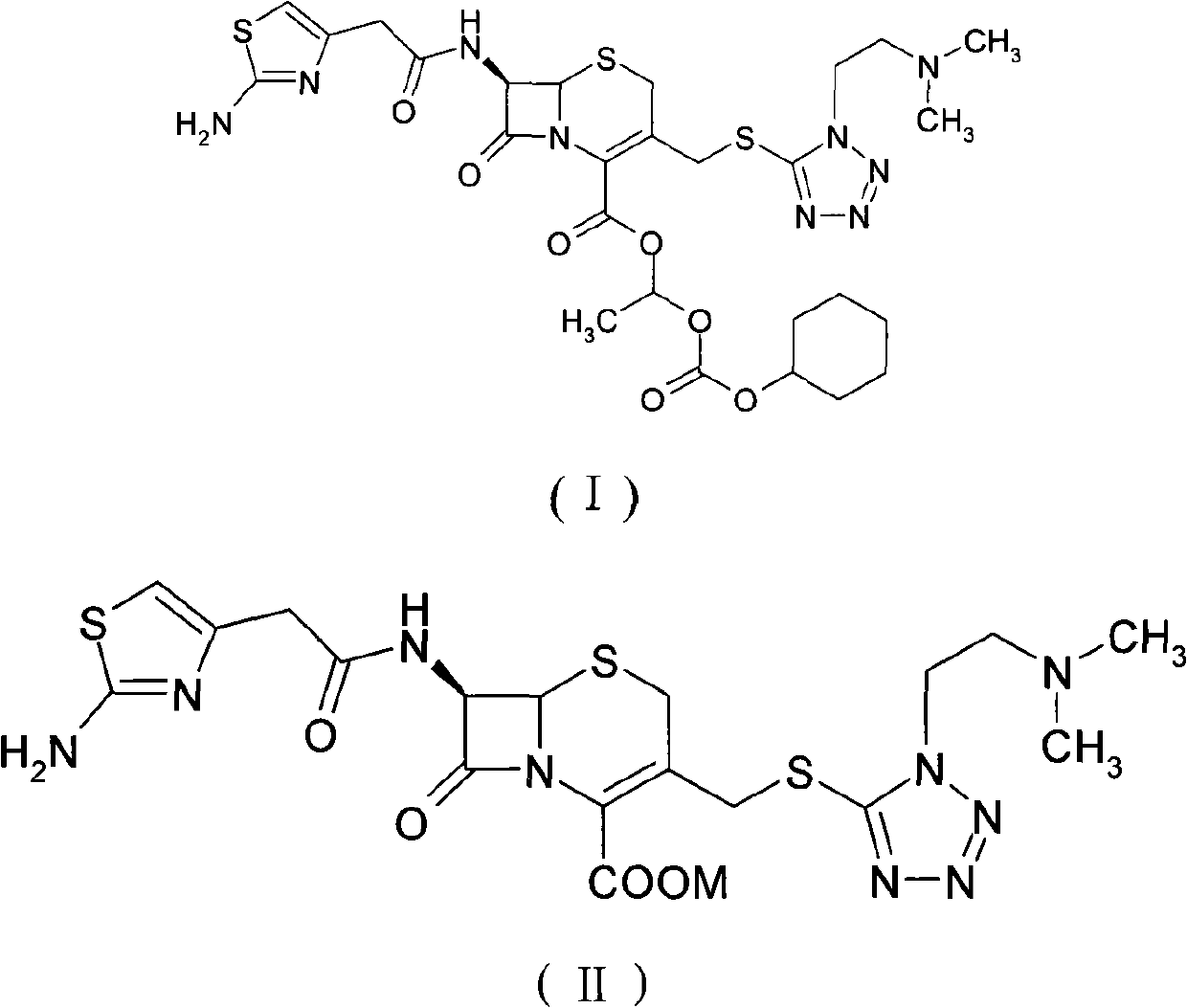
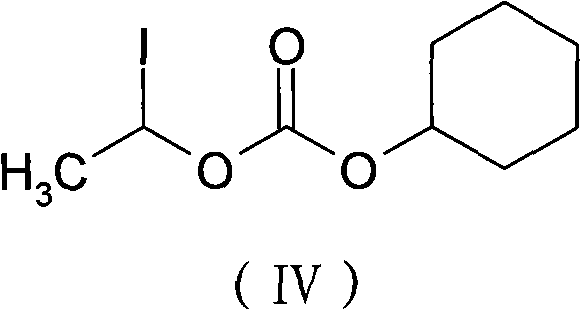
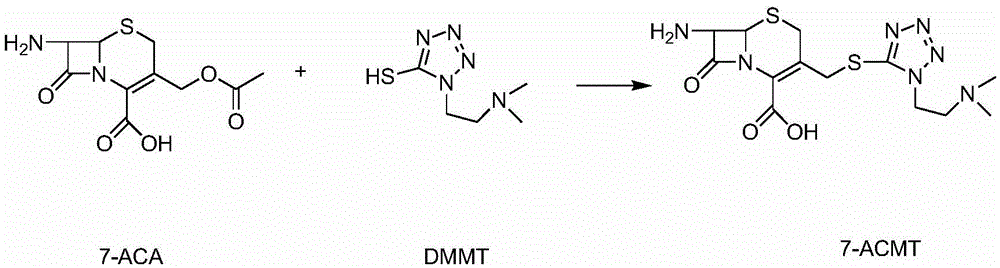
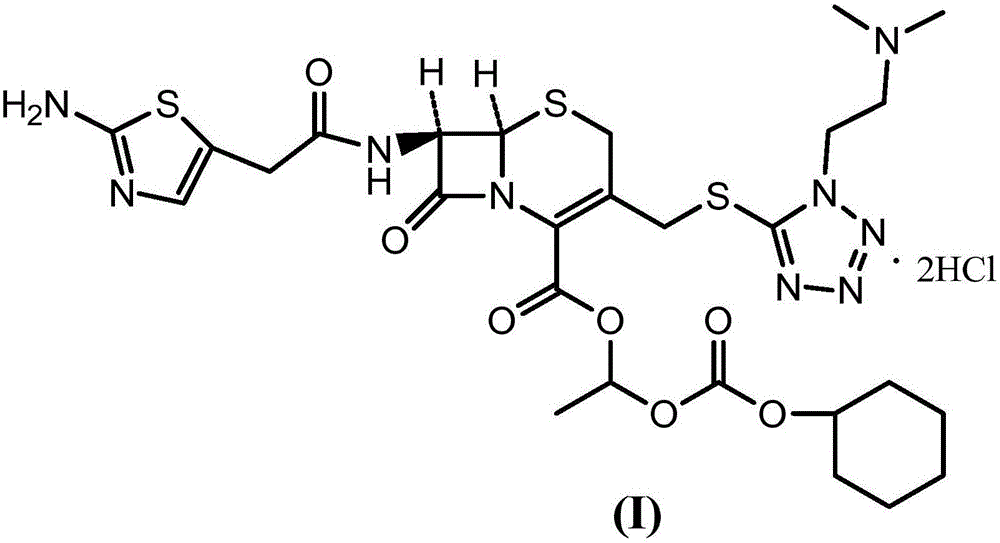
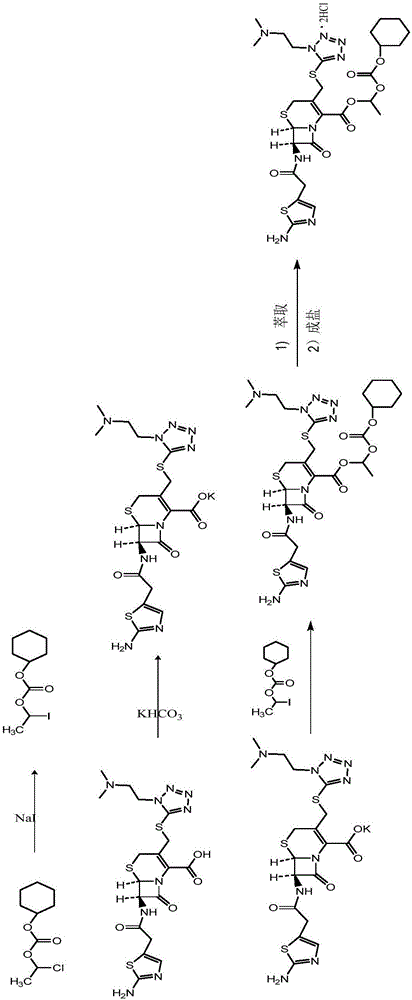
The invention provides a novel preparation technology of cefotiam hexetil hydrochloride represented by formula (I). The cefotiam hexetil hydrochloride is produced by following steps: cefotiam hydrochloride is taken as a raw material; the cefotiam hydrochloride together with an iodo-substitued substance (1-Iodoethyl cyclohexyl carbonate) are subjected to esterification reaction to obtain cefotiam hexetil in the presence of micronized carbonate; an organic solvent is added after completion of the reaction, and then insoluble alkali and salts are removed by filtration; the solution is extracted, subjected to salt forming reaction and refined, and then the cefotiam hexetil hydrochloride with high purity is obtained. The preparation technology of the invention is simple to operate. The cefotiam does not need to be pre-treated to be potassium or sodium salt and be separated. Especially extraction, crystallization and pulping are employed in the post-processing purification of the product, so that the product with high yield and purity can be obtained without purification by chromatographic columns, repeated adjustment by acid and alkali, or freeze drying. The yield of the technology is about 60%, the cost is low, and the technology is suitable for industrialized production with large scale. [0]
Owner:迈洋致达(北京)科技有限公司
Cefotiam hydrochloride compound in new path
InactiveCN101633666AReduce usageEasy to synthesizeAntibacterial agentsOrganic chemistryAcetic acidCefotiam Hydrochloride
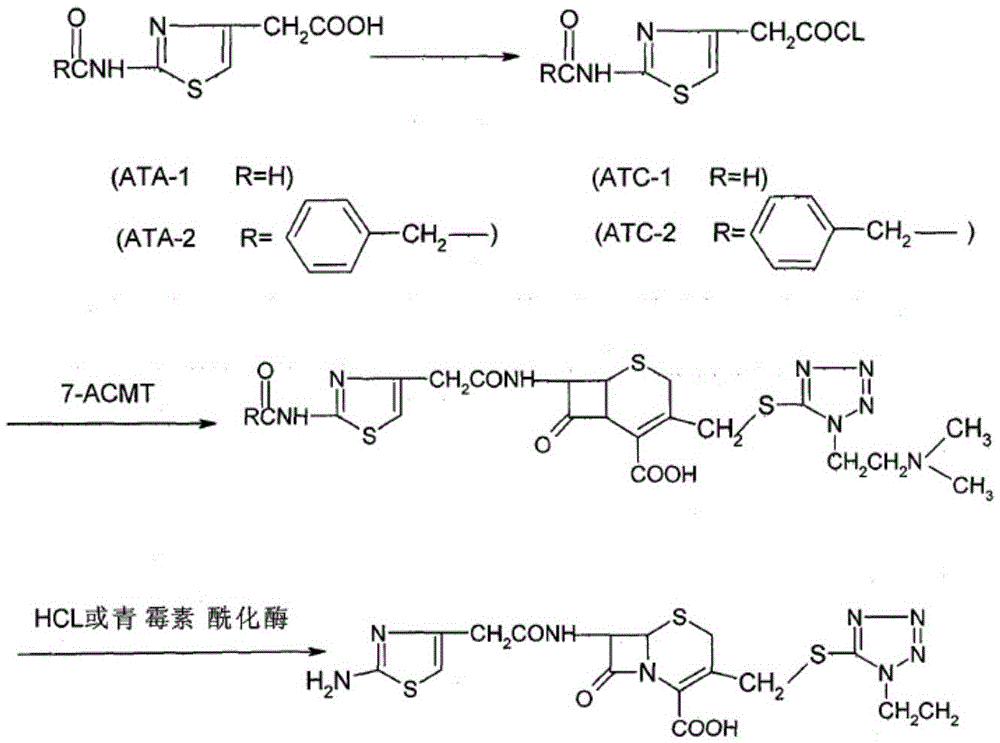
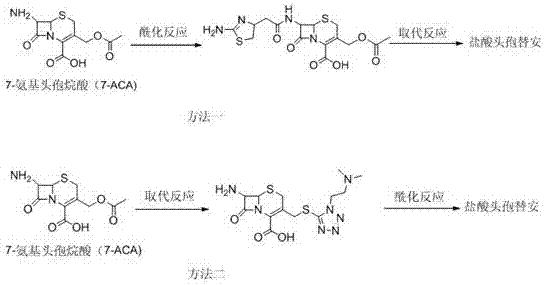
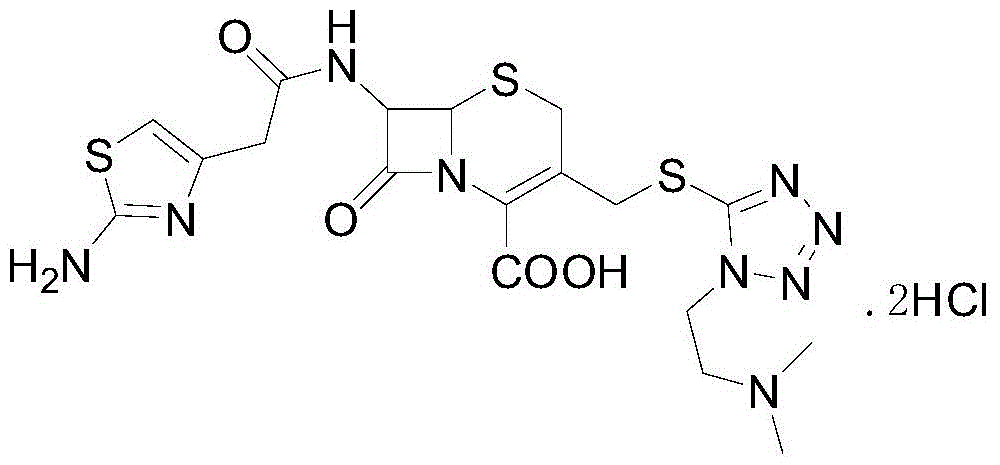
The invention relates to a cefotiam hydrochloride compound in a new path. The preparation method comprises the following steps: allowing 2-aminothiazole-4-acetic acid to react with methanoic acid to generate 2-formylaminothiazol-4-acetic acid, adding 7-ACMT and triethylamine, taking N, N-diisopropylethylamine and dimethylfomamide as solvent, taking p-toluene sulfonylchloride as a catalyst, stirring to react, and then adding hydrochloric acid to obtain the cefotiam hydrochloride.
Owner:HAINAN LINGKANG PHARMA CO LTD
Cefotiam hydrochloride/anhydrous sodium carbonate medicinal composition suspension injection and new use thereof
InactiveCN101912361AHigh parcel rateCommon low priceOrganic active ingredientsInorganic non-active ingredientsCefotiam HydrochlorideSodium carbonate anhydrous
