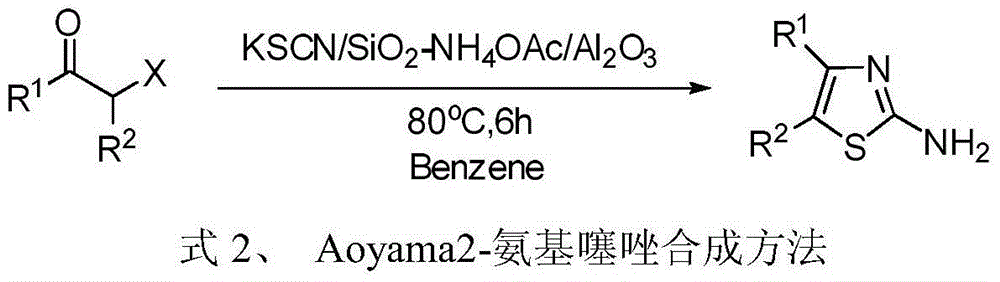
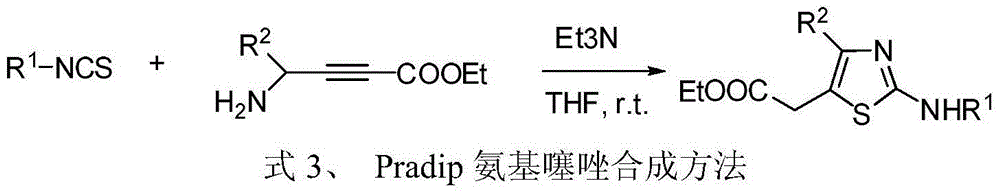
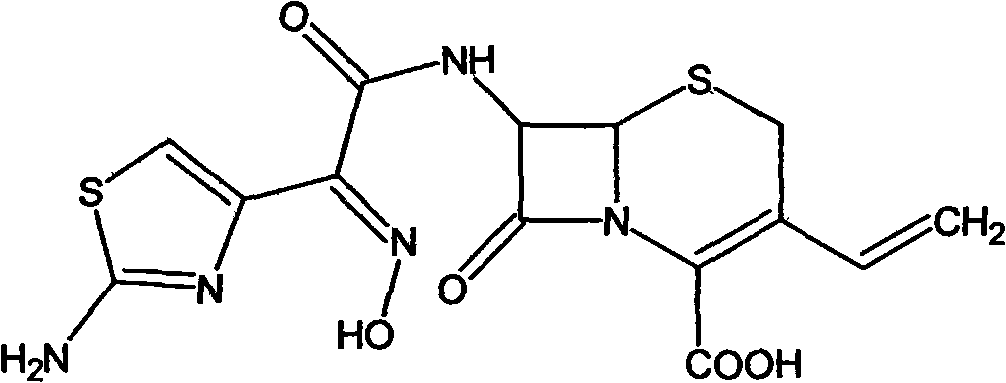
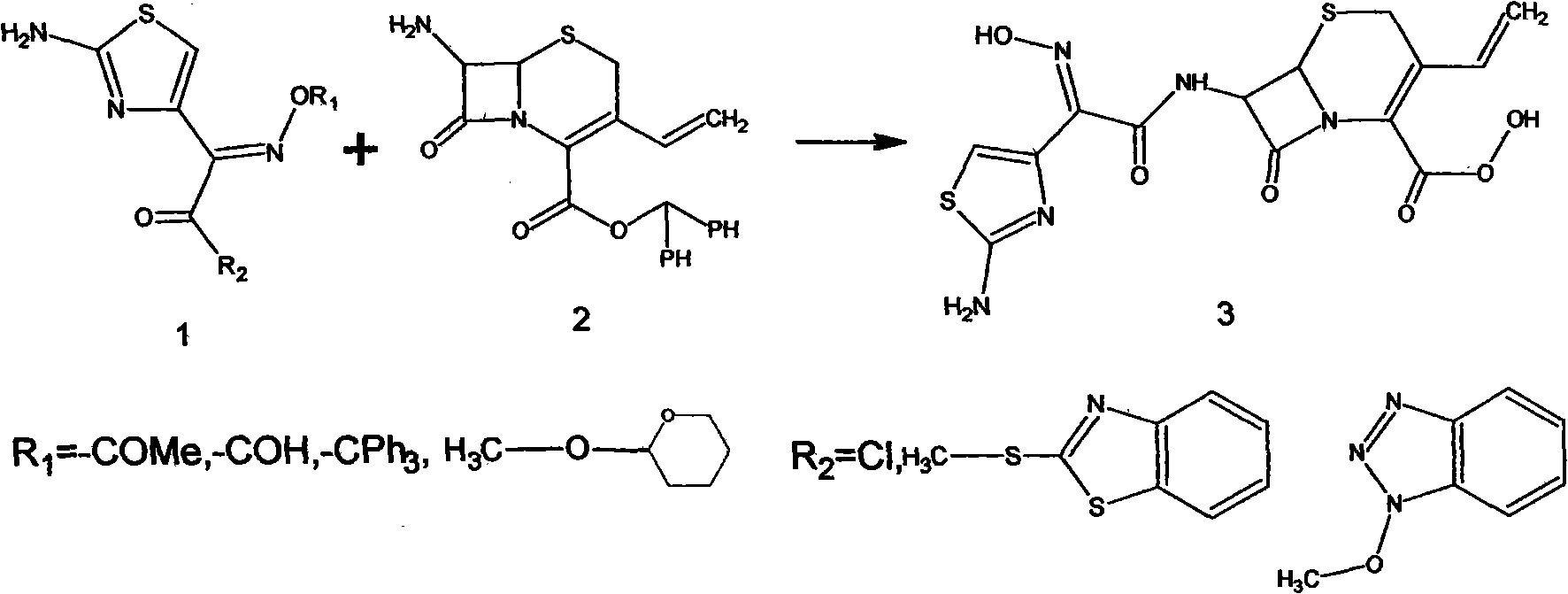
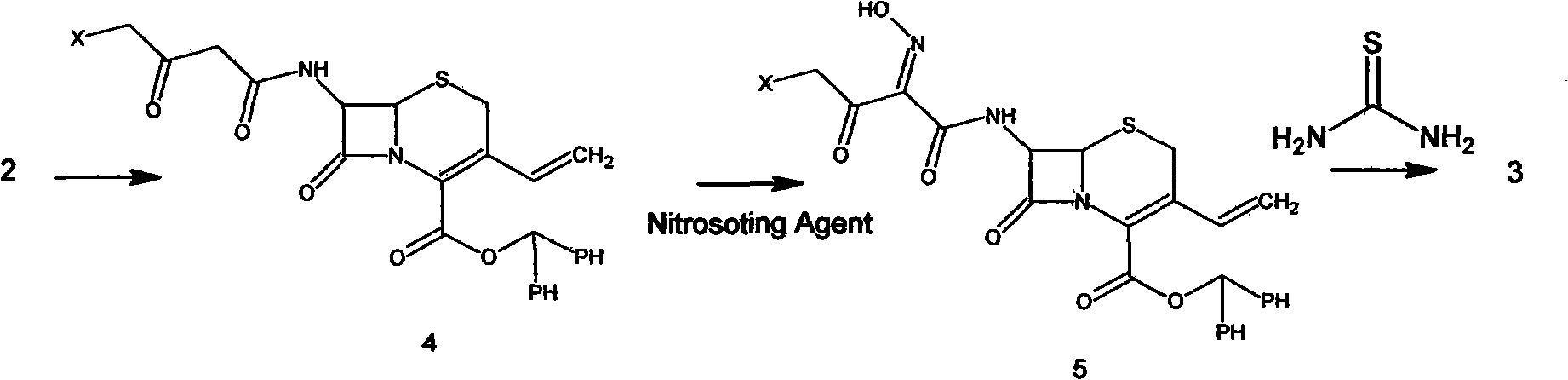
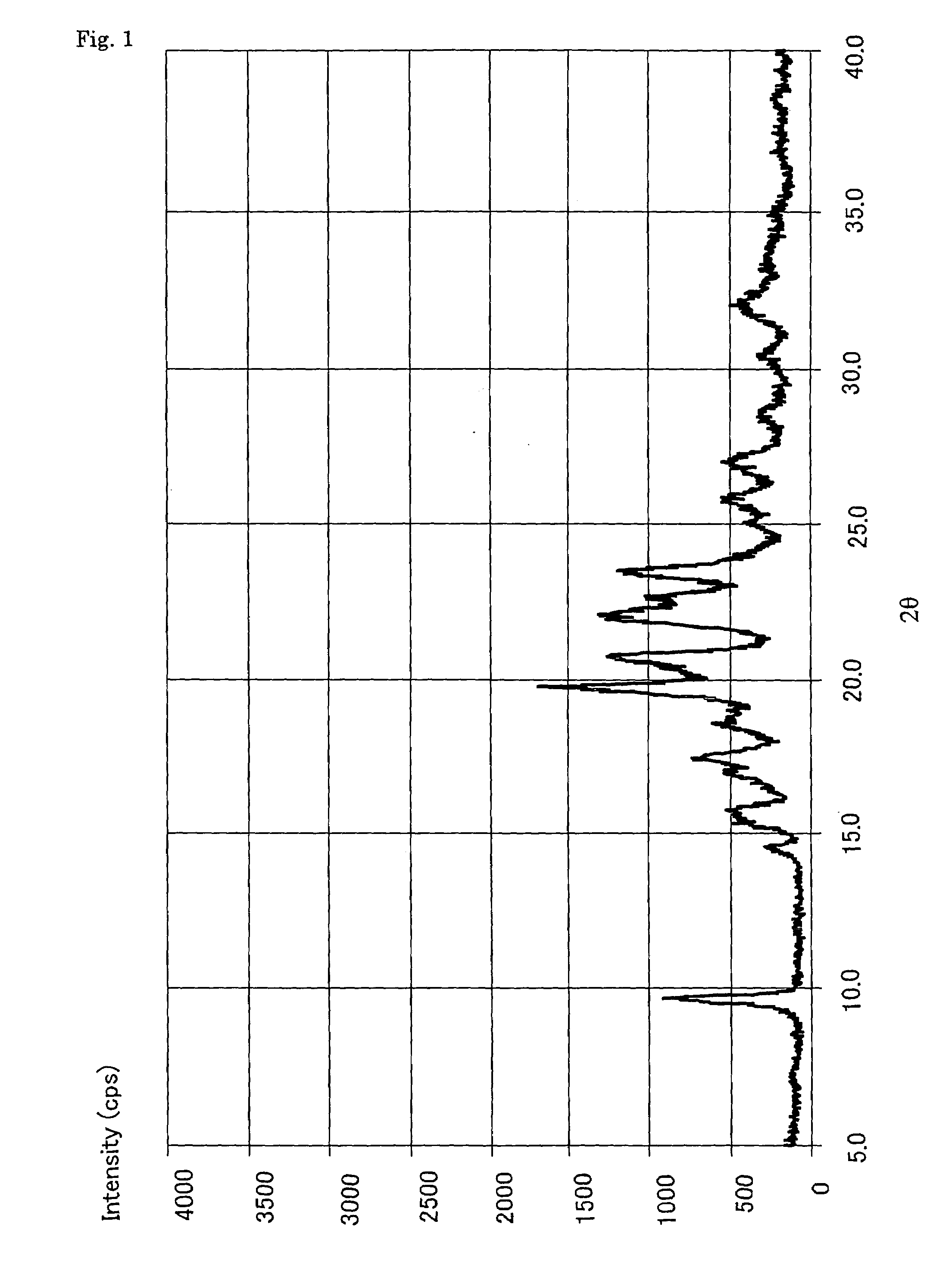
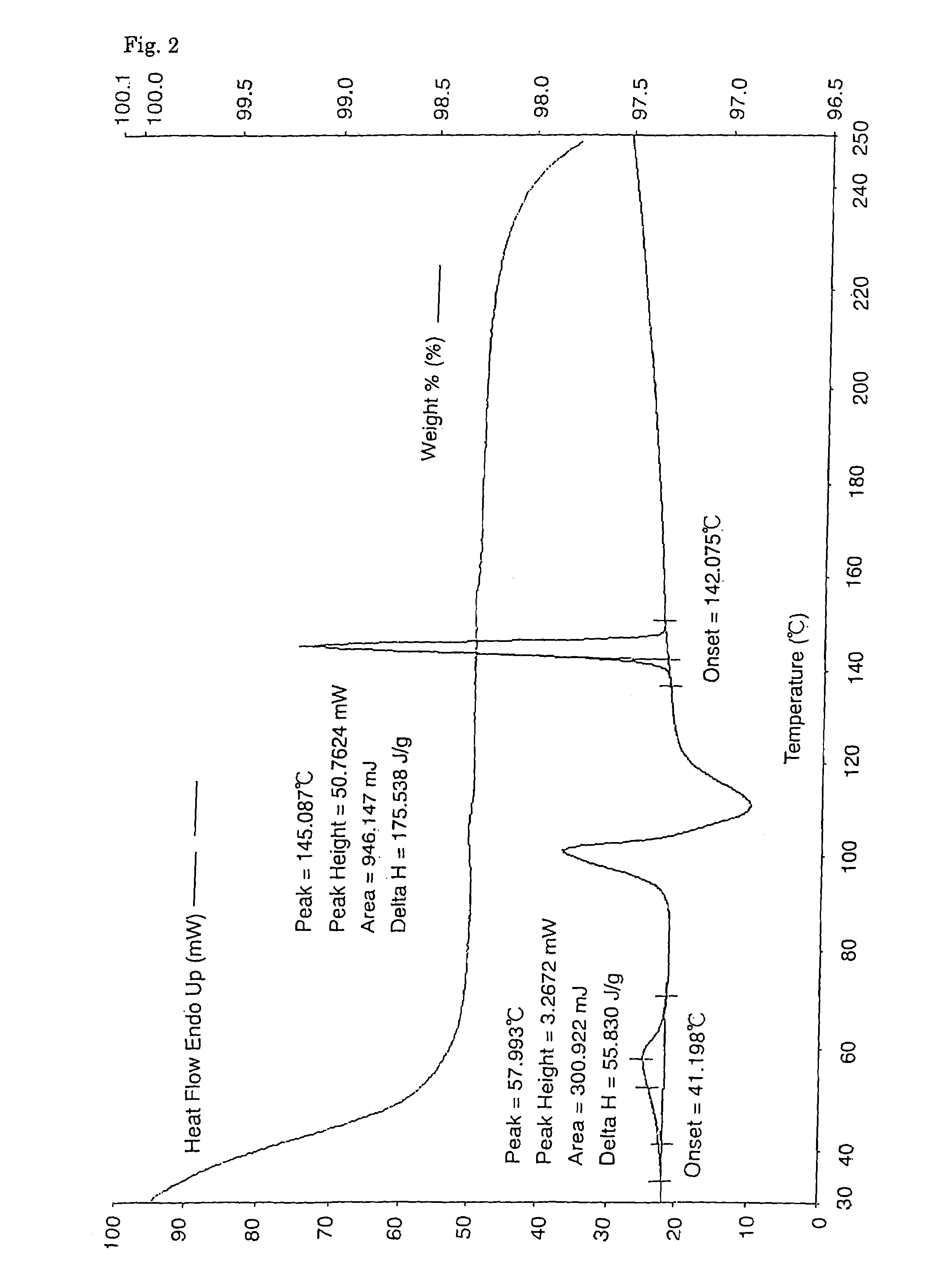
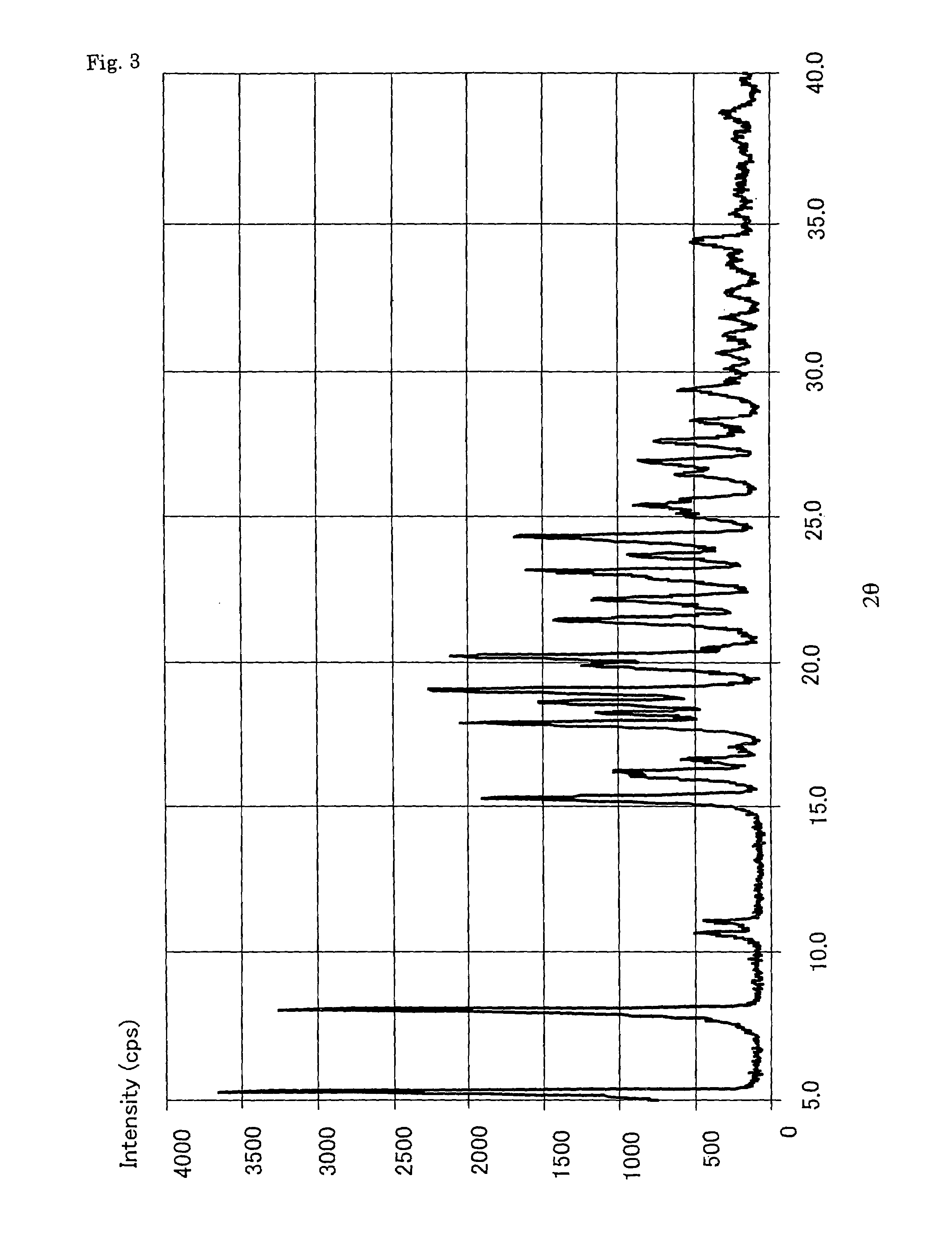
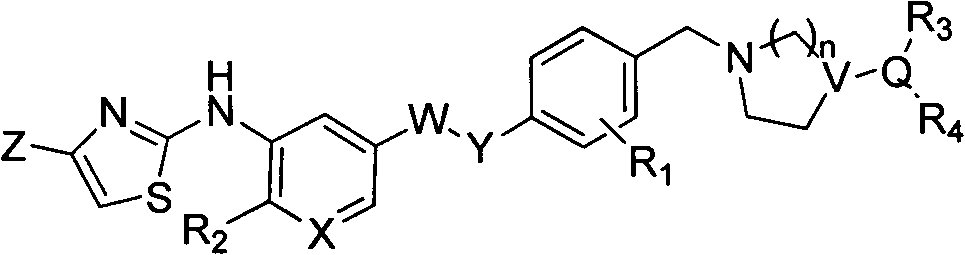
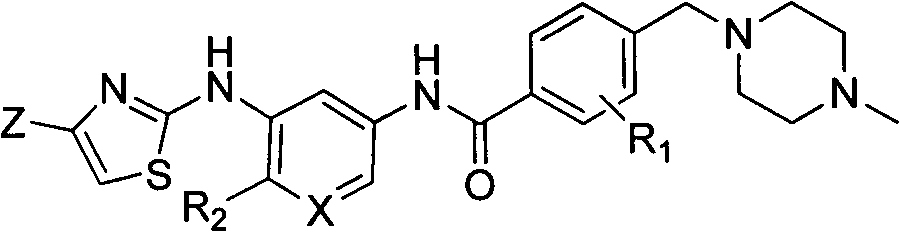
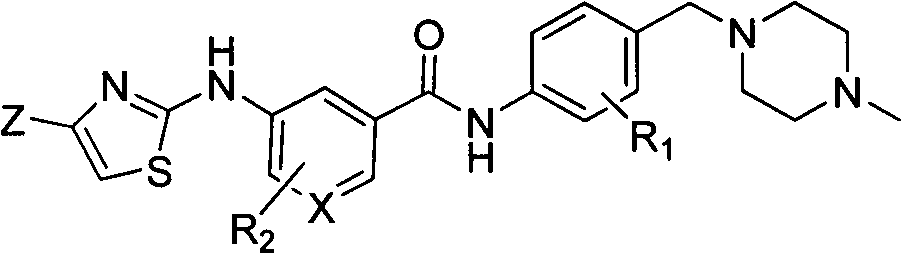
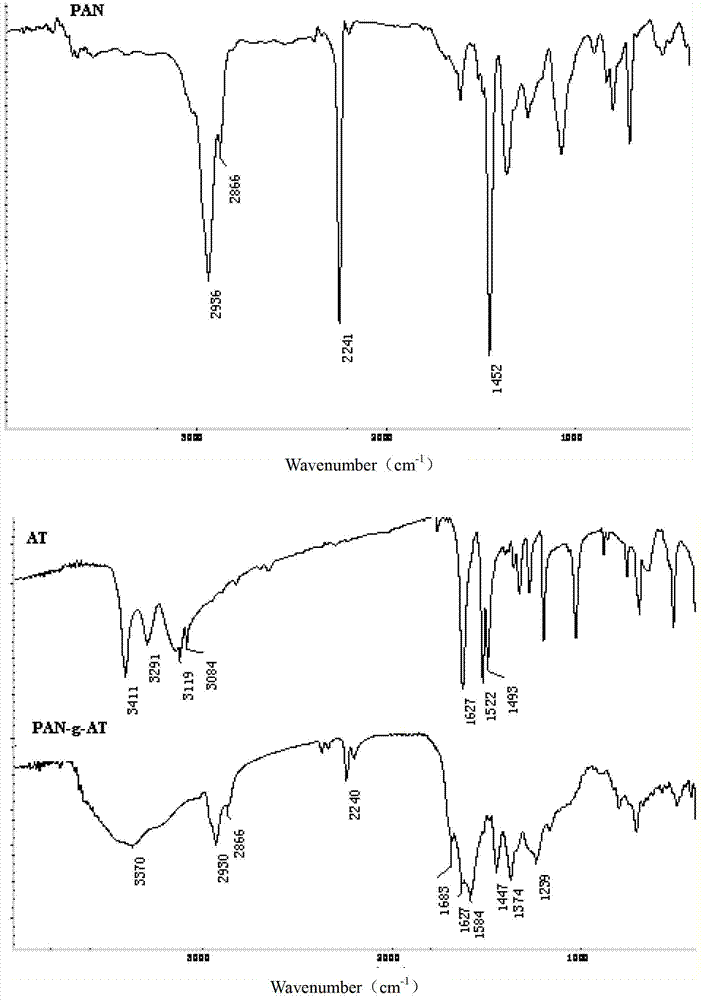
