Patents
Literature
51 results about "Acotiamide Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
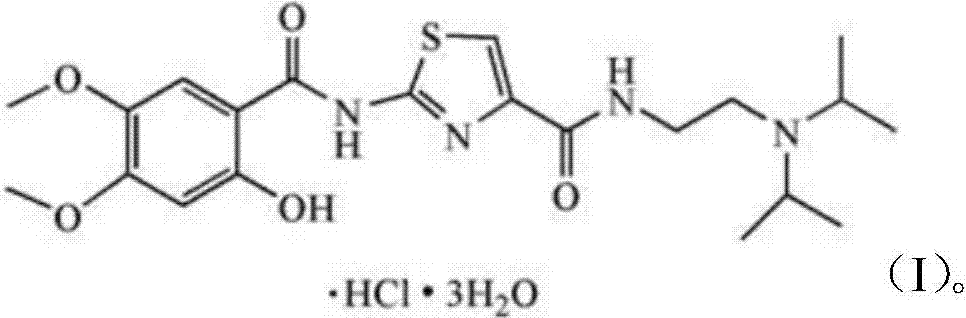
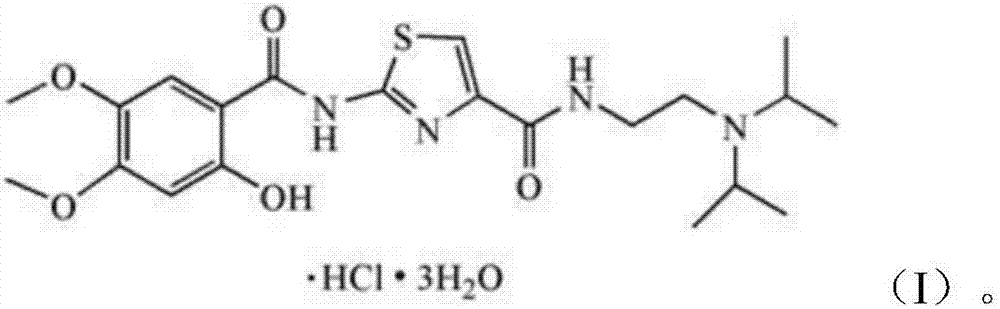
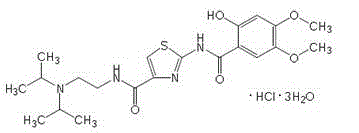
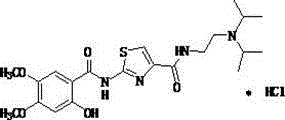
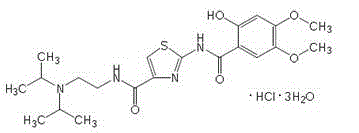
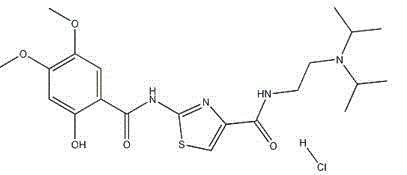
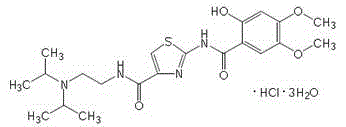
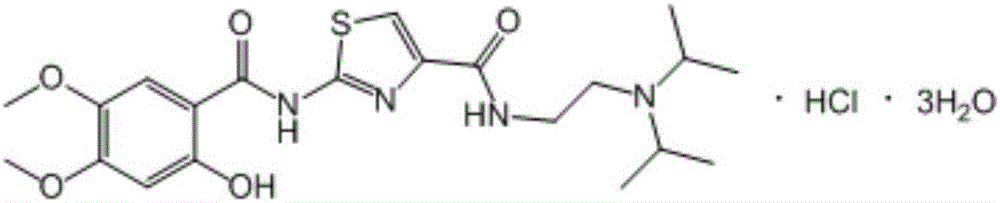
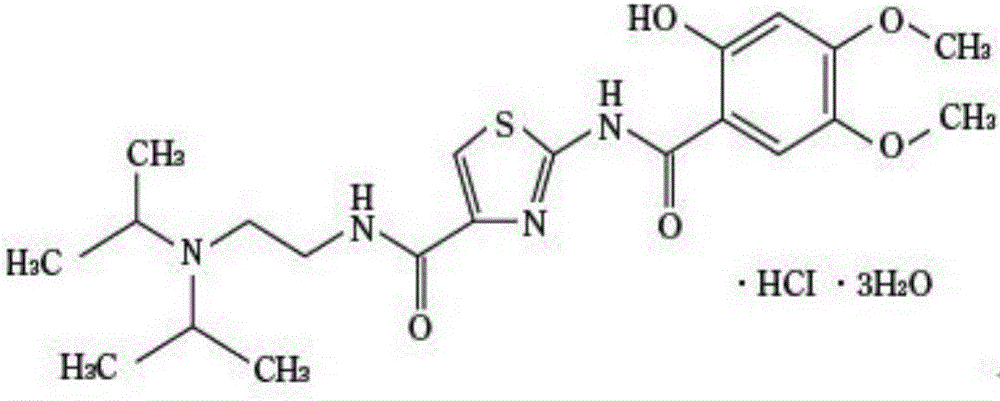
The hydrochloride salt form of acotiamide, a prokinetic agent with gastrointestinal (GI) motility-enhancing activity. Although the exact mechanism by which acotiamide exerts its effect has yet to be fully elucidated, this agent appears to inhibit acetylcholinesterase (AchE), an enzyme responsible for the breakdown of acetylcholine (Ach). Increased Ach concentrations lead to an improvement of gastric emptying and GI motility and eventually to a reduction of dyspepsia symptoms.
Method for preparing acotiamide hydrochloride
The invention discloses a method for preparing a compound of formula (5). The method comprises the following steps: 1, reacting a compound of formula (2) with triphosgene, diphosgene or phosgene in a non-polar solvent in the presence of an organic alkali to obtain a product, reacting the obtained product with a compound of formula (3) to obtain a compound of formula (1); and 2, reacting the compound of formula (1) with a compound of formula (6) in 1,4-dioxane to obtain a substance, reacting the substance with HCl to form a salt which is the compound of formula (5), wherein R in the compound of formula (3) and the compound of formula (5) is a methyl group or an ethyl group.
Owner:CHANGZHOU NO 4 PHARMA FACTORY +2
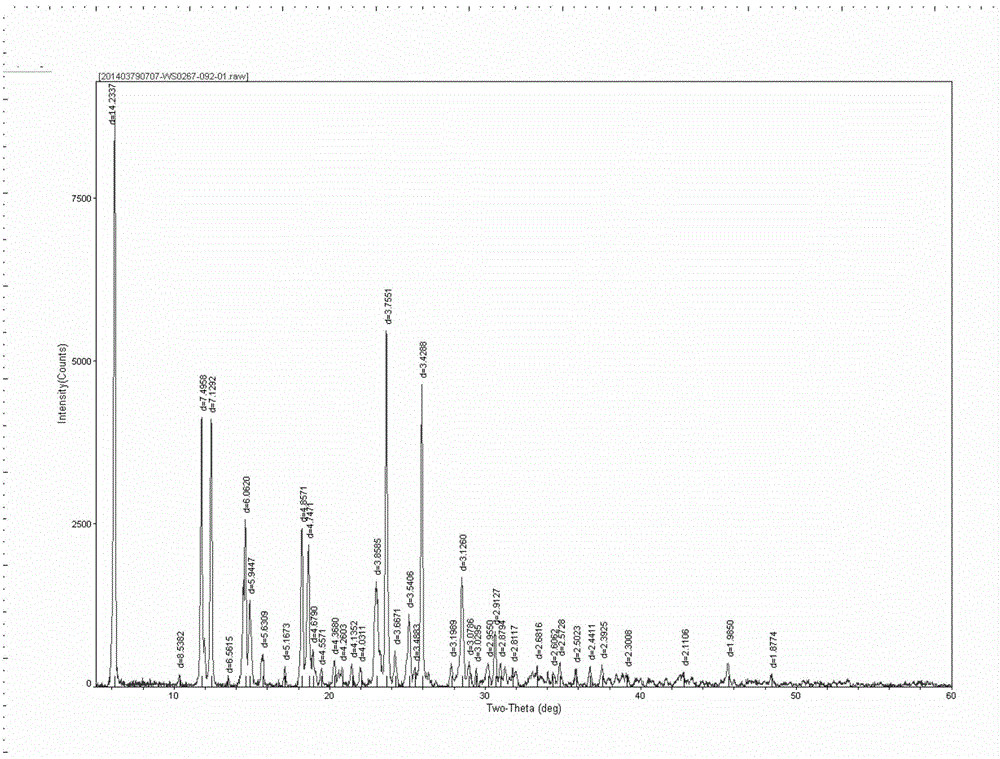
Method for preparing acotiamide hydrochloride trihydrate
ActiveCN103709120AMild reaction conditionsImprove stabilityOrganic chemistryBulk chemical productionAcotiamide HydrochlorideMedicinal chemistry
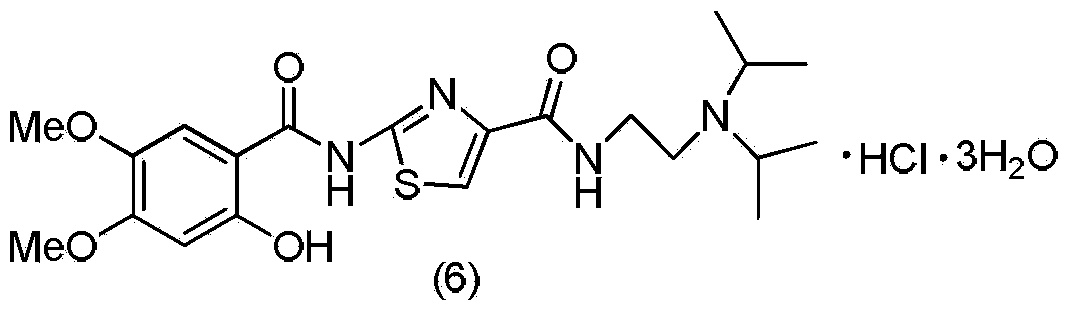
The invention discloses a method for preparing an acotiamide hydrochloride trihydrate. The method comprises the following steps: 1, reacting a compound of a formula (1) and N,N-diisopropylethylamine (2) to obtain a compound (3); 2, performing two-site amino deprotection on the compound (3) to obtain a compound (4); 3, condensing the compound (4) and an acyl chloride derivative to obtain a compound (5); 4, further preparing the compound (5) to obtain the acotiamide hydrochloride trihydrate (6).
Owner:CHINA RESOURCES SAIKE PHARMA
One-pot method for preparing acotiamide hydrochloride
ActiveCN104045606AReduce pollutionOvercome the shortcomings of cumbersome industrial operations and not suitable for industrial productionOrganic chemistryThiazoleSolvent
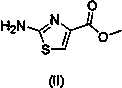
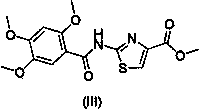
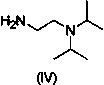
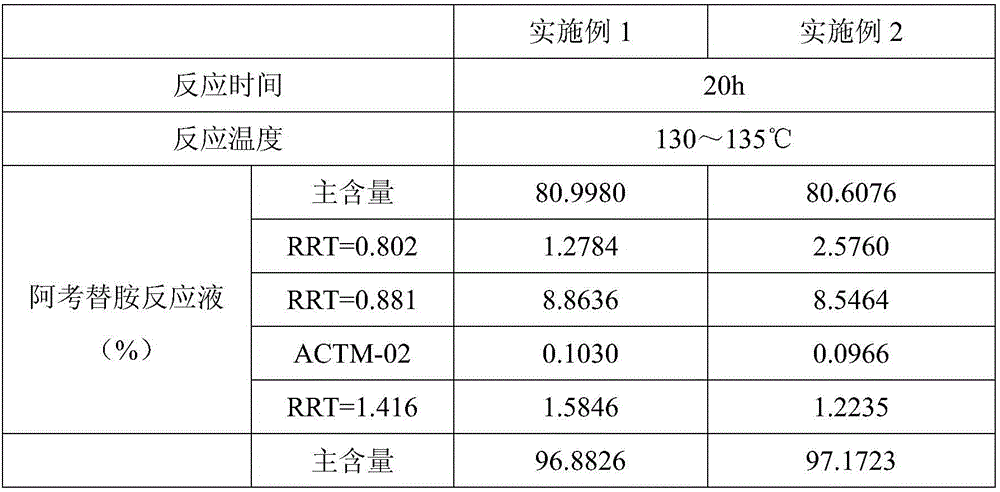
The invention relates to a one-pot method for preparing an acotiamide hydrochloride (a compound V). The method comprises the following steps: by taking 2,3,5-trimethoxybenzoic acid as shown in a formula (I) and 2-aminothiazole-4-methyl formate as shown in a formula (II) as raw materials, carrying out a condensation reaction to obtain a compound III; selecting an appropriate solvent and controlling a reaction condition, directly carrying out an aminating reaction between an intermediate III which is not separated and purified and N,N-diisoprylamino ethylamine as shown in a formula (IV), and finally obtaining the acotiamide hydrochloride (the compound V) by use of the one-pot method. The one-pot method for preparing the acotiamide hydrochloride has the advantages that the reaction steps are reduced, the operation process is simplified, and the production efficiency is improved; besides, the method is safe and environmental friendly, and suitable for industrial production. The formulas (I, II, III, IV and V) are as shown in the specification.