The invention provides cefotiam hydrochloride / anhydrous sodium carbonate medicinal composition suspension injection and new use thereof. The preparation is prepared from 166 to 282.2 weight parts of biodegrading agent, 6.64 to 26.56 weight parts of emulsifying agent, 9.96 to 26.56 weight parts of stabilizer, 13.28 to 49.8 weight parts of additive, 83 weight parts of cefotiam hydrochloride and 17 weight parts of anhydrous sodium carbonate. The invention further discloses new use of the cefotiam hydrochloride suspension injection for treating subacute thyroiditis in clinic.
Owner:HAINAN YONGTIAN PHARMA INST
Method for preparing cefotiam hydrochloride
ActiveCN104356146AImprove efficiencyImprove labor efficiencyOrganic chemistryAfter treatmentCefotiam Hydrochloride
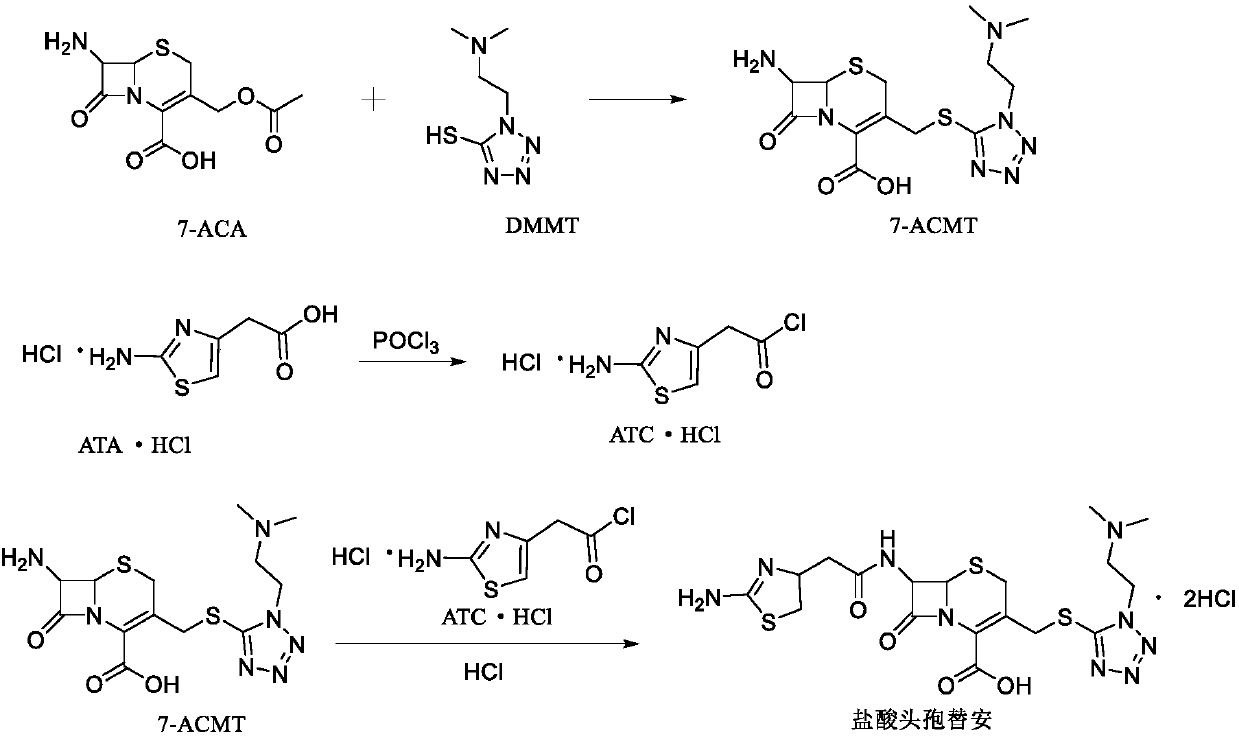
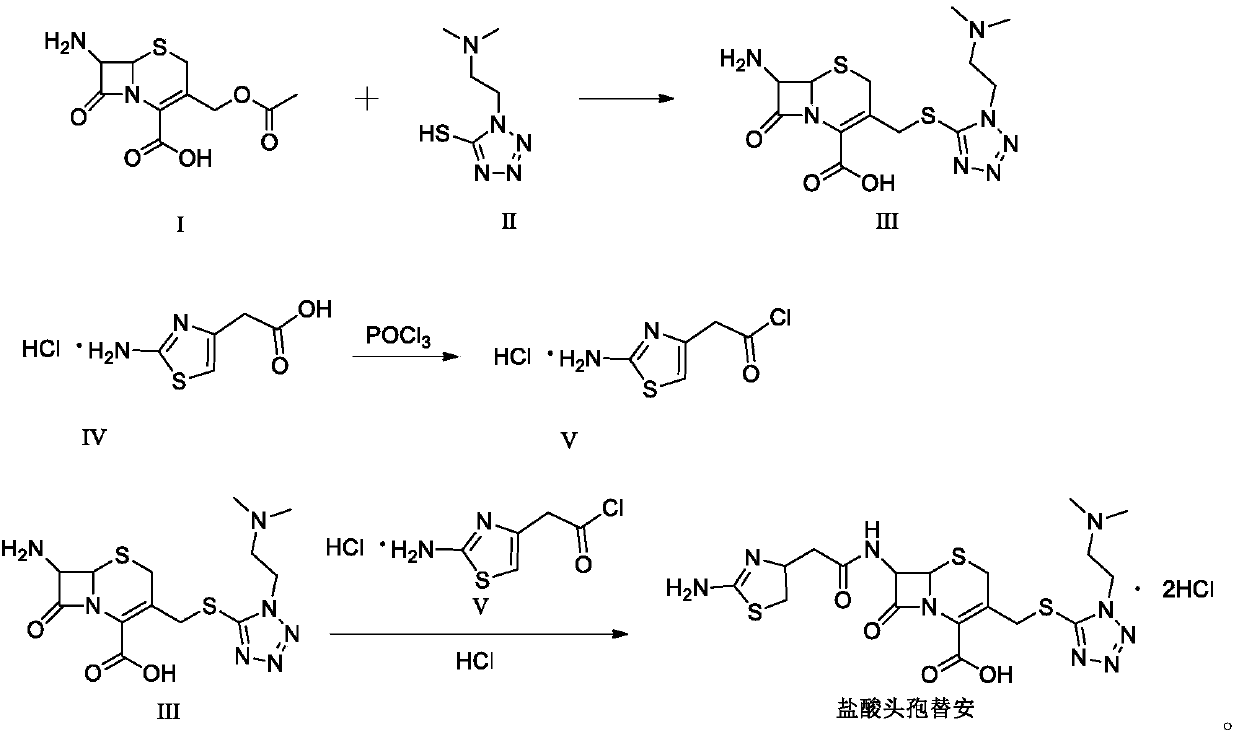
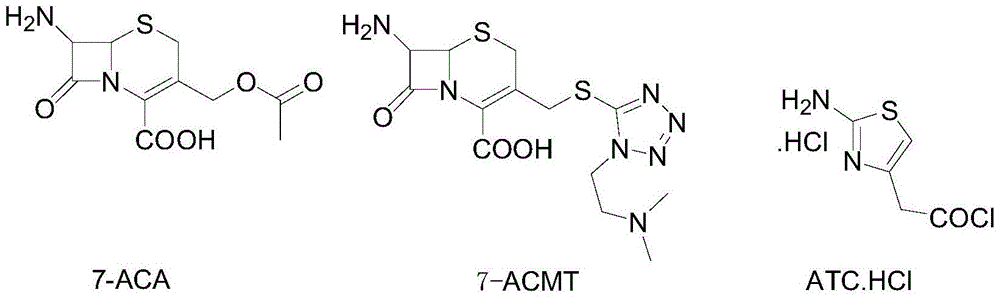
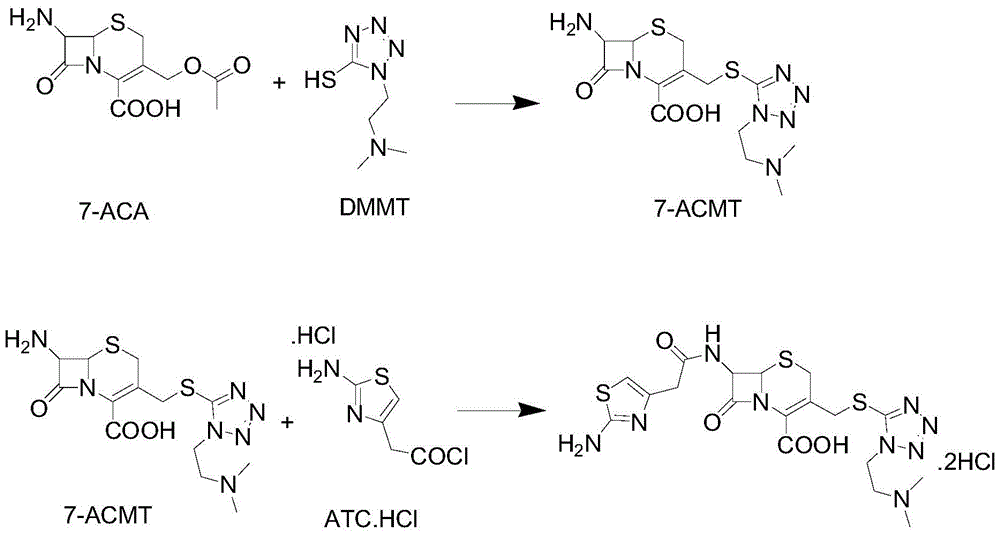
The invention discloses a method for preparing cefotiam hydrochloride. The method comprises the following steps: carrying out a condensation reaction by using 7-ACA and DMMT as raw materials, a boron trifluoride acetonitrile complex as a catalyst and acetonitrile as a reaction solvent to prepare a 7-ACMT reaction liquid; carrying out a 'one-pot' acylation on the reaction liquid with ATC.HCl after treatment of direct water addition and alkali regulation without separating and purifying 7-ACMT; extracting and separating organic impurities from the acylation reaction liquid by acidification and organic solvent, and adding hydrophilic solvent to separate high-purity cefotiam hydrochloride. According to the method, two reactions are carried out in one step, the equipment using efficiency and labor efficiency can be improved, the labor intensity is reduced, the product yield is improved on the premise of guaranteeing the product quality, and the production cost is reduced; all impurity separating operations are carried out after the acylation reaction, product loss caused by intermediate separating operation can be reduced, and the product purity can be guaranteed.
Owner:ZHEJIANG ZHEBANG PHARMA
Method for preparing cefotiam hexetil hydrochloride by cefotiam hydrochloride