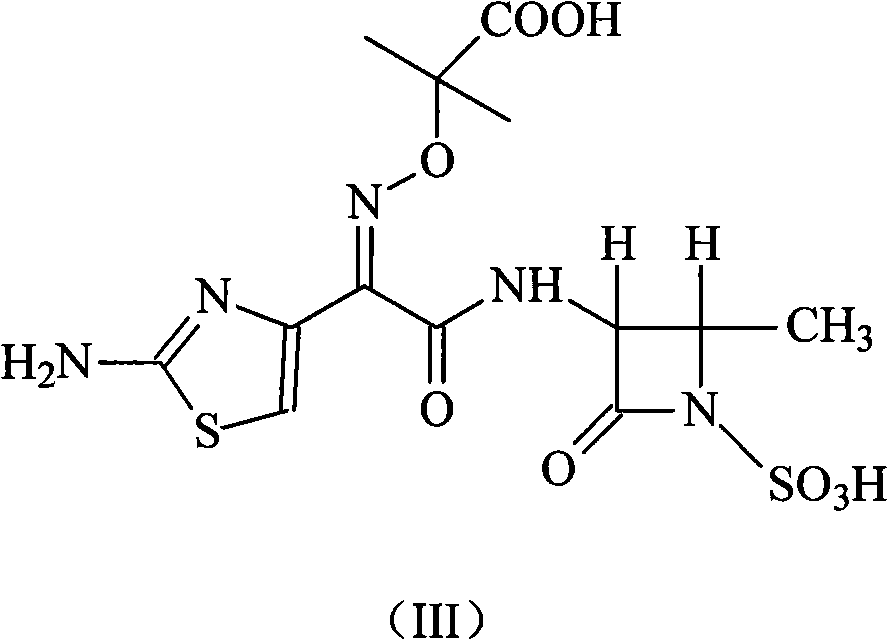
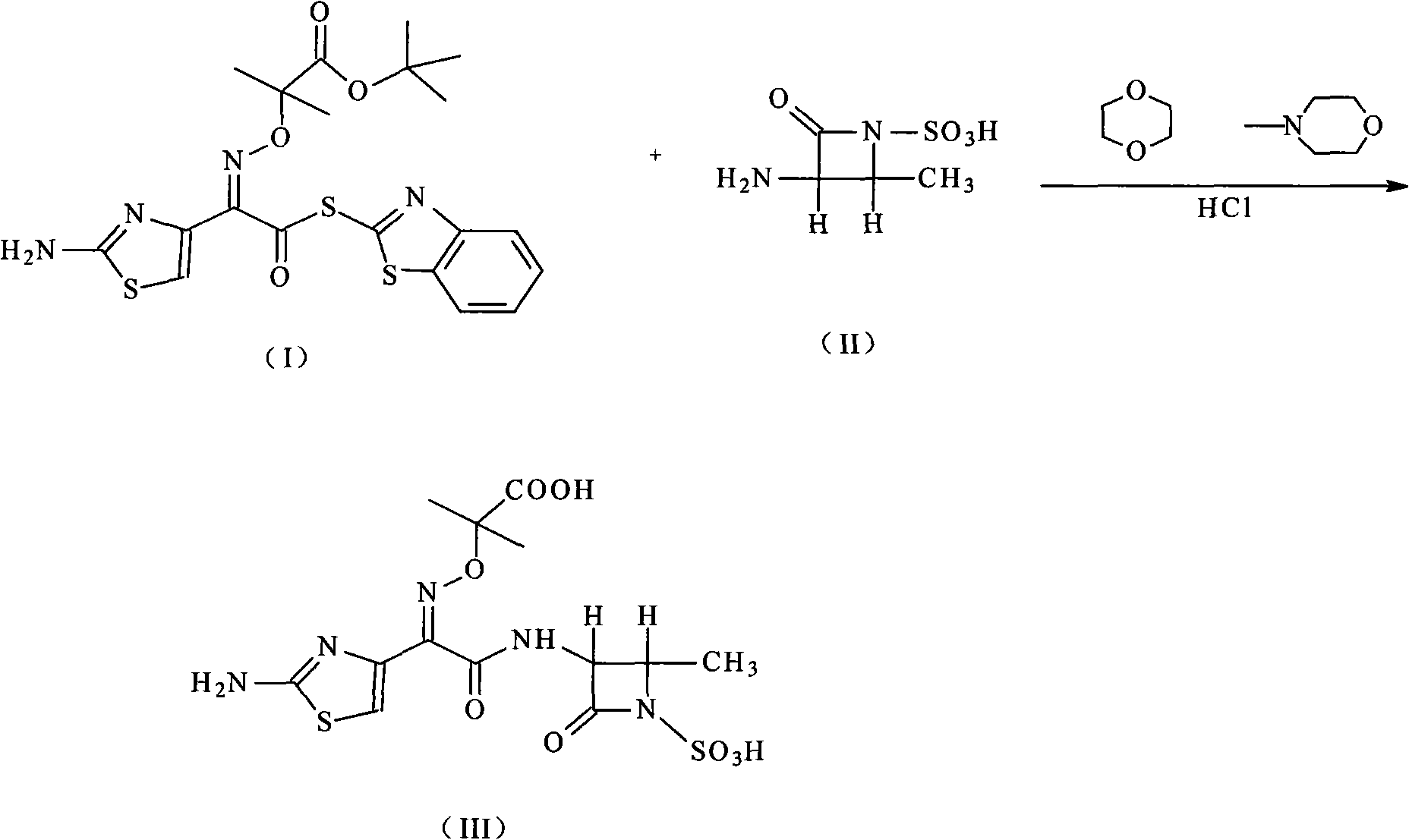
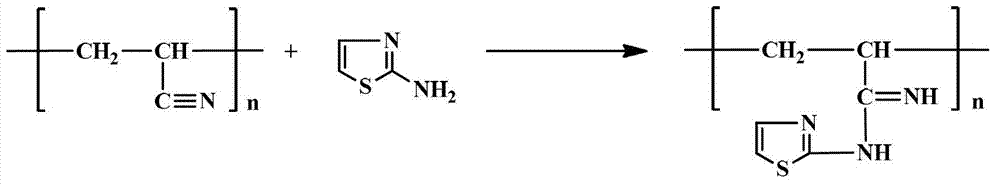Patents
Literature
206 results about "2-aminothiazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
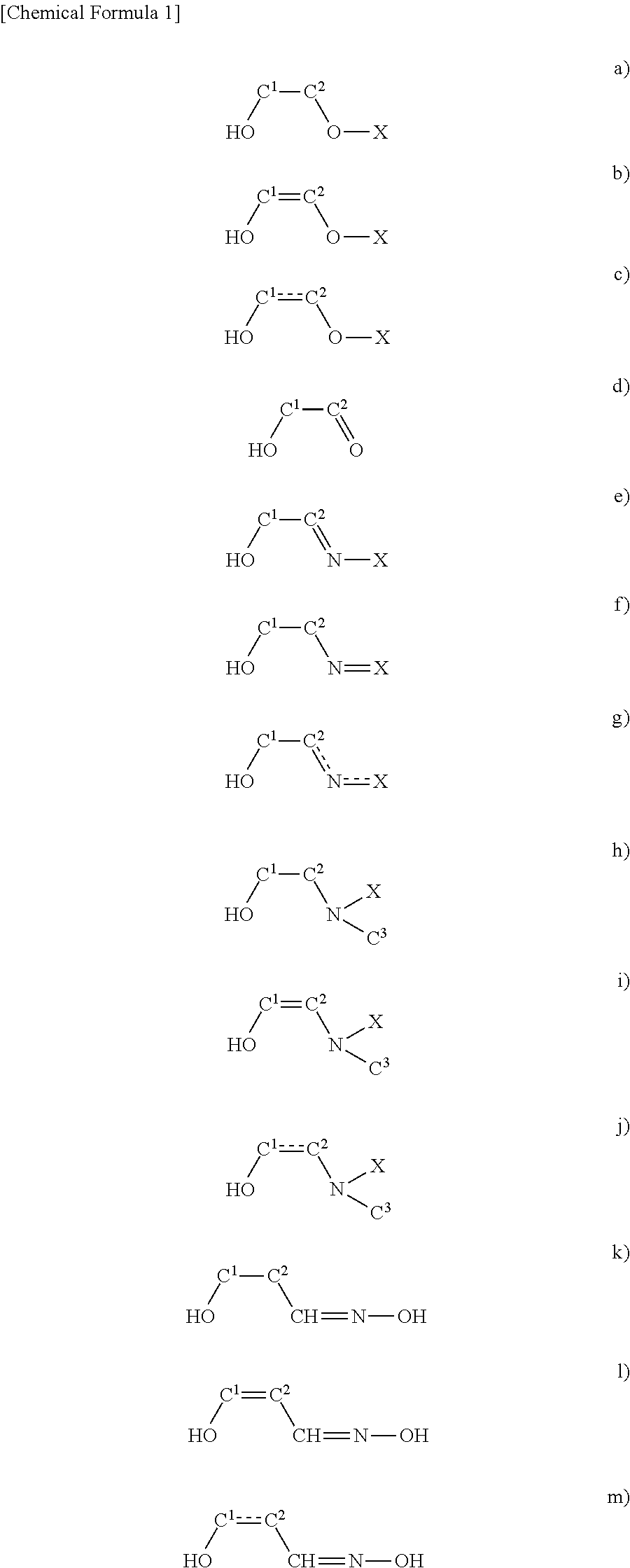
2-AMINOTHIAZOLE reacts violently when nitrated with nitric or nitric-sulfuric acids. It is also incompatible with strong oxidizing agents, strong acids, acid chlorides and acid anhydrides. (NTP, 1992) from CAMEO Chemicals. Literature.