Owner:HANGZHOU XINBOSI BIOMEDICAL CO LTD
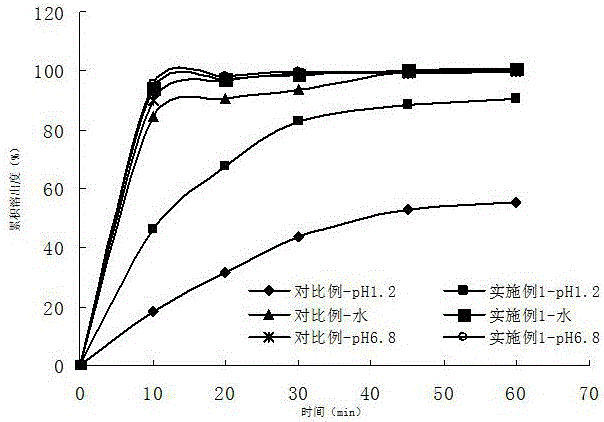
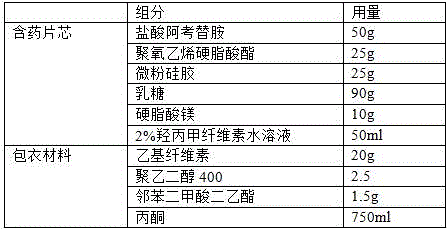
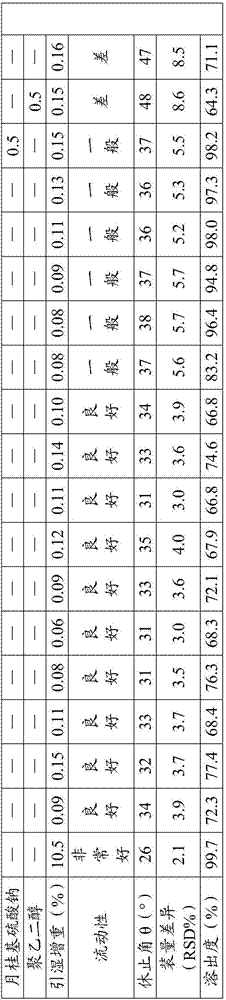
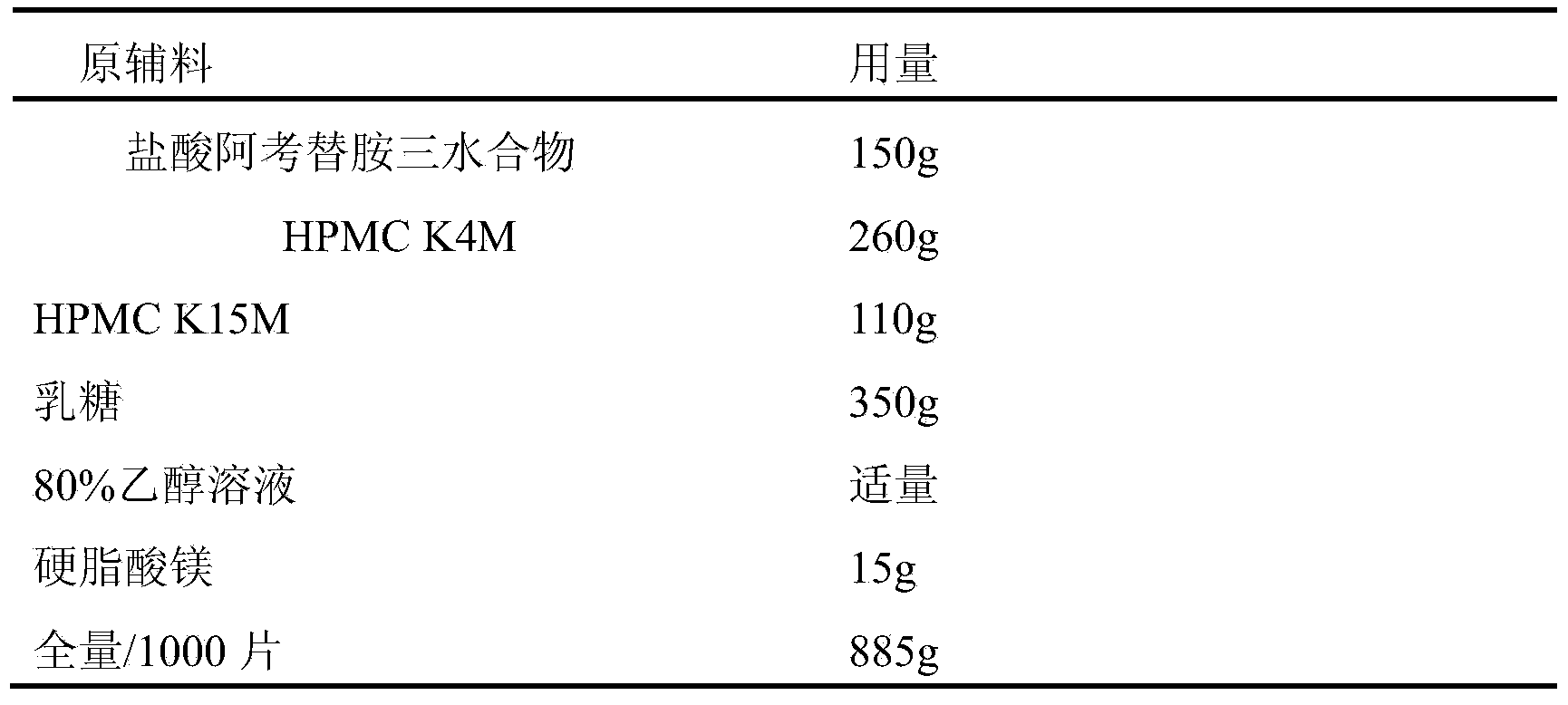
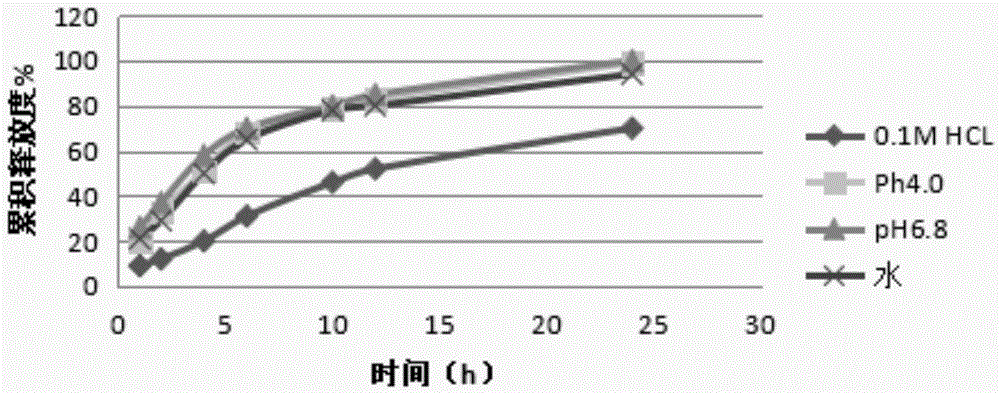
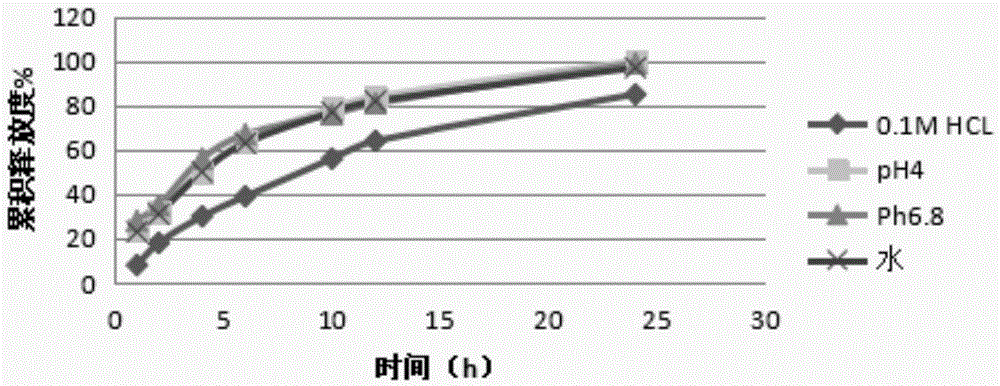
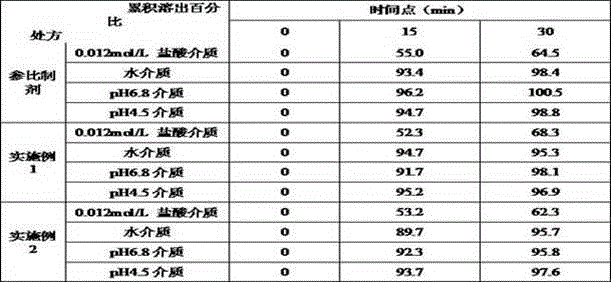
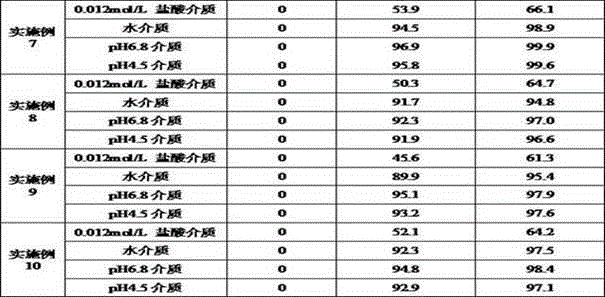
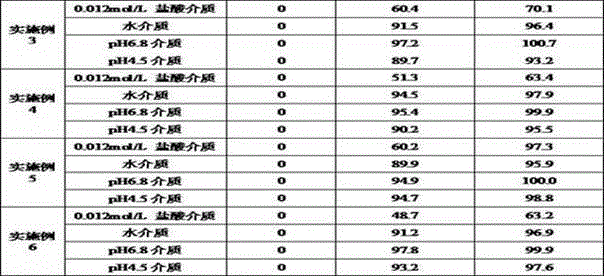
Acotiamide hydrochloride sustained release tablet and preparation method thereof
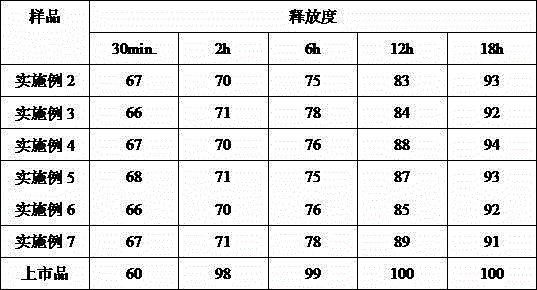
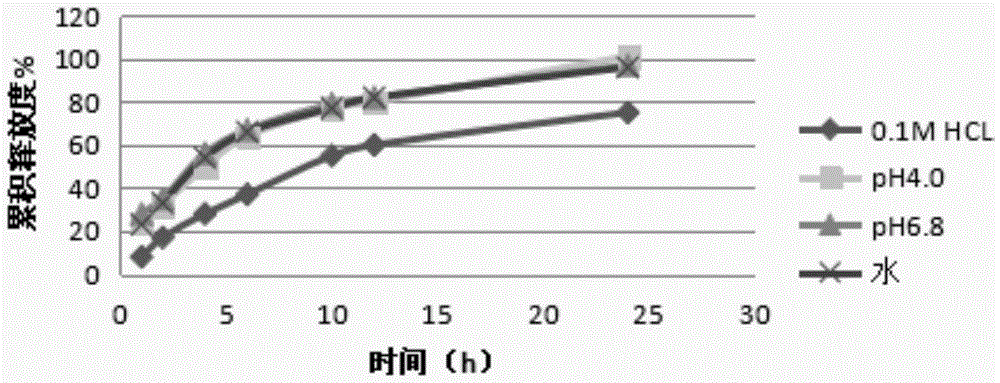
The invention discloses an acotiamide hydrochloride sustained release tablet and a preparation method thereof. The sustained release tablet, in unit dose package, comprises 150mg of acotiamide hydrochloride trihydrate, 200 to 300mg of sustained-release material, 300 to 400mg of stabilizer, adhesive, lubricant and coating material. The sustained release tablet has 12-hour sustained release effect and good chemical stability. The releasing rate of the sustained release tablet is good in repeatability from laboratory to mass production stages, and is basically unchanged through 6 month stability inspection.
Owner:ANHUI PIOM PHARMA
Synthetic method of acotiamide hydrochloride
ActiveCN103665023AHigh selectivityLess side effectsGroup 4/14 element organic compoundsSugar derivativesSide effectAcotiamide Hydrochloride
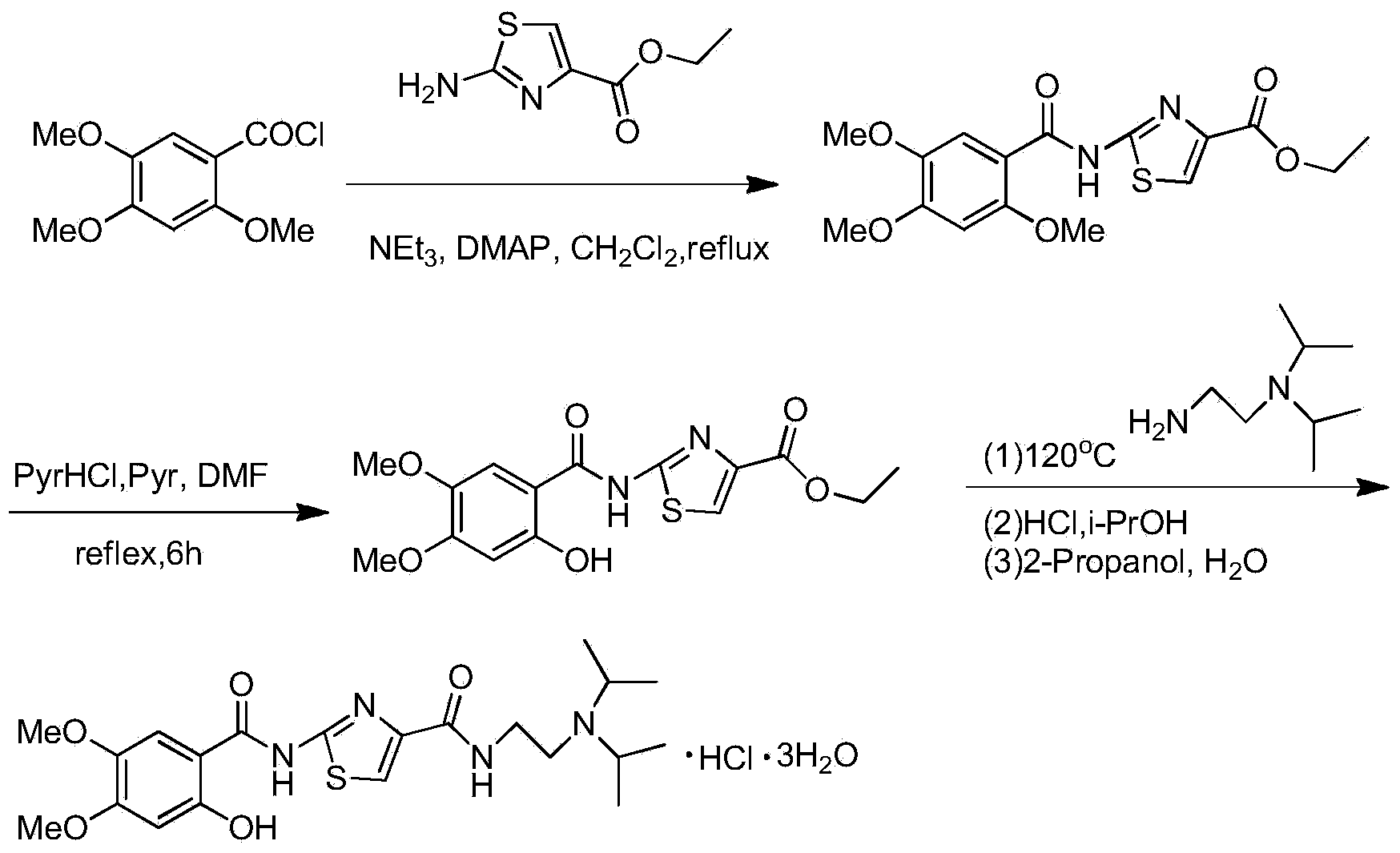
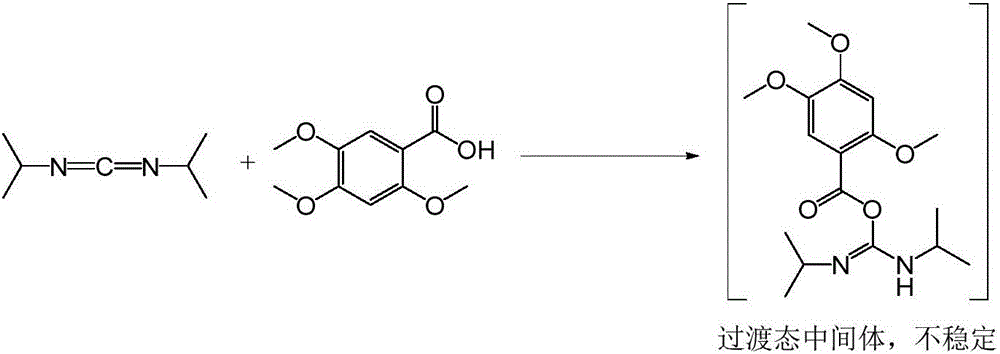
The invention relates to a synthetic method of acotiamide hydrochloride. The synthetic route of the acotiamide hydrochloride comprises the following steps: protecting hydroxyl vicinal to carboxyl in a formula (1) compound to obtain a formula (2) compound, performing acylating chlorination on the formula (2) compound to obtain a formula (3) compound, condensing the formula (3) compound with a formula (4) compound to obtain a formula (5) compound, condensing the formula (5) compound with a formula (6) compound to obtain a formula (7) compound, removing protection of 2-site hydroxyl of a benzene ring from the formula (7) compound, and salifying the compound to obtain the acotiamide hydrochloride. The synthetic method has the benefits that the formula (2) compound is not extracted but directly used for the next reaction to form the formula (5) compound in a one-pot method; the method is high in selectivity, low in side effect and high in yield; in the meantime, the formula (5) compound to the formula (8) compound can be prepared with the one-pot method; the method is high in the protection removal selectivity, low in side effect, less in the operation steps, and easy for realizing industrialization.