The invention discloses a method for preparing cefotiam hexetil hydrochloride by cefotiam hydrochloride, belonging to medicines. The method comprises the steps of leading the cefotiam hydrochloride and potassium bicarbonate or sodium bicarbonate to react to prepare cefotiam, wherein the cefotiam hydrochloride is produced in an industrialized scale manner andthe charging mol ratio of the cefotiam hydrochloride and potassium bicarbonate or sodium bicarbonate is 1:2.5-10; then reacting with carbonic acid 1-iodinated cyclohexyl ethyl ester to obtain cefotiam hexetil; and conducting purification and hydrochloric acid acidization to obtain cefotiam hexetil hydrochloride. According to the method, especially the cefotiam is prepared by reacting cefotiam hydrochloride with 2.5-10 times of mol ratio of potassium bicarbonate or sodium bicarbonate in water, the prepared cefotiam hexetil hydrochloride has high purity, the total quantity of related substances in the prepared product is smaller than 1.5% by adopting a HPLC (high performance liquid chromatograp) method for detection, the yield rate is larger than 90%, and the production cost is low, thus being beneficial to industrialized manufacturing.
Owner:西安万隆制药股份有限公司
Cefotiam-hydrochloride-containing medicine preparation and preparation method thereof
InactiveCN102824310AWon't happenAvoid spray hazardAntibacterial agentsPowder deliveryFluid replacementBottle
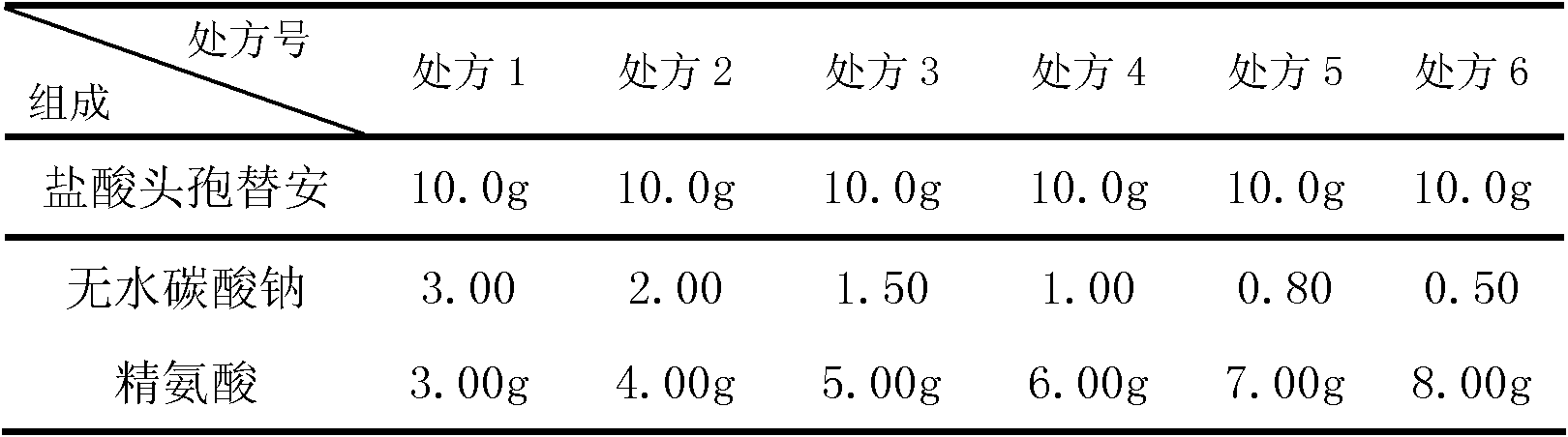
The invention discloses a cefotiam-hydrochloride-containing medicine preparation which is composed of an active component cefotiam hydrochloride, basic amino acid, and anhydrous sodium carbonate. The weight ratio of cefotiam hydrochloride (calculated according to cefotiam) to basic amino acid to anhydrous sodium carbonate is 1:0.4-0.6:0.05-0.15. A preparation method provided by the invention is substantially advantaged in that: (1) vacuum pumping is not needed after product sub-packaging, such that processes are reduced; (2) when the medicine is dissolved, the generation of a large amount of carbon dioxide is prevented, such that the danger for cefotiam medicine liquid to be sprayed from a bottle is avoided; (3) when the cefotiam preparation is diluted into various fluid replacements (including glucose solutions, electrolyte solutions or amino acid preparations), the amount of generated carbon dioxide is extremely low, such that clarity observation of intravenous infusion is not affected; (4) the dosage of anhydrous sodium carbonate is low, such that the possibility of alkalosis is avoided; and (5) the dosage of sodium ions is reduced, such that the preparation is suitable for patients with cardiac or renal insufficiency, edema patients, neonates, pregnant women, and the aged. Therefore, the application scope of the preparation is expended.