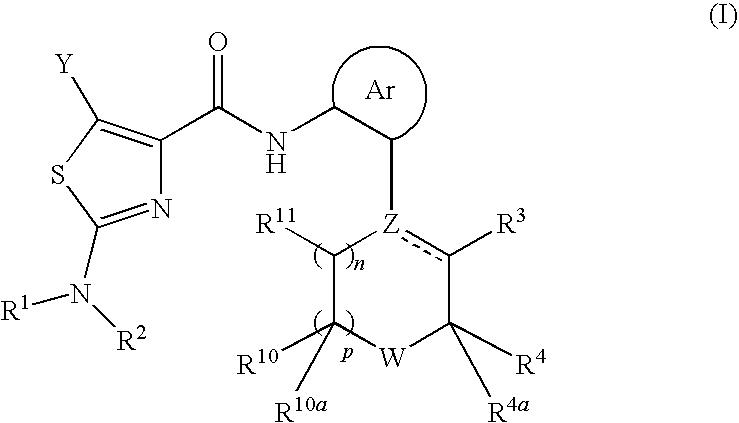
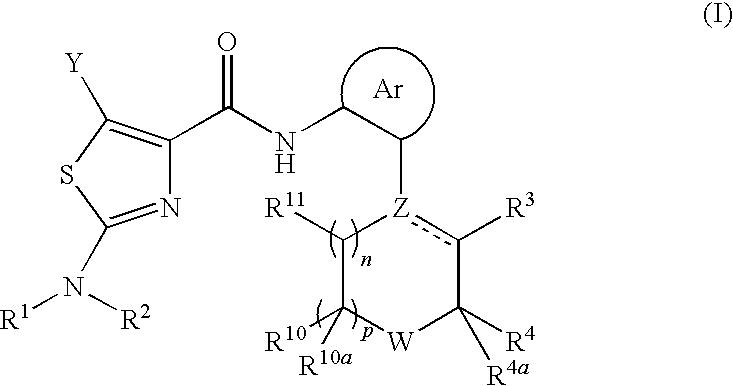
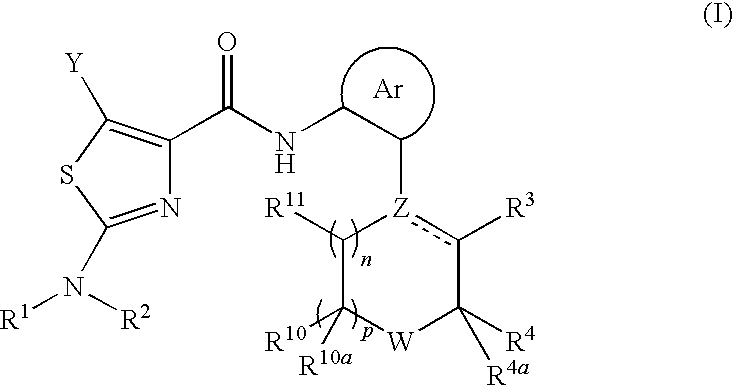
Process for preparing 2-aminothiazole-5-aromatic carboxamides as kinase inhibitors
The invention relates to processes for preparing compounds having the formula (I) and crystalline forms thereof, wherein Ar is aryl or heteroaryl, L is an optional alkylene linker, and R2, R3, R4, and R5, are as defined in the specification herein, which compounds are useful as kinase inhibitors, in particular, inhibitors of protein tyrosine kinase and p38 kinase.
Owner:BRISTOL MYERS SQUIBB HLDG IRELAND UNLTD
Process for preparing 2-aminothiazole-5-aromatic carboxamides as kinase inhibitors
ActiveUS7491725B2Efficient preparationHigh yieldBiocideOrganic active ingredientsArylProtein-Tyrosine Kinases
The invention relates to processes for preparing compounds having the formula,and crystalline forms thereof, wherein Ar is aryl or heteroaryl, L is an optional alkylene linker, and R2, R3, R4, and R5, are as defined in the specification herein, which compounds are useful as kinase inhibitors, in particular, inhibitors of protein tyrosine kinase and p38 kinase.
Owner:BRISTOL MYERS SQUIBB CO
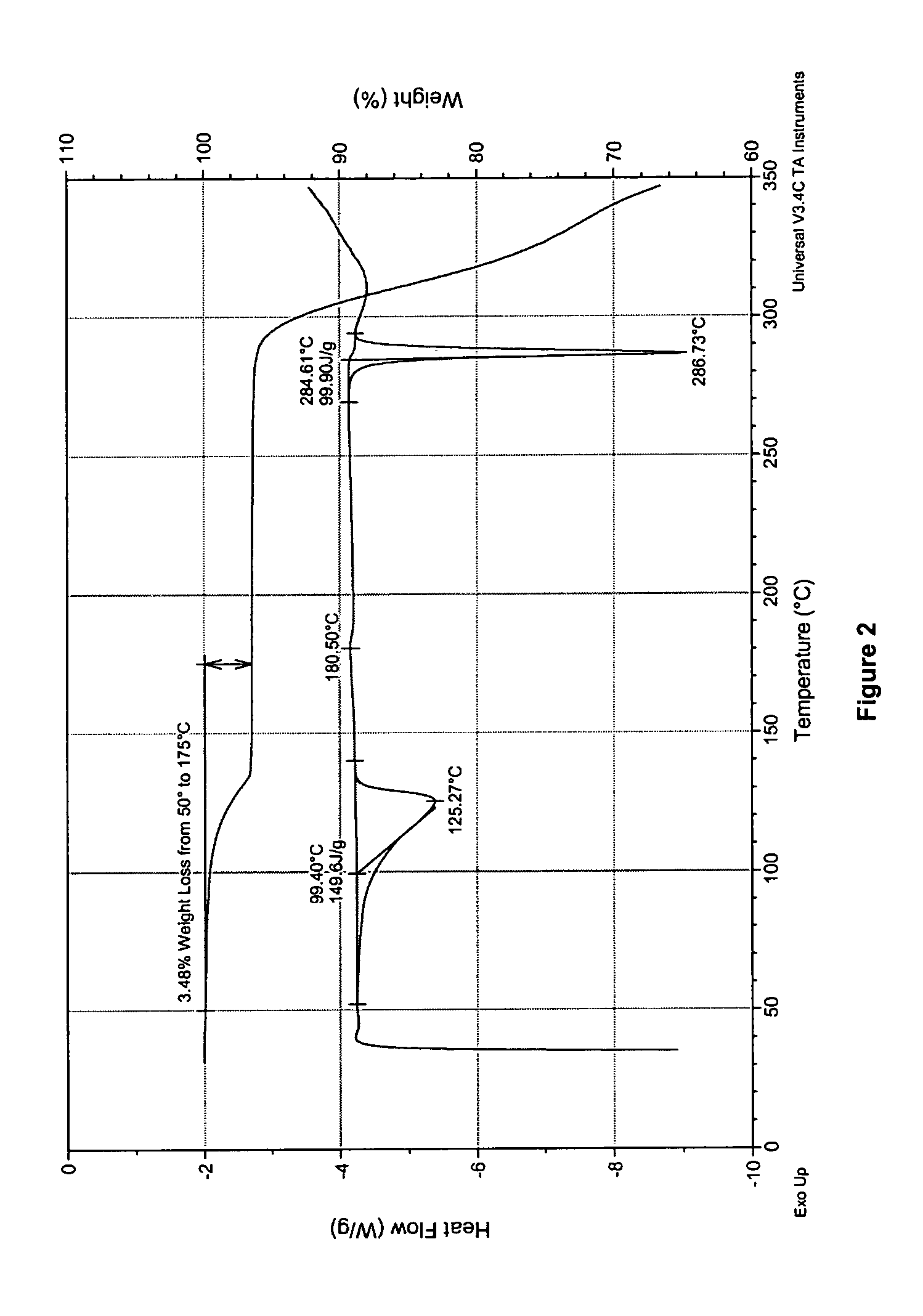
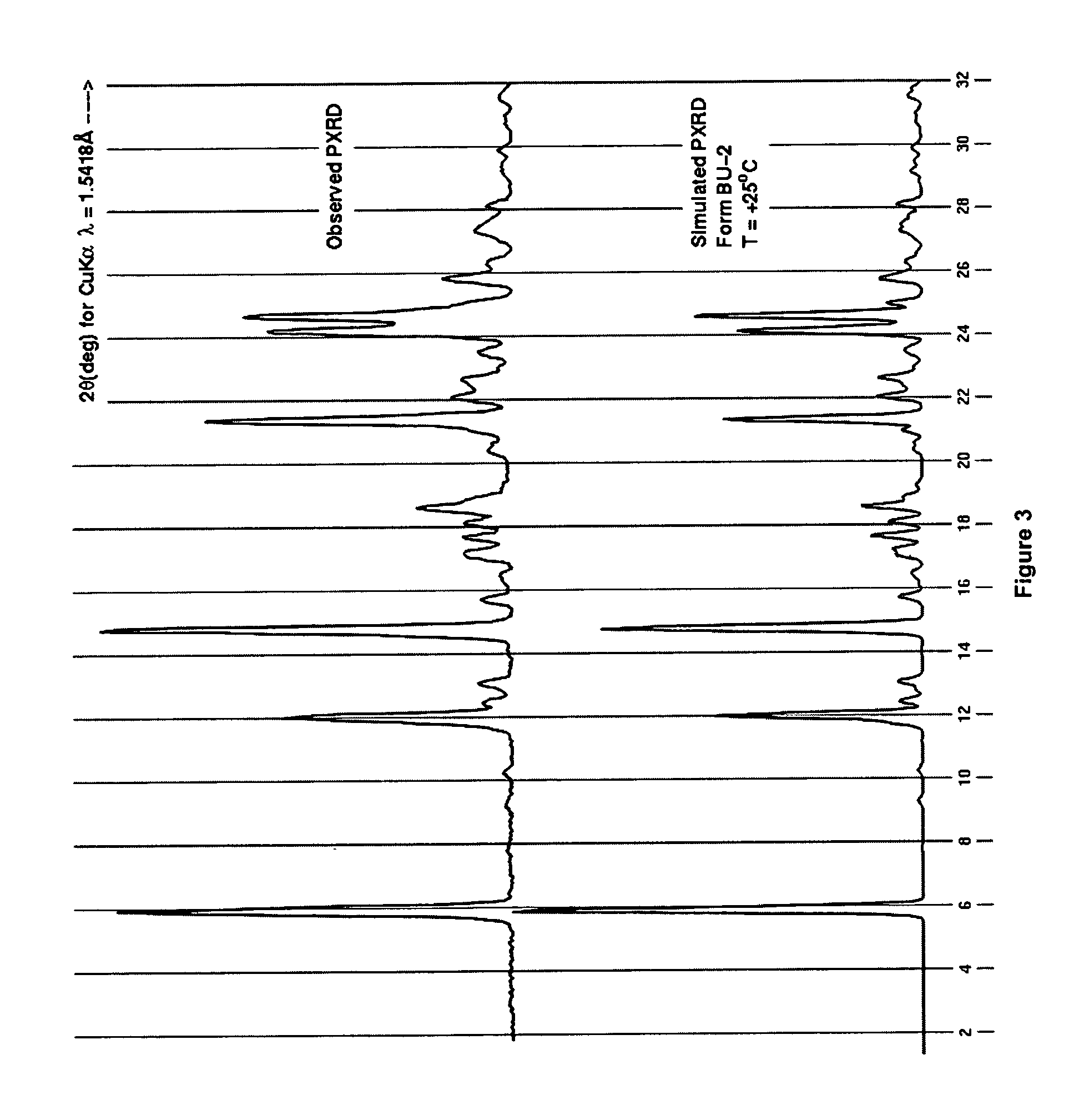
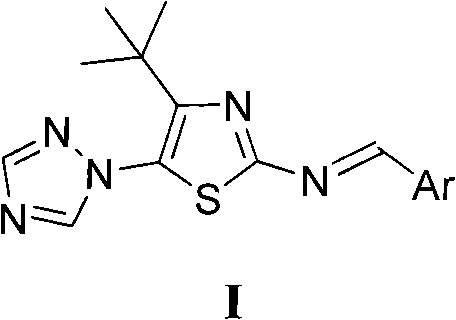
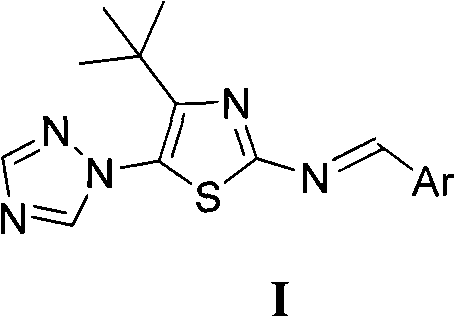
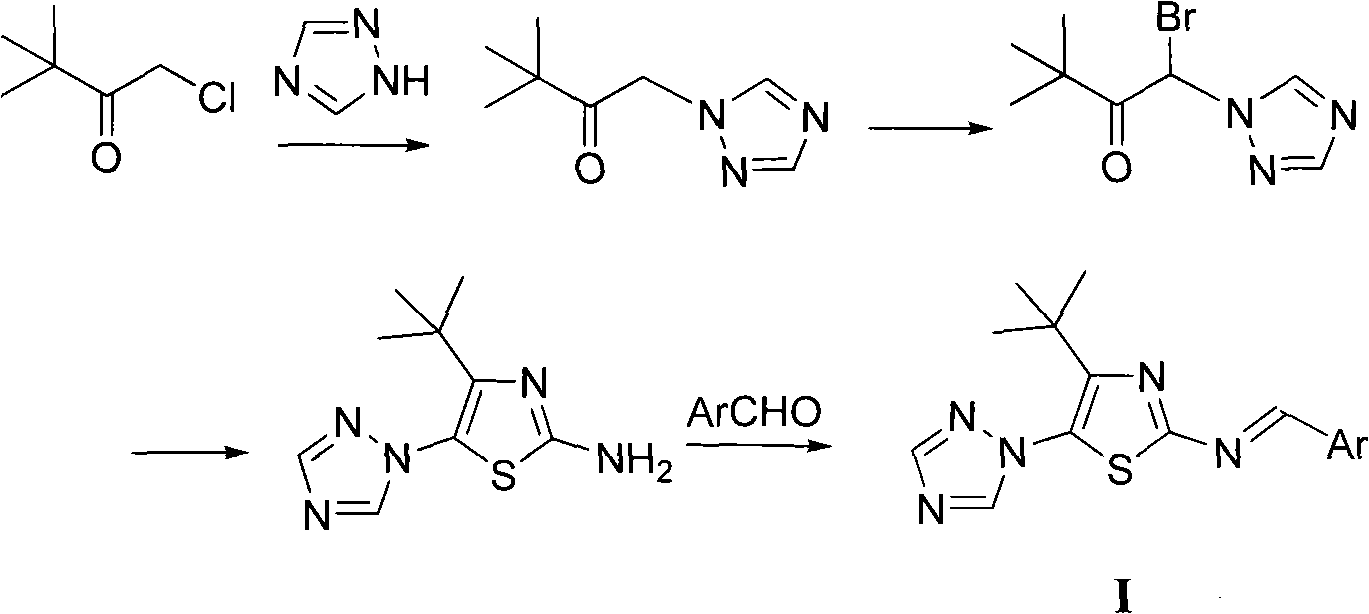
4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-benzylimino thiazole and preparation method and application thereof
The invention discloses 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-benzylimino thiazole (I) having the chemical structural formula shown rightwards. A method for preparing the 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-benzylimino thiazole is as follows: the step of reflux reaction is carried out on the 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-aminothiazole and aryl aldehyde in benzene, thereby preparing the 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-benzylimino thiazole. The 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-benzylimino thiazole can be used for preparing bactericide.
Owner:HUNAN UNIV
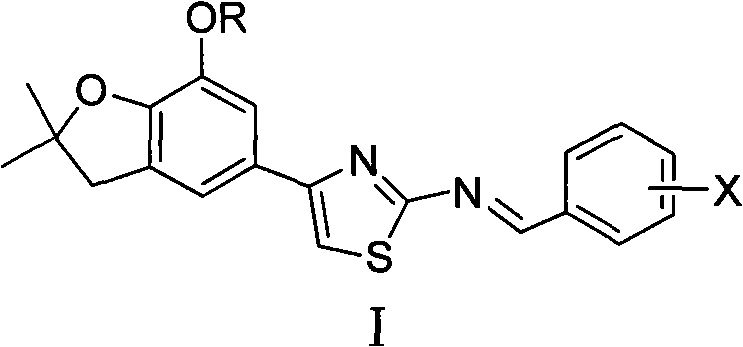
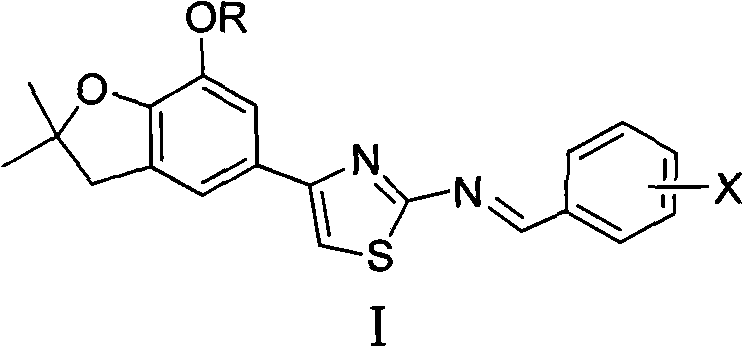
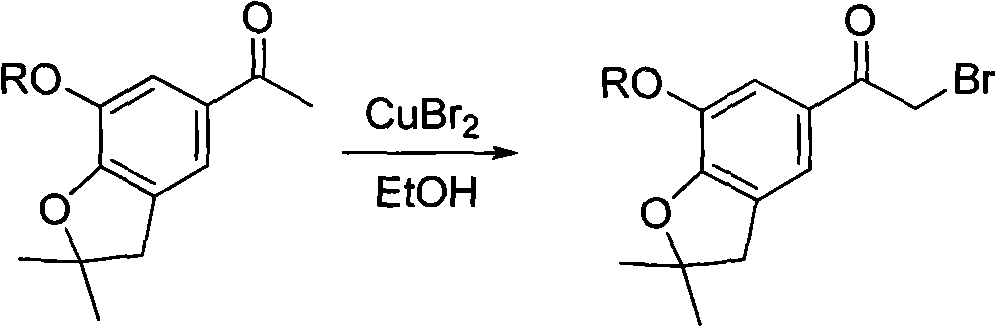
4-(benzofuran-5-yl)-2-benzal aminothiazole and application of 4-(benzofuran-5-base)-2-benzal aminothiazole as antineoplastic agent
The invention discloses 4-(benzofuran-5-yl)-2-benzal aminothiazole as shown in a chemical structural formula I. The preparation method of the 4-(benzofuran-5-yl)-2-benzal aminothiazole is as follows: 1-(7-hydroxy / alkoxy-2,2-dimethyl-2,3-dihydro-benzofuran-5-yl) butanone is subject to bromination and reacts with thiourea to obtain 4-(7-hydroxy / alkoxy-2,2-dimethyl-2,3-dihydro-benzofuran-5-yl)-2-aminothiazole which reacts with aromatic aldehyde to prepare the 4-(benzofuran-5-yl)-2-benzal aminothiazole. The 4-(benzofuran-5-yl)-2-benzal aminothiazole has good activity inhabiting activity on Hela cells, human liver cancer cells (Bel 7402 cells) and lung carcinoma cells (A549 cells) and can be used for preparing the antineoplastic agent.