Owner:CHINA RESOURCES SAIKE PHARMA
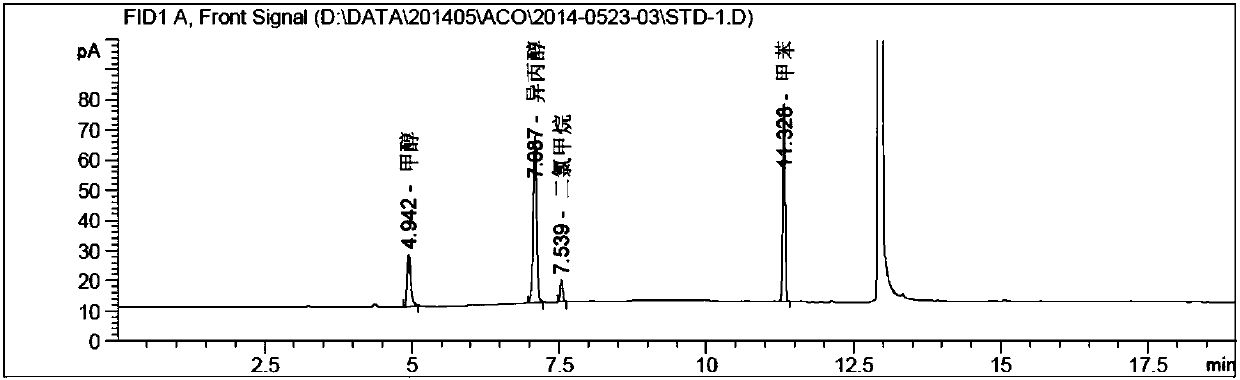
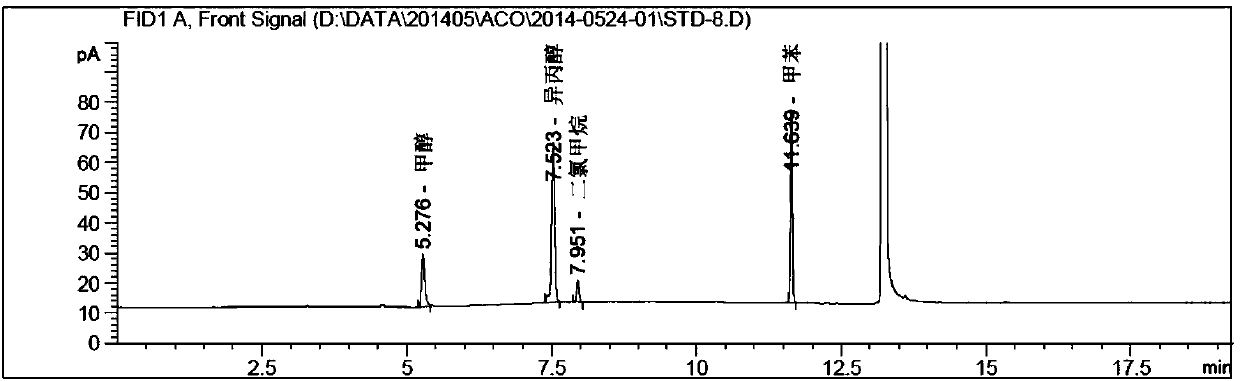
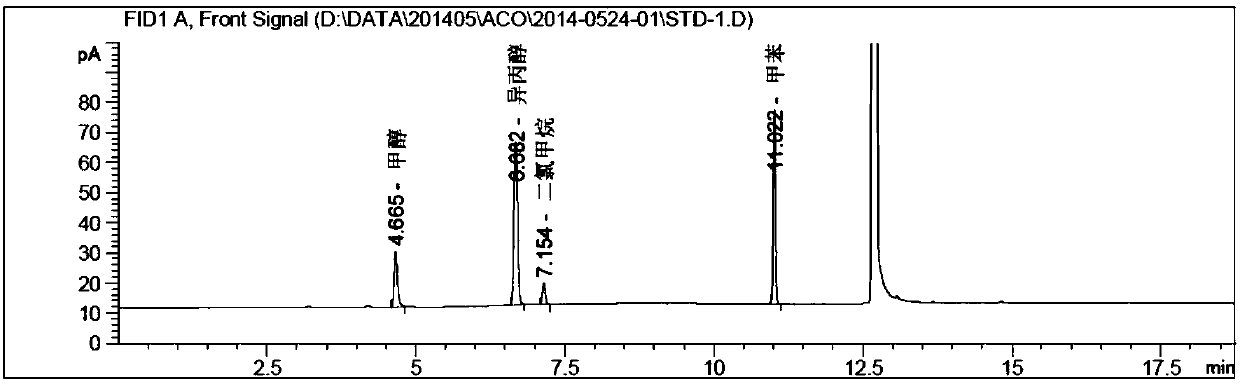
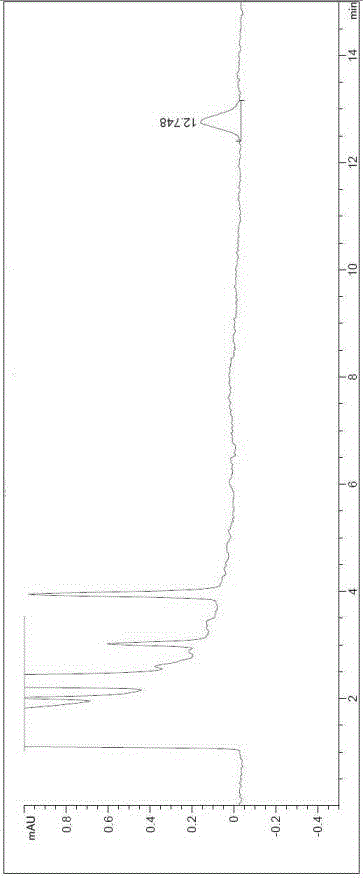
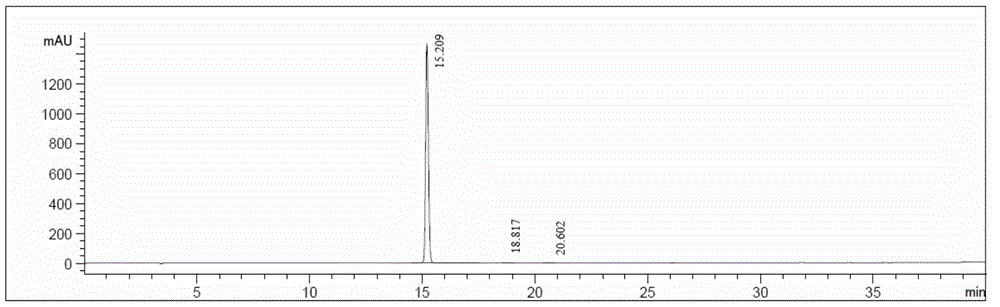
Method for determining solvents residual in acotiamide hydrochloride bulk drug
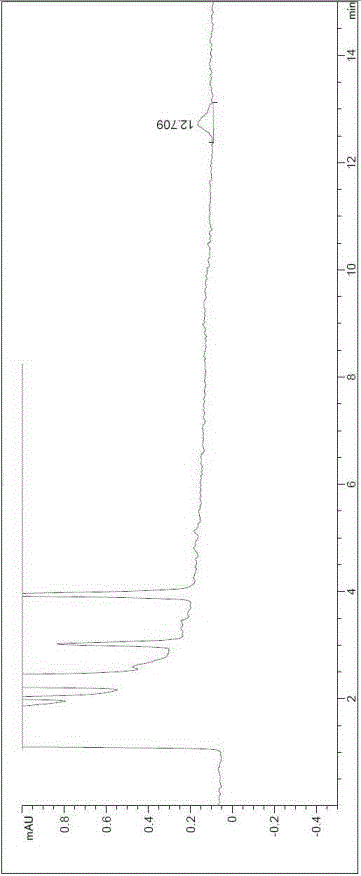
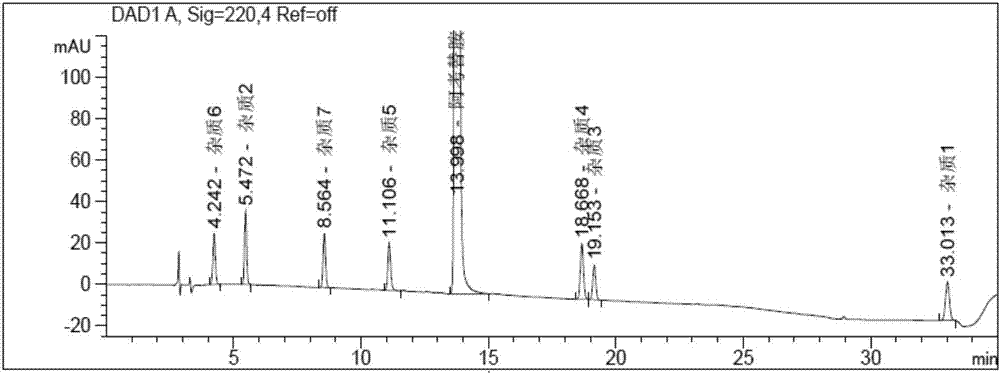
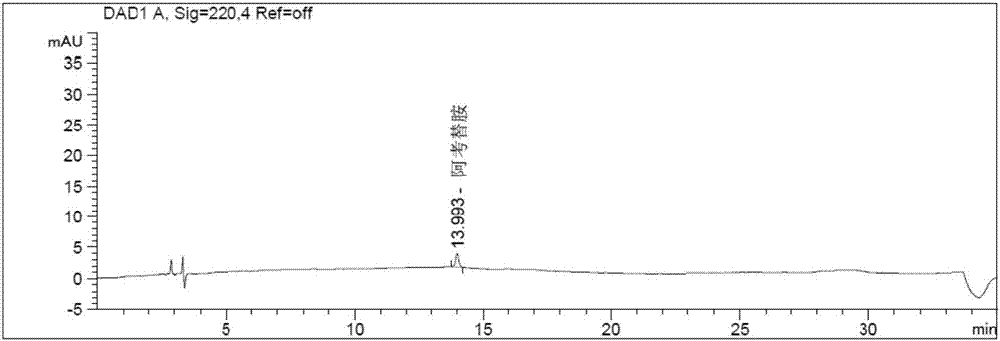
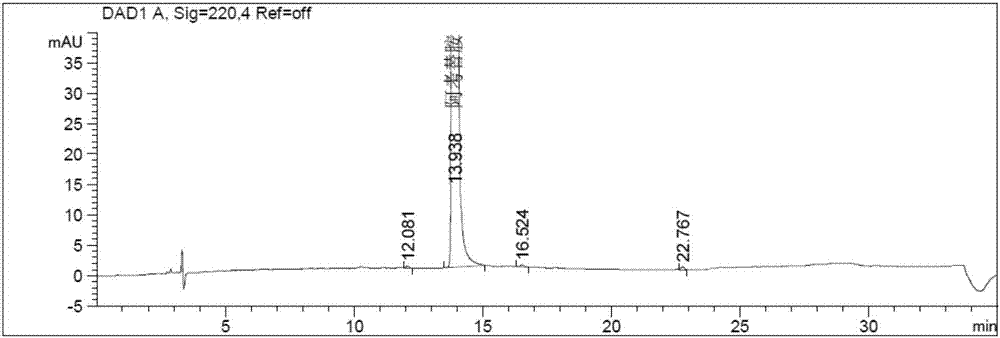
The invention discloses a method for determining solvents residual in an acotiamide hydrochloride bulk drug. The method adopts the following analysis conditions: the chromatographic column adopts a capillary chromatographic column with 6% cyanopropylphenyl 94% polydimethylsiloxane as the stationary phase; a reference substance solution is injected multiple times in a split flow manner, a sample solution is injected in the split flow manner, the carrier gas is nitrogen, the introduction is carried out by using a headspace injector, and detection is performed after temperature programming; and the detector adopts an FID detector. Methanol, isopropanol, dichloromethane and toluene in the acotiamide hydrochloride bulk drug are rapidly and efficiently separated under same chromatographic conditions through the method in order to effectively control the quality of acotiamide hydrochloride. The detection method has the advantages of strong specificity, high detection sensitivity, high precision, high accuracy, convenience in operation, and effective control of the product quality.
