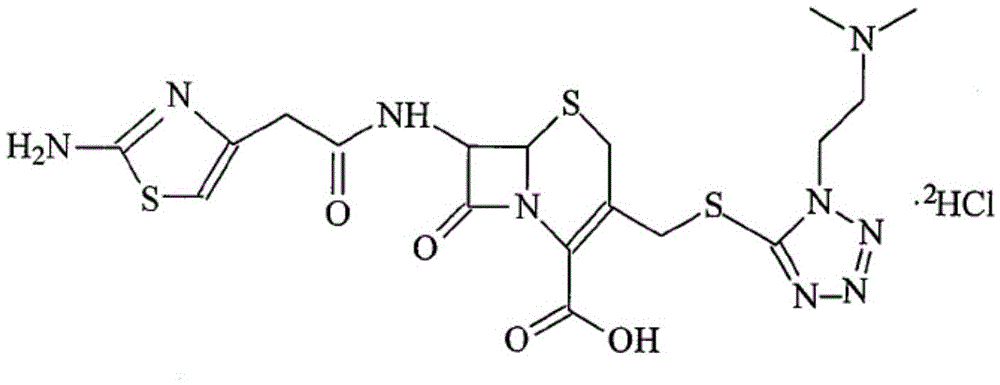
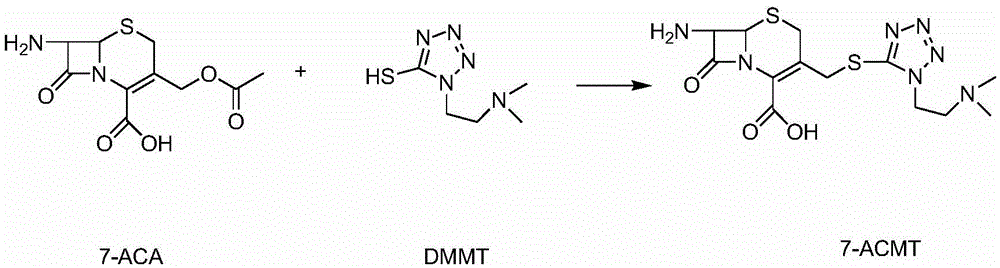
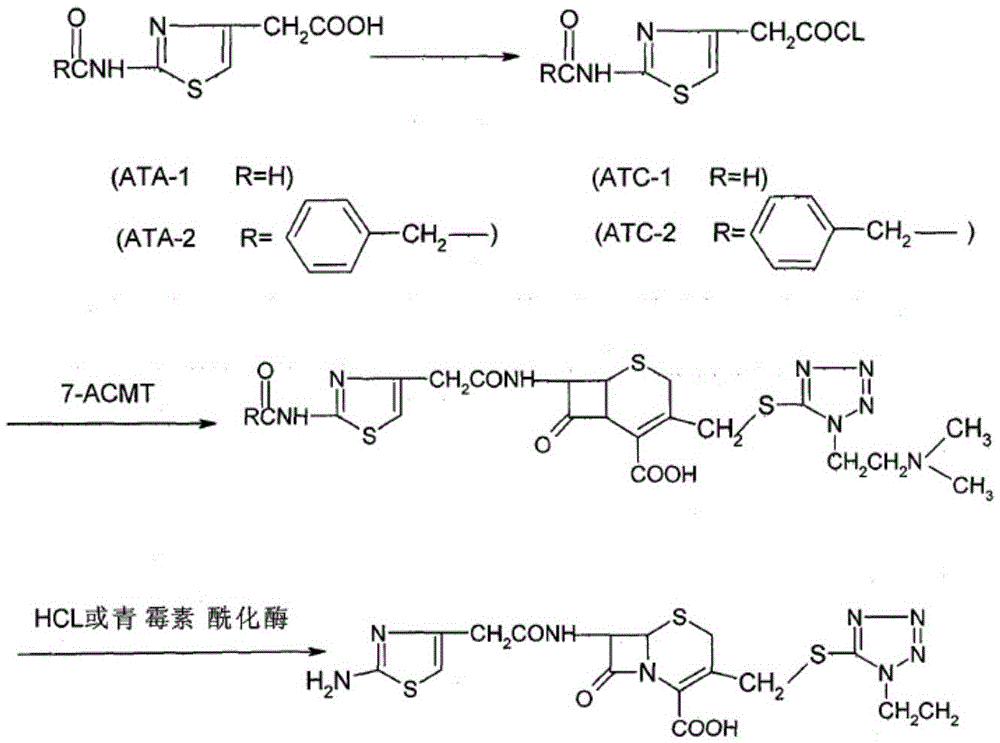
Cefotiam hydrochloride for reducing anaphylaxis and preparation thereof
A technology for cefotiam hydrochloride and allergic reaction, applied in the field of antibiotics, can solve the problems of low yield, difficult transportation, unsuitable for industrial production and the like, and achieves the effects of high product yield, simple reaction process and low impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Dissolve 7-ACMT385g in 2L of acetone, add 300g of tert-butyldimethylsilyl chloride and 202g of triethylamine, stir the reaction at room temperature, TLC detects that the reaction is complete, cool to 0°C, and remove the solvent to obtain formula 1 compound.