Owner:HUNAN UNIV
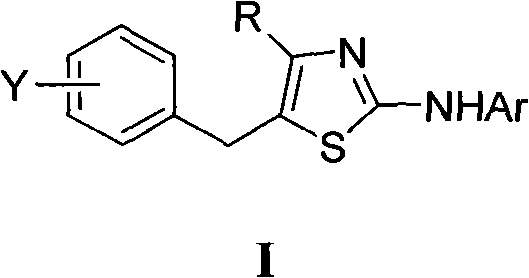
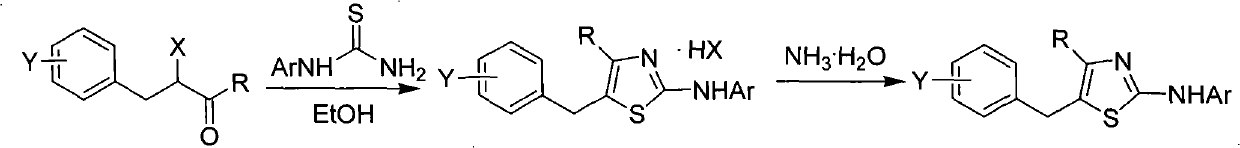
5-benzyl-4-alkyl-2-aminothiazole as well as preparation and application of 5-benzyl-4-alkyl-2-aminothiazole
InactiveCN102070556AHas antitumor activityOrganic active ingredientsOrganic chemistryChemical structureAryl
The invention discloses 5-benzyl-4-alkyl-2-aminothiazole as shown in a chemical structure formula I and pharmaceutically acceptable salts of the 5-benzyl-4-alkyl-2-aminothiazole as shown in the specification. A preparation method of the 5-benzyl-4-alkyl-2-aminothiazole is as follows: heating 2-haslogen-1-aryl alkyl ketone, alkyl thiourea and ethanol, stirring, and reacting for a certain time; filtering, drying to obtain5-benzyl-4-alkyl-2-aminothiazole salts; and filtering the obtained filter cake and neutralizing with ammonia water, and then obtaining 5-benzyl-4-alkyl-2-aminothiazole. The invention also discloses application of the 5-benzyl-4-alkyl-2-aminothiazole or salts thereof in preparing antitumor medicaments.
Owner:HUNAN UNIV
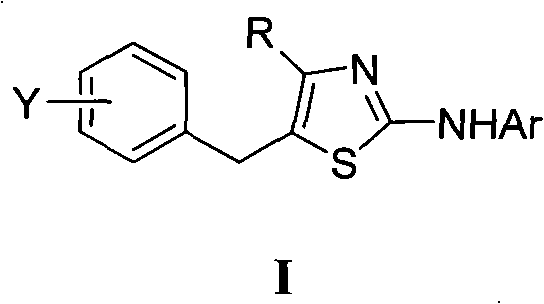
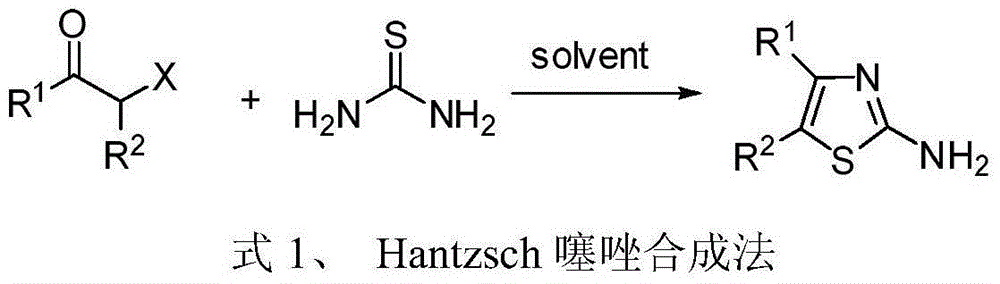
4, 5-disubstituted-2-aminothiazole compound and preparation method thereof
InactiveCN104151262ALow priceReduce pollutionOrganic chemistryAntineoplastic agentsRotary evaporatorPotassium thiocyanate
The invention discloses a 4, 5-disubstituted-2-aminothiazole compound. The structural formula is shown in the specification, wherein R1 is 4-tolyl, 4-chlorophenyl, 4-methoxyphenyl, 4-nitrobenzene or propoxy, and R2 is phenyl, 4-tolyl, 4-fluorophenyl, 4-methoxyphenyl, 2-furyl, isopropyl, 4-nitrophenyl or n-propyl. The invention simultaneously provides a preparation method of the 4, 5-disubstituted-2-aminothiazole compound. The preparation method comprises the following steps: enabling an olefin azide type compound and potassium thiocyanate to react at the temperature of 75-85 DEG C in the presence of a solvent and a metal catalyst, concentrating an obtained reaction solution, then extracting with water and ethyl acetate, washing an obtained organic layer, then drying and concentrating by a rotary evaporator; performing silica gel column chromatography on an obtained concentrate to obtain the 4, 5-disubstituted-2-aminothiazole compound.
Owner:ZHEJIANG UNIV
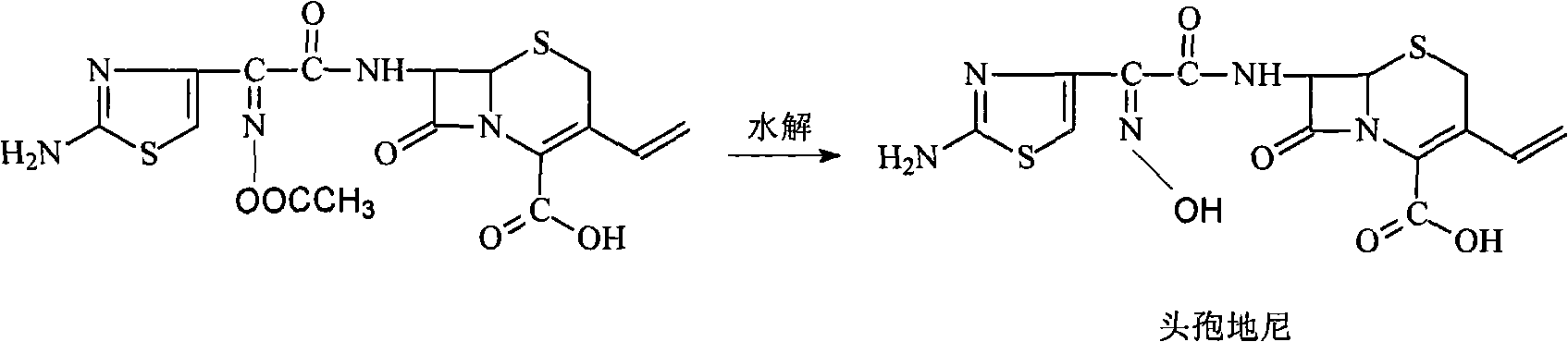
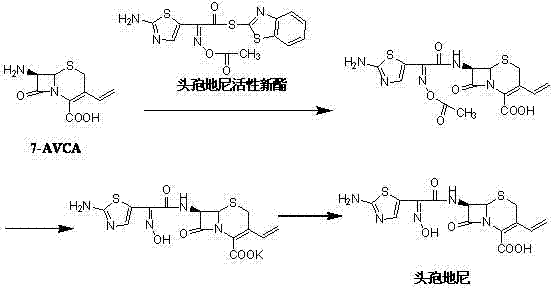
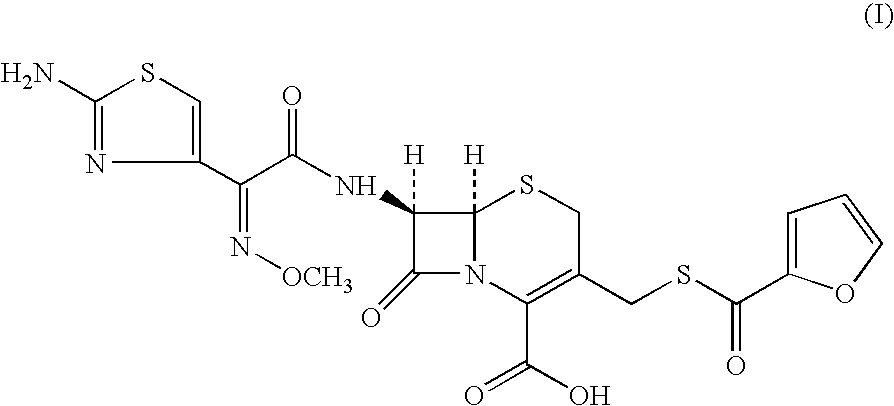
Preparation method of cefdinir
ActiveCN101565427AEasy to recycleReduce pollutionAntibacterial agentsOrganic chemistryOrganic baseCarboxylic acid
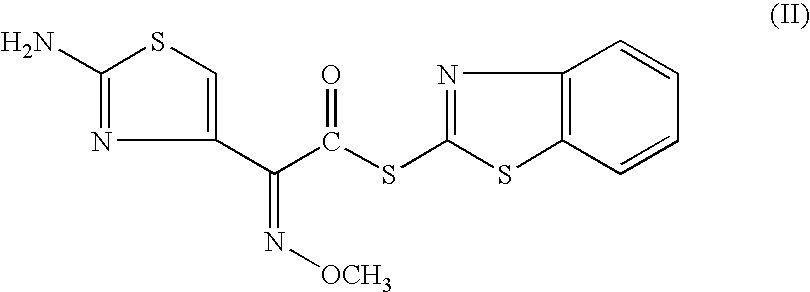
The invention relates to a preparation method of cefdinir, comprising the following steps: reacting 7-amino-3-vinyl-8-oxy-5-thia-1- nitric heterocyclic dicyclo[4.2.0]octyl-2-en-2-carboxylic acid with (Z)-2-(2-aminothiazole-4-yl)-2-acetoxy imino thioacetic acid (S-2-benzothiazole)ester in the presence of organic base at low temperature; extracting, adjusting p H value, preparing the intermediate of the cefdinir, removing the ester-group protective group of the intermediate of the cefdinir to obtain the cefdinir. The preparation method uses the low-temperature reaction technique, capable of increasing the reaction yield and reducing the impurities generated by the high temperature reaction. The hydrolysis and crystallization process is very easily controlled. The used alcohols, ketones or esters solvent is easily recovered, thus the production cost and the three-wastes drain are reduced, therefore the pollution to the environment is reduced. The preparation method of cefdinir is suitable for large-scale production.
Owner:ZHEJIANG ANGLIKANG PHARMA
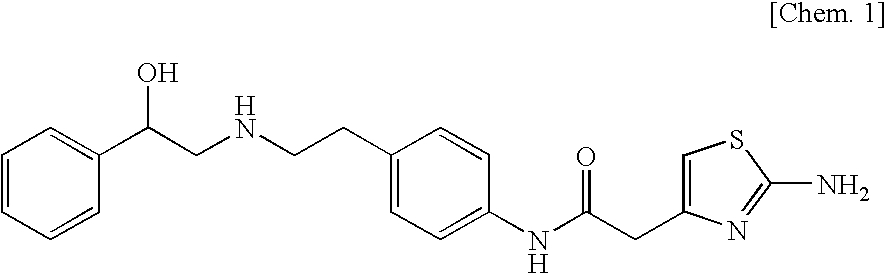
Alpha-form or beta-form crystal of acetanilide derivative
ActiveUS7342117B2Surely and simply obtainedImprove solubilityOrganic active ingredientsBiocideAdditive ingredientDiabrezide
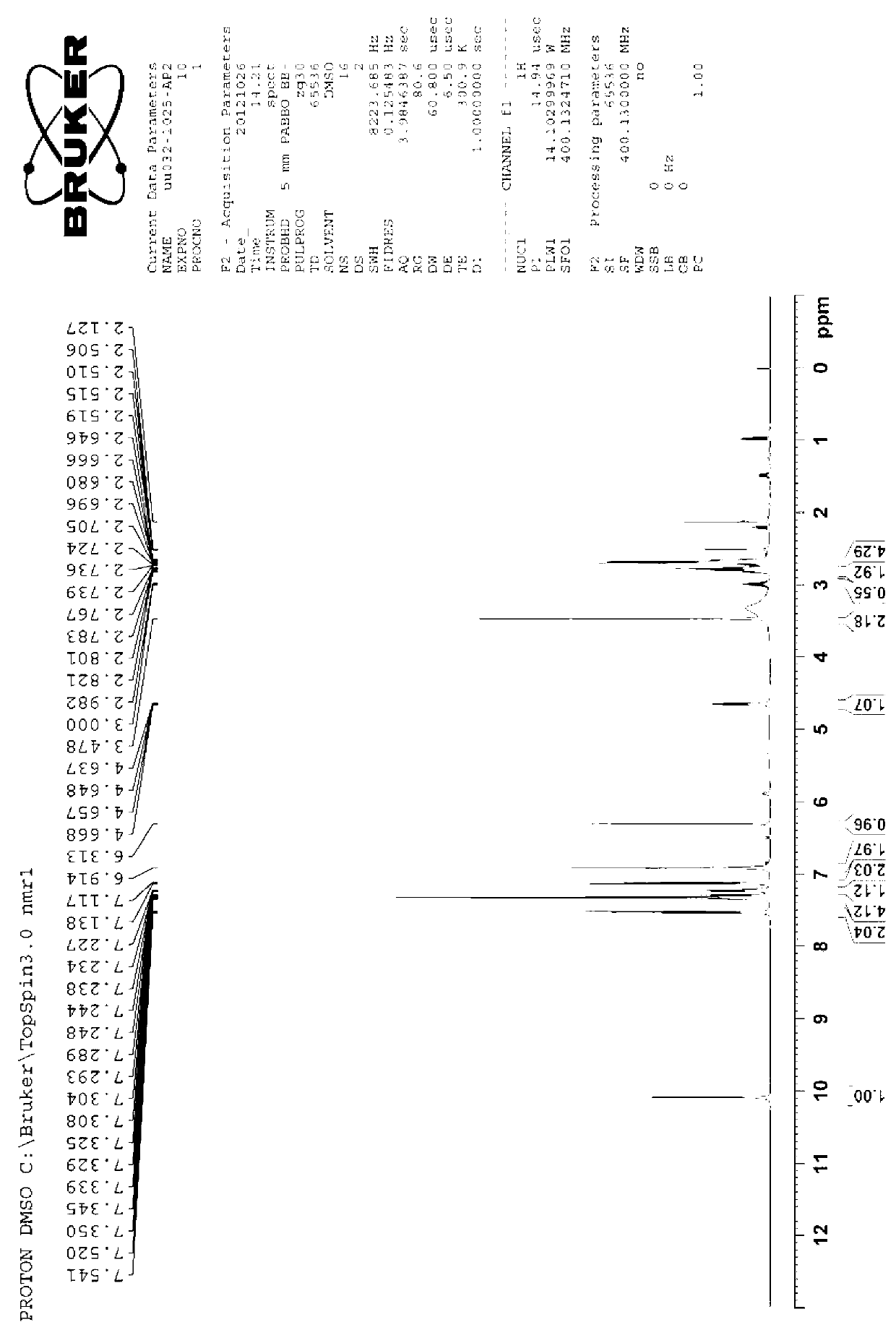
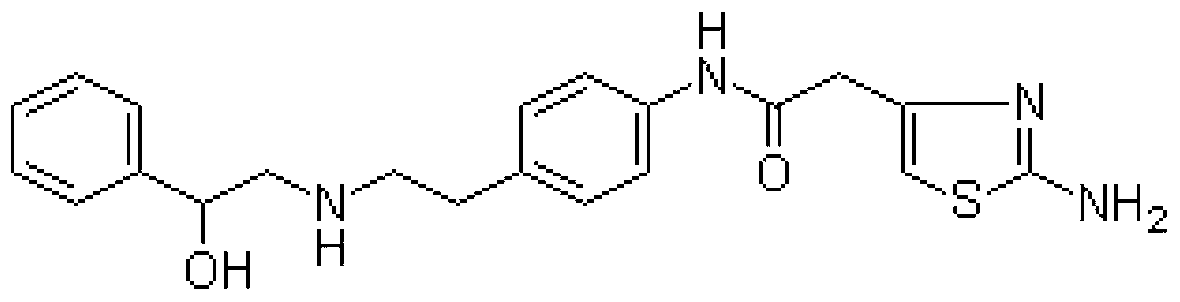
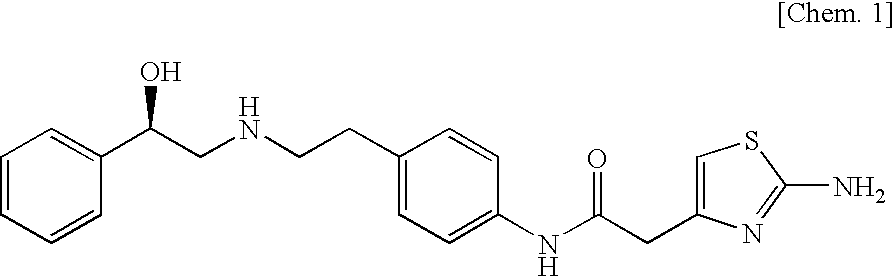
To provide novel crystals useful as an ingredient for the production of a diabetes remedy. The invention is concerned with α-form crystal and β-form crystal of (R)-2-(2-aminothiazol-4-yl)-4′-[2-[(2-hydroxy-2-phenyleth-yl)amino]ethyl]acetanilide. The α-form crystal does not exhibit hygroscopicity and has stability such that it can be used as a medicine, and is useful for mass synthesis in the industrial production. The β-form crystal does not relatively exhibit hygroscopicity and is also useful as a production intermediate of the α-form crystal.