Owner:WATERSTONE PHARMA WUHAN
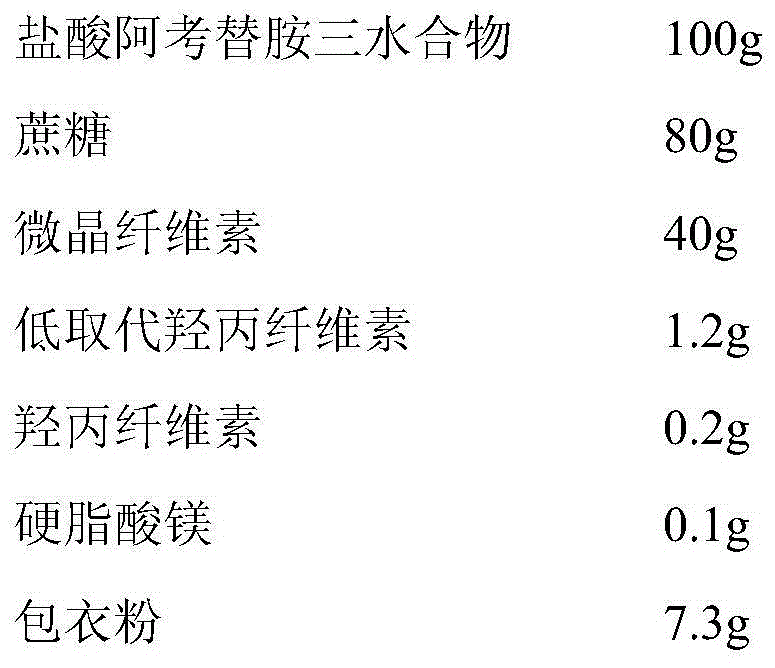
Acotiamide hydrochloride medicinal preparation and preparation method thereof
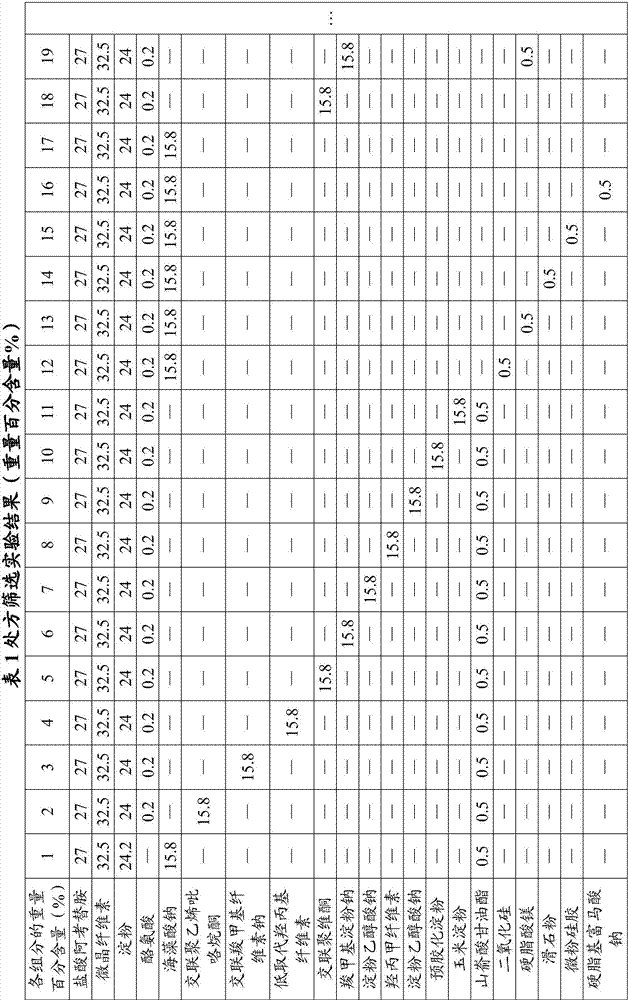
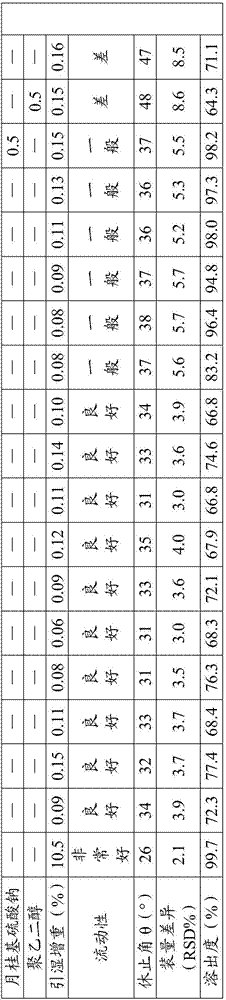
The invention provides an acotiamide hydrochloride medicinal preparation and a preparation method thereof. The acotiamide hydrochloride medicinal preparation comprises acotiamide hydrochloride and / or a hydrate thereof, at least one diluent, at least one disintegrating agent, at least one adhesive and at least one lubricant, the diluent includes a first diluent and is a hydrophilic instant component, and a weight ratio of the hydrophilic instant component to acotiamide hydrochloride and / or the hydrate thereof is 2:5-4:5. The acotiamide hydrochloride medicinal preparation is prepared by introducing a specific proportion of the hydrophilic instant component to a medicinal preparation and by mixing the hydrophilic instant component among the above acotiamide hydrochloride effective component. When the acotiamide hydrochloride medicinal preparation is dissolved and a material accumulation phenomenon appears, an external layer hydrophilic instant auxiliary material is treated as a pore forming agent, and an internal layer hydrophilic instant auxiliary material is treated as capillaries in a raw material with the dissolving of the external layer hydrophilic instant auxiliary material, and can guide a dissolution medium to rapidly enter the raw material in order to increase the dissolution and improve the dissolution speed.
Owner:SHIJIAZHUANG NO 4 PHARMA
Acotiamide compound
InactiveCN104447611AHigh purityHigh optical purityOrganic active ingredientsOrganic chemistryAcotiamideAcotiamide Hydrochloride
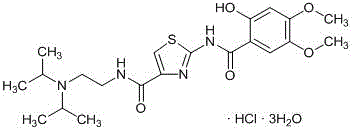
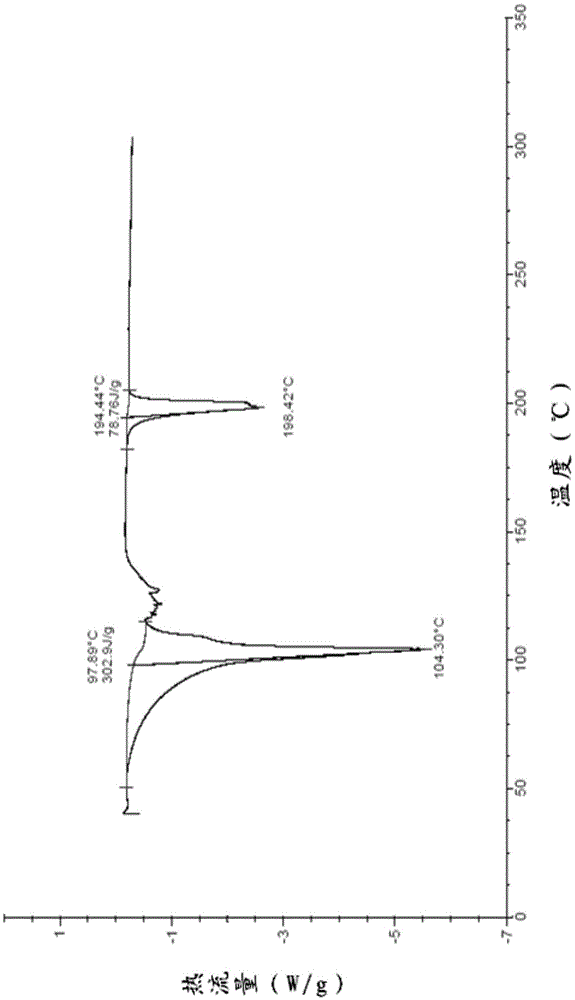
The invention belongs to the technical field of medicine and relates to an Acotiamide hydrochloride hydrate crystal form and a preparation method thereof. The Acotiamide hydrochloride contains trihydrate and has the advantages of chemical purity of 99.9%, the largest impurity content less than 0.1%, optical purity of 99.95%ee and good stability. The invention also relates to a use of the Acotiamide hydrochloride hydrate composition in preparation of drugs for treating stomach reception disorder.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Preparation method of acotiamide hydrochloride intermediate
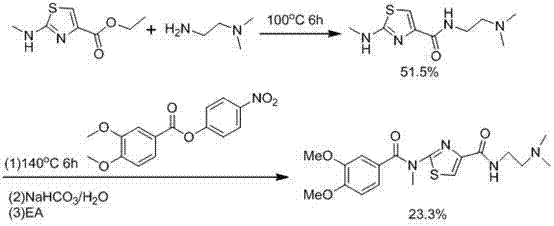
The invention relates to a preparation method of an acotiamide hydrochloride intermediate. The preparation method comprises that 2,4,5-trimethoxybenzoic acid and ethyl 2-aminothiazol-4-formate as initial raw materials undergo a condensation reaction under the action of a condensing agent TBTU and an acid binding agent DIEA to produce a desired product. The preparation method is free of a highly corrosive solvent, can be operated simply, is suitable for industrial production and realizes a high yield and good purity.
Owner:JIANGSU HANSOH PHARMA CO LTD
Acotiamide hydrochloride trihydrate refining method and acotiamide hydrochloride trihydrate preparation method
ActiveCN105753810AEasy to removeLiquid phase with high purityOrganic chemistryOrganic solventPotassium hydroxide
The invention discloses an acotiamide hydrochloride trihydrate refining method and an acotiamide hydrochloride trihydrate preparation method.The acotiamide hydrochloride trihydrate refining method includes adding crude acotiamide into an inorganic base aqueous solution with uniformly mixing, adding an organic solvent with stirring to crystallize, filtering and drying to obtain acotiamide hydrochloride sodium salt or acotiamide hydrochloride potassium salt, wherein inorganic base refers to sodium hydroxide, potassium hydroxide, sodium carbonate or potassium carbonate; adding the acotiamide hydrochloride sodium salt or acotiamide hydrochloride potassium salt into a hydrous isopropanol solution with uniformly mixing, adding hydrochloric acid to acidize a mixture to a pH value being 1-2, stirring to crystallize, heating to dissolve, decreasing the temperature to crystallize, and performing decompression drying so as to obtain acotiamide hydrochloride trihydrate.The acotiamide hydrochloride trihydrate refining method has the advantages that the acotiamide is prepared into the sodium salt or potassium salt, the sodium salt or potassium salt is refined, and accordingly impurities before and after a main peak can be removed effectively, and the final product, namely the acotiamide hydrochloride trihydrate, is above 99.8% in purity and below 0.10% in individual impurities.
Owner:浙江新赛科药业有限公司
High-performance liquid chromatography analytical method for N1,N1-diisopropyl ethylenediamine
PendingCN105021740AAccurate methodGood reproducibilityComponent separationEthylenediamineTest sample
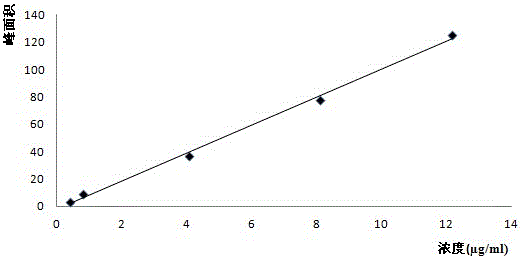
The invention relates to a high-performance liquid chromatography analytical method for N1,N1-diisopropyl ethylenediamine. The analytical method is characterized by including the following steps that 1, N1,N1-diisopropyl ethylenediamine standard solutions of different concentrations are prepared, excess 2,4-dinitrofluorobenzene is added into the standard solutions for a derivatization reaction, after the reaction is completed, high-performance liquid chromatography analysis is carried out on reaction solutions, and the DNB-N1,N1-diisopropyl ethylenediamine peak area corresponds to a N1,N1-diisopropyl ethylenediamine concentration drawing standard curve; 2, a test sample is prepared into a solution, excess 2,4-dinitrofluorobenzene is added into the solution to react, after the reaction is completed, high-performance liquid chromatography analysis is carried out on the reaction solution, and the content of N1,N1-diisopropyl ethylenediamine in the test sample is calculated. The analytical method is accurate, high in repeatability and sensitivity, and capable of being used for detecting N1,N1-diisopropyl ethylenediamine in the producing and placing processes of acotiamide hydrochloride and tablets thereof and detecting N1,N1-diisopropyl ethylenediamine in other samples.