Owner:SHANGHAI NEW ASIA PHARMA
Preparation method of cefotiam hydrochloride for injection
ActiveCN104337768AImprove stabilityAntibacterial agentsOrganic active ingredientsCefotiam HydrochlorideReagent
The invention discloses a preparation method of cefotiam hydrochloride for injection. A recrystallization method is adopted, an appropriate reagent is selected for carrying out soaking treatment, and high-purity cefotiam hydrochloride can be prepared. The preparation method of cefotiam hydrochloride for injection has the advantages that technology is simple and technological conditions are strictly controlled, so that a pharmaceutical preparation with high stability and good quality can be obtained.
Owner:SICHUAN PHARMA
Pharmaceutical composition containing cefotiam hydrochloride compound and preparation method thereof
ActiveCN102204915AImprove qualityEfficient removalAntibacterial agentsOrganic active ingredientsDecompositionFreeze-drying
The invention provides a pharmaceutical composition containing a cefotiam hydrochloride compound, which is a powder injection and is composed of, by weight, 3-10 parts of cefotiam hydrochloride, 0.1-0.5 parts of sodium carbonate and 20-150 parts of mannitol. The invention also provides a method for preparing the pharmaceutical composition, comprising steps of: mixing the cefotiam hydrochloride and the mannitol to produce freeze-drying powders, making soda ash into a freeze-drying powder, and mixing the freeze-drying powders, followed by drying. According to the invention, the stability of the pharmaceutical composition powder injection is raised, the storage time of the pharmaceutical composition is prolonged, and simultaneously medication waste and medication hidden troubles caused by the medicine instability are avoided. The preparation method provided by the invention, wherein cefotiam hydrochloride and sodium carbonate are respectively prepared into freeze-drying powders and then the freeze-drying powders are mixed, avoids the influence of CO2 released from the dissolved sodium carbonate on the decomposition of the cefotiam hydrochloride, reduces the content of impurities in the freeze-drying powder injection, makes the best use of active ingredients, and is suitable for large scale production.
Owner:福建康成医药有限公司
Preparation method of cefotiam hexetil hydrochloride
InactiveCN102424687AReduce usageSimple purification methodOrganic chemistryCefotiam HydrochloridePurification methods
The invention provides a preparation method of cefotiam hexetil hydrochloride. The method comprises: subjecting cefotiam hydrochloride and 1-iodoethyl cyclohexyl carbonate to an esterification reaction so as to obtain a cefotiam hexetil crude product, which is then purified into hydrochloride, thus obtaining cefotiam hexetil hydrochloride. In the esterification reaction, potassium carbonate is added, and cefotiam hydrochloride, potassium carbonate and 1-iodoethyl cyclohexyl carbonate are in a reaction mole ratio of 1:2.2-2.5:2.5-3. The method provided in the invention can reach a yield of 66% and make the isomer ratio less than 2%, or even to 1.3%. Employment of the preparation method in the invention can avoid usage of a chromatographic column and simplify the purification method, so that the method can be more suitable for industrial production needs.
Owner:HUNAN FANGSHENG PHARMACEUTICAL CO LTD
Monohydrater cefotiam hydrochloride compound and pharmaceutical composition thereof
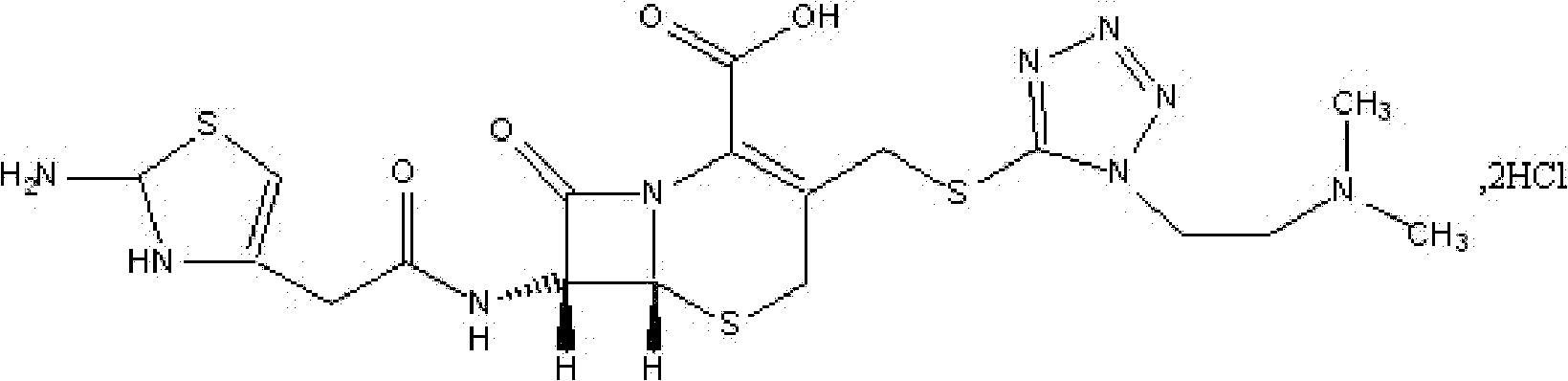
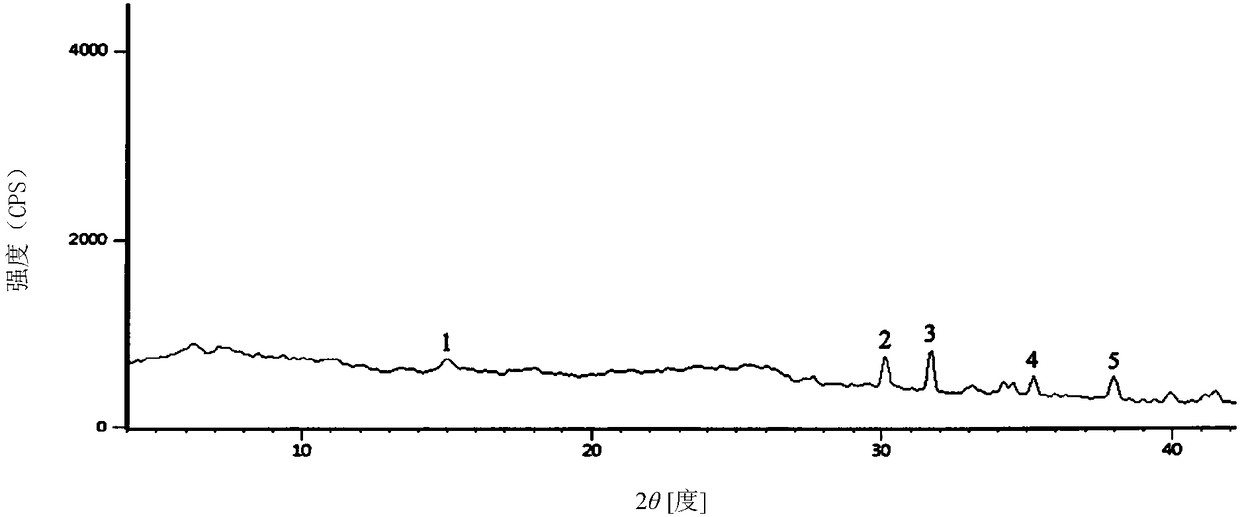
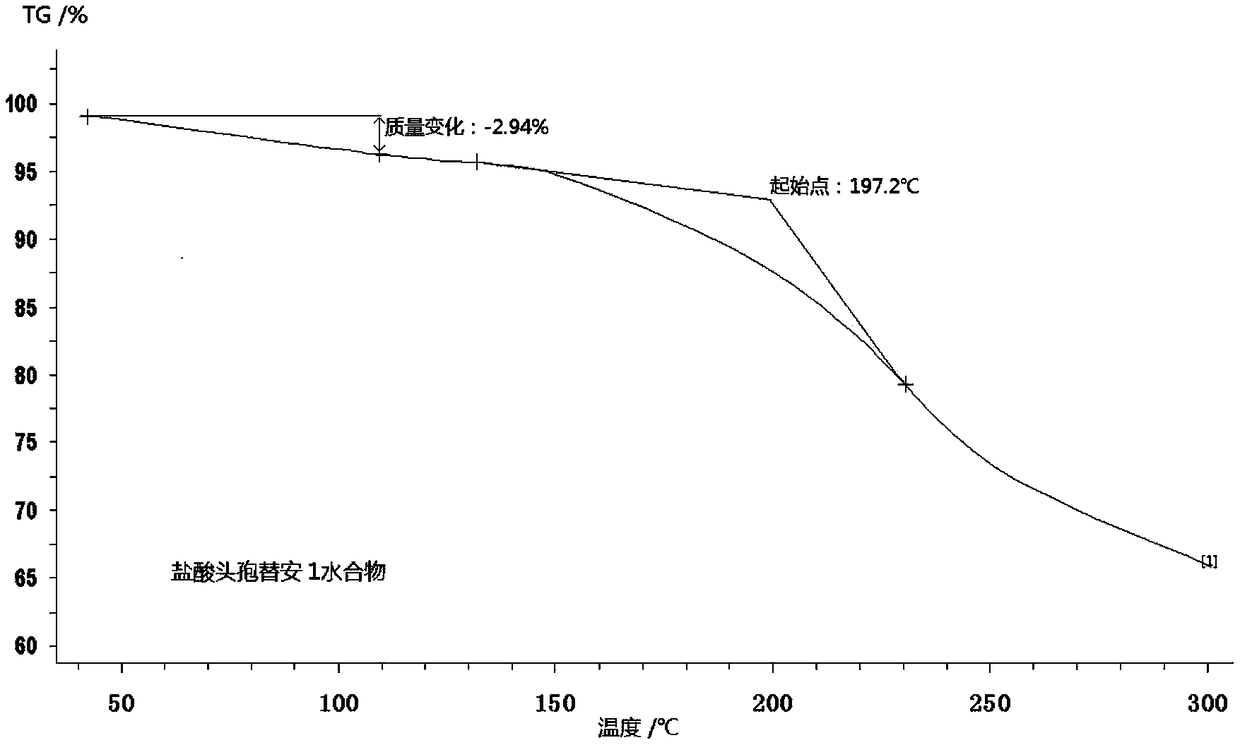
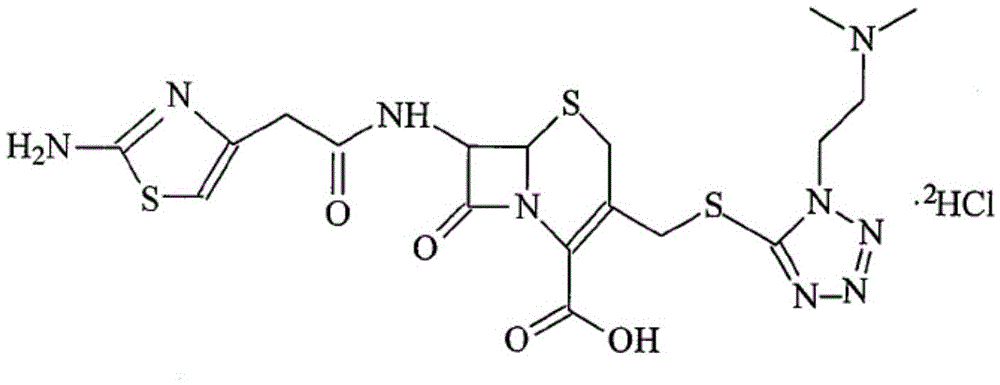
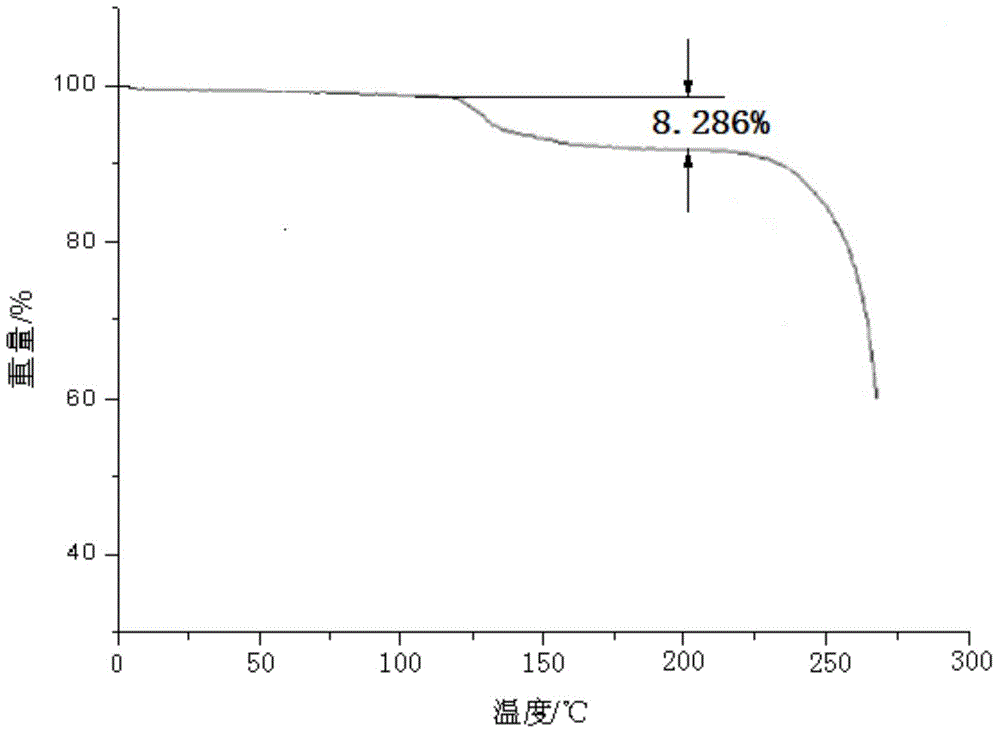
