Synthesis method of cefotiam hydrochloride
A technology of cefotiam hydrochloride and a synthesis method, applied in the field of drug synthesis, can solve the problems of low yield, difficult operation, difficult content assurance, etc., and achieve the effects of shortening the reaction time, speeding up the reaction rate, and increasing the purity of the product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
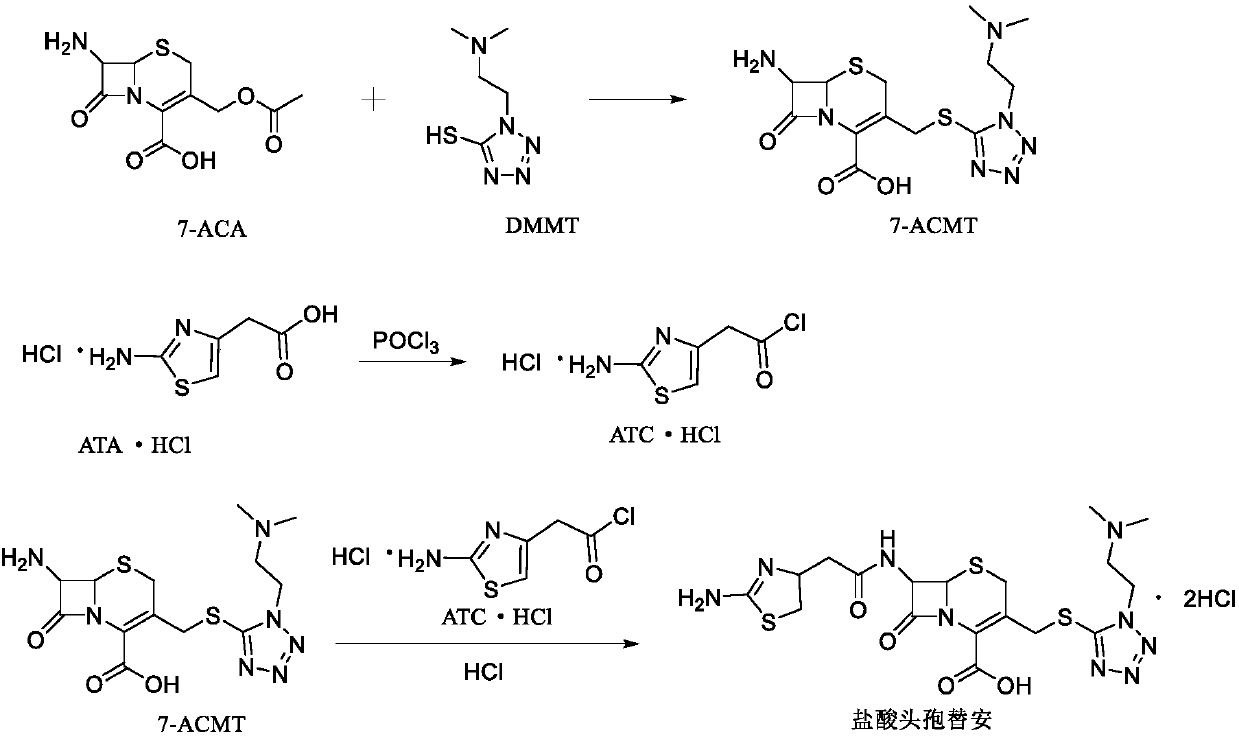
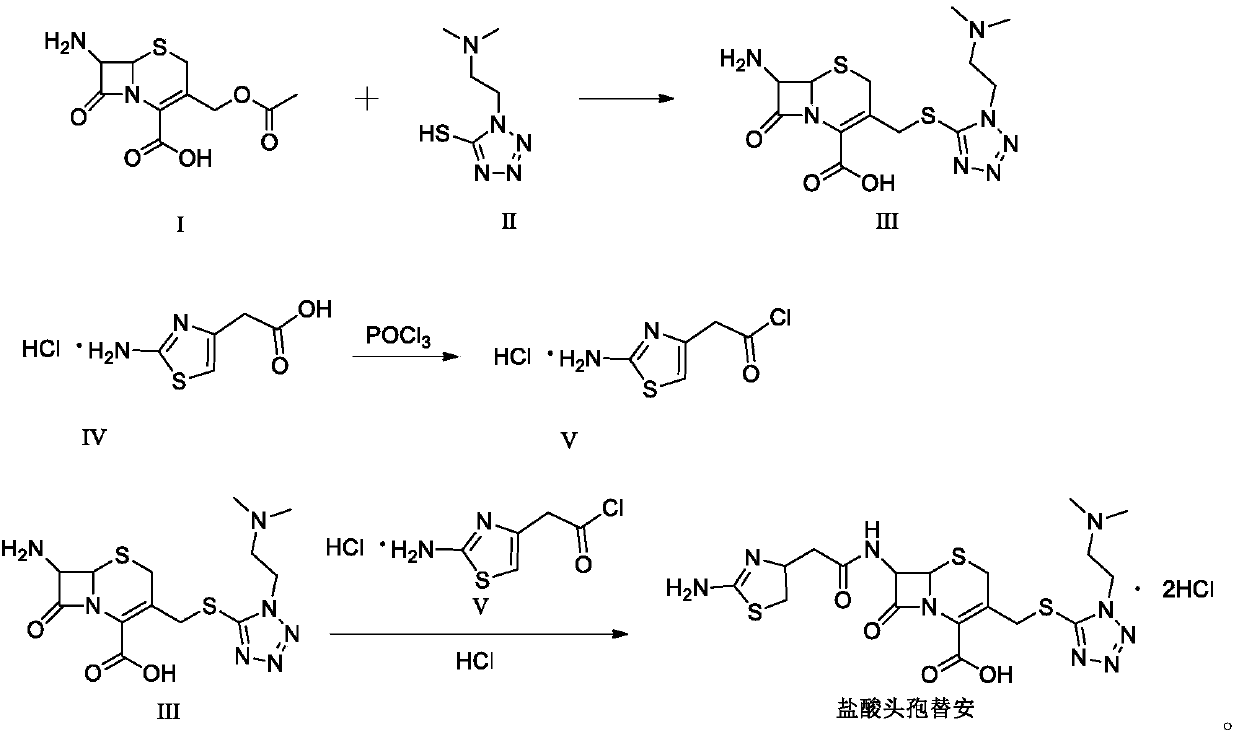
[0032] (1) Add 60mL of dimethyl carbonate and 7.06g of citric acid to a 500mL three-necked flask, add 6.36g of DMMT while stirring, add 10.00g of 7-ACA, and slowly add boron trifluoride-dimethyl carbonate complex 34.82 g, control the temperature at 20-30°C, and monitor the reaction by HPLC after 1h. Add 1.28g of sodium dithionite, stir for 10min, transfer to water, add 80mL of isopropanol, slowly add concentrated ammonia water dropwise to adjust the pH to 3.0, and control the dropping time After 30-60 minutes, cool down to 0-10°C to crystallize for 1 hour. Suction filtration yielded 18.82 g of 7-ACMT wet product, with a purity of 99.4% by HPLC and a maximum of 0.08% impurity.
[0033] (2) Add 150mL of dichloromethane to a 500mL three-necked flask, add 20.00g of aminothiazole acetic acid hydrochloride, cool down to -5~5°C, add 31.62g of phosphorus oxychloride, and slowly add N,N-diisopropyl Add 13.32 g of ethylamine, raise the temperature to 10-20° C. after adding, add 2.00 g ...
Embodiment 2
[0036] (1) Add 60mL of dimethyl carbonate and 9.86g of 2-hydroxysuccinic acid into a 500mL three-necked flask, add 6.36g of DMMT while stirring, add 10.00g of 7-ACA, and slowly add boron trifluoride-dimethyl carbonate complex Compound 40.62g, temperature control 