Owner:ASTELLAS PHARMA INC
2-aminothiazoles compound
The invention relates to the technical field of medicinal chemistry, in particular to a 2-aminothiazoles compound or acceptable slat in pharmaceutical science, a preparing method thereof, pharmaceutical composition comprising the same, and application thereof in antineoplastic drug preparation.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
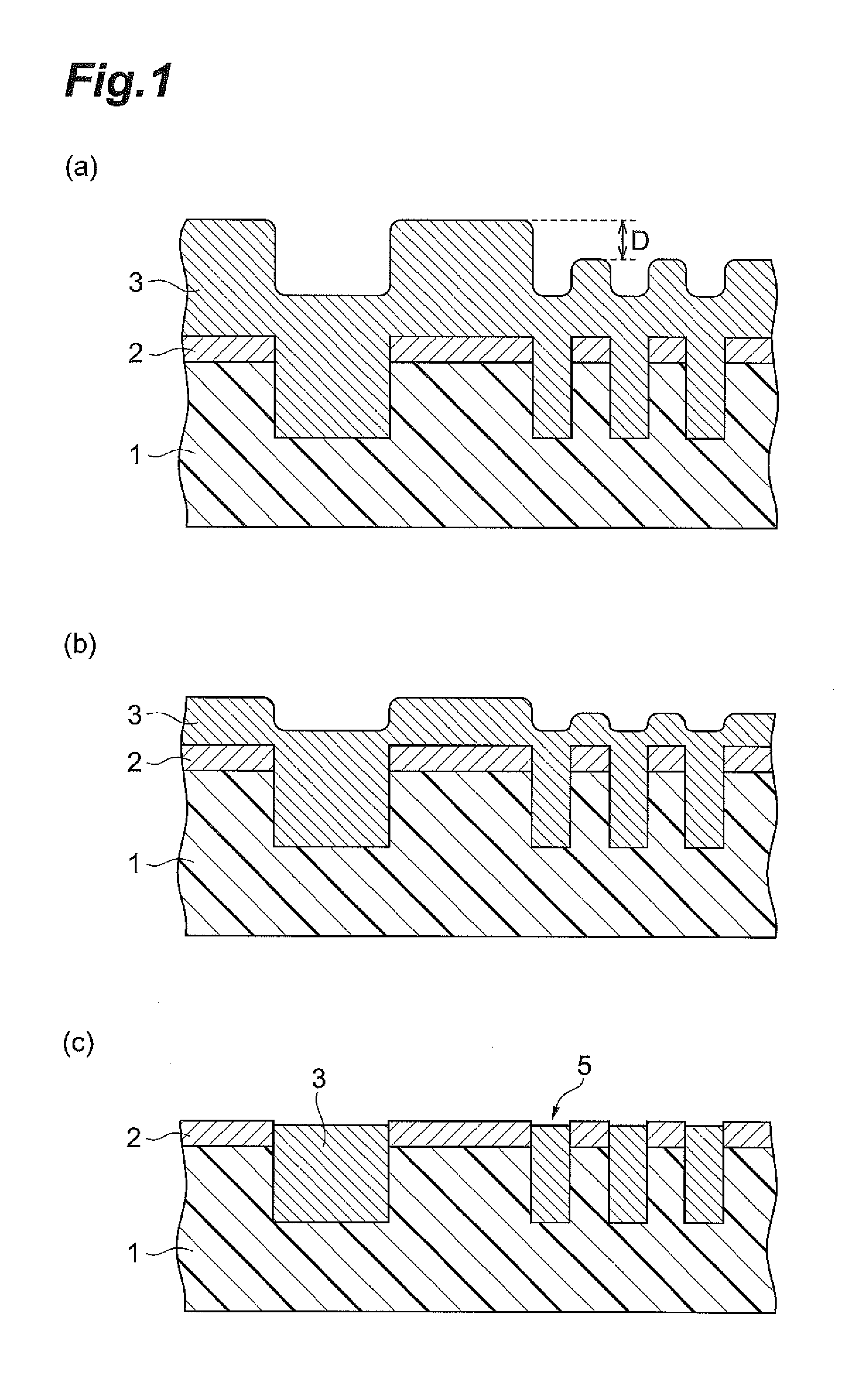
Polishing solution for cmp and polishing method using the polishing solution
ActiveUS20110275217A1Good water solubilitySatisfactory maintenance of dispersibilityOther chemical processesSemiconductor/solid-state device manufacturingMetallurgySilicon oxide
The polishing solution for CMP of the invention comprises abrasive grains, a first additive and water, wherein the first additive is at least 1,2-benzoisothiazole-3(2H)-one or 2-aminothiazole. The polishing method of the invention is a polishing method for a substrate having a silicon oxide film on the surface, and the polishing method comprises a step of polishing the silicon oxide film with a polishing pad while supplying the polishing solution for CMP between the silicon oxide film and the polishing pad.
Owner:RESONAC CORP
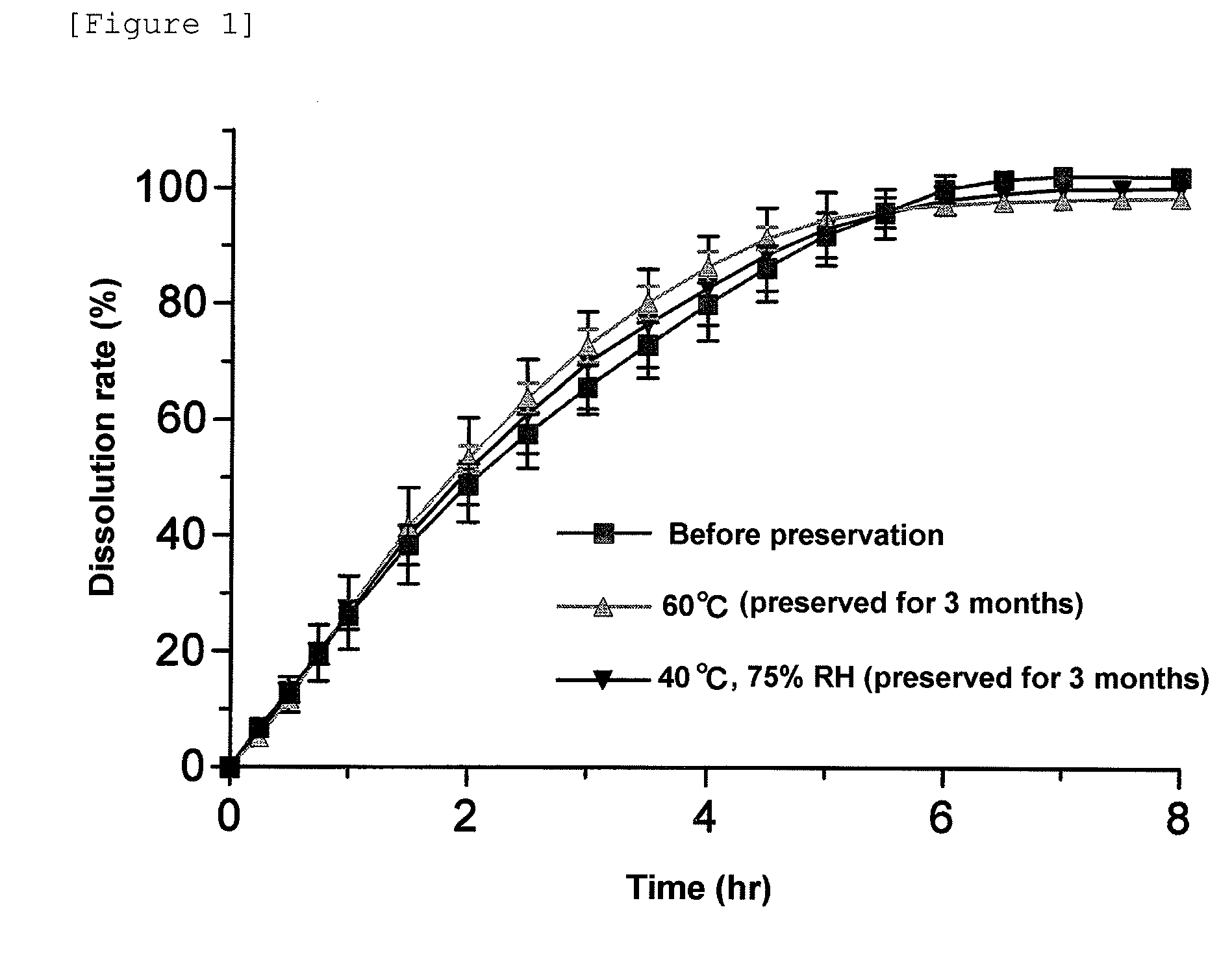
Pharmaceutical composition for modified release
InactiveUS20100144807A1Slow changeReduced stateOrganic active ingredientsBiocideSolubilityAcetic acid
A pharmaceutical composition for modified release, comprising (1) (R)-2-(2-aminothiazol-4-yl)-4′-[2-[(2-hydroxy-2-phenylethyl)amino]ethyl]acetic acid anilide, or a pharmaceutically acceptable salt thereof, (2) at least one additive which ensures penetration of water into the pharmaceutical composition and which has a solubility such that the volume of water required for dissolving 1 g of the additive is 5 mL or less, and (3) a hydrogel-forming polymer having an average molecular weight of approximately 100,000 or more, or a viscosity of 12 mPa·s or more at a 5% aqueous solution at 25° C. is disclosed.
Owner:ASTELLAS PHARMA INC
2-aminothiazole-4-carboxylic amides as protein kinase inhibitors
The present invention relates to novel Anilinopiperazine Derivatives of formula (I), compositions comprising the Anilinopiperazine Derivatives, and methods for using the Anilinopiperazine Derivatives for treating or preventing a proliferative disorder, an anti-proliferative disorder, inflammation, arthritis, a central nervous system disorder, a cardiovascular disease, alopecia, a neuronal disease, an ischemic injury, a viral disease, a fungal infection, or a disorder related to the activity of a protein kinase.
Owner:MERCK SHARP & DOHME LLC
Synthesis method of mirabegron
InactiveCN103193730ALow costRealize safe and clean productionOrganic chemistryBulk chemical productionSynthesis methodsPhenethyl alcohol
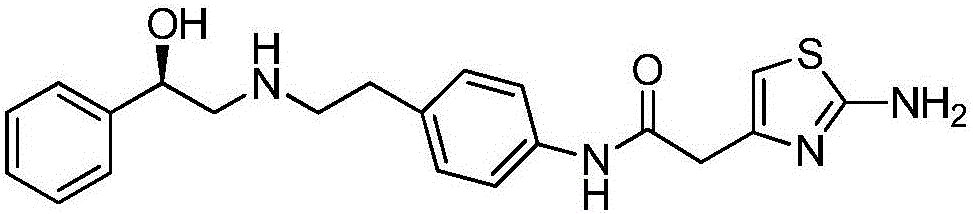
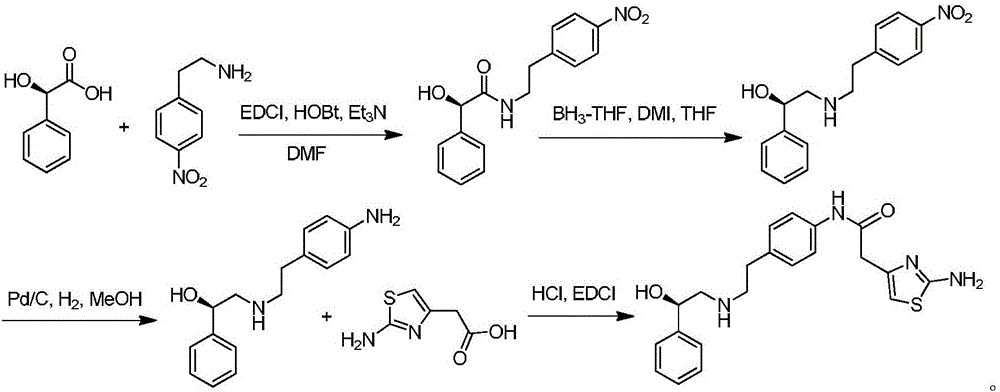
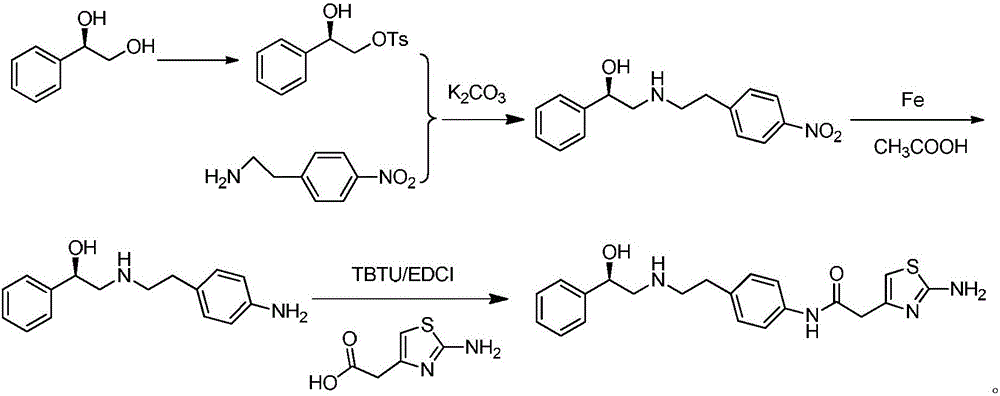
The invention provides a synthesis method of mirabegron and belongs to the technical field of medicine synthesis. The synthesis method solves the problems that the synthesis method of the mirabegron is low in product yield and is not suitable for large-scale industrialized production in the prior art. The synthesis method comprises the following steps: 1) amino protection: reacting 2-aminothiazole-5-acetic acid with an amino protective agent to obtain a mirabegron intermediate product A; 2) condensation reaction: performing condensation reaction on the mirabegron intermediate product A and 4-amino phenethyl alcohol to obtain a mirabegron intermediate product B; 3) oxidation reaction: performing oxidation reaction on the mirabegron intermediate product B and an oxidant to obtain a mirabegron intermediate product C; and 4) reductive amination and protecting group removal: reacting the mirabegron intermediate product C with (R)-2-amino-1-phenethyl alcohol and removing the protecting group from the mirabegron intermediate product C to obtain the mirabegron. The synthesis method of the mirabegron is low in cost, high in product yield and suitable for large-scale industrialized production.