[0030] (2) Dissolve 204 g of 2-formylaminothiazole-4 acetyl chloride in 2L of dichloromethane, then add 598 g of the compound of formula 1 obtained in step (1), stir and react at room temperature for 3 hours, and TLC detects that the reaction is complete. The solvent is removed under reduced pressure to obtain the compound of formula 1 .
[0031] (3) The compound of formula 2 prepared in step (2) was dissolved in 2L of acetone, then an excess of aqueous hydrochloric acid was added, heated to 35° C. for 3 hours, crystals were precipitated after the reaction was complete, washed, and vacuum-dried to obtain cefotiam hydrochloride Salt 550kg, yield is 92%.
[0032] Determination of content: according to size excl...
Embodiment 2
[0038] (1) Dissolve 7-ACMT385g in 2L ethyl acetate, add 315g tert-butyldimethylsilyl chloride and 212g triethylamine, stir the reaction at room temperature, TLC detects that the reaction is complete, cool to -5°C, and remove the solvent Compound of formula 1 is obtained.
[0039] (2) Dissolve 204 g of 2-formylaminothiazole-4 acetyl chloride in 2 L of chloroform, then add 592 g of the compound of formula 1 obtained in step (1), stir and react at room temperature for 2.5 hours, TLC detects that the reaction is complete, and depressurizes Removal of the solvent affords the compound of formula 1 .
[0040] (3) Dissolve the compound of formula 2 prepared in step (2) in 2L of methyl isobutyl ketone, then add excess hydrochloric acid aqueous solution, heat to 40°C and react for 2.5 hours, after the reaction is complete, crystals are precipitated, washed, and vacuum-dried to obtain cephalosporin Thiam hydrochloride 545g, the yield is 91%.
[0041] The content of cefotiam hydrochlori...
Embodiment 3
[0043] (1) Dissolve 7-ACMT385g in 2L tetrahydrofuran, add 308g tert-butyldimethylsilyl chloride and 207g triethylamine, stir the reaction at room temperature, TLC detects that the reaction is complete, cool to 5°C, and remove the solvent to obtain formula 1 compound.
[0044](2) Dissolve 204 g of 2-formylaminothiazole-4 acetyl chloride in 2L of acetone, then add 596 g of the compound of formula 1 obtained in step (1), stir and react at room temperature for 2.5 hours, TLC detects that the reaction is complete, and depressurizes Removal of the solvent affords the compound of formula 1 .
[0045] (3) The compound of formula 2 prepared in step (2) was dissolved in 2L of acetone, then an excess of hydrochloric acid aqueous solution was added, heated to 45° C. for 2 hours, crystals were precipitated after the reaction was complete, washed, and vacuum-dried to obtain cefotiam hydrochloride Salt 538g, the yield is 90%.
[0046] The content of cefotiam hydrochloride in Example 3 was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com