The invention discloses a monohydrate cefotiam hydrochloride compound and a preparing method thereof. One mole of cefotiam hydrochloride contains one mole of water, and an x-ray diffraction spectrogram of the compound has characteristic peaks at the positions with the diffraction angles 2theta of 14.82-15.22 degrees, 29.92-30.32 degrees, 31.48-31.88 degrees, 35.01-35.41 degrees and 37.79-38.19 degrees. 7-amino-cephalosporanic acid (7ACA) serving as a starting material is condensed with 1-(2-dimethylaminoethyl)-5-thiotetrazole under the catalytic effect of organic solvents dimethyl carbonate boron trifluoride and the like to prepare an intermediate at a C-3 site; then 2-(2-aminothiazol-4-yl) acetic acid hydrochloride reacts with methylene chloride and concentrated hydrochloric acid gas to prepare acyl chloride at a 7 site, and the one-water cefotiam hydrochloride compound is synthesized. The operation is simple and environmentally friendly, the reactants are easily obtained, the reaction conditions are mild, and the yield is high. The one-water cefotiam hydrochloride compound has low hygroscopicity and impurity content, good fluidity and thermodynamic stability and wider applicationprospects.
Owner:宁应
Medicine cefotiam hydrochloride composition for treating bacterial infection
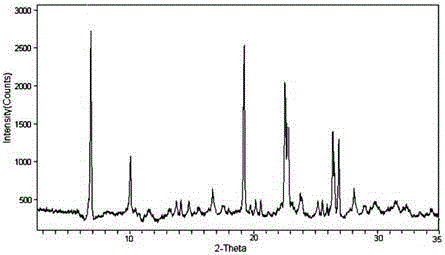
InactiveCN105193819AAntibacterial agentsOrganic active ingredientsSodium Chloride InjectionMoisture absorption
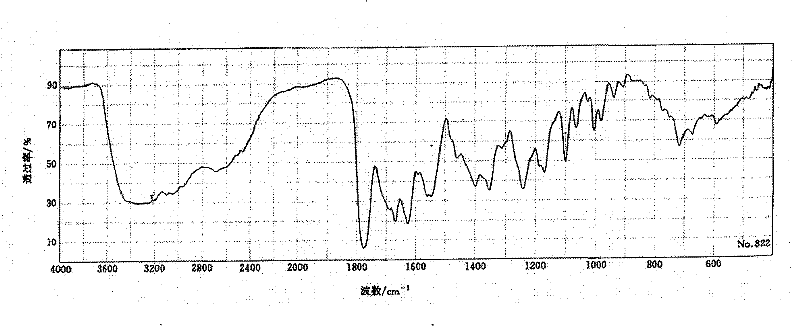
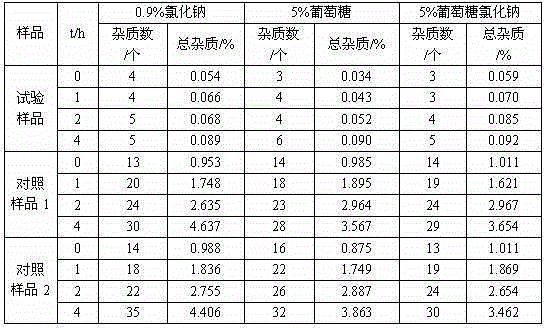
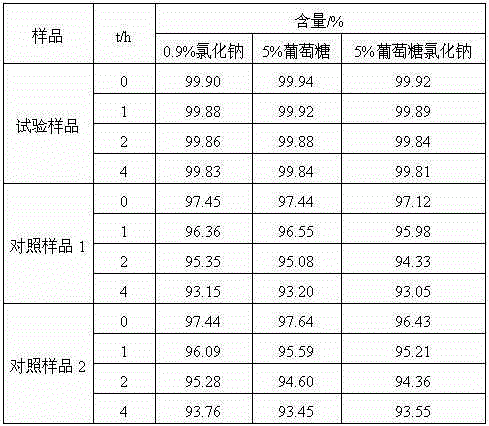
The invention relates to a medicine cefotiam hydrochloride composition for treating the bacterial infection, and belongs to the technical field of medicines. The composition is composed of cefotiam hydrochloride and sodium dihydrogen phosphate. The cefotiam hydrochloride is a crystal. An X-ray powder diffraction pattern obtained through measurement by means of Cu-K alpha ray is shown in a figure 1. The novel crystal form of the hydroxyfasudil is different from a crystal form structure in the prior art. According to experimental verification, surprisingly, it is found that the novel crystal-form compound is high in purity, good in mobility and stability, low in polymer content and not prone to moisture absorption, the obtained powder injection is matched with a 0.9% sodium chloride injection, 5% glucose injection and 5% sodium chloride and dextrose injection and the stability of the solution is good after the solution is placed at the room temperature for 4 h.
Owner:QINGDAO HUAZHICAO PHARMA CO LTD
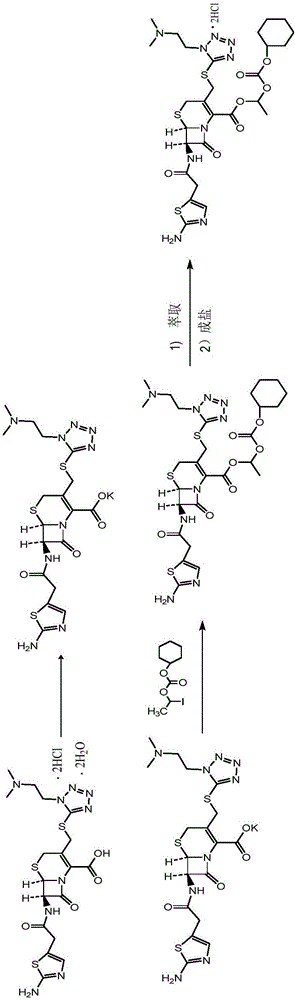
Preparation methods of high-purity cefotiam hexetil and dihydrochloride of high-purity cefotiam hexetil
ActiveCN101993449ASimple manufacturing methodSuitable for industrial productionOrganic chemistryDevitrificationCefotiam Hydrochloride
The invention provides a method for preparing high-purity cefotiam hexetil as shown in formula (I). The method is as follows: cefotiam hydrochloride taken as a raw material reacts with carbonic acid-1-iodine ethyl ester cyclohexyl in an organic solvent in the presence of carbonates to obtain the high-purity cefotiam hexetil. The invention also provides a method for preparing dihydrochloride of the cefotiam hexetil, comprising the following steps: the cefotiam hexetil is dissolved in a reaction solvent containing hydrogen chloride and a devitrification solvent to carry out crystallization for 1-2h at the temperature of 5-30 DEG C. The methods of the invention are simple and practicable, are suitable for industrial production, special equipment is not required, and the cost is low; and the cefotiam hexetil and the dihydrochloride of the cefotiam hexetil have high purity, low impurity content and high yield.