Owner:SUZHOU UUGENE BIOPHARMA
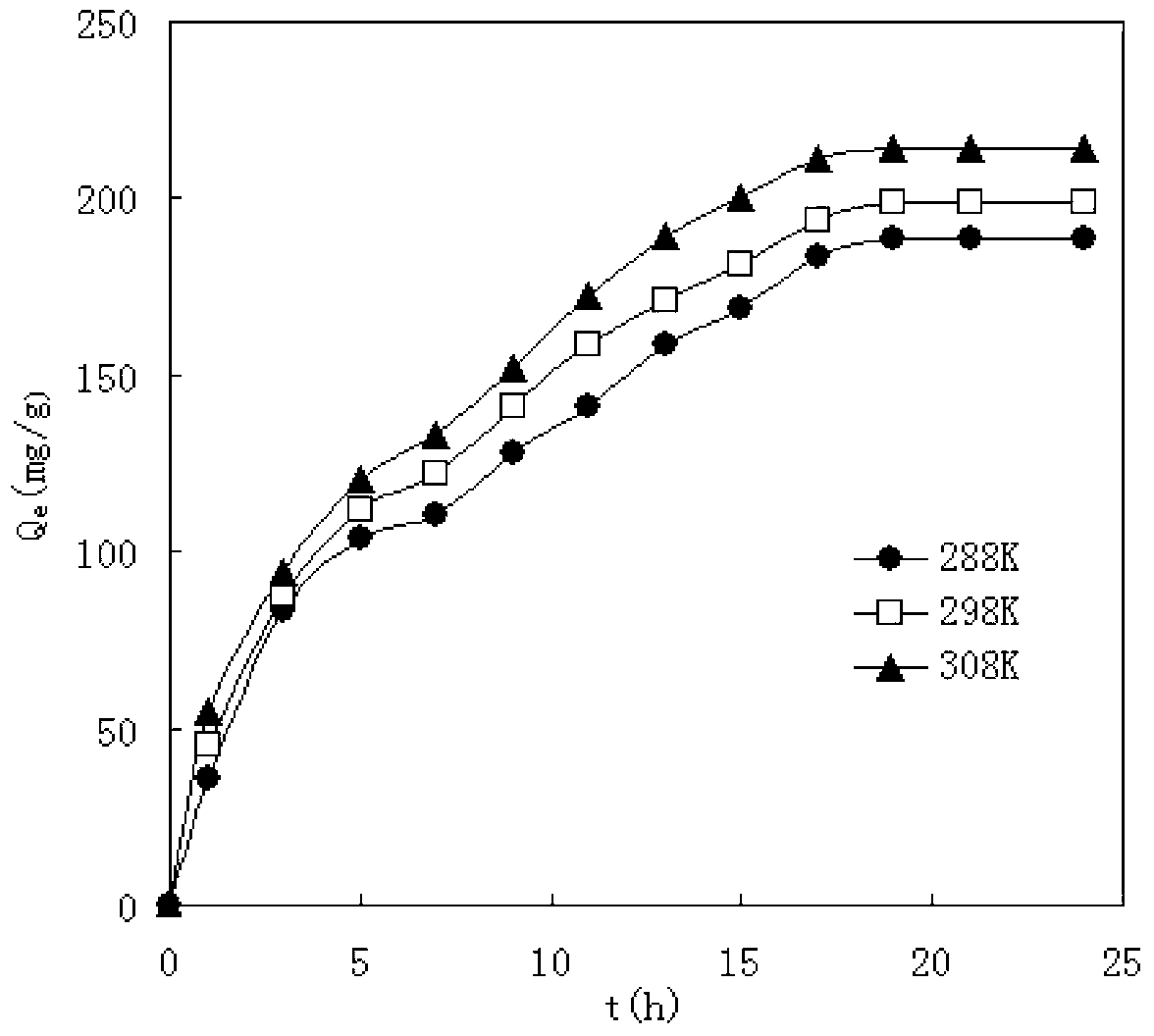
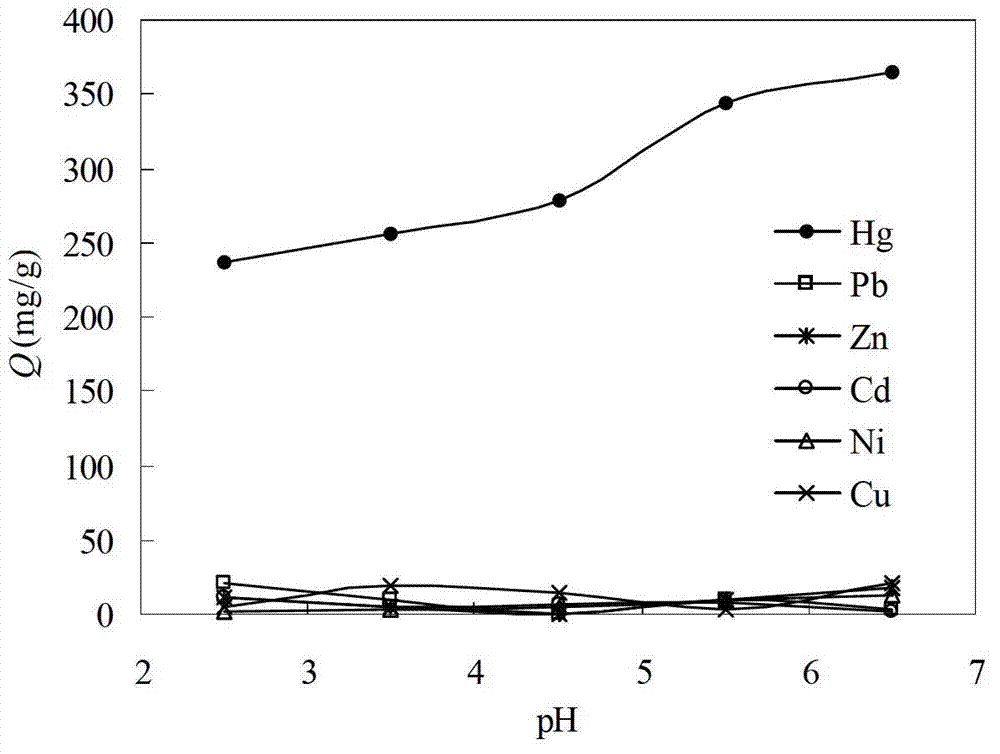
Preparation method of modified magnetic chitosan microsphere heavy metal ion adsorbent
InactiveCN103263895AWide variety of sourcesLow priceOther chemical processesAlkali metal oxides/hydroxidesPreferential adsorptionSorbent
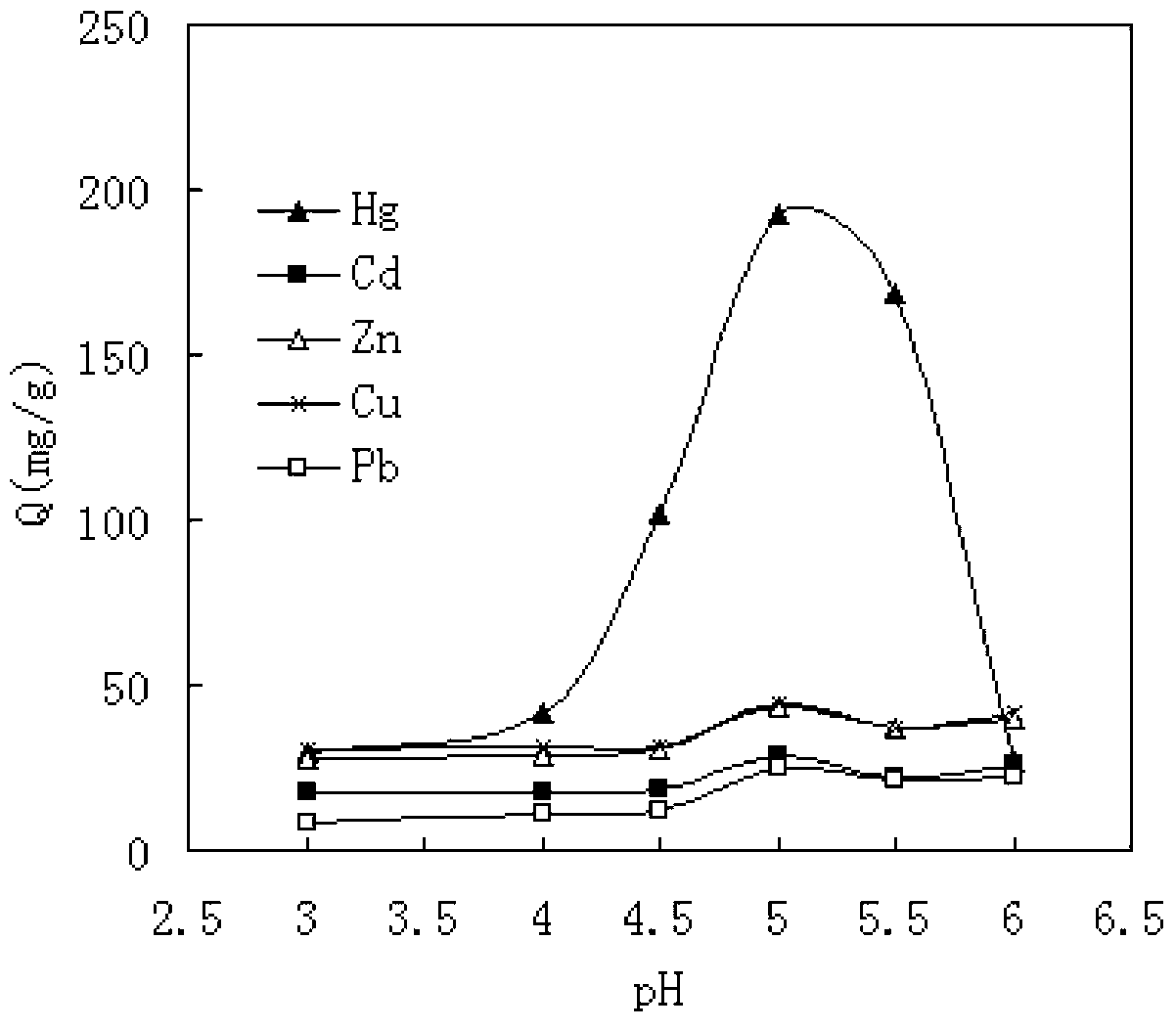
The invention relates to a preparation method of a modified magnetic chitosan microsphere heavy metal ion adsorbent, and in particular relates to a modified magnetic chitosan material which has good selectivity and adsorptive property for heavy metal ions in food, and can be recycled, and a preparation method of the modified magnetic chitosan material. Specifically, the invention discloses the preparation method of the modified magnetic chitosan microsphere heavy metal ion adsorbent. The method comprises the steps of preparing magnetic chitosan microspheres by taking chitosan powder as a raw material, further preparing the magnetic chitosan microspheres into hydroxypropyl chlorine magnetic chitosan microspheres, and carrying out chemical grafting on the hydroxypropyl chlorine magnetic chitosan microspheres by taking the hydroxypropyl chlorine magnetic chitosan microspheres as parents and 2-aminothiazole as ligands to obtain the modified magnetic chitosan microsphere heavy metal ion adsorbent. The modified magnetic chitosan microsphere heavy metal ion adsorbent prepared by the method has preferential adsorption for Hg2+ ions.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
Novel synthesis method of mirabegron
ActiveCN103304511ALow costRealize safe and clean productionOrganic chemistryBulk chemical productionSynthesis methodsPhenethyl alcohol
The invention provides a novel synthesis method of mirabegron, and belongs to the technical field of drug synthesis. The problems that the yield of synthesized mirabegron is low in the prior art, and the method is unsuitable for large-scale industrial production are solved. The synthesis steps are as follows: 1) performing amino protection, namely reacting 2-aminothiazole-4-acetic acid with an amino protective agent to obtain a mirabegron intermediate product A; 2) performing condensation reaction, namely performing the condensation reaction on the mirabegron intermediate product A and the 4-aminophenethanol to obtain the mirabegron intermediate product B; 3) performing oxidizing reaction, namely performing the oxidizing reaction on the mirabegron intermediate product B and an oxidizing agent to obtain the mirabegron intermediate product C; 4) removing protective agent while performing reductive amination, namely reacting the mirabegron intermediate product C with (R)-2-amino-1-phenethanol under the effect of a reducing agent, and meanwhile removing the protective group on the mirabegron intermediate product C to obtain the mirabegron. The novel synthesis method of mirabegron provided by the invention is low in cost, high in product yield and suitable for large-scale industrial production.
Owner:江苏欣德瑞医药科技有限公司
Preparation method of polyacrylonitrile chelating resin adsorbent
InactiveCN102773081AAtom utilization is highReflect the characteristics of green chemistryOther chemical processesMicrosphereSorbent
The invention discloses a preparation method of a polyacrylonitrile chelating resin adsorbent. The method includes firstly, adding polyacrylonitrile microspheres to reaction solvents for immersion; secondly, adding 2-aminothiazole which serves as a ligand and metallic sodium which serves as a catalytic agent to the swelling polyacrylonitrile microspheres for reaction under protection of nitrogen; and thirdly, using the reaction solvents to immerse modified polyacrylonitrile chelating resins, washing the modified polyacrylonitrile chelating resins until washing liquids are colorless, sequentially using deionized water, alcohol and diethyl ethers to wash the modified polyacrylonitrile chelating resins for a plurality of times, then using distilled water to wash the modified polyacrylonitrile chelating resins, sequentially performing alkali wash, water washing, acid washing and water washing, and drying the polyacrylonitrile chelating resins to constant weight at the temperature between 40 DEG C and 60 DEG C to obtain the polyacrylonitrile chelating resin adsorbent. The polyacrylonitrile chelating resin adsorbent prepared by the method can absorb Hg (II) ions in a higher selectivity mode.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
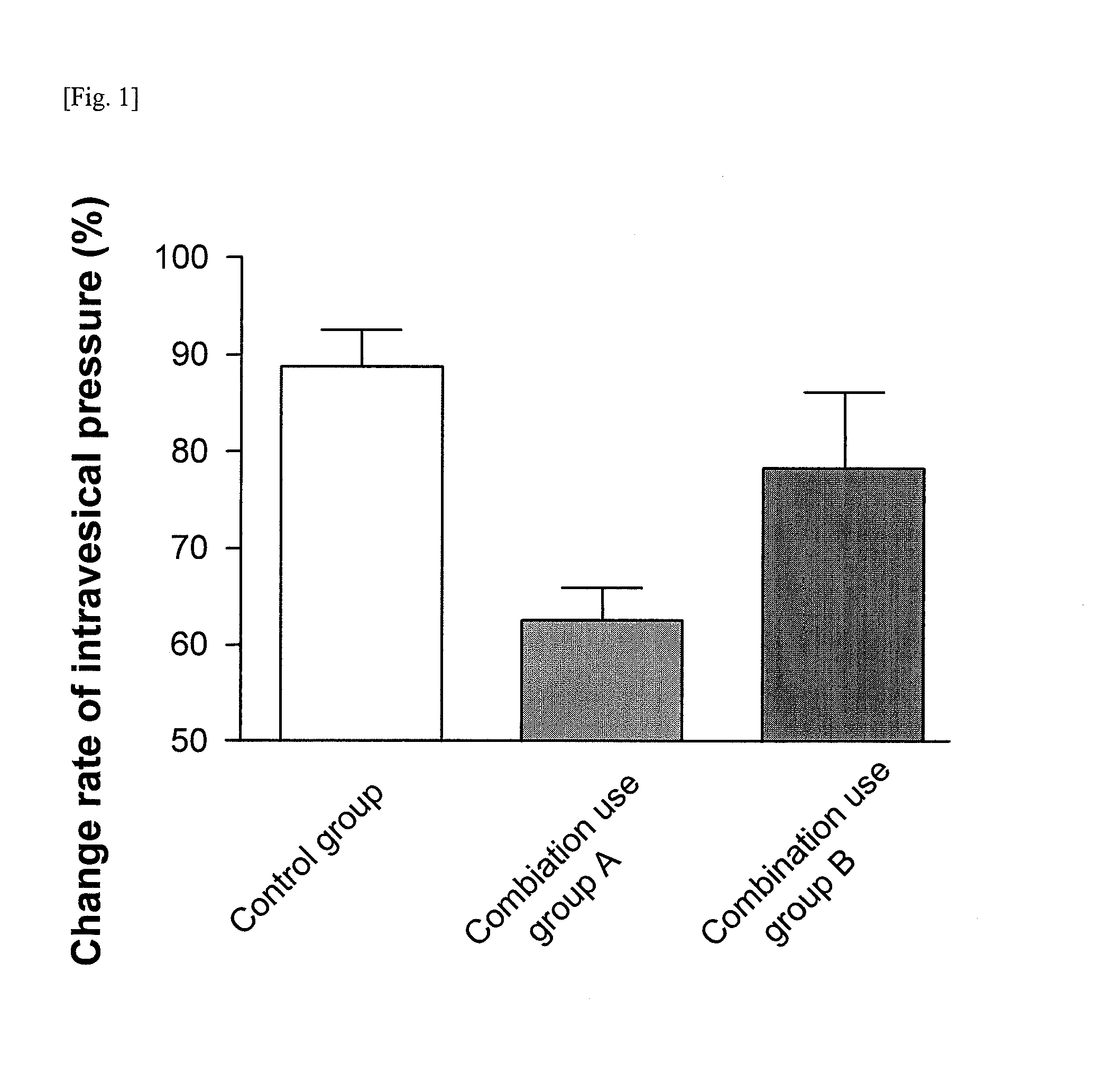
Pharmaceutical composition for treating overactive bladder
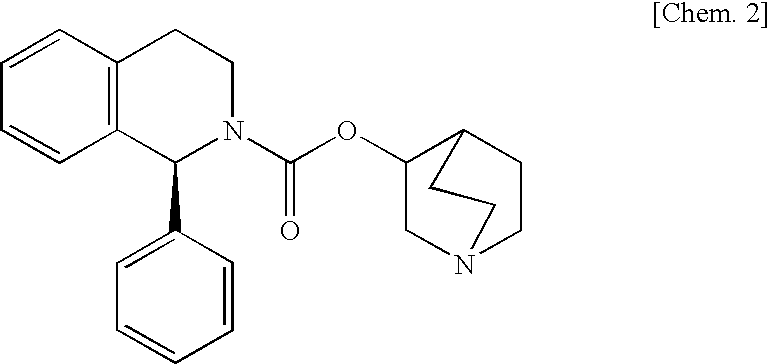
[Problems] To provide a pharmaceutical composition which is useful as a therapeutic agent for overactive bladder.[Means for Solution] A pharmaceutical composition comprising (R)-2-(2-aminothiazol-4-yl)-4′-{2-[(2-hydroxy-2-phenylethyl)amino]ethyl}acetanilide or a pharmaceutically acceptable salt thereof and (3R)-quinuclidin-3-yl (1S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline-2-carboxylate or a pharmaceutically acceptable salt thereof, as active ingredients, in particular a pharmaceutical composition for improving various symptoms accompanying overactive bladder, such as urinary urgency, pollakiuria and / or urinary incontinence
Owner:ASTELLAS PHARMA INC
New preparation method of Cefdinir
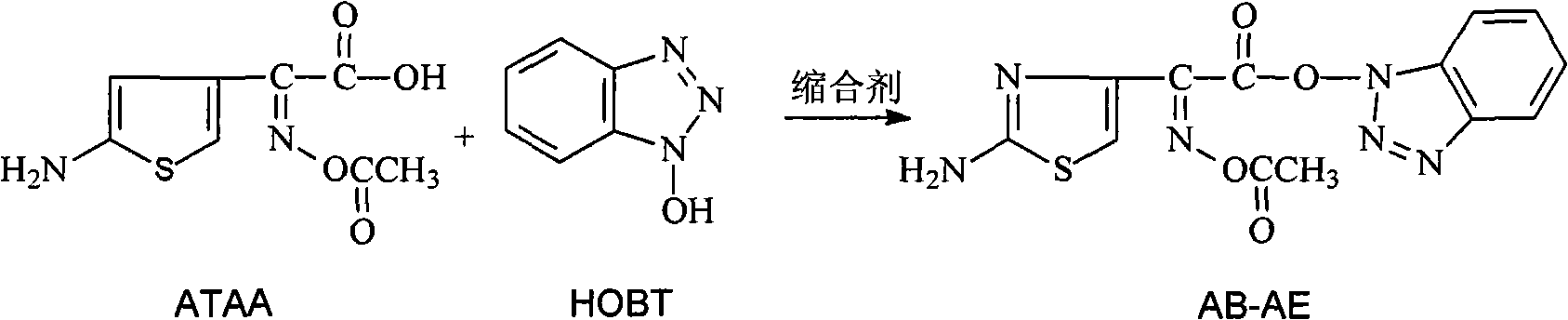
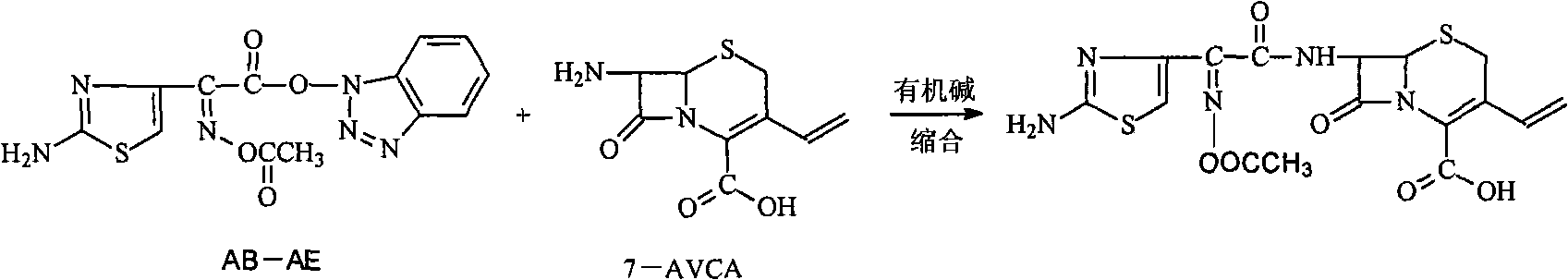
The invention provides a new preparation method of Cefdinir, in particular to a method for preparing Cefdinir from an intermediate of a new active ester. The preparation method comprises the following steps: using (Z)-2-(2-aminothiazole-4-yl)-2-acetoxyiminoacetic acid (ATAA) and 1-hydroxybenzotrizole (HOBT) to be subject to dehydrolysis condensation to generate a new active ester, i.e. 1-[(Z)-2-(2-amino-4-thiazyl)-2-(acetoxyimino) acetoxy] benzotrizole (AB-AE), then using the AB-AE and 7-amino-vinyl-3-cephalosporin-4-carboxylic acid (7-AVCA) as raw materials to be subject to the condensation reaction, and then hydrolyzing to prepare the Cefdinir. The invention has the advantages that the AB-AE has good stability and is convenient to store, the yield of the synthesized Cefdinir is more than 90%, and the process is more applicable to the industrial production.