Owner:HANGZHOU XINBOSI BIOMEDICAL CO LTD
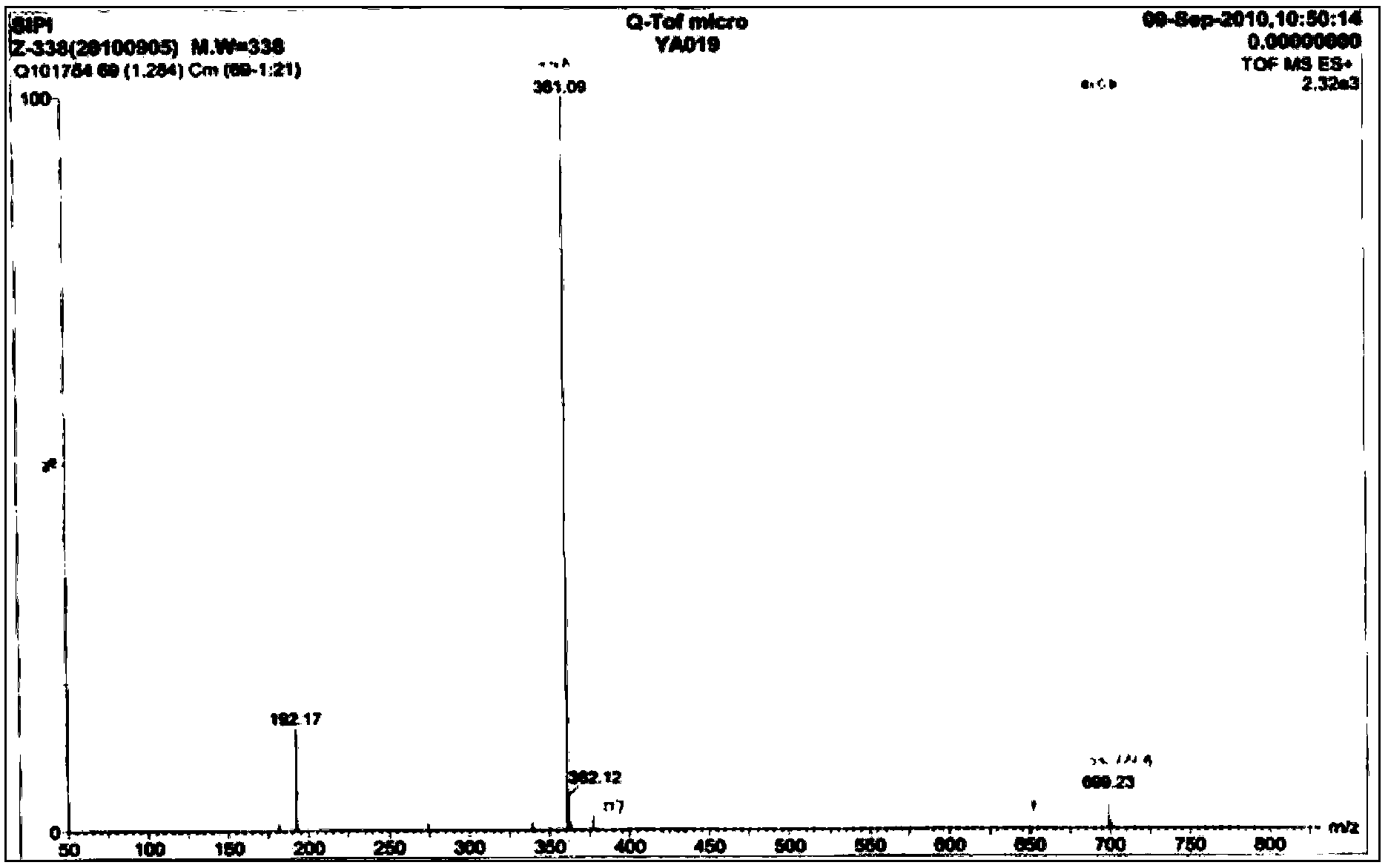
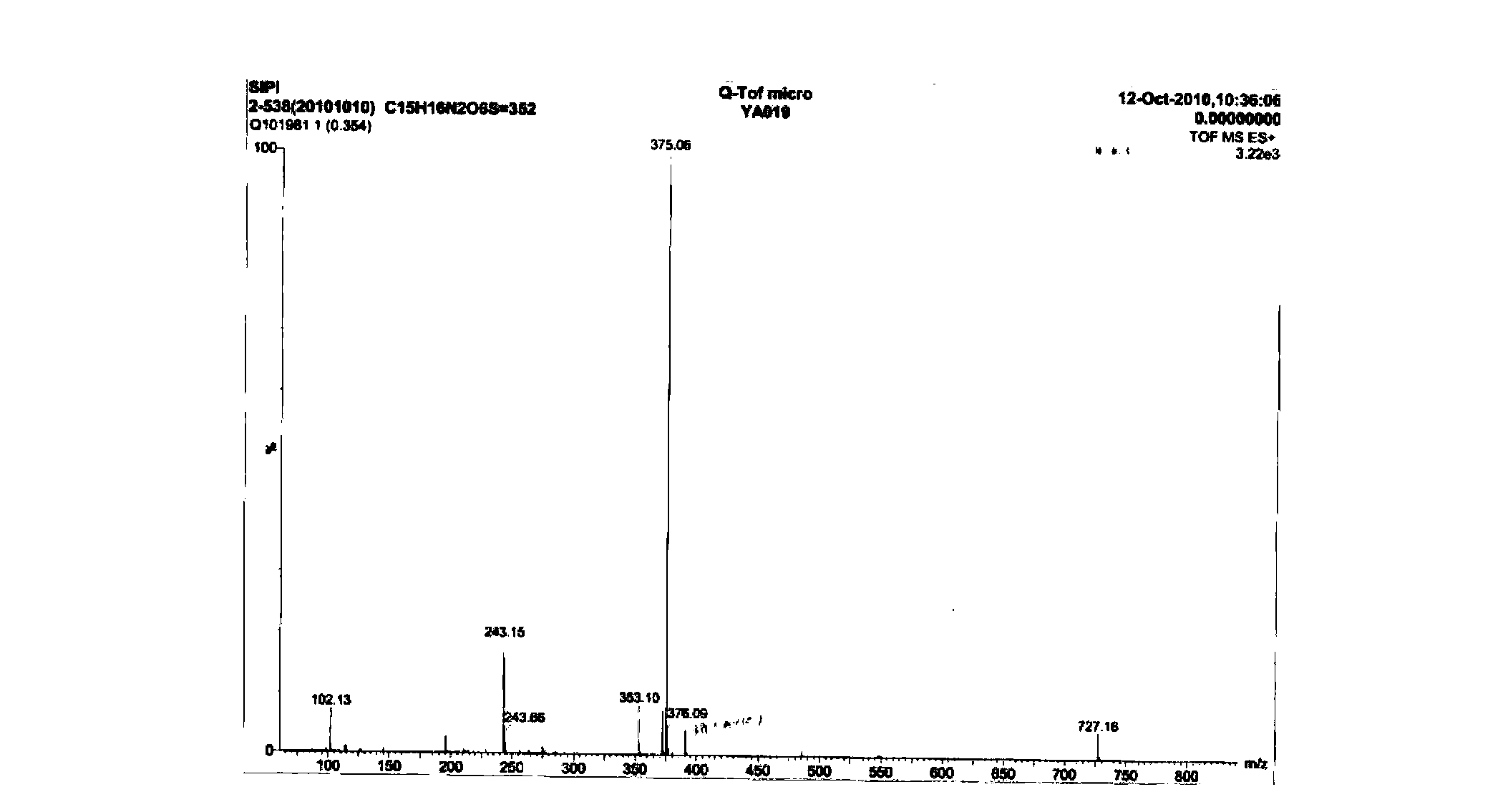
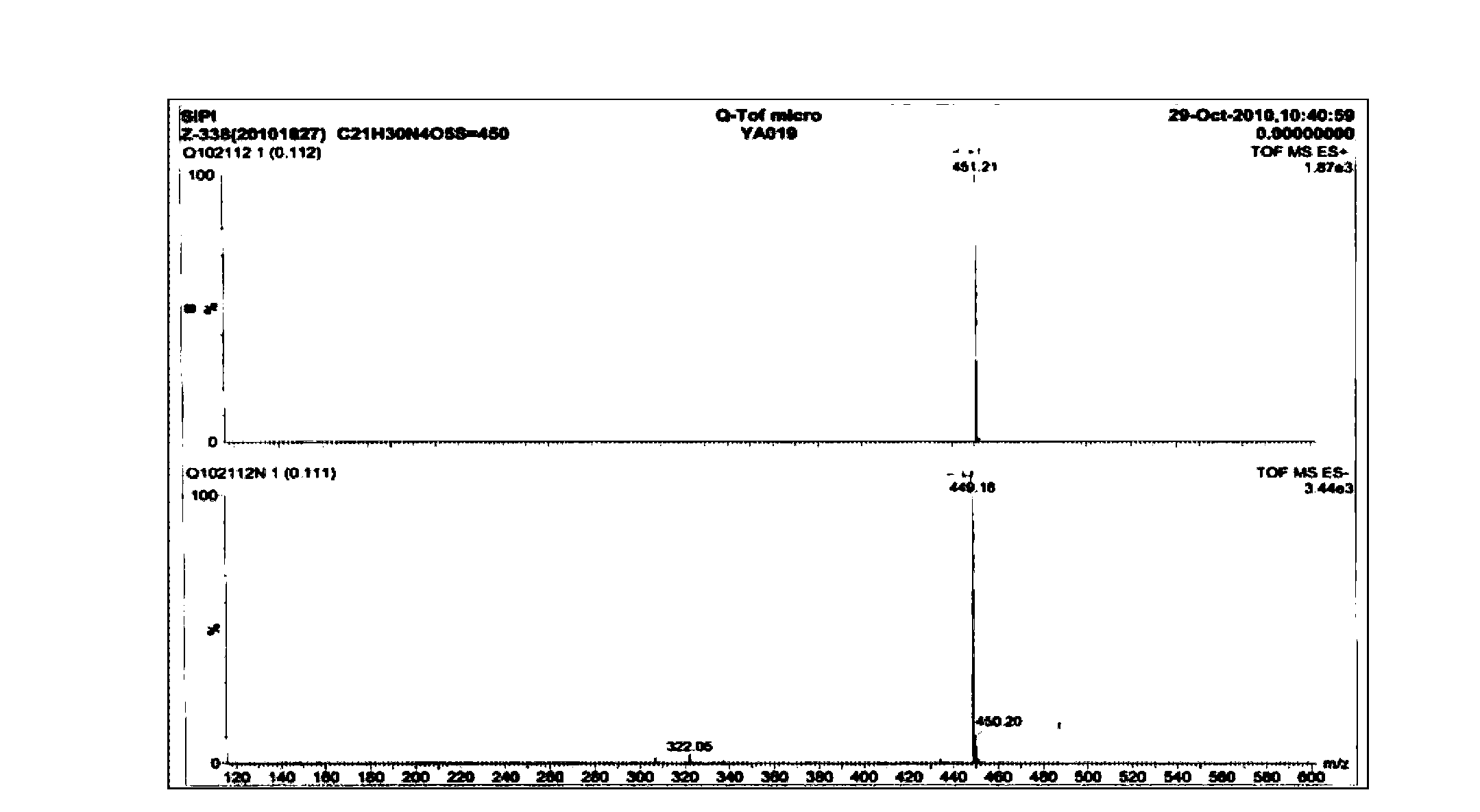
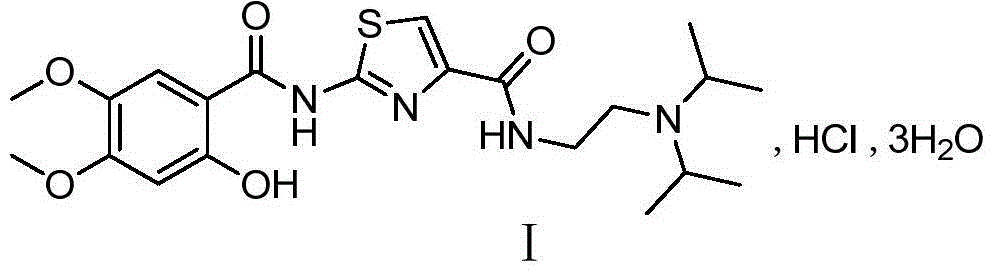
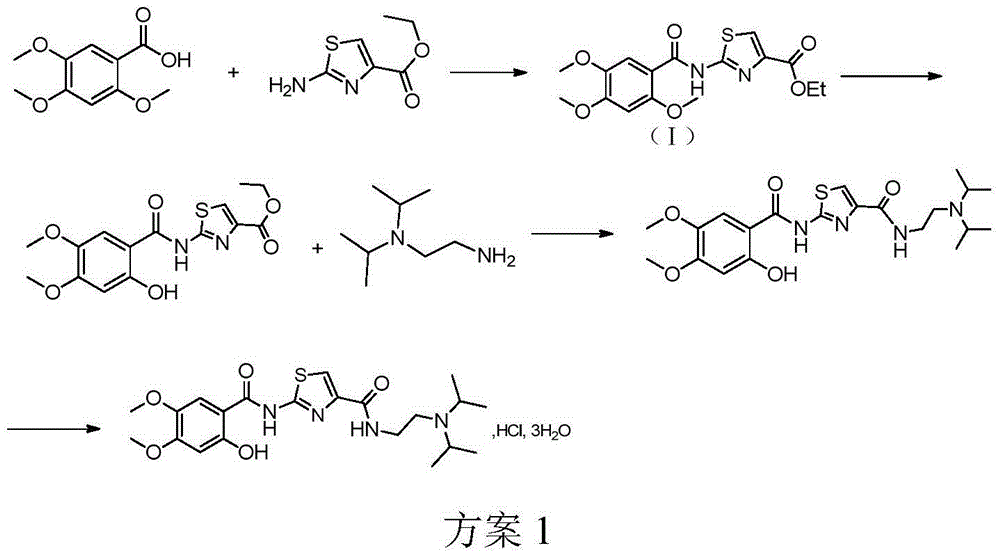
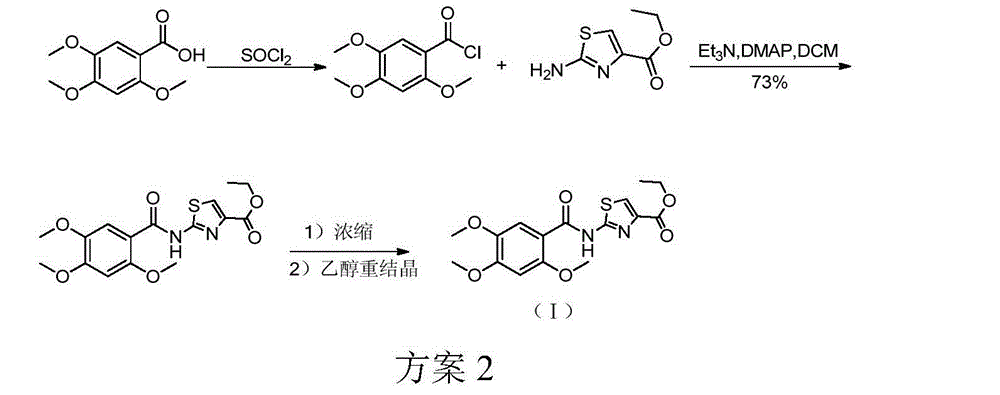
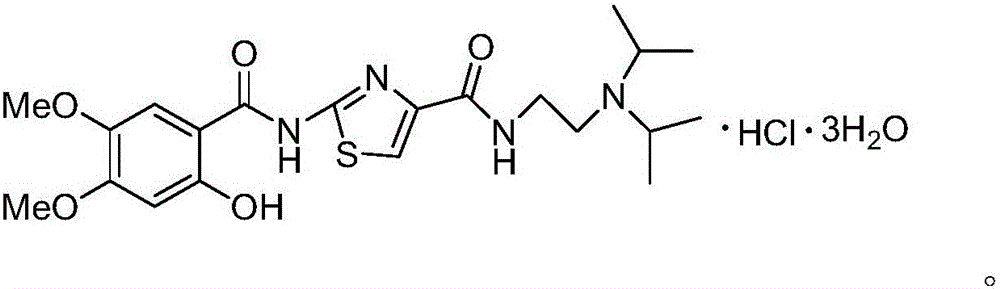
6,7-dimethoxy-benzo[d][1,3]dioxin-2,4-dione and preparation method thereof
The invention discloses a compound of formula (1) and a preparation method thereof. The compound of the formula (1) is used for preparing the intermediate of acotiamide hydrochloride (Z-338).
