One-pot method for preparing acotiamide hydrochloride
A technology for acotiamide hydrochloride and salt formation, which is applied in the field of preparation of acotiamide hydrochloride, can solve problems such as cumbersome industrial operations, high operating requirements, environmental problems, etc., and achieve the goal of overcoming cumbersome industrial operations and simplifying Operation process, the effect of improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0037] Example 1: Preparation of Acotiamide Hydrochloride
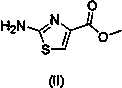
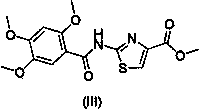
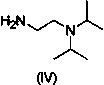
[0038] Add 2,4,5-trimethoxybenzoic acid (20 g, 94.3 mmol) and 200 ml N,N-dimethylformamide into a 500 ml reaction flask. Add TBTU (30.88 g, 113.2 mmol), add N,N - Diisopropylethylamine (14.59g, 113.2mmol), stirred at room temperature for 2 hours. Add methyl 2-aminothiazole-4-carboxylate (14.92 g, 94.3 mmol), DMAP (2.30 g, 18.9 mmol), heat to 75°C, and stir for 24 hours. join in N,N -Diisopropylethylenediamine (27.16g, 188.6mmol), heated to 140°C and stirred for 10 hours. After cooling, add 400ml of n-butanol, stir, and let stand to separate layers. The upper layer was taken, washed with 400ml of saturated saline, and allowed to stand to separate layers. The upper layer was taken, and 120 ml of isopropanol hydrogen chloride was added dropwise at low temperature to precipitate a solid. Filter under reduced pressure, and put the filter cake into a blast drying oven at 60° C. for 1 hour to dry. 28.5 g of acot...
example 2
[0040] Example 2: Preparation of Acotiamide Hydrochloride
[0041] Add 2,4,5-trimethoxybenzoic acid (20g, 94.3mmol) to a 500ml reaction bottle, 200ml N,N - Dimethylacetamide. Add TBTU (30.88g, 113.2mmol), add N,N- diisopropylethylamine (14.59g, 113.2mmol)), stirred at room temperature for 2 hours. Add methyl 2-aminothiazole-4-carboxylate (14.92g, 94.3mmol), DMAP (2.3g, 18.9mmol), heat to 75°C, and stir for 24 hours. join in N,N -Diisopropylethylenediamine (27.16g, 188.6mmol), heated to 140°C and stirred for 10 hours. After cooling, add 400ml of n-butanol, stir, and let stand to separate layers. The upper layer was taken, washed with 400ml of saturated saline, and allowed to stand to separate layers. Take the upper layer, add 120 ml of isopropanol hydrogen chloride dropwise at low temperature, precipitate solid, filter under reduced pressure, and put the filter cake into a blast drying oven at 60°C for 1 hour to dry. 31.7 g of acotiamide hydrochloride (compound V)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com