Owner:LIVZON PHARM GRP INC +1
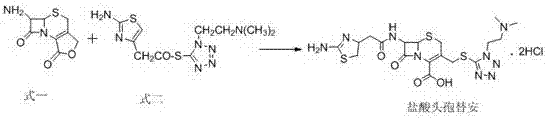
Synthesis method of cefotiam hydrochloride
The invention belongs to the technical field of medicines and discloses a synthesis method of cefotiam hydrochloride. The synthesis method comprises the steps: carrying out a condensation reaction ina mixed solvent by taking 7-aminocephalosporanic acid and 1-[2-(dimethylamino)ethyl]-1H-tetrazole-5-thiol as raw materials and a high-concentration boron trifluoride and dimethyl carbonate complexingcompound as a catalyst to prepare a three-position intermediate; and then, reacting the three-position intermediate with (2-aminothiazole-4-yl)acetyl chloride to obtain cefotiam hydrochloride. The method provided by the invention is stable and soft in reaction and few in byproducts, and the yield and purity of the produced cefotiam hydrochloride are relatively ideal.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Cefotiam hydrochloride for reducing anaphylaxis and preparation thereof
ActiveCN105646539AHigh purityThe reaction process is simpleAntibacterial agentsPowder deliveryCefotiam HydrochlorideAntibiotic Drugs
The invention relates to an antibiotic drug, in particular to cefotiam hydrochloride for reducing anaphylaxis. The compound has the advantages of high yield, high purity and the like, and is suitable for industrial production, and product stability and anaphylaxis reducing and clinic application of a preparation are all obviously improved.
Owner:福安药业集团庆余堂制药有限公司 +1
Preparation method for cefotiam hydrochloride
The invention provides a preparation method for cefotiam hydrochloride. The preparation method is characterized by comprising the following steps; adopting boron trifluoride complex to catalyze condensation reaction of 7-aminocephalosporanic acid and 1-(2-dimethyl aminoethyl)-1H-5-tetrazole-thione in case of taking dimethyl carbonate as a solvent; without crystallizing and separating, taking purewater as a reaction solvent and performing condensation on the pure water and aminothiazole acetyl chloride hydrochloride to form cefotiam; and performing hydrochloric acid acidification and crystallization on cefotiam to obtain cefotiam hydrochloride. A solvent used in the preparation method can be recycled, so that environment-friendly pressure is greatly reduced, environmental pollution is small, and indexes of cefotiam hydrochloride are improved while cost is reduced.
Owner:SHANGHAI NEW ASIA PHARMA +1
Cefotiam hydrochloride compound, method for preparing same and pharmaceutical composition with cefotiam hydrochloride compound
ActiveCN104926835AImprove liquidityImprove stabilityAntibacterial agentsOrganic active ingredientsCefotiam HydrochlorideSodium Chloride Injection
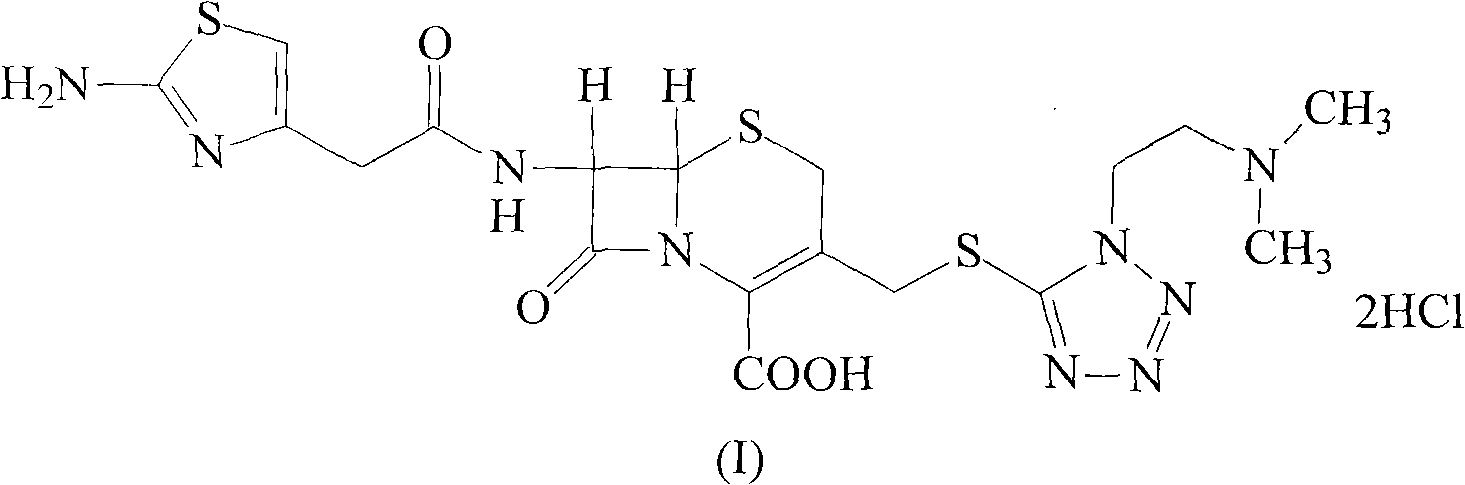
The invention belongs to the technical field of medicine, and particularly relates to a cefotiam hydrochloride compound, a method for preparing the same and a pharmaceutical composition with the cefotiam hydrochloride compound. The cefotiam hydrochloride compound is cefotiam hydrochloride trihydrate, and a structural formula of the cefotiam hydrochloride compound is shown. The cefotiam hydrochloride compound, the method and the pharmaceutical composition have the advantages that crystals of the cefotiam hydrochloride trihydrate are good in flowability, injection cefotiam hydrochloride made of the crystals of the cefotiam hydrochloride trihydrate, 0.9% sodium chloride injection, 5% glucose injection and 5% glucose and sodium chloride injection can be compatible with one another to obtain liquor, and the liquor is excellent in stability after being placed at the room temperature for 4h.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Method for preparing cefotiam hexetil hydrochloride
InactiveCN106349256AAvoid purification processAvoid stabilityOrganic chemistryDevitrificationCefotiam Hydrochloride
The invention discloses a method for preparing cefotiam hexetil hydrochloride. The method comprises the following steps: preparing cefotiam hexetil free alkali through choride iodination and one-pot esterification method by taking stable raw materials such as cefotiam hexetil hydrochloride and chlorinated carbonate as initial materials; performing low-temperature concentration, devitrification and other processes, thereby obtaining the purified cefotiam hydrochloride free alkali; and finally, carrying out a hydrochloride formation reaction, thereby obtaining the cefotiam hexetil hydrochloride. The method has the characteristics of high reaction stability and controllability, low product impurity content, simplicity in industrialized production and the like.
Owner:CHENGDU BRILLIANT PHARMA CO LTD
High-purified cefotiam hydrochloride compound
InactiveCN101787037BHigh purityImprove product qualityOrganic chemistryCefotiam HydrochlorideOrganic chemistry
The invention provides a cefotiam hydrochloride compound, which is highly purified and finally obtained by the way of achieving the refine and purification purposes through a specifically designed method of acid-base conversion and macroporous absorption resin adsorption, thus optimizing the quality of preparation products and the safety of clinical medication.