Owner:CHANGZHOU NO 4 PHARMA FACTORY +2
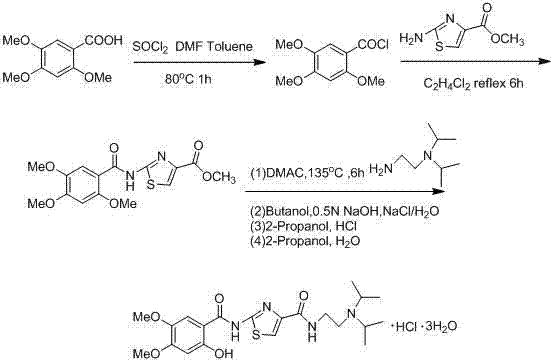
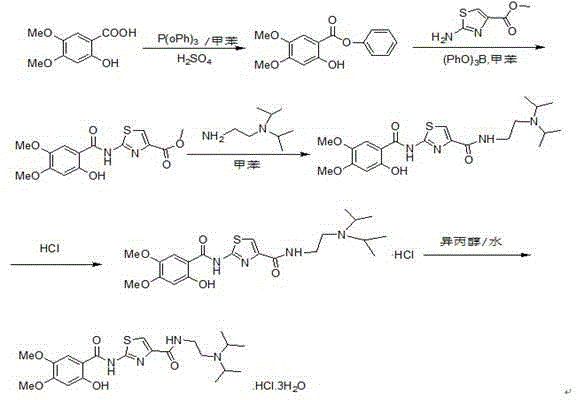
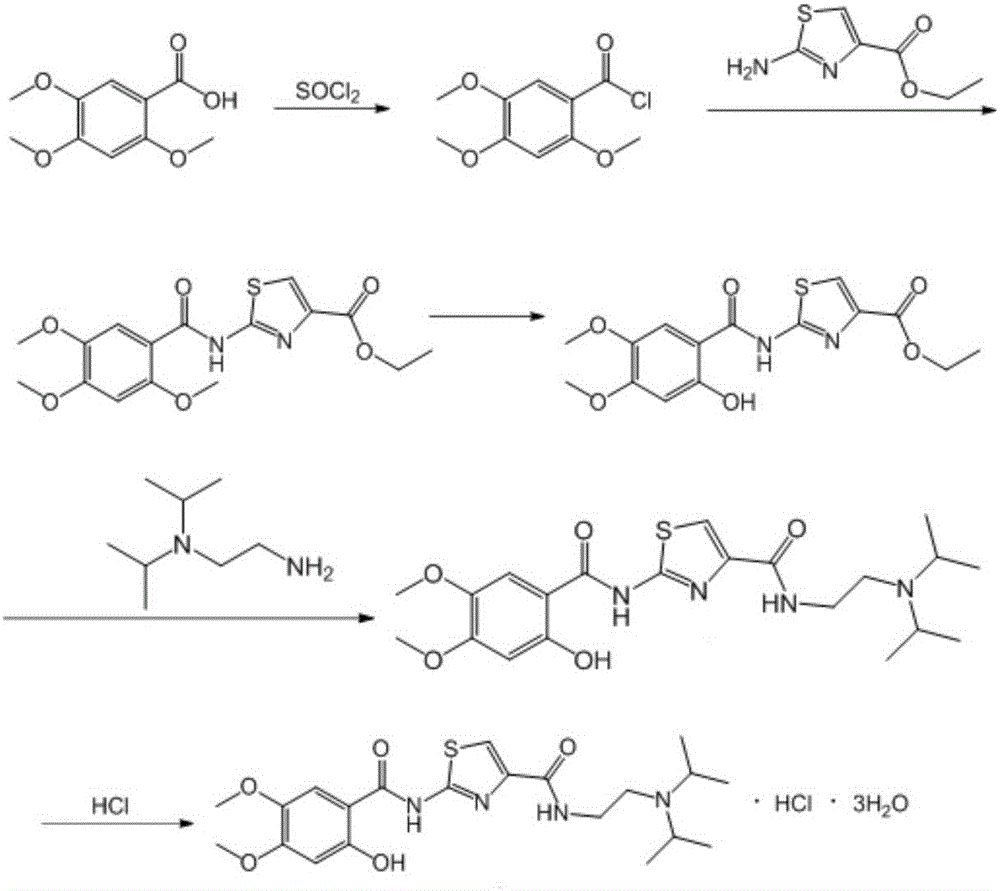
Preparation method of acotiamide hydrochloride
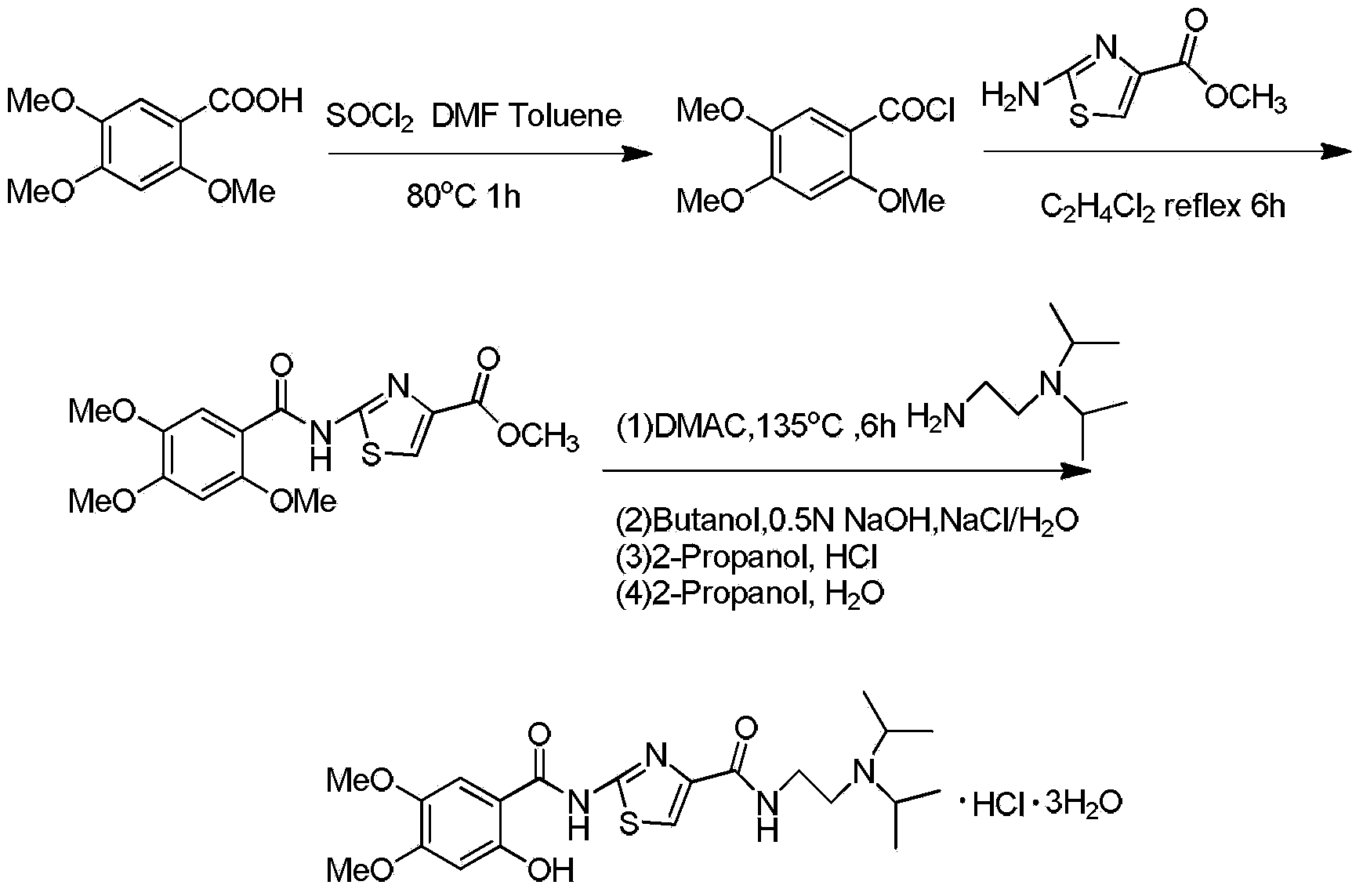
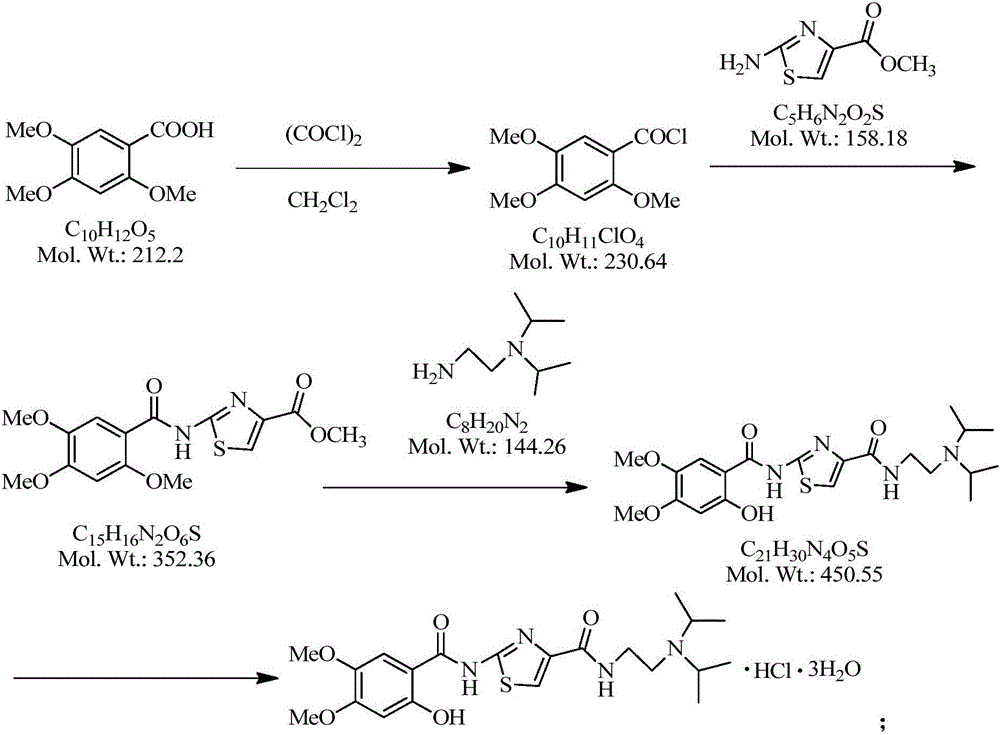
The invention discloses a preparation method of acotiamide hydrochloride. The preparation method is characterized by comprising the following steps: 1, making 2-hydroxy-4, 5-dimethoxybenzoic acid react with SOCl<2>, and obtaining a midbody, namely 2-hydroxy-4, 5-dimethoxy benzoyl chloride; 2, making the product obtained in step 1 react with 2-aminothiazole-4-formic acid alkyl ester, and obtaining 2-[(2-hydroxy-4,5-dimethoxy benzoyl chloride) amidogen]-1,3-thiazole-4-formic acid alkyl ester; 3, making the product obtained in step 2 react with N, N-diisopropyl ethanediamine, and obtaining acotiamide; 4, introducing hydrogen chloride directly for reaction without conducting separation, and obtaining the acotiamide hydrochloride after post-treatment is conducted on the product. The preparation method has the advantages of being high in purity and yield, low in cost, simple to operate and the like.
Owner:山东富创医药科技有限公司 +1
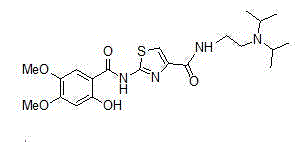
Preparation methods for acotiamide and hydrochloride thereof
The invention discloses preparation methods for acotiamide and hydrochloride thereof; 2[(2-hydroxy-4,5-dimethoxybenzoyl)amino]-1,3-thiazole-4-carboxylic acid methyl ester and N,N-diisoprylamino ethylamine are subjected to a reaction in a molten state to obtain N-[2-(diisopropylamino)ethyl]-2-(2-hydroxy-4,5-dimethoxybenzoyl)amino]-1,3-thiazole-4-carboxamide, and hydrochloric acid is dropwise added to N-[2-(diisopropylamino)ethyl]-2-(2-hydroxy-4,5-dimethoxybenzoyl)amino]-1,3-thiazole-4-carboxamide in methanol to prepare acotiamide hydrochloride trihydrate. The preparation methods reduce the use of toluene, and are economical and convenient, complete in reaction, and safe in process; at the same time, toluene residues in the final products and the amount of an impurity 2[(2-hydroxy-4,5-dimethoxybenzoyl)amino]-4-carboxyl-1,3-thiazole are reduced, and the product quality is improved; and the crude products are easy to refine and purify, and the reaction yield is high.
Owner:REGENEX PHARMA LTD
Method for determining related substances in acotiamide hydrochloride bulk drug and preparation thereof by using HPLC
The invention discloses a method for determining the related substances in an acotiamide hydrochloride bulk drug and a preparation thereof by using HPLC, wherein the chromatographic conditions of the method comprise that the chromatographic column is an octyl silane bonded silica gel column, and the mobile phase is the mixed solution of a phosphate buffer liquid and a first organic solvent. According to the present invention, with the method, the related substances in the acotiamide hydrochloride bulk drug and the preparation thereof can be rapidly and efficiently separated under the same chromatographic conditions so as to effectively control the quality of the bulk drug and the preparation; and the detection method has advantages of strong specificity, high detection sensitivity, high precision, high accuracy, easy operation, and effective control of product quality.
Owner:WATERSTONE PHARMA WUHAN
Acotiamide hydrate crystal form and its preparation method and use
InactiveCN104447612AHigh purityHigh optical purityOrganic active ingredientsOrganic chemistryAcotiamide HydrochlorideImpurity
The invention belongs to the technical field of medicine and relates to an Acotiamide hydrochloride hydrate crystal form and a preparation method thereof. The Acotiamide hydrochloride contains trihydrate and has the advantages of chemical purity of 99.9%, the largest impurity content less than 0.1%, optical purity of 99.95%ee and good stability. The invention also relates to a use of the Acotiamide hydrochloride hydrate composition in preparation of drugs for treating stomach reception disorder.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Acotiamide hydrochloride preparation and application thereof
The invention discloses an acotiamide hydrochloride preparation and application thereof. The acotiamide hydrochloride preparation is prepared from acotiamide hydrochloride, lactose, microcrystalline cellulose and a pharmaceutically acceptable carrier. The prepared acotiamide hydrochloride preparation has good mobility, stability and dissolution rate so as to be suitable for industrial mass production. The acotiamide hydrochloride preparation is a pharmaceutical composition treating functional dyspepsia (FD), is reasonable in proportion, can quickly release drugs, and can generate excellent curative effects to diseases.
Owner:FOSHAN TENGRUI MEDICINE TECH CO LTD
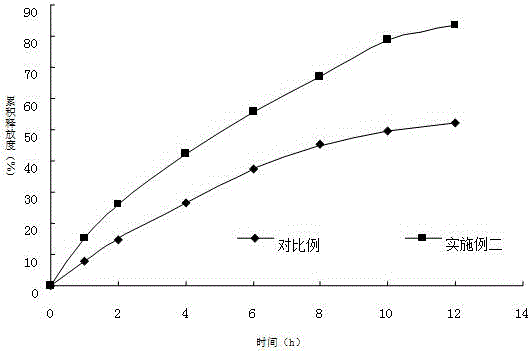
Acotiamide hydrochloride sustained-release drug and preparation method thereof
InactiveCN105055363AAvoid sudden releaseTo achieve the purpose of sustained releaseOrganic active ingredientsDigestive systemSustained release drugCurative effect
The invention discloses an acotiamide hydrochloride sustained-release drug. The acotiamide hydrochloride sustained-release drug only needs to be taken once every morning. The acotiamide hydrochloride sustained-release drug has the advantages of quick effect, good sustained-release effect, good curative effect and less adverse reactions. A collagen membrane is utilized for coating acotiamide hydrochloride and other pharmaceutically acceptable auxiliary materials, wherein a weight ratio of acotiamide hydrochloride to the collagen membrane is 1:(1-1.5).
