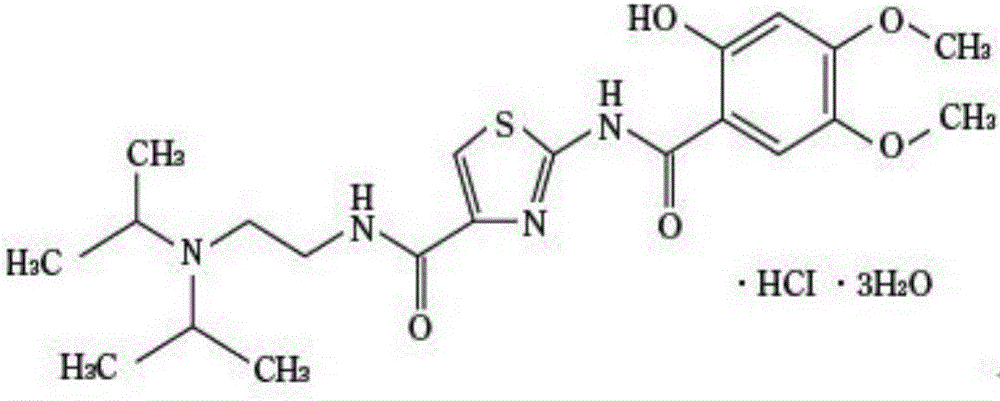
Acotiamide hydrochloride controlled release tablet and preparation method thereof
A technology of acotiamide hydrochloride and sustained-release tablets, applied in the field of medicine, can solve the problems of low release rate and poor stability in the early stage, and achieve the effects of improving the release rate, high product stability, and no solvent residue.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: Composition and preparation method of acotiamide hydrochloride sustained-release tablet
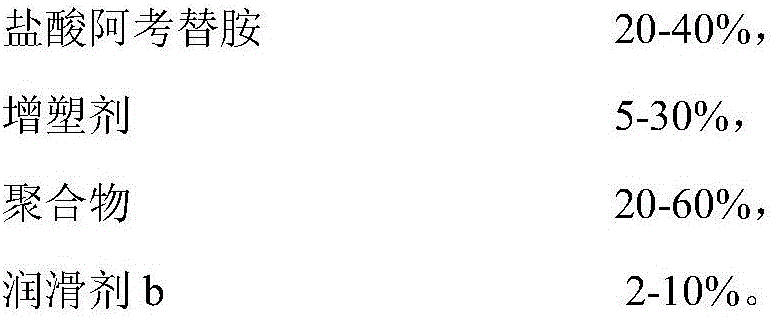
[0052] A, the composition of acotiamide hydrochloride sustained-release tablet
[0053]
[0054]
[0055] B, wherein each composition and corresponding weight percent in the acotiamide hydrochloride solid dispersion are as follows:
[0056]
[0057] C. Preparation method
[0058] (1) Acotiamide hydrochloride, xylitol, sorbitol, hypromellose, povidone K30, and poloxamer are pulverized respectively, and after crossing an 80 mesh sieve, they are mixed uniformly by weight percentage in B to prepare into a physical mixture;
[0059] (2) Set the extrusion temperature of the twin-screw hot-melt extruder to 80°C, start the screw when the preset temperature is reached, add the physical mixture in step (1) into the extruder, and extrude through the screw strips;
[0060] (3) Pulverizing the strip in step (2), crossing a 40-mesh sieve to obtain a solid dispersion wi...
Embodiment 2
[0064] Embodiment 2: Composition and preparation method of acotiamide hydrochloride sustained-release tablet
[0065] A, the composition of acotiamide hydrochloride sustained-release tablet
[0066]
[0067]
[0068] B, wherein each composition and corresponding weight percent in the acotiamide hydrochloride solid dispersion are as follows:
[0069]
[0070] C, preparation method: same as embodiment 1.
Embodiment 3
[0071] Embodiment 3: Composition and preparation method of acotiamide hydrochloride sustained-release tablet
[0072] A, the composition of acotiamide hydrochloride sustained-release tablet
[0073]
[0074] B, wherein each composition and corresponding weight percent in the acotiamide hydrochloride solid dispersion are as follows:
[0075]
[0076] C. Preparation method
[0077] (1) Acotiamide hydrochloride, poloxamer, crospovidone, povidone K30, and polyethylene glycol are pulverized respectively, and after passing through a 60-mesh sieve, they are uniformly mixed according to the weight percentage in B to prepare physical mixture;
[0078] (2) Set the extrusion temperature of the twin-screw hot-melt extruder to 130°C, start the screw when the preset temperature is reached, add the physical mixture in step (1) into the extruder, and extrude through the screw strips;
[0079] (3) Pulverizing the strip in step (2), crossing a 24-mesh sieve to obtain a solid dispersio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com