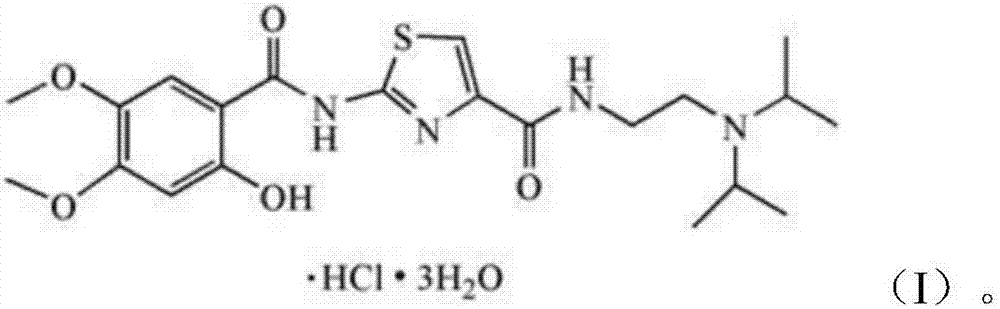
Acotiamide hydrochloride composite capsule
A technology of acotiamide hydrochloride and a composition, applied in the field of pharmaceutical preparations, can solve the problems of poor fluidity of acotiamide hydrochloride capsules, no further prompts, obvious differences in filling amount and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
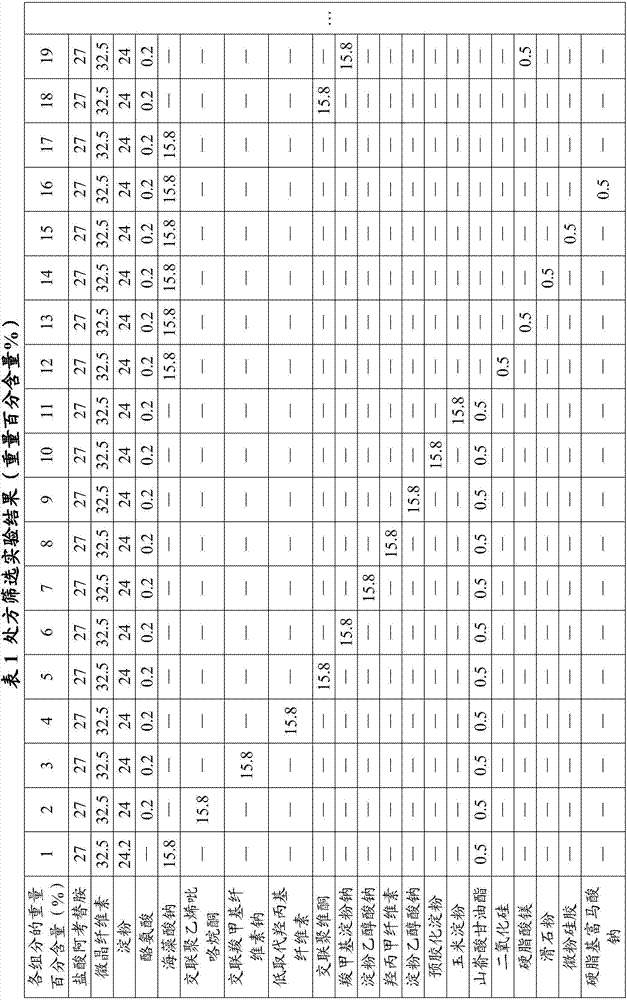
[0051] Experimental Example 1: Prescription Screening Experiment
[0052]
[0053]
[0054] Since acotiamide hydrochloride is easy to absorb moisture and is unstable to heat and humidity, the dry granulation method is preferred to prevent the influence of the temperature of the water agent on the raw and auxiliary materials. The specific preparation method is as follows: crush the raw and auxiliary materials separately, pass through a 80-mesh sieve, and mix evenly Send it to a dry granulator for granulation, 18-mesh granulation, and capsule filling.
[0055] There are too many screening experimental data, and only some important experimental data are listed here. Due to the poor fluidity of acotiamide hydrochloride and starch, the loading capacity is poorly controlled, which brings inconvenience to production. The inventor found through a large number of experimental screenings that sodium alginate was used as a disintegrating agent, and glyceryl behenate was added as a...
experiment example 2
[0056] Experimental Example 2: Screening experiment of dosage of glyceryl behenate and sodium alginate
[0057] This experimental example is used as a dosage screening experiment of glyceryl behenate and sodium alginate when preparing acotiamide hydrochloride capsules to control the weight percentage of each raw material: acotiamide hydrochloride 25%-30%, micro 30%-35% of crystalline cellulose, 22%-26% of starch, and 0.1%-0.3% of tyrosine, on the basis of which the weight percent content of glyceryl behenate and sodium alginate is adjusted.
[0058] The amount of disintegrating agent sodium alginate has a great influence on the disintegration time and dissolution rate, and if the amount is too small, it cannot meet the requirements of disintegration and dissolution. Sodium alginate aqueous solution is a viscous disintegrating agent, the larger the dosage, the slower the disintegration and dissolution rate. Therefore, the inventors selected 14%-18% by weight after a large numb...
experiment example 3
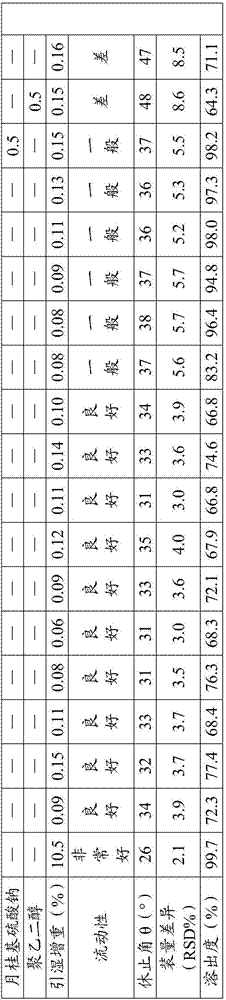
[0064] Experimental Example 3: Performance Testing
[0065] According to the prescription provided by the present invention and the comparison of the performance of the acotiamide hydrochloride capsules prepared by the preparation method and the performance of the prior art acotiamide hydrochloride capsules are shown in Table 3:
[0066] Table 3 performance test results
[0067]
[0068] As can be seen from the above table, the acotiamide hydrochloride capsules of the present invention have high dissolution rate, good fluidity, low loading difference, significantly reduced hygroscopicity, and its performance is significantly better than that of the existing preparation acotiamide hydrochloride.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Outer diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com