Owner:HAINAN LINGKANG PHARMA CO LTD
Cefotiam hydrochloride crystal compound and preparation method thereof
InactiveCN107383065AAchieve one-time crystallizationHigh purityOrganic chemistry methodsBulk chemical productionCefotiam HydrochlorideOrganic solvent
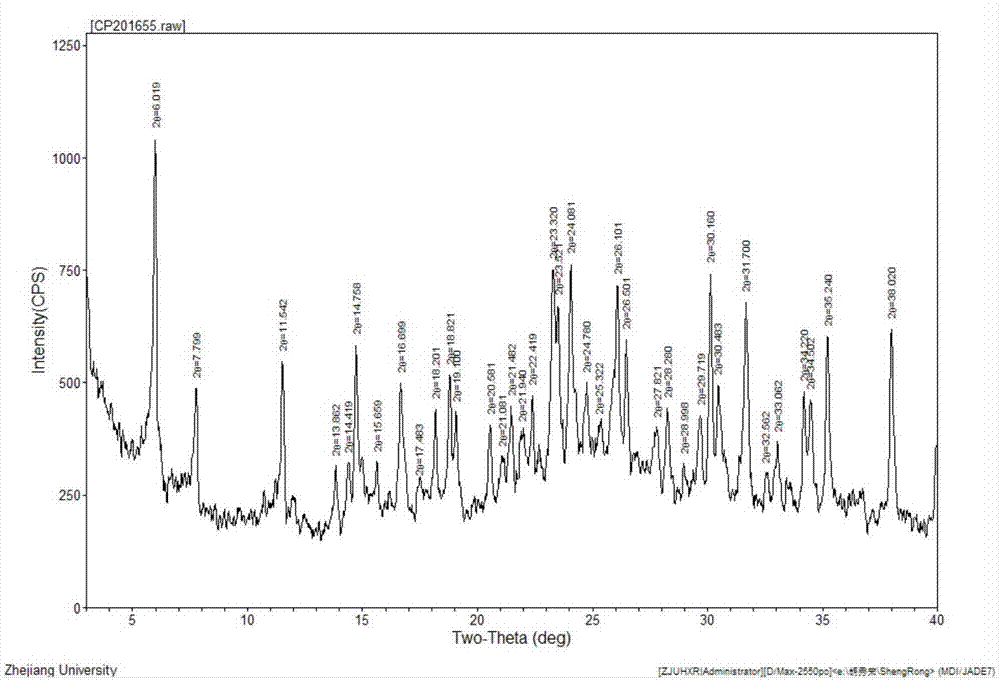
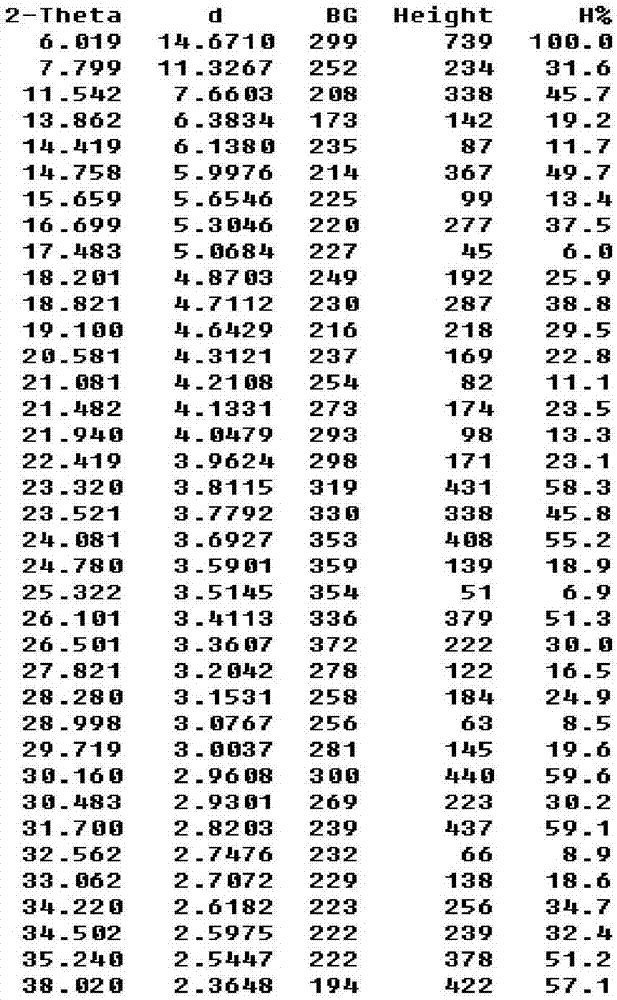
The invention relates to the field of medicines and primarily relates to a cefotiam hydrochloride crystal compound and a preparation method thereof. The invention relates to a crystal form of cefotiam hydrochloride. The cefotiam hydrochloride crystal compound is characterized by being measured by a Cu-Kalpha ray. The primary characteristic peaks in an X-ray powder diffraction pattern are displayed when 2theta are 6.019 degrees, 11.542 degrees, 14.758 degrees, 16.699 degrees, 18.821 degrees, 19.100 degrees, 23.320 degrees, 23.521 degrees, 24.081 degrees, 26.101 degrees, 26.501 degrees, 30.160 degrees, 30.483 degrees, 31.700 degrees, 34.220 degrees, 34.502 degrees, 35.240 degrees and 38.020 degrees. The crystal form of cefotiam hydrochloride provided by the invention is high in sample purity, good in stability, low in water content, low in residue of an organic solvent, uniform in size distribution, good in flowability and suitable for package. The method provided by the invention is high in yield of production process and simple and easy to control.
Owner:ZHEJIANG YONGNING PHARMA
New cefotiam hydrochloride synthesis method and applications of cefotiam hydrochloride in sterile powder injection
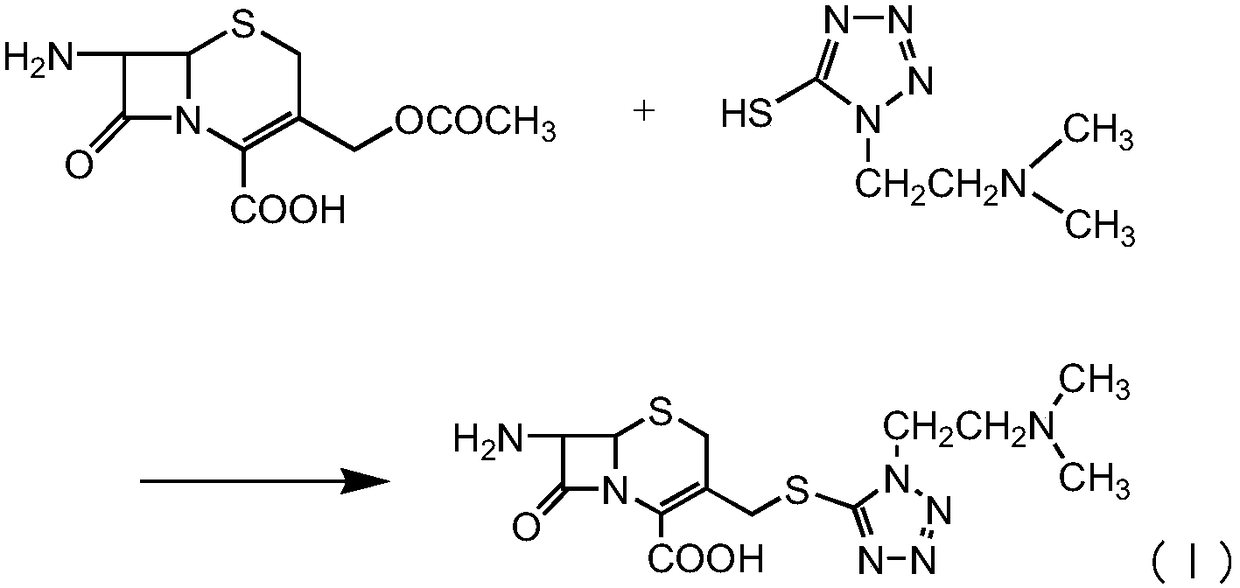
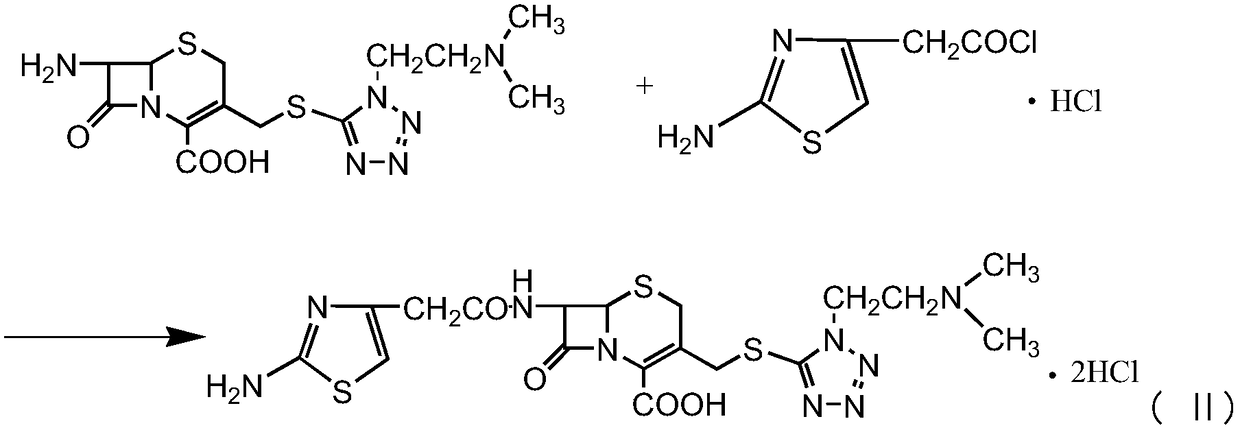
InactiveCN107488185AHigh purityLow purityAntibacterial agentsPowder deliveryCefotiam HydrochlorideSynthesis methods
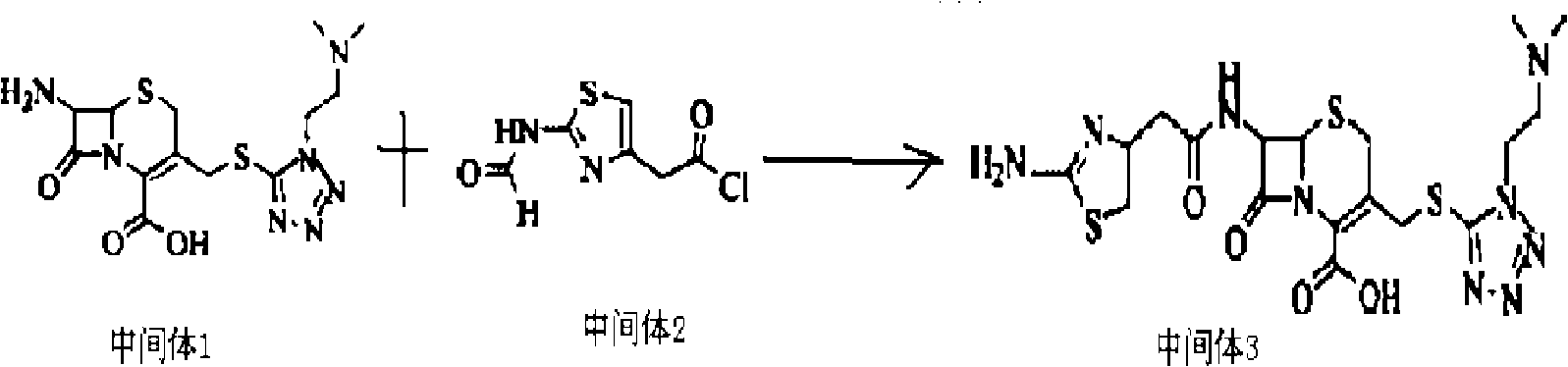
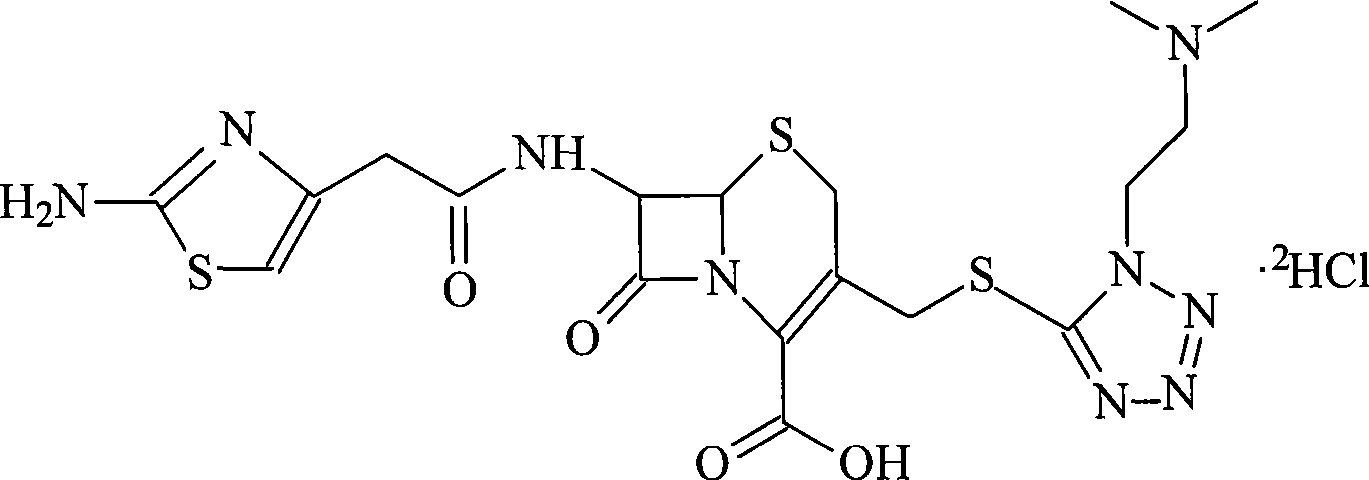
The invention discloses a new cefotiam hydrochloride synthesis method, which comprises that a compound represented by a formula 1 and a compound represented by a formula 2 are subjected to a reaction to prepare the cefotiam hydrochloride. According to the present invention, the method has advantages of short reaction steps, simple and convenient operation, high yield, atom economy, low cost and environment friendliness; and the cefotiam hydrochloride prepared through the synthesis method has good quality, and can be used for preparing the cefotiam hydrochloride powder injection for clinical use.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD
Method for preparing cefotiam hydrochloride
The invention relates to a method for preparing cefotiam hydrochloride. The method comprises the following steps: performing double trisphenylation on 2-aminothiazol-4-acetic acid (called ATA for short) used as raw material to obtain triphenylmethyl 2-triphenylaminothiazol-4-acetate (called 2TrATA for short), connecting the 2TrATA with 7-ACMT to obtain triphenyl cefotiam (called TrCEFO for short),and finally removing a triphenyl group to obtain the target product cefotiam hydrochloride (called CEFO for short). The preparation method has the advantages of simple and easily available raw materials, simplicity in post-treatment operation, and easiness in industrial production.
Owner:CHONGQING CHANGJIE MEDICINE CHEM +1
A kind of preparation method of cefotiam hydrochloride
