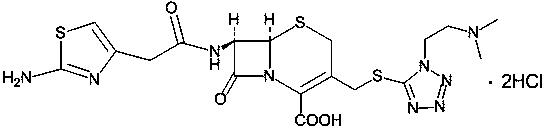
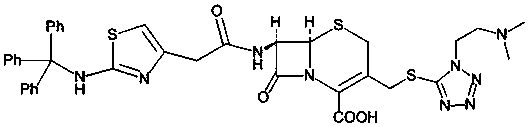
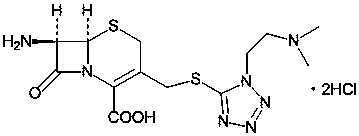
Method for preparing cefotiam hydrochloride
A technology of cefotiam hydrochloride and concentrated hydrochloric acid, applied in the field of drug synthesis, can solve the problems of difficult operation, low yield, difficult reaction control and the like, and achieve the effects of simple operation, high purity and simplified reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1.2 Synthesis of TrATA
[0042] Dissolve 2-aminothiazole-4-acetic acid (ATA) (10.0g, 0.0632mol) in 200ML acetonitrile, add triphenylbromethane (51.07g, 0.158mol), add diisopropylethylamine (24.5g, 0.1896mol), react at 25°C for 5h, TLC monitors the reaction is complete, add 100ml of purified water to quench the reaction, extract the reaction solution with dichloromethane (200ML×2), combine the organic layer and wash with saturated brine (100ML×2) Dry over anhydrous magnesium sulfate, filter and spin dry under reduced pressure to obtain 2TrATA, 36.70 g of off-white solid, yield 90.8%, HPLC purity 97.6%.
[0043] 2. Synthesis of TrCEFO
[0044] Suspend 7-ACMT (10.0g, 0.0218mol) in tetrahydrofuran 200ML as a solvent, cool down to 0°C, and slowly add diisopropylethylamine (8.45g, 0.0654mol) dropwise. After the addition is complete, the system becomes clear. The obtained clear solution was added dropwise to the tetrahydrofuran solution of 2TrATA (15.4g, 0.0239mol dissolved ...
Embodiment 2
[0048] 1. Synthesis of 2TrATA
[0049] Dissolve 2-aminothiazole-4-acetic acid (ATA) (5.80g, 0.0366mol) in 100ML acetonitrile, add triphenylbromethane (29.62g, 0.0916mol), add diisopropylethylamine (24.5g, 0.1896mol), reacted at 40°C for 3 hours, TLC monitored the reaction to be complete, added 80ml of purified water to quench the reaction, extracted the reaction solution with dichloromethane (100ML×2), combined the organic layer and washed it with saturated brine (100ML×2) Dry over anhydrous magnesium sulfate, filter and spin dry under reduced pressure to obtain 2TrATA, 21.17 g of off-white solid, yield 90%, HPLC purity 97.5%.
[0050] 2. Synthesis of TrCEFO
[0051] Suspend 7-ACMT (5.50g, 0.0149mol) in tetrahydrofuran 100ML as a solvent, cool down to 0°C, and slowly add diisopropylethylamine (5.81g, 0.0449mol) dropwise. After the addition is complete, the system becomes clear. The obtained clear solution was added dropwise to 2TrATA in THF solution (10.62g, 0.0164mol dissol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com