Owner:ZHEJIANG YONGNING PHARMA
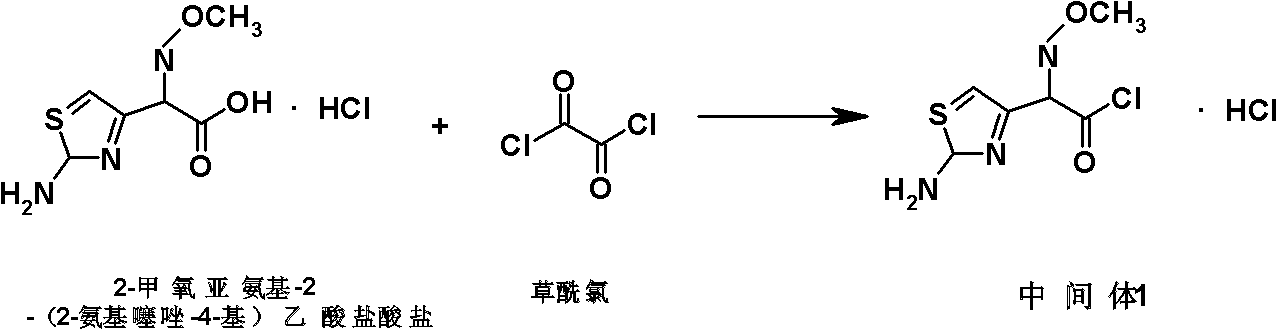
Preparation method of cefepime hydrochloride
ActiveCN101935325ASimple processAvoid the phenomenon of inhomogeneous crystal form and poor fluidityOrganic chemistryCefepime hydrochlorideBetaine
The invention discloses a preparation method of cefepime hydrochloride, comprising the following steps of: reacting oxalyl chloride with 2-methoxyimino-2-(2-aminothiazole-4-yl) acetic acid hydrochloride to obtain a midbody I, i.e. 2-methoxyimino-2-(2-aminothiazole-4-yl) acetyl chloride hydrochloride; mixing silanized 7-aminoce-phalosporanic acid and silanized N-methylpyrrolidine, and reacting to obtain a midbody II, i.e. hydriodic acidification (6R, 7R)-7-amino-3-[(1-methyl-1-tetrahydro pyrrolidine) methyl]-3-cephem-4-formic betaine, in the presence of trimethyl idodine silicon hydride, isopropanol and an aqueous solution of hydrogen iodide; dissolving the midbody II into dichloromethane, sequentially adding trimethylchlorosilane and hexamethyldisilazane for reaction, and then adding the midbody I and triethylamine to react to prepare the cefepime hydrochloride. The cefepime hydrochloride prepared by the method has the advantages of uniform crystal form, good flowability and simple process and is suitable for industrialized production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Method for preparation of EHATA
The invention discloses a new method for preparing ethyl 2-(2-aminothiazole-4-yl)-2-hydroxyimino acetate. The method for preparing ethyl 2-(2-aminothiazole-4-yl)-2-hydroxyimino acetate comprises following steps: taking diketene as raw material and producing intermediate product 4-chloracetyl acetidin through chlorination and esterification, and preparing final product through nitrosation and cyclization reaction. The invention is characterized in that it employs cheaper raw material and phase transition catalysis technology, which simplifies operation process, reduces reaction phase transition, decreases production cost and three- waste pollution, and its industry application value is great.
Owner:SHANDONG HUIHAI PHARMA & CHEM
2-aminothiazole-4-carboxylic amides as protein kinase inhibitors
The present invention relates to novel Anilinopiperazine Derivatives of formula (I), compositions comprising the Anilinopiperazine Derivatives, and methods for using the Anilinopiperazine Derivatives for treating or preventing a proliferative disorder, an anti-proliferative disorder, inflammation, arthritis, a central nervous system disorder, a cardiovascular disease, alopecia, a neuronal disease, an ischemic injury, a viral disease, a fungal infection, or a disorder related to the activity of a protein kinase.
Owner:MERCK SHARP & DOHME LLC
Compound of aztreonam and a synthetic method thereof
InactiveCN101514200AEasy to handleImprove responseAntibacterial agentsOrganic chemistryAcetic acidIminodiacetic acid
The invention provides a compound of aztreonam and a synthetic method thereof. In the method, general solvents are used, suitable amines are chosen, (2-aminothiazole-4-group)-2-(tert-butoxycarbonyl)-iminodiacetic acid isopropoxide acid-2-ester mercaptobenzothiazole and (3-S-trans form)-3-amino-4-methyl-2-oxo-1-sulfonic acid azetidine are used as intermediates; therefore, the method simplifies reaction and has good effect on depriving protecting groups by the mixed aqueous solution of acetic acid and hydrochloric acid.
Owner:HAINAN LINGKANG PHARMA CO LTD
One-pot method for preparing acotiamide hydrochloride
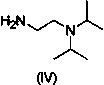
ActiveCN104045606AReduce pollutionOvercome the shortcomings of cumbersome industrial operations and not suitable for industrial productionOrganic chemistryThiazoleSolvent
The invention relates to a one-pot method for preparing an acotiamide hydrochloride (a compound V). The method comprises the following steps: by taking 2,3,5-trimethoxybenzoic acid as shown in a formula (I) and 2-aminothiazole-4-methyl formate as shown in a formula (II) as raw materials, carrying out a condensation reaction to obtain a compound III; selecting an appropriate solvent and controlling a reaction condition, directly carrying out an aminating reaction between an intermediate III which is not separated and purified and N,N-diisoprylamino ethylamine as shown in a formula (IV), and finally obtaining the acotiamide hydrochloride (the compound V) by use of the one-pot method. The one-pot method for preparing the acotiamide hydrochloride has the advantages that the reaction steps are reduced, the operation process is simplified, and the production efficiency is improved; besides, the method is safe and environmental friendly, and suitable for industrial production. The formulas (I, II, III, IV and V) are as shown in the specification.
Owner:HANGZHOU XINBOSI BIOMEDICAL CO LTD
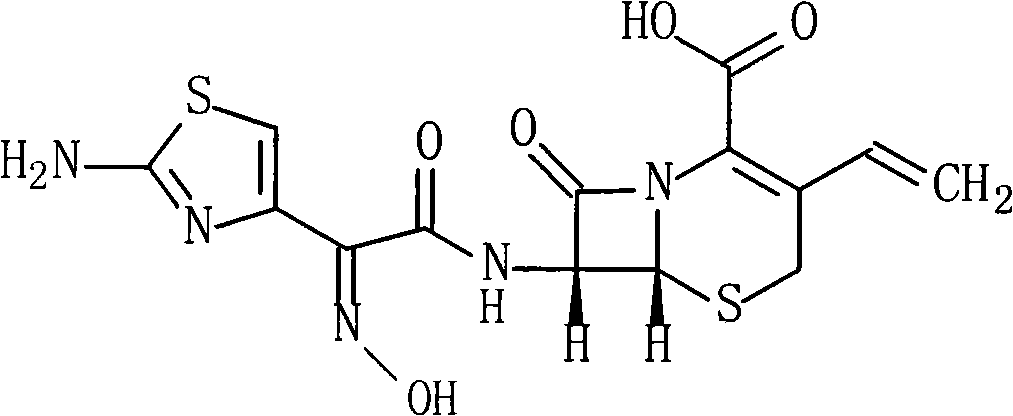
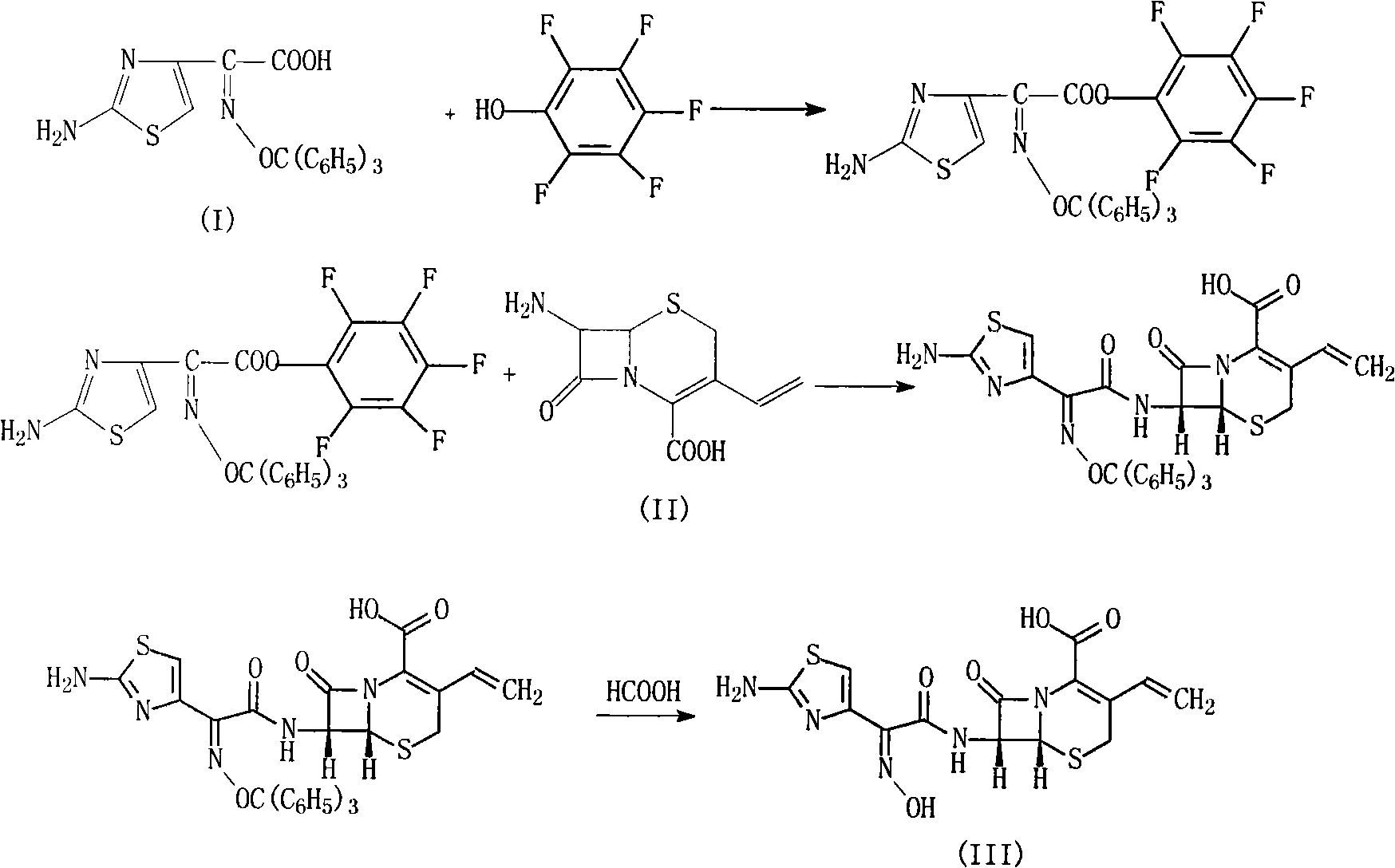
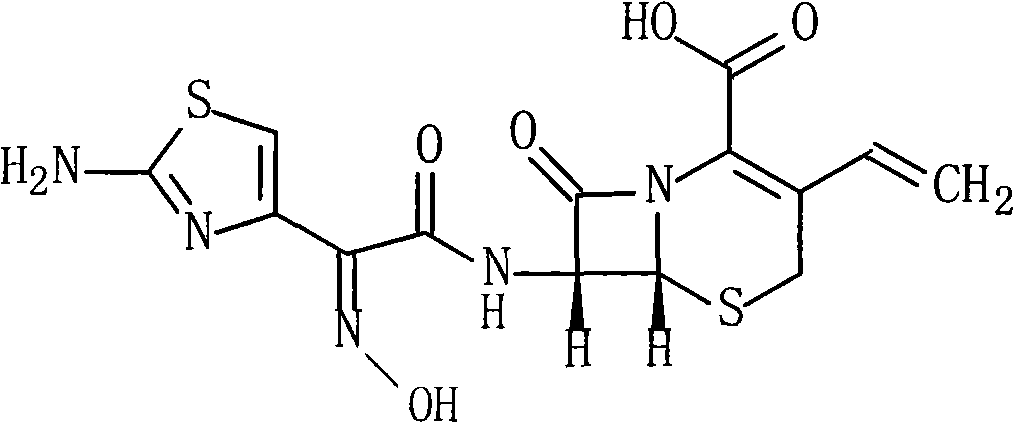
Cefdinir compound and new preparation method thereof
The invention discloses a cefdinir compound and a new preparation method thereof. The method prepares the cefdinir by performing reaction on (Z)-2-(2-aminothiazole-4-group)-2-triphenylmethyl iminoacetic acid serving as an initial raw material, pentafluorophenol serving as an activating group and 7-AVCA.