The invention discloses a method for preparing cefotiam hydrochloride. The method comprises the following steps: carrying out a condensation reaction by using 7-ACA and DMMT as raw materials, a boron trifluoride acetonitrile complex as a catalyst and acetonitrile as a reaction solvent to prepare a 7-ACMT reaction liquid; carrying out a 'one-pot' acylation on the reaction liquid with ATC.HCl after treatment of direct water addition and alkali regulation without separating and purifying 7-ACMT; extracting and separating organic impurities from the acylation reaction liquid by acidification and organic solvent, and adding hydrophilic solvent to separate high-purity cefotiam hydrochloride. According to the method, two reactions are carried out in one step, the equipment using efficiency and labor efficiency can be improved, the labor intensity is reduced, the product yield is improved on the premise of guaranteeing the product quality, and the production cost is reduced; all impurity separating operations are carried out after the acylation reaction, product loss caused by intermediate separating operation can be reduced, and the product purity can be guaranteed.
Owner:ZHEJIANG ZHEBANG PHARMA
Antibacterial cefotiam hydrochloride drug composition
The invention discloses an antibacterial cefotiam hydrochloride drug composition, belongs to the technical field of medicines. The drug composition comprises cefotiam hydrochloride and arginine, wherein cefotiam hydrochloride is crystalline, and the X-ray powder diffraction pattern measured by Cu-K-alpha rays is shown in the figure 1 (in the description). The new crystalline form of cefotiam hydrochloride provided by the invention is different from the crystal structure in the prior art, by experimental verification, the purity of the crystalline compound is high, the fluidity is good, the stability is good, the polymer content is low, hygroscopicity is avoided, and the stability of solution is good after being left to stand for 4 h at room temperature, wherein the solution is obtained by compounding the prepared powder injection with 0.9% sodium chloride injection, 5% glucose injection, and 5% sodium chloride and dextrose injection.
Owner:QINGDAO HUAZHICAO PHARMA CO LTD
Cefotiam hydrochloride pharmaceutical composition for injection and preparation method thereof
InactiveCN103446047ANot easy to cause poisoningGuaranteed to be stable and safeAntibacterial agentsOrganic active ingredientsSodium bicarbonateCefotiam Hydrochloride
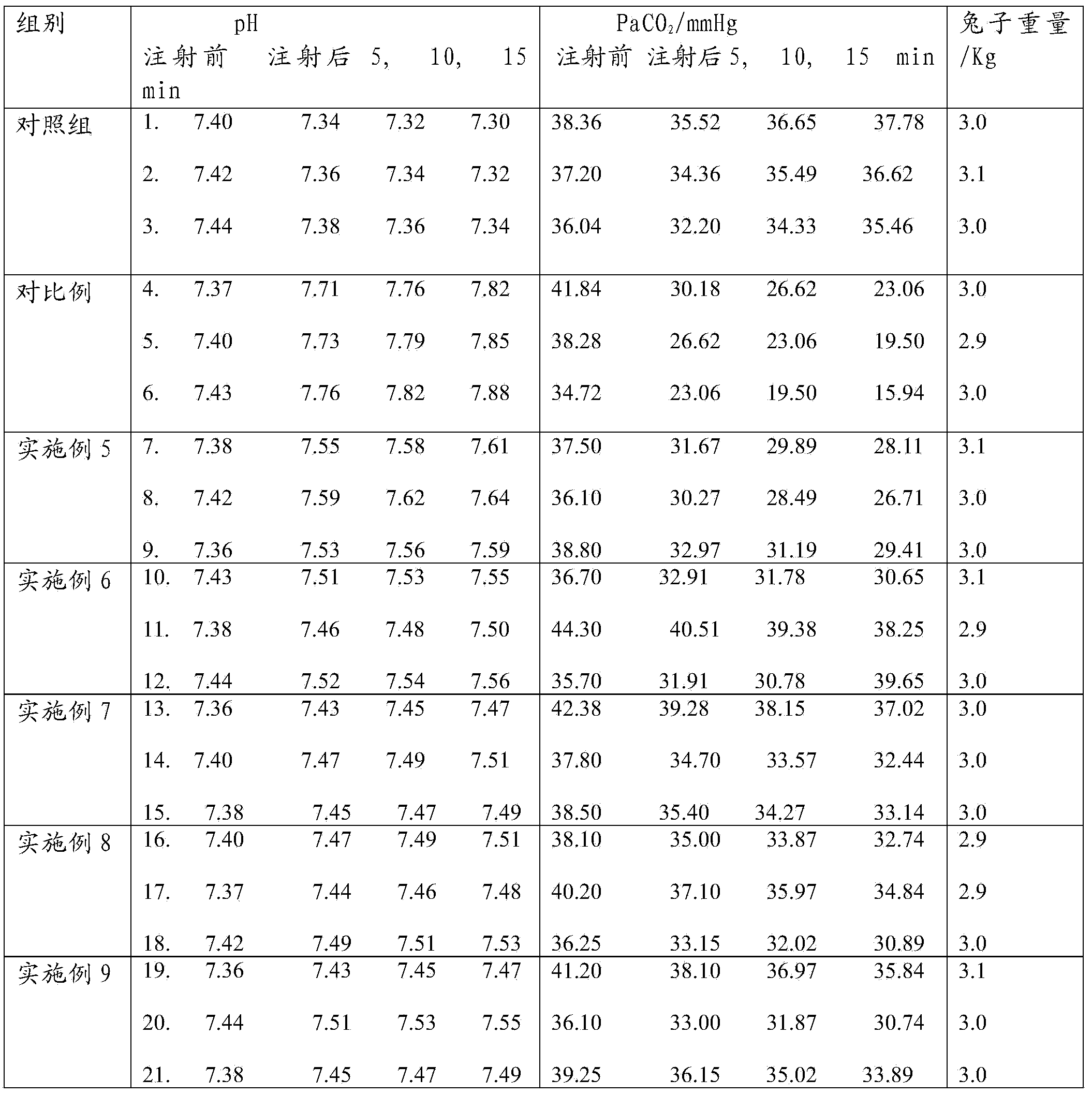
The invention discloses a cefotiam hydrochloride pharmaceutical composition for injection and a preparation method thereof. The cefotiam hydrochloride pharmaceutical composition for injection comprises the following components in parts by weight: 500 parts of cefotiam hydrochloride, (121-x) parts of anhydrous sodium bicarbonate and 2x parts of anhydrous sodium carbonate, wherein x is not less than 1 and not more than 120. The cefotiam hydrochloride pharmaceutical composition for injection disclosed by the invention can be used for solving the problems in the prior art that CO2 partial pressure in blood is extremely low and pH stability is low after cefotiam hydrochloride injection is injected to a human body and the problem that intoxication is easily caused after long-term use of the cefotiam hydrochloride injection.
Owner:HANGZHOU TONGSHENG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com