Owner:叶澄
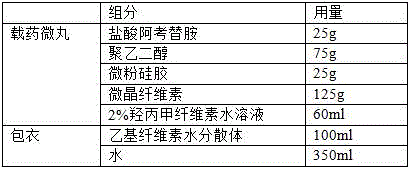
Acotiamide hydrochloride sustained-release pellet and preparation method thereof
InactiveCN105476963AEfficient can effectively adjust the release rateEffective regulation of release rateOrganic active ingredientsDigestive systemSustained release pelletsDrug release rate
The invention belongs to the field of medicine, and discloses an acotiamide hydrochloride sustained-release pellet and a preparation method thereof. The acotiamide hydrochloride sustained-release pellet consists of the following components in parts by weight: 2-50 parts of acotiamide hydrochloride, 20-95 parts of a thinner, 1-10 parts of a binding agent, 1-30 parts of a sustained-release material, 0-5 parts of a pore-forming agent, 0-5 parts of a plasticizer, 0-20 parts of an anti-sticking agent and 0-5 parts of a light-screening agent. By mixing the thinner, the binding agent, the sustained-release material and the acotiamide hydrochloride according to certain proportions, a drug release rate can be effectively regulated, and the acotiamide hydrochloride can be slowly released in vivo so as to generate a stable blood concentration and to avoid adverse reactions caused by relatively great fluctuation of the blood concentration in vivo in the case that the acotiamide hydrochloride dissolves rapidly in vivo. The preparation method of the acotiamide hydrochloride sustained-release pellet is simple to operate, low in production cost and high in efficiency, and the preparation method is applicable to the industrial production of the acotiamide hydrochloride sustained-release pellets.
Owner:BEIJING COLLAB PHARMA
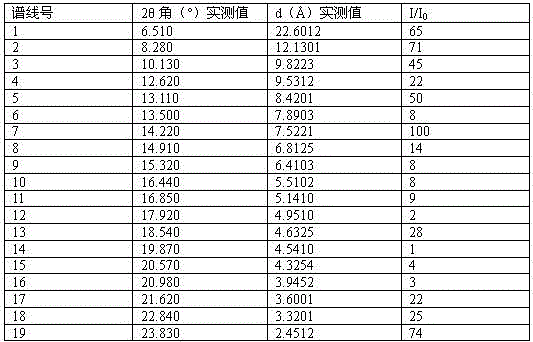
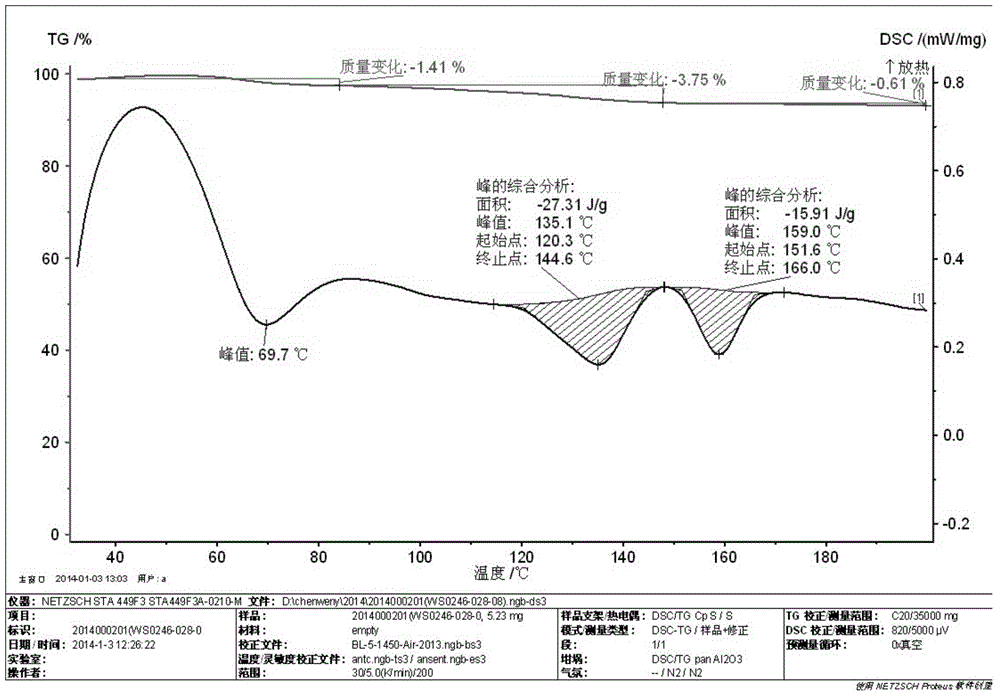
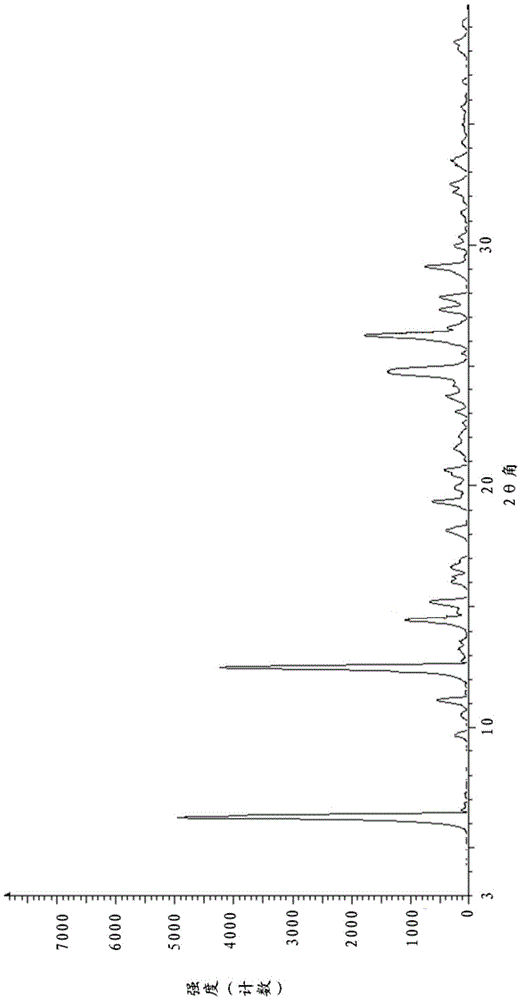
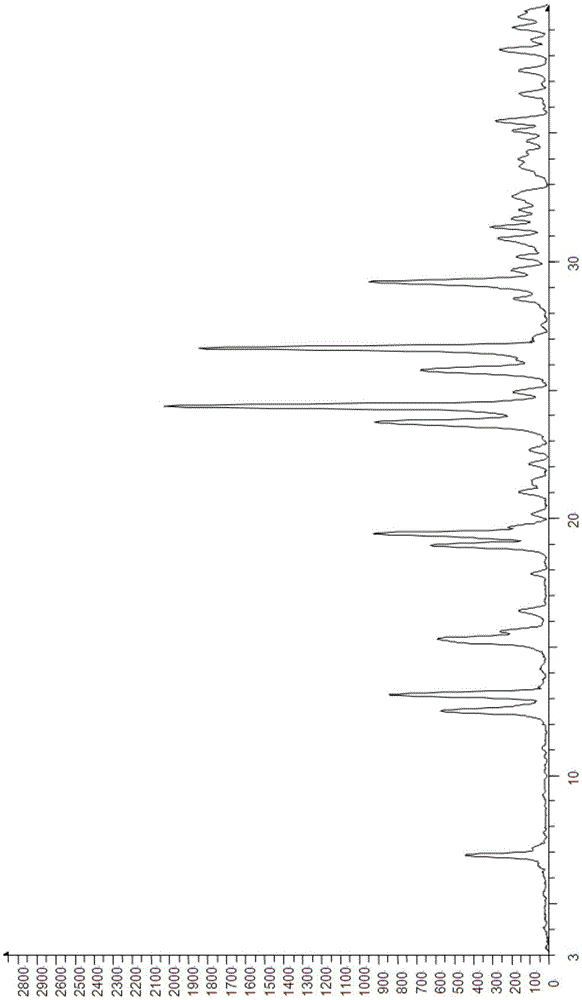
Crystalline form I of acotiamide hydrochloride hydrate, preparation method therefor and use thereof
InactiveCN105237493AImprove solubilityHigh yieldOrganic active ingredientsOrganic chemistryOrganic solventDissolution
The present invention relates to a crystalline form I of an acotiamide hydrochloride hydrate, a preparation method therefor and use thereof. The preparation method comprises: mixing acotiamide with an organic solvent so as to obtain a first mixture containing the acotiamide and the organic solvent; adding a salt-forming solvent into the first mixture to carry out a salt-forming reaction so as to obtain a second mixture containing an acotiamide hydrochloride; filtering the second mixture, and washing and drying a filter cake by an organic solvent so as to obtain a third mixture containing a crude product of the acotiamide hydrochloride; dissolving the third mixture in an aqueous organic solvent and performing slowly cooling crystallization after heating dissolution, so as to obtain a fourth mixture of an acotiamide hydrochloride hydrate crystal; and separating the crystal from the fourth mixture, and heating the separated crystal at 50-90 DEG C so as to obtain the acotiamide hydrochloride hydrate. The method is mild in reaction condition and simple in process and can be used for efficiently obtaining a highly pure acotiamide hydrochloride hydrate and a stable crystalline form thereof.
Owner:WATERSTONE PHARMA WUHAN
Acotiamide hydrochloride intermediate, and synthesis technique and application thereof
The invention provides an acotiamide hydrochloride intermediate, and a synthesis technique and application thereof, belonging to the technical field of drug synthesis. The synthesis technique comprises the following steps: (1) mixing 2,4,5-trimethoxy benzoic acid, ethyl 2-aminothiazolyl-4-formate, 1-hydroxybenzotriazole, an acid binder and an organic solvent; (2) adding diisopropyl carbodiimide into the mixture in the step (1); and (3) after the step (2) finishes, carrying out reaction while controlling the temperature of 25-50 DEG C, thereby obtaining the acotiamide hydrochloride intermediate. The synthesis technique avoids using sulfoxide chloride, improves the operating environment, has the advantages of short reaction time, fewer side reactions, simple purification treatment, high yield (the optimal yield can reach 68%) and high purity (98.8%), and is suitable for industrialized scale-up production.
Owner:SUZHOU SIXTH PHARMA PLANT OF JIANGSU WUZHONG PHARMA GROUP
Acotiamide hydrochloride controlled release tablet and preparation method thereof
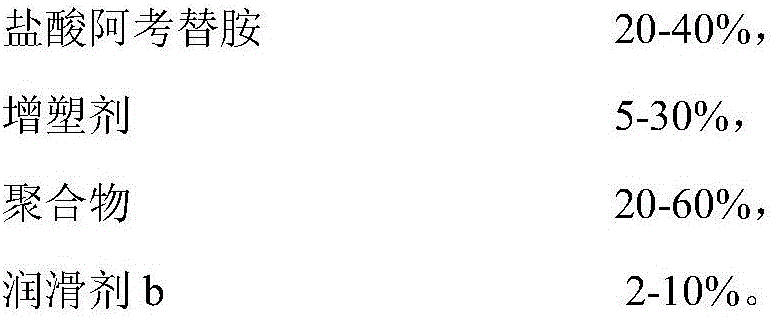
InactiveCN106511291AGood water solubilityFacilitated releaseOrganic active ingredientsDigestive systemSolventPharmacology
The invention relates to an acotiamide hydrochloride controlled release tablet and a preparation method thereof, and belongs to the field of medical preparations. The controlled release tablet comprises an acotiamide hydrochloride solid dispersion and pharmaceutical excipients, wherein the acotiamide hydrochloride solid dispersion comprises acotiamide hydrochloride, a polymer, a plasticizer and a lubricant b; and the pharmaceutical excipients include a controlled release material, a stabilizer, a disintegrating agent, an adhesive, a lubricant a and a coating material. The preparation method comprises the steps of firstly performing hot-melt extrusion on acotiamide hydrochloride to obtain the acotiamide hydrochloride solid dispersion; secondly adding the solid dispersion to the pharmaceutical excipients to prepare a mixture; thirdly, preparing the mixture into a tablet; and finally preparing the tablet into a coated tablet by using the coating material. According to the scheme, the early release degree of acotiamide hydrochloride is increased, the controlled release effect can last for 24 hours, and the tablet has very high stability; and in addition, the preparation method is simple in process and free of solvent residues, and other impurities are not introduced in the whole process, so that the preparation method is suitable for industrial production.
Owner:JINAN KANGHE MEDICAL TECH
Acotiamide hydrochloride dihydrate crystal, and preparation method and applications thereof
ActiveCN105481791AEasy to prepareShort preparation cycleOrganic active ingredientsOrganic chemistrySolubilityAcotiamide Hydrochloride
The invention belongs to the field of crystal drug, and especially relates to an acotiamide hydrochloride dihydrate crystal, and a preparation method and applications thereof. The chemical properties of the acotiamide hydrochloride dihydrate crystal are stable; no obvious hygroscopicity is detected in influence factor tests and acceleration tests, and crystal form is not changed; and the content of related substance is low. The solubility of the acotiamide hydrochloride dihydrate crystal is slightly higher, and the dissolution rate of prepared tablets is slightly higher. The preparation method of the acotiamide hydrochloride dihydrate crystal is simple; no complex equipment is needed; preparation period is short; and product yield is 83% or higher.
Owner:BEIJING COLLAB PHARMA
Oral tablet of acotiamide hydrochloride trihydrate and preparation method thereof
ActiveCN105769784AReduce volumeEasy to acceptOrganic active ingredientsDigestive systemPatient complianceAcotiamide Hydrochloride
The invention discloses o an oral tablet of acotiamide hydrochloride trihydrate and a preparation method thereof. The acotiamide hydrochloride trihydrate disclosed by the invention adopts a water insoluble filler as the first filler, and aims to still maintain the original particle shape when drug particles contact a dissolution medium and other solutions so as to prevent acotiamide hydrochloride from gathering, thus achieving a good dissolution effect. At the same time, through additional adding of a second filler and a disintegrating agent, the material is ensured with good compressibility and dissolution performance. According to the acotiamide hydrochloride tablet and the preparation method provided by the invention, the operation is simple, the dosage of auxiliary materials is significantly reduced, the tablet weight is small, and while the dissolution rate is guaranteed, the patient compliance is also improved.
Owner:REGENEX PHARMA LTD
6,7-dimethoxy-benzo[d][1,3]dioxene-2,4-dione and its preparation method
The invention discloses a compound of formula (1) and a preparation method thereof. The compound of the formula (1) is used for preparing the intermediate of acotiamide hydrochloride (Z-338).
Owner:CHANGZHOU NO 4 PHARMA FACTORY +2
Acotiamide hydrochloride pharmaceutical composition and preparation method thereof
InactiveCN106344517AImprove stabilityImprove solubilityPowder deliveryOrganic active ingredientsSolubilityPolyethylene glycol
The invention discloses a cotiamide hydrochloride pharmaceutical composition and a preparation method thereof, comprising dissolving acotiamide hydrochloride and polyethylene glycol in ethanol, adding the solution to pharmaceutically acceptable excipients lactose or One or several compositions of microcrystalline cellulose are dried under reduced pressure to make acotiamide hydrochloride pharmaceutical composition. The obtained acotiamide hydrochloride pharmaceutical composition can be mixed with other auxiliary materials, and then directly compressed to make tablets, or filled into gelatin capsules to make capsules, or packed into bags to make granules. The acotiamide hydrochloride pharmaceutical composition prepared by the invention can significantly improve the solubility of the drug, increase the dissolution rate of the drug, improve the bioavailability of the drug, has a simple preparation process, has no special requirements for equipment, and is suitable for large-scale production.