20~30℃, react after 1h, HPLC monitors the reaction is over, add sodium dithionite 2.56g, stir for 10min and transfer to water, add isopropanol 80mL, slowly add concentrated ammonia water to adjust the pH to 4.5, control The dropping time is 30-60 minutes, and the temperature is lowered to 0-10°C for 1 hour of crystallization. Suction filtration yielded 18.65 g of 7-ACMT wet product, with a purity of 98.9% by HPLC and a maximum of 0.09% impurity.
[0037] (2) Add 150mL of dichloromethane to a 500mL three-necked flask, add 20.00g of aminothiazole acetic acid hydrochloride, cool down to -5~5°C, add 31.62g of phosphorus oxychloride, and slowly add N,N-dimethyl 7.53g of formamide, after the addition, the temperature was ra...
Embodiment 3
[0040] (1) Add 60mL of dimethyl carbonate and 6.62g of acetic acid into a 500mL three-necked flask, add 6.36g of DMMT while stirring, add 10.00g of 7-ACA, and slowly add 46.42g of boron trifluoride-dimethyl carbonate complex, Control the temperature at 20-30°C and react. After 1 hour, HPLC monitors that the reaction is over. Add 0.64g of sodium dithionite, stir for 10 minutes, transfer to water, add 80mL of isopropanol, slowly add concentrated ammonia water to adjust the pH to 4.0, and control the dropping time for 30~ After 60 minutes, the temperature was lowered to 0-10°C for 1 hour of crystallization. Suction filtration yielded 18.82 g of 7-ACMT wet product, with a purity of 99.4% by HPLC and a maximum of 0.08% impurity.
[0041] (2) Add 150mL of dichloromethane to a 500mL three-necked flask, add 20.00g of aminothiazole acetic acid hydrochloride, cool down to -5~5°C, add 31.62g of phosphorus oxychloride, and slowly add N,N-dimethyl 8.97 g of acetamide was added. After the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com