Owner:HAINAN MEIDA PHARMA
Remedy for overactive bladder comprising acetic acid anilide derivative as the active ingredient
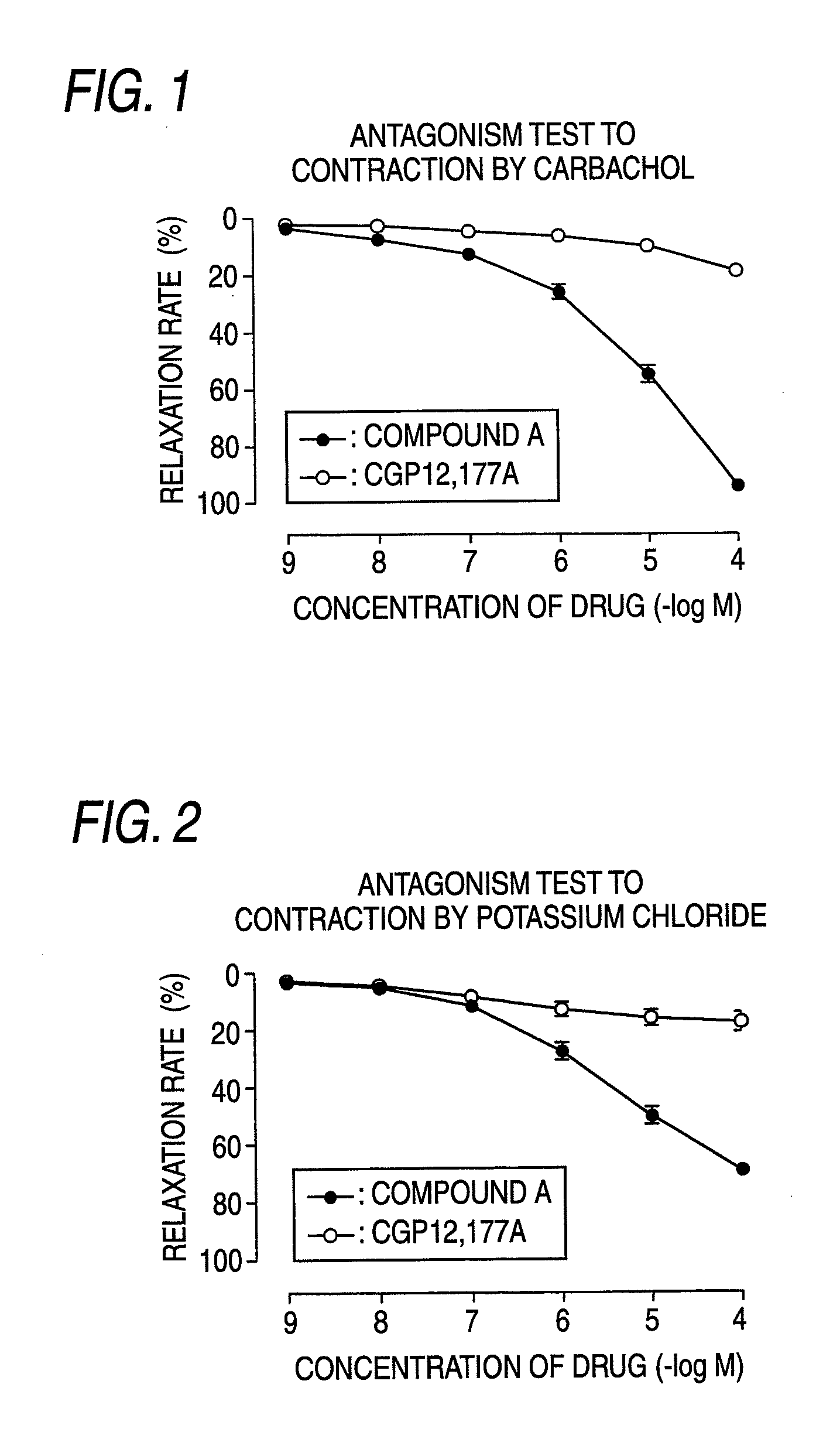
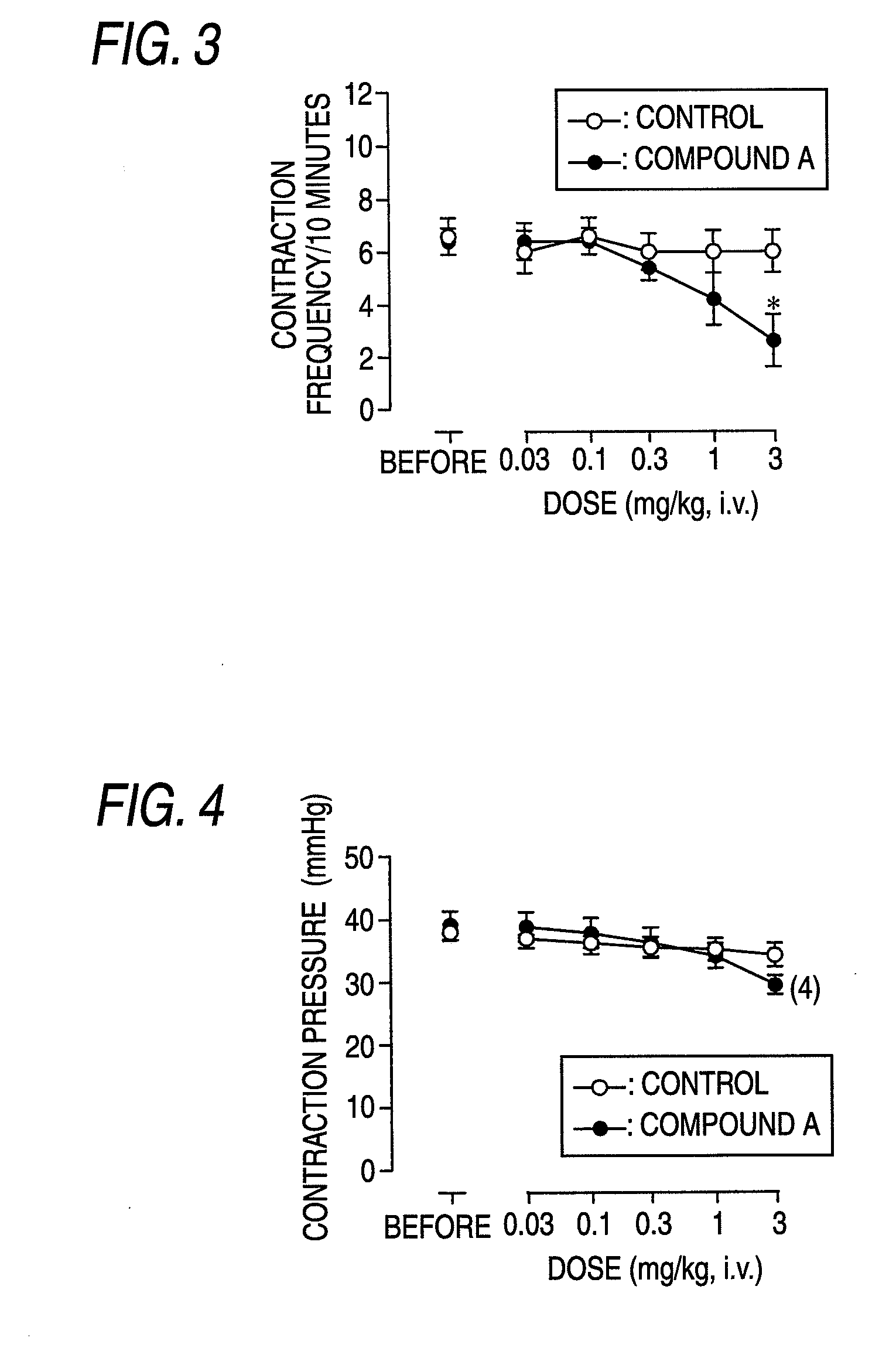
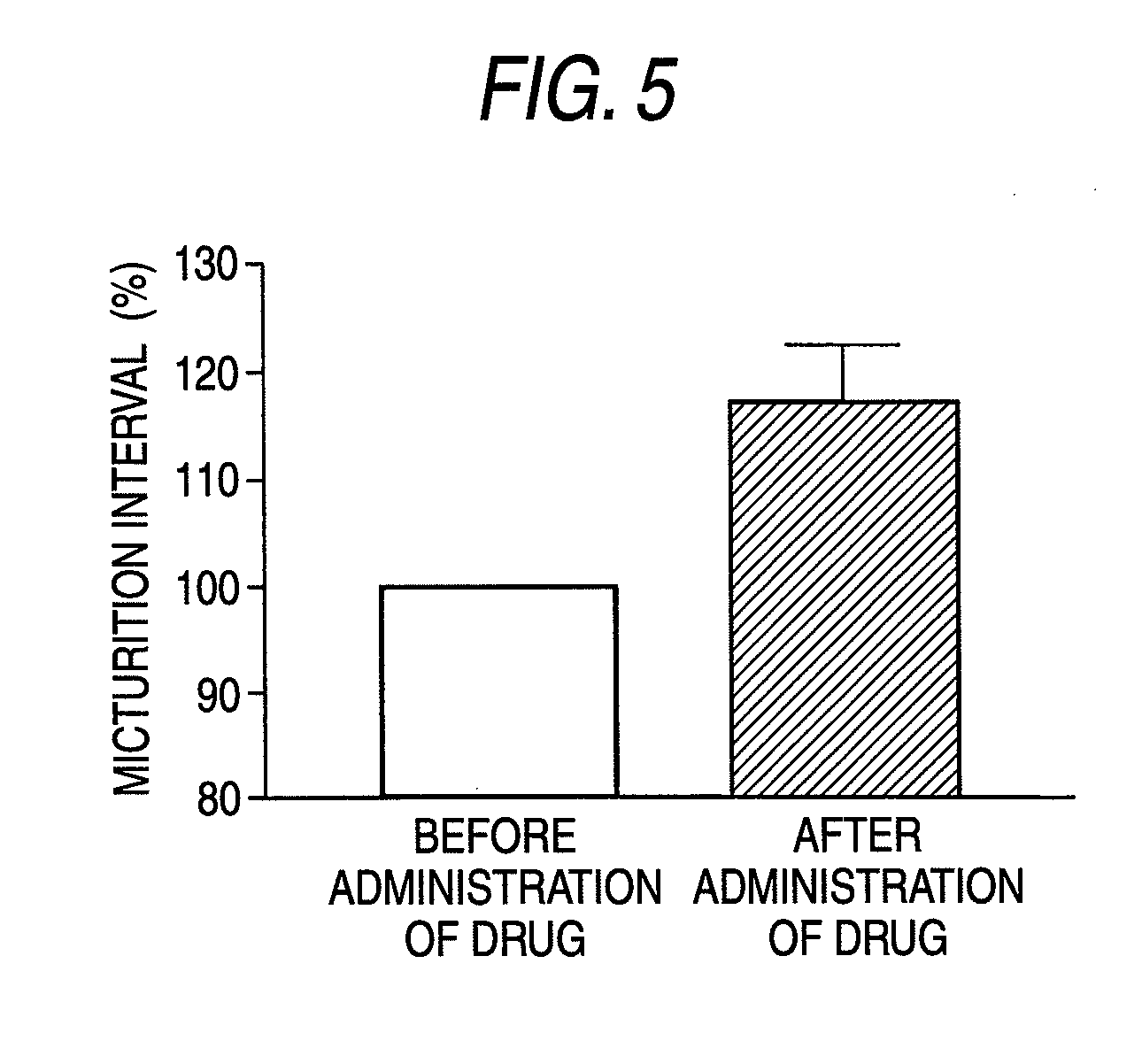
InactiveUS20090093529A1Strong bladder relaxation actionDecreases contraction frequency of contractionBiocideOrganic active ingredientsMeasurement testBULK ACTIVE INGREDIENT
(R)-2-(2-aminothiazol-4-yl)-4′-[2-[(2-hydroxy-2-phenylethyl)amino]ethyl]acetic acid anilide or its salt shows a potent bladder relaxation effect in “isolated rat bladder smooth muscle relaxation test”, dose-dependently lowers the contraction frequency of rhythmic bladder contractions in “rat rhythmic bladder contraction measurement test” and, moreover, prolongs the urination intervals in “urination functions measurement test on cyclophosphamide-induced overactive bladder model rat”. Owing to these effects, the above compound is useful as a remedy for overactive bladder.
Owner:ASTELLAS PHARMA INC
Preparation method of cefdinir
InactiveCN102516261AThe size is easy to controlEasy to wash offOrganic chemistryCarboxylic acidAlkaline hydrolysis
The invention relates to a preparation method of cefdinir. According to the invention, under the effect of triethylamine, 7-amino-3-vinyl-8-oxo-5-thia-1-azabicyclo[4.2.0]octa-2-alkene-2carboxylic acid is subject to a reaction with (Z)-2-(2-aminothiazole-4-group)-2-acetyl oxyimino thioacetic acid(S-2-benzothiazole), such that a cefdinir ester liquid is obtained; the cefdinir ester liquid is extracted; an organic solvent is added to the cefdinir ester liquid; acetyl is removed by alkaline hydrolysis; sylvite of a weak acid is added to the liquid, the pH value is controlled, such that cefdinir sylvite is obtained; the sylvite is dissolved by using water; an organic solvent is added to the solution; the pH value is regulated, such that cefdinir is obtained. With the method, yield and quality are substantially improved; crystal form of the product is stable; and the method is suitable for industrialized productions.
Owner:ZHEJIANG GUOBANG PHARMA
2-aminothiazole-4-carboxylic amides as protein kinase inhibitors
The present invention relates to novel Anilinopiperazine Derivatives of formula (I), compositions comprising the Anilinopiperazine Derivatives, and methods for using the Anilinopiperazine Derivatives for treating or preventing a proliferative disorder, an anti-proliferative disorder, inflammation, arthritis, a central nervous system disorder, a cardiovascular disease, alopecia, a neuronal disease, an ischemic injury, a viral disease, a fungal infection, or a disorder related to the activity of a protein kinase.
Owner:MERCK SHARP & DOHME LLC
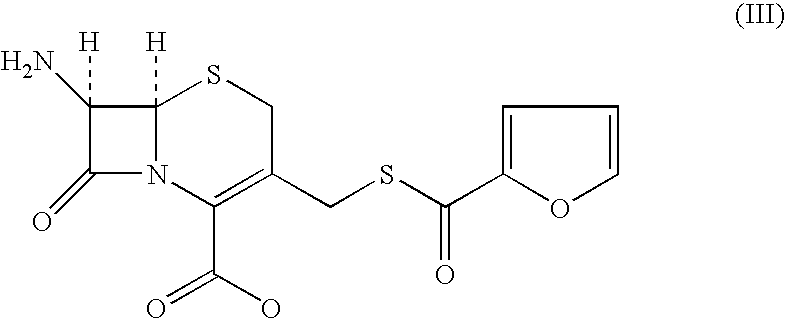
Method for manufacture of ceftiofur
A process for preparation of ceftiofur of formula (I)having purity greater than 97% is disclosed. The process comprises reacting [2-(2-aminothiazol-4-yl)]-2-syn-methoxyimino acetic acid-2-benzothiazolyl thioester of formula (II),with 7-amino-3-(2-furanylcarbonylthiomethyl)-3-cephem-4-carboxylic acid of formula (III)in the presence of a mixture of an water-immiscible inert organic solvent and water and in the presence of a organic base and isolating ceftiofur of formula (I) substantially free of impurities by,a) adding water to the reaction mixture and selectively partitioning the impurities in the organic phase and ceftiofur (I) in the form of a salt with the base in the aqueous phase,b) acidifying the aqueous phase containing ceftiofur (I) in the form of a salt with the base in the presence of a mixture containing a water-miscible and a water-immiscible organic solvent and in the presence of a saturated aqueous solution of an alkali or alkaline earth containing salt, to partition ceftiofur (I) in the organic phase, andc) isolating ceftiofur (I) of high purity and substantially free of impurities by evaporation of the organic solvent or precipitation by addition of a anti-solvent.
Owner:LUPIN LTD
Preparation method of cefdinir
InactiveCN103319503AGood for pH adjustmentDissolution Control and ConditioningOrganic chemistrySulfurNitrogen
The invention discloses a preparation method of cefdinir, which comprises following steps: (1) letting 7-amino-3-vinyl-8-oxo-5-sulfur heterocyclic-1-aza-bicycle [4.2.0] symplectic-2-alkene-2-carboxylic acid (7-AVCA) react with (Benzothiazol-2-yl)-(Z)-2-trityloxyimino-2-(2-aminothiazole-4-yl)-thioacetate with the existence of Tributylamine in a dichloromethane system to obtain a cefdinir ester solution, then conducting steaming to remove the dichloromethane in order to obtain a sepia and thick cefdinir ester solid; (2) purifying the cefdinir ester solid obtained in (1) to obtain the cefdinir. In the invention, a cefdinir synthetic product with high recovery rate and high purity can be produced in the method without the application of a counter solvent. The preparation process of the product has the advantages that reaction conditions are gentle, operation processes are simple and pollutions are fewer etc. Therefore, the method is applicable in industrial production.
Owner:CHENGDU BRILLIANT PHARMA CO LTD
Efficient synthesis method of mirabegron
InactiveCN106083758AThe reaction route is simpleLower reaction costOrganic chemistryPalladium on carbonHydrazine compound
The invention discloses an efficient synthesis method of mirabergron. The method comprises the specific steps that nitrophenylacetonitrile is subjected to a nitroreduction and nitrile group reduction reaction in hydrazine hydrate under the catalysis effect of a catalyst of palladium on carbon hydrogenation to obtain ethylamine; (R)-1-phenyl-1,2-ethanediol and methylsulfonyl chloride react under the catalysis effect of a basic catalyst of piperidine or triethylamine to obtain (R)-1-phenyl-1-hydroxy-2-mesyl-ethane; the (R)-1-phenyl-1-hydroxy-2-mesyl-ethane and the ethylamine react under the catalysis effect of a basic catalyst of potassium carbonate or triethylamine to obtain (R)-2-((4-amino phenyl ethyl amine)-1-phenethyl alcohol; the (R)-2-((4-amino phenyl ethyl amine)-1-phenethyl alcohol and 2-aminothiazole-4-ethyl acetate are subjected to a condensation reaction under the effect of potassium methoxide to obtain mirabergron. The method is simple and easy to implement, the raw materials are cheap and easy to obtain, the reaction efficiency is high, and the repeatability is good.
Owner:HENAN NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com