Owner:福建生物工程职业技术学院
Acotiamide hydrochloride membrane controlled slow release preparation and preparation method thereof
InactiveCN106491558AGuaranteed releaseImprove solubilityOrganic active ingredientsDigestive systemAcotiamide HydrochlorideBiochemistry
The invention provides an acotiamide hydrochloride membrane controlled slow release preparation and a preparation method thereof. A solid dispersion technology is used for preparing an acotiamide hydrochloride intermediate; then, a slow release material is used for coating to prepare the membrane controlled slow release preparation. The slow release preparation has a good slow release effect.
Owner:福建生物工程职业技术学院
Preparation method for acotiamide hydrochloride composition capsule
InactiveCN107536824AImprove stabilityImprove liquidityOrganic active ingredientsDigestive systemDissolutionAcotiamide Hydrochloride
The invention relates to the field of pharmaceutical preparations, and particularly discloses a preparation method for an acotiamide hydrochloride composition capsule. The preparation method includesthe steps of adding acotiamide hydrochloride, microcrystalline cellulose, starch, tyrosine, sodium alginate and glyceryl behenate into 5% of hydroxypropyl methylcellulose solution, conducting capsulation after wet granulation, and obtaining the acotiamide hydrochloride composition capsule. The preparation method is simple, and solves the technical problem that the acotiamide hydrochloride composition capsule obtained by the existing technology of wet granulation is easily-hygroscopic, not high in dissolution rate, poor in stability and the like; therefore, the prepared capsule is not easy to absorb moisture and agglomerate, and has high fluidity and a high dissolution rate, which is beneficial to the safe use and long-term storage of clinical drugs.
Owner:甘宜玲
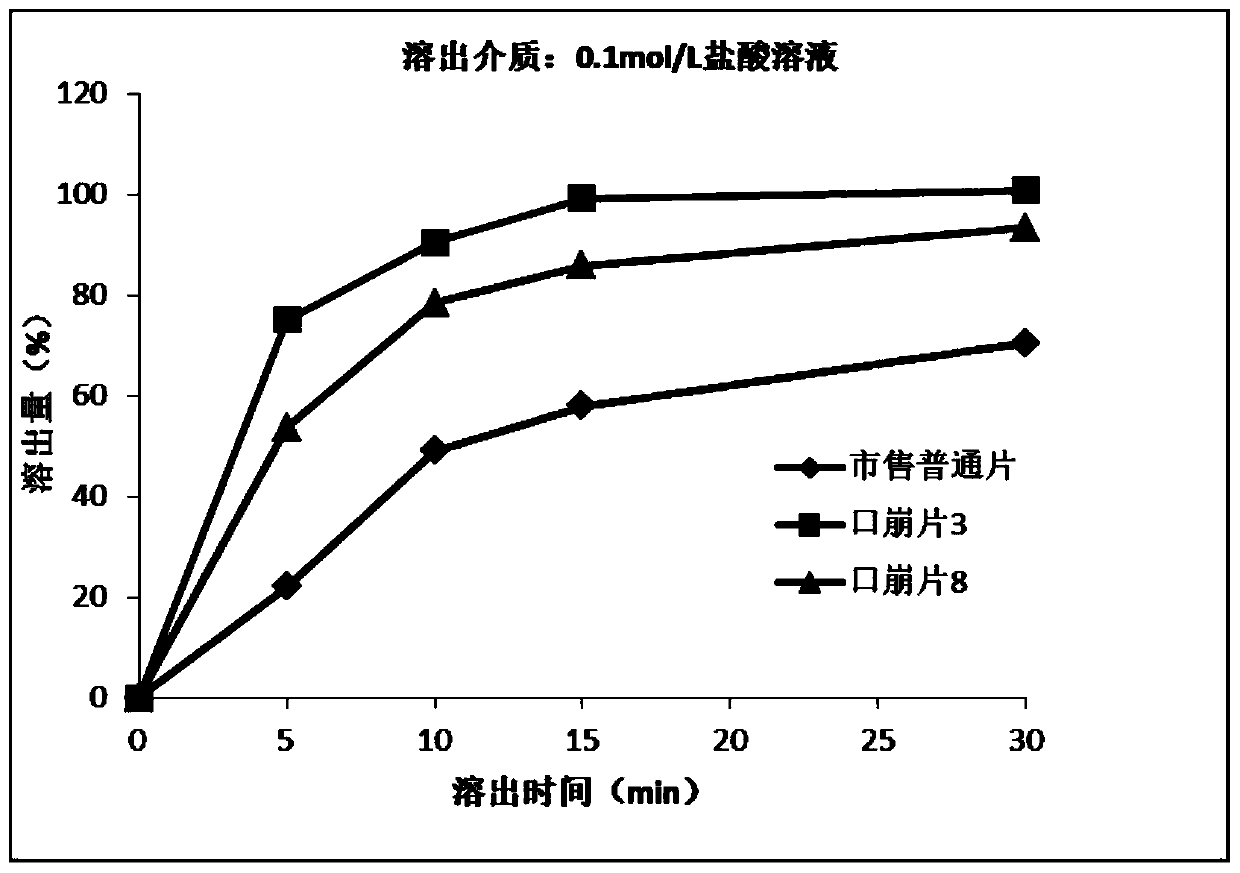
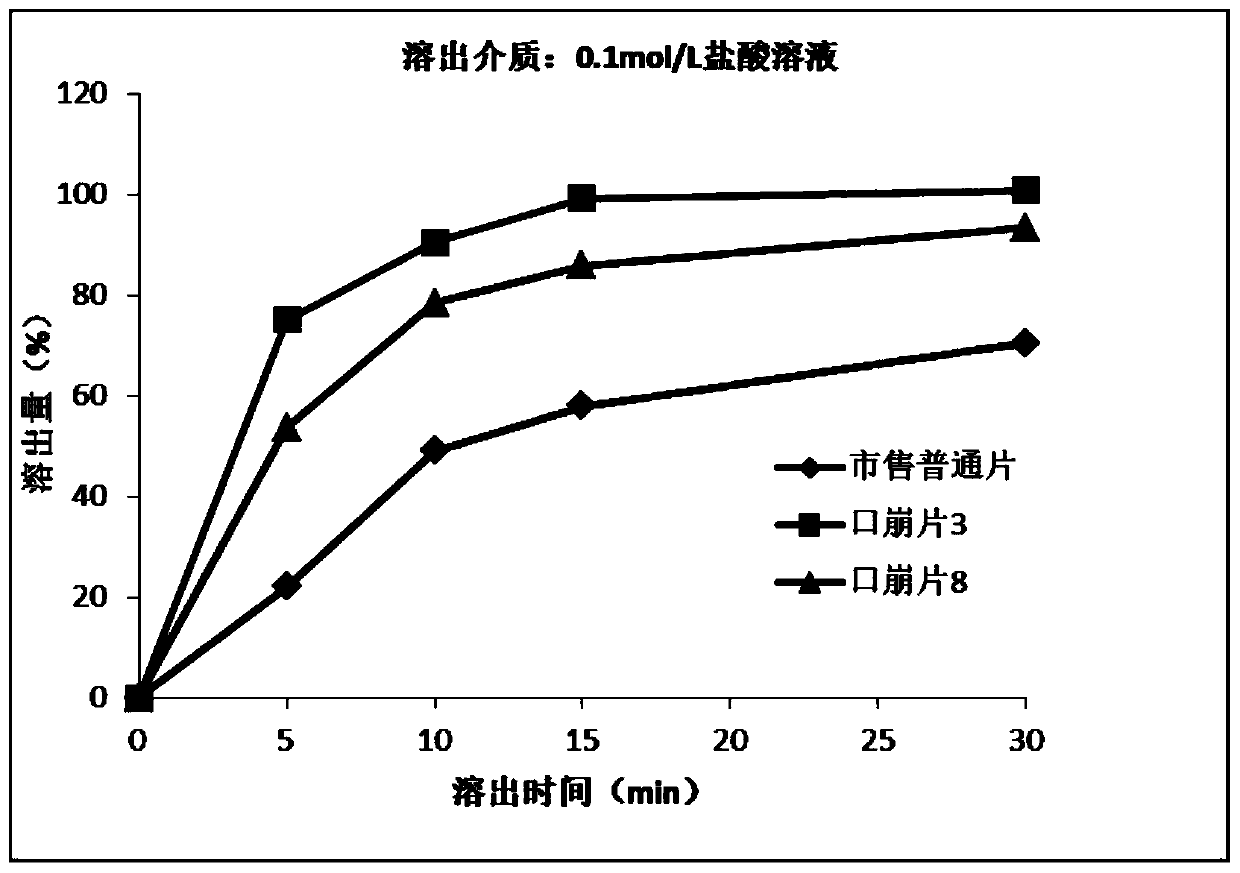
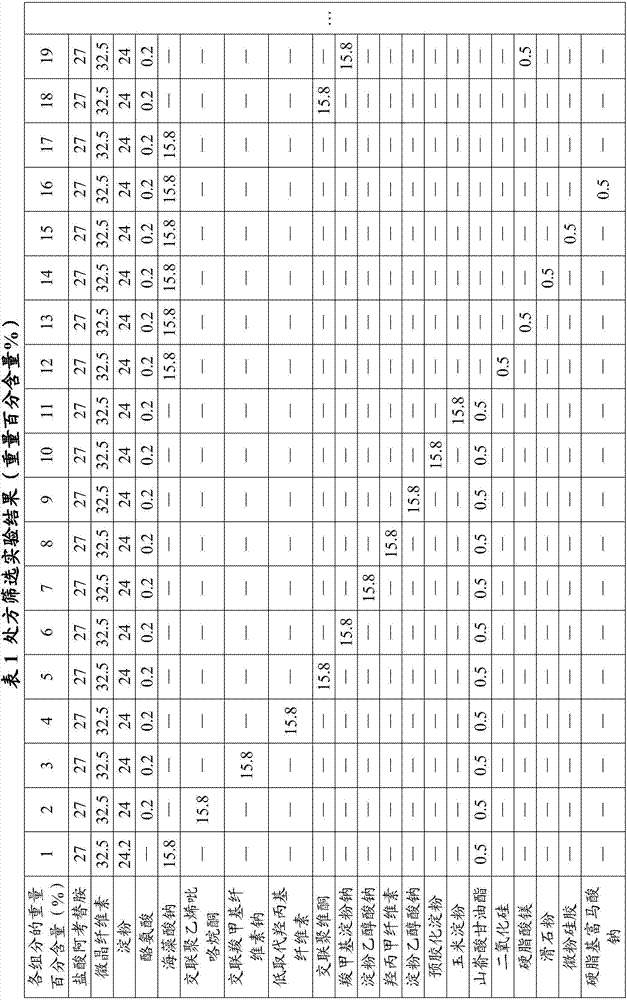
Oral disintegrating acotiamide hydrochloride tablet and preparation method thereof
ActiveCN109999003ADissolution rate is fastMask bitternessOrganic active ingredientsDigestive systemOlder peopleAcotiamide Hydrochloride
The invention discloses an oral disintegrating acotiamide hydrochloride tablet and a preparation method thereof. The oral disintegrating acotiamide hydrochloride tablet comprises, by weight, the following components: 40-90% of acotiamide hydrochloride bitter taste masking pellets, 4.5-49.9% of a filler, 4-10% of a disintegrating agent, 0.5-1.0% of a lubricant, 0-2.0% of a corrigent and 0-0.2% of essence, wherein the acotiamide hydrochloride bitter taste masking pellets are prepared from, by weight, 55-65% of acotiamide hydrochloride, 20-35% of blank pellet cores, 1-5% of a binder, 6-12% of bitter taste masking coating material and 0.7-1.2% of a pore-forming agent. The oral disintegrating acotiamide hydrochloride tablet can be rapidly disintegrated into fine particles in the oral cavity, does not need to be taken by water, is comfortable and cool in taste after being put into the mouth, does not have obvious gritty feeling, is pleasant in taste, is convenient for patients to swallow, isparticularly suitable for old people, children and people with dysphagia and can improve the medication compliance of patients and increase the populations using the tablet.
Owner:HUNAN WARRANT PHARMA +2
Acotiamide hydrochloride composite capsule
InactiveCN107496373AImprove liquidityHigh dissolution rateOrganic active ingredientsDigestive systemCelluloseTyrosine
The invention relates to the field of pharmaceutical preparations, and particularly discloses an acotiamide hydrochloride composite capsule. The acotiamide hydrochloride composite capsule is prepared from acotiamide hydrochloride, microcrystal cellulose, starch, tyrosine, sodium alginate and glyceryl behenate. The acotiamide hydrochloride composite capsule preferably selects the acotiamide hydrochloride, microcrystal cellulose, starch, tyrosine, sodium alginate and glyceryl behenate as the composition of the acotiamide hydrochloride, and the synergistic effect can improve the stability, mobility and dissolution rate of the acotiamide hydrochloride, can reduce the moisture absorption performance and can facilitate the safe application and long-term storage of clinical drugs.
Owner:甘宜玲
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


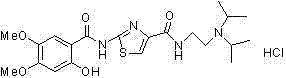



![6,7-dimethoxy-benzo[d][1,3]dioxin-2,4-dione and preparation method thereof 6,7-dimethoxy-benzo[d][1,3]dioxin-2,4-dione and preparation method thereof](https://images-eureka.patsnap.com/patent_img/ab860e37-6654-4118-9e2c-f05796b76a75/HDA00001625226900011.PNG)
![6,7-dimethoxy-benzo[d][1,3]dioxin-2,4-dione and preparation method thereof 6,7-dimethoxy-benzo[d][1,3]dioxin-2,4-dione and preparation method thereof](https://images-eureka.patsnap.com/patent_img/ab860e37-6654-4118-9e2c-f05796b76a75/BDA00001625226800021.PNG)
![6,7-dimethoxy-benzo[d][1,3]dioxin-2,4-dione and preparation method thereof 6,7-dimethoxy-benzo[d][1,3]dioxin-2,4-dione and preparation method thereof](https://images-eureka.patsnap.com/patent_img/ab860e37-6654-4118-9e2c-f05796b76a75/BDA00001625226800022.PNG)

![6,7-dimethoxy-benzo[d][1,3]dioxene-2,4-dione and its preparation method 6,7-dimethoxy-benzo[d][1,3]dioxene-2,4-dione and its preparation method](https://images-eureka.patsnap.com/patent_img/0ca425b7-b333-431d-8faf-d6b3897e147d/HDA00001625226900011.PNG)
![6,7-dimethoxy-benzo[d][1,3]dioxene-2,4-dione and its preparation method 6,7-dimethoxy-benzo[d][1,3]dioxene-2,4-dione and its preparation method](https://images-eureka.patsnap.com/patent_img/0ca425b7-b333-431d-8faf-d6b3897e147d/BDA00001625226800021.PNG)
![6,7-dimethoxy-benzo[d][1,3]dioxene-2,4-dione and its preparation method 6,7-dimethoxy-benzo[d][1,3]dioxene-2,4-dione and its preparation method](https://images-eureka.patsnap.com/patent_img/0ca425b7-b333-431d-8faf-d6b3897e147d/BDA00001625